Effect of Different Peat Substitute Substrates on the Growth and Quality of Seedlings of Handroanthus chrysanthus (Jacq.) S.O. Grose
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Determination of Physical Properties of Matrix
2.3. Determination of Growth Characters and Biomass
2.4. Determination of Root Parameters
2.5. Determination of Photosynthetic Parameters and Relative Chlorophyll Content
2.6. Optimization of Substrate Formulation
2.7. Data Processing and Statistical Analysis
3. Results
3.1. Analysis of Variance of Physical Properties of Mixed Substrates
3.2. Effects of Different Substrates on Growth Characteristics and Chlorophyll Content of H. chrysanthus
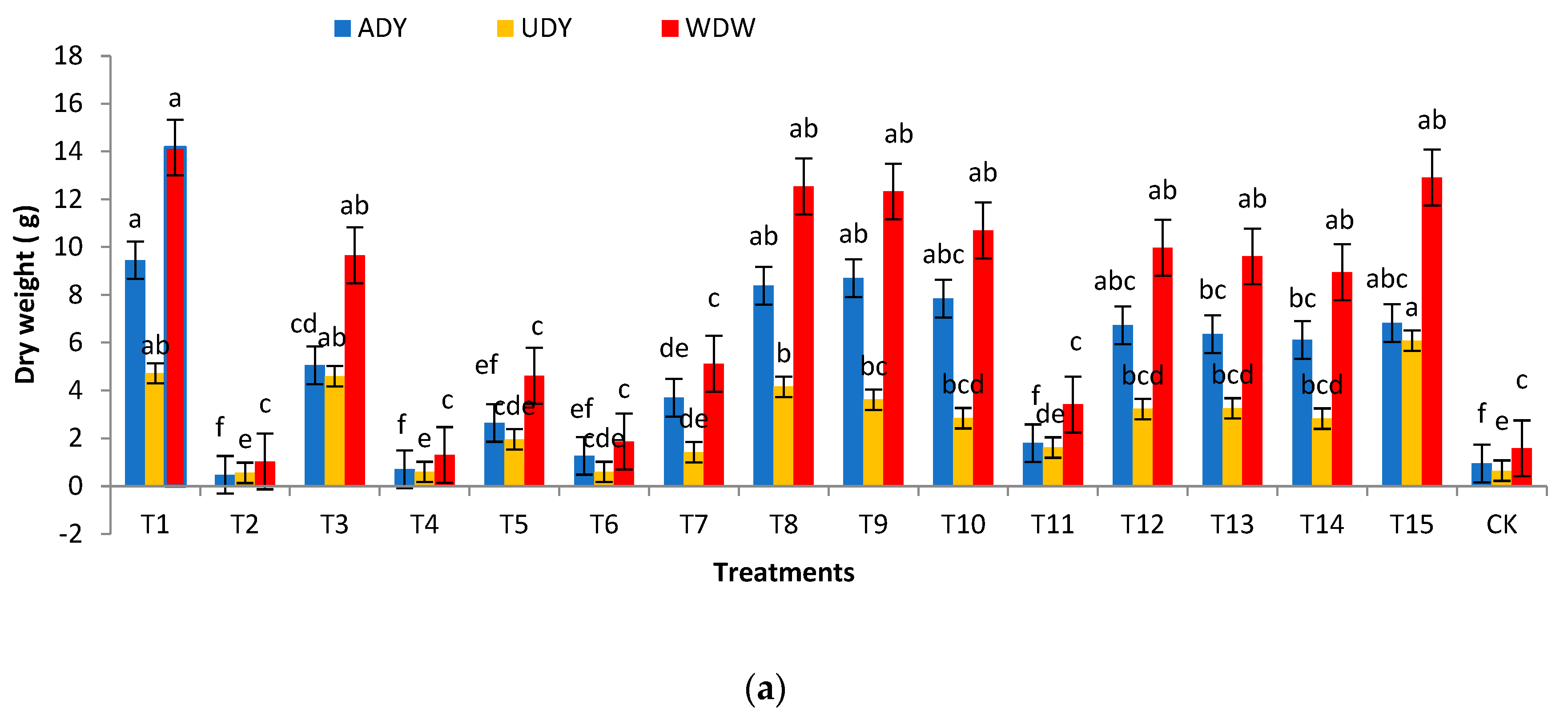
3.3. Effects of Different Substrates on the Biomass of H. chrysanthus
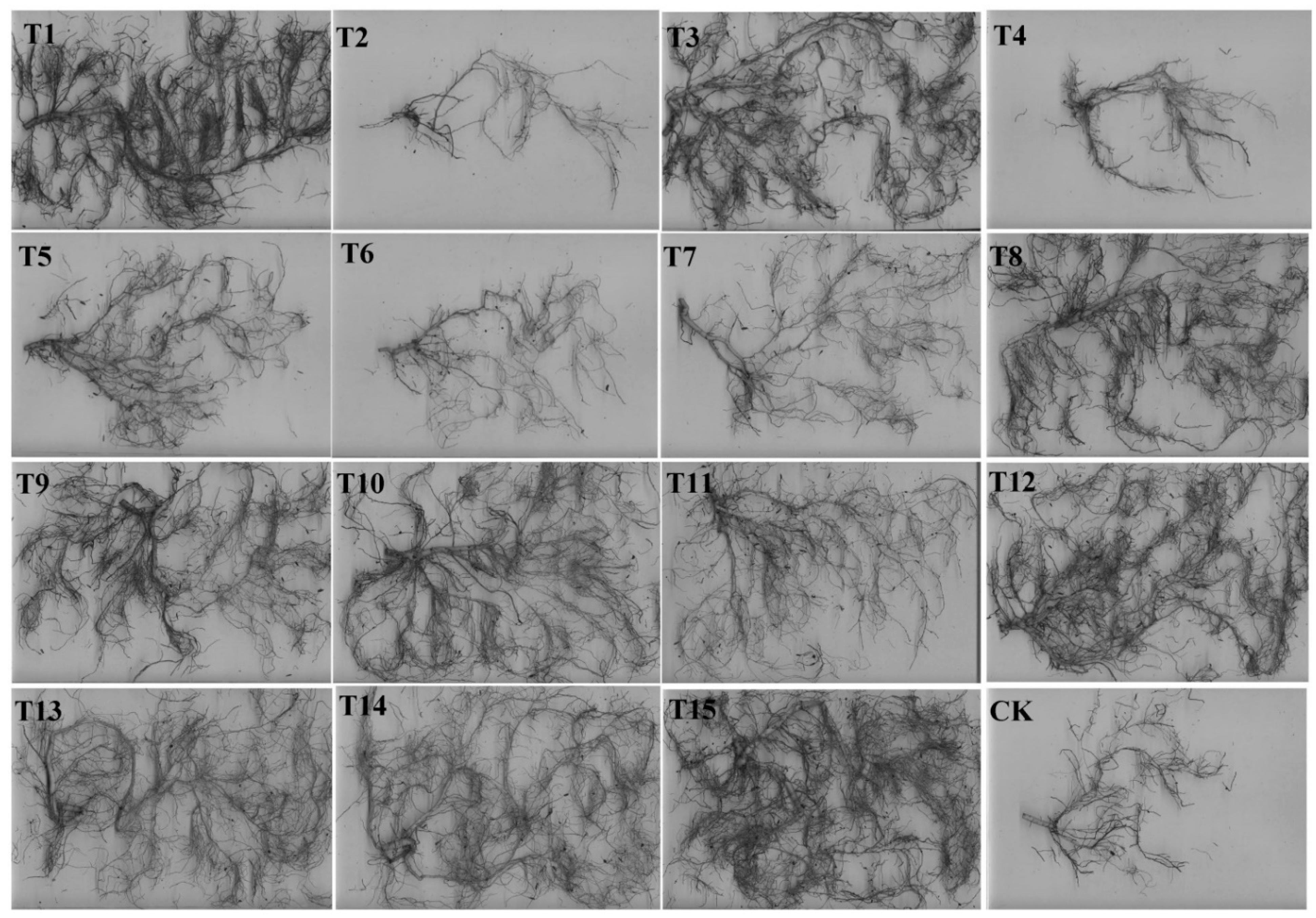
3.4. Effects of Different Substrates on the Root Indices of H. chrysanthus
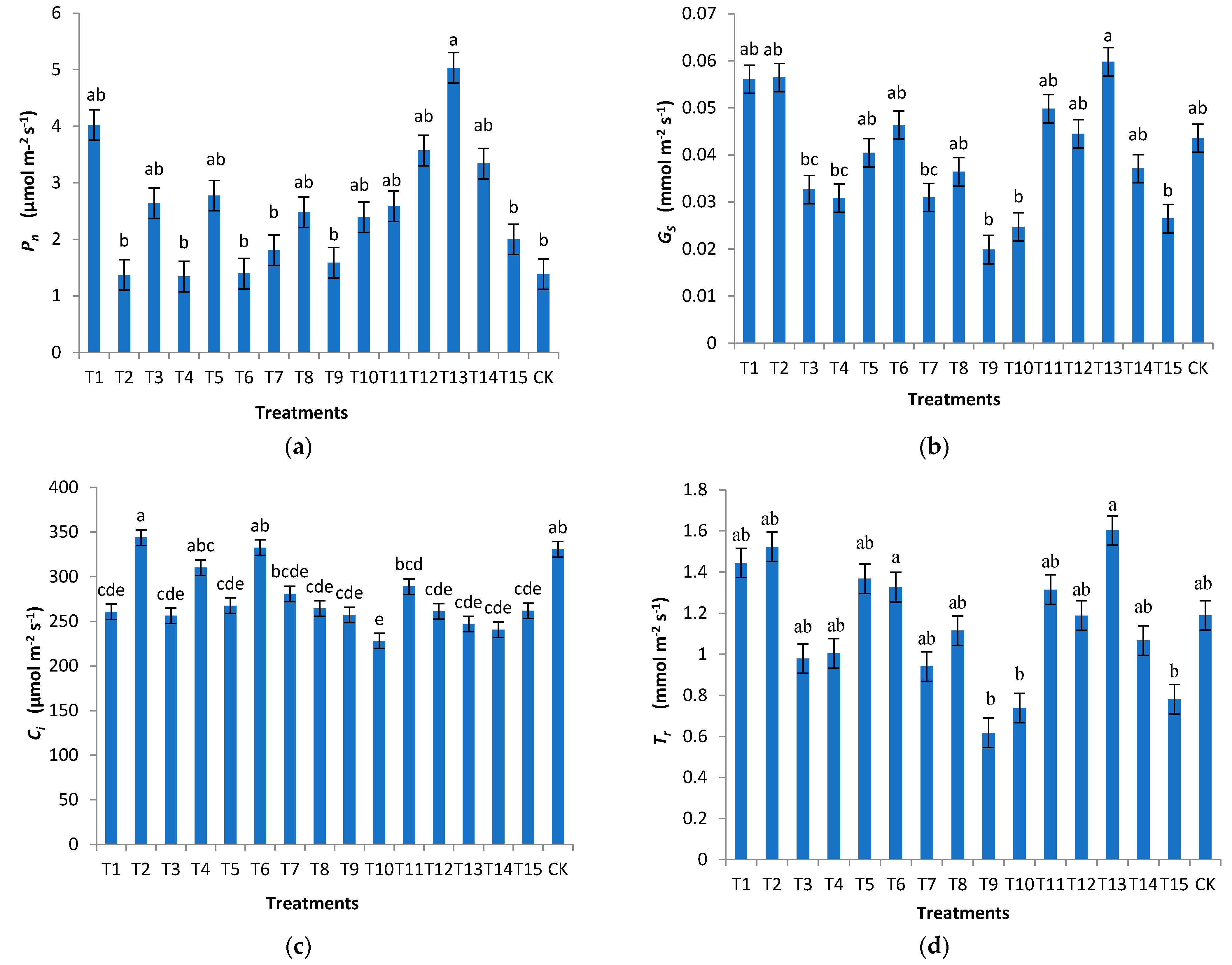
3.5. Effects of Different Substrates on the Photosynthesis of H. chrysanthus
3.6. Comprehensive Evaluation of Different Substrates on the Seedlings of H. chrysanthus
3.7. Optimization and Analysis of Substrate Ratio
R2 = 0.9360,
4. Discussion
4.1. Effects of Different Substrates on the Growth of H. chrysanthus
4.2. Effects of Different Substrates on the Biomass and Root of H. chrysanthus
4.3. Effects of Different Substrates on the Photosynthesis of H. chrysanthus
4.4. Effects of Different Substrates on the Comprehensive Evaluation and Matrix Formula Optimization for H. chrysanthus
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alwyn, H. Bignoniaceae: Part II (Tribe Tecomeae). Flora Neotrop. Monogr. 1992, 25, 1–373. [Google Scholar] [CrossRef]
- Grose, S.O.; Olmstead, R.G. Evolution of a charismatic neotropical clade: Molecular phylogeny of Tabebuia S.L. Crescentieae, and allied genera (Bignoniaceae). Syst. Bot. 2007, 32, 650–659. [Google Scholar] [CrossRef]
- Grose, S.O.; Olmstead, R.G. Taxonomic revisions in the polyphyletic genus Tabebuia S. I. (Bignoniaceae). Syst. Bot. 2009, 32, 660–670. [Google Scholar] [CrossRef]
- George, W.; Derra, L.R. A Tropical Garden Flora; Bishop Museum Press: Honolulu, HI, USA, 2005. [Google Scholar]
- Wang, X. Botanical Garden tree species of Tabebuia chrysanthus. Guangdong For. Sci. Technol. 2007, 23, 115–116. [Google Scholar] [CrossRef]
- Yuan, L.L.; Wang, S.P.; Liu, N.; Zhang, Q.M.; Ren, H.; Lei, Z.X.; Pan, G.B.; Luo, J. The ecological and biological characteristics and sowing propagation techniques of Tabebuia chrysanthus. Ecol. Sci. 2014, 33, 984–990. [Google Scholar] [CrossRef]
- Joselin, J.; Brintha, T.S.S.; Florence, A.R.; Jeeva, S. Phytochemical evaluation of Bignoniaceae flowers. J. Chem. Pharm. Res. 2013, 5, 106–111. [Google Scholar]
- Cai, D.C.; Fang, L.; Shang, X.H.; Zhang, P.J. Research status and development prospects of Tabebuia. Eucalyptus Sci. Technol. 2016, 33, 46–50. [Google Scholar] [CrossRef]
- Pinto, J.R.; Marshall, J.D.; Dumroese, R.k.; Davis, A.S.; Cobos, D.R. Establishment and growth of container seedlings for reforestation: A function of stocktype and edaphic conditions. Forest Ecol. Manag. 2011, 261, 1876–1884. [Google Scholar] [CrossRef]
- South, D.B.; Harris, S.W.; Barnett, J.P.; Hainds, M.J.; Gjerstad, D.H. Effect of container type and seedling size on survival and early height growth of Pinus palustris seedlings in Alabama, U.S.A. Forest Ecol. Manag. 2005, 204, 385–398. [Google Scholar] [CrossRef]
- Caron, J.; Nkongolo, V.K.N. Aeration in growing media: Recent developments. Acta Hortic. 1999, 481, 545–552. [Google Scholar] [CrossRef]
- Fonteno, W.C. Problems & considerations in determining physical properties of horticultural substrates. Acta Hortic. 1993, 342, 197–204. [Google Scholar] [CrossRef]
- Raviv, M.; Wallach, R.; Silber, A.; Bar-Tal, A. Substrates and their analysis. In Hydroponic Production of Vegetables and Ornamentals; Savvas, D., Passam, H., Eds.; Embryo Publications: Athens, Greece, 2002; pp. 25–102. [Google Scholar]
- Bragg, N. A Review of Peat Reserves and Peat Usage in Horticulture and Alternative Materials–Report for the Horticultural Development Company (HDC); HDC: Petersfield, UK, 1990. [Google Scholar]
- Yamani, A.; Achmad, B. Peat soil as an alternative soil substrate and its effect on balangeran (Shorea belangeran) seedling growth. Int. J. Biosci. 2019, 14, 188–196. [Google Scholar] [CrossRef]
- Schmilewski, G. The Role of Peat in Assuring the Quality of Growing Media. Mires Peat 2008, 3, 2. Available online: http://www.mires-and-peat.net/pages/volumes/map03/map0302.php (accessed on 3 August 2022).
- Huang, B.H. Substrate optimization for plug seedlings of Tabebuia chrysanthus. J. Minnan Normal Univ. (Nat. Sci.) 2016, 94, 29–32. [Google Scholar]
- Alexander, P.D.; Bragg, N.C.; Meade, R.; Padelopoulos, G.; Watts, O. Peat in horticulture and conservation: The UK response to a changing world. Mires Peat 2008, 3, 8. Available online: http://www.mires-and-peat.net/pages/volumes/map03/map0308.php (accessed on 3 August 2022).
- Cleary, J.; Roulet, N.T.; Moore, T.R. Greenhouse gas emissions from Canadian peat extraction, 1990–2000: A life cycle analysis. Ambio 2005, 34, 456–461. [Google Scholar] [CrossRef]
- Silber, A.; Bar-Tal, A. Nutrition of substrate-grown plants. In Soilless Culture: Theory and Practice; Raviv, M., Lieth, J.H., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 291–339. [Google Scholar] [CrossRef]
- Alexander, P.D.; Bragg, N.C. Defining sustainable growing media for sustainable UK horticulture. Acta Hortic. 2014, 1034, 219–224. [Google Scholar] [CrossRef]
- Wood, B.W. Pecan production in North America. Southwest. Entomol. 2003, 51, 1–19. [Google Scholar]
- Chen, L.H.; Yang, X.M.; Raza, W.; Luo, J.; Zhang, F.G.; Shen, Q.R. Solid-state fermentation of agro-industrial wastes to produce bioorganic fertilizer for the biocontrol of Fusarium wilt of cucumber in continuously cropped soil. Bioresour. Technol. 2011, 102, 3900–3910. [Google Scholar] [CrossRef]
- Medina, E.; Paredes, C.; Pérez-Murcia, M.D.; Bustamante, M.A.; Moral, R. Spent mushroom substrates as component of growing media for germination and growth of horticultural plants. Bioresour. Technol. 2009, 100, 4227–4232. [Google Scholar] [CrossRef]
- Ostos, J.C.; López-Garrido, R.; Murillo, J.M.; López, R. Substitution of peat for municipal solid waste- and sewage sludge-based composts in nursery growing media: Effects on growth and nutrition of the native shrub Pistacia lentiscus L. Bioresour. Technol. 2008, 99, 1793–1800. [Google Scholar] [CrossRef]
- Teng, F.; Liu, Y.; Hu, J.W.; Sun, Q.Y.; Wan, F.F.; Yang, C.; Zhang, J. Effect of different mushroom slag compost on growth and nutrient accumulation of Pinus tabulaeformis containerized transplantings. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2016, 40, 184–190. [Google Scholar] [CrossRef]
- He, S.M.; Zhou, P.H.; Xin, G.M.; Zhao, Q.Z.; Yang, H.; Fu, M.J. Study on the selection of tomato seedling medium based on fungus residue. Agric. Sci. J. Yanbian Univ. 2020, 42, 43–50. [Google Scholar] [CrossRef]
- Shang, X.H.; Xie, Y.J.; Peng, Y.; Zhang, P.J. The effect of matured compost eucalyptus-bark media on Eucalyptus seedlings. J. Cent. South Univ. For. Technol. 2011, 31, 33–38. [Google Scholar] [CrossRef]
- Meerow, A.W. Growth of two subtropical ornamentals using coir (coconut mesocarp pith) as a peat substitute. HortScience 1994, 29, 1484–1486. [Google Scholar] [CrossRef]
- Meerow, A.W. Growth of two tropical foliage plants using coir dust as a container medium amendment. Hortic. Technol. 1995, 5, 237–239. [Google Scholar] [CrossRef]
- Zhang, M.W. Study on the effect of new cocoon matrix and peat substrate on pakchoi cultivation. Mod. Agric. Sci. Technol. 2019, 1, 73. [Google Scholar] [CrossRef]
- Nicolas, J.S. Biomass utilization of rice wastes and by-products: A rapid rural appraisal in selected Philippine farming villages. Energy Sustain. Dev. 1994, 1, 30–31. [Google Scholar] [CrossRef]
- Petrovic, A.; Cvetkoviae, N.; Trajkovic, S.; Ibric, S.; Popadic, D.; Djuric, Z. Mixture design evaluation of drug release from matrix tablets containing carbomer and HPMC. J. Control Release 2006, 16, e104–e106. [Google Scholar] [CrossRef]
- Lian, Z.H. Principles and Techniques of Soilless Culture; China Agriculture Press: Beijing, China, 1994. [Google Scholar]
- Hou, J.W.; Dong, L.H. Evaluation of growing medium composition on marigold (Tagetes erecta L.) seedling quality. Acta Hort. 2011, 891, 237–240. [Google Scholar] [CrossRef]
- Wang, C.N.; Du, C.; Yang, Z.M.; Wang, H.L.; Shang, L.J.; Liu, L.L.; Yang, Z.Y.; Song, S.Y.; Amanullah, S. Study on the cultivation of seedlings using buds of potato (Solanum tuberosum L.). Peer J. 2022, 10, e13804. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.R. Soilless Culture, 2nd ed.; China Agriculture Press: Beijing, China, 2011. [Google Scholar]
- Barretta, G.E.; Alexandera, P.D.; Robinsonb, J.S.; Bragg, N.C. Achieving environmentally sustainable growing media for soilless plant cultivation systems—A review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef]
- Landis, T.D.; Tinus, R.W.; Mcdonald, S.E. Containers and growing media//LANDIS T D. In The Container Tree Nursery Manual; Department of Agriculture: Washington, DC, USA, 1990. [Google Scholar]
- Liu, Y. Principles and Methodology for Seedling Quality Modification and Control; China Forestry Press: Beijing, China, 1999. [Google Scholar]
- Liu, L.M. Brief analysis of container seedling medium. J. South China Agric. Univ. 2015, 24, 68–69. [Google Scholar] [CrossRef]
- Guo, S.R. Research Progress, Current Exploitations and Developing Trends of Solid Cultivation Medium. Trans. CSAE 2005, 12, 1–4, (In Chinese with English Abstract). Available online: https://www.doc88.com/p-1955427437060.html (accessed on 10 August 2022).
- Guo, J. Studies on the Differences in Biological Characters of Seeds, Seedlings and Salt-Tolerance of Melia azedarach L. from Different Provenances. Master’s Thesis, Nanjing Forestry University, Nanjing, China, 2008. [Google Scholar]
- Sun, C.X.; Feng, M.L.; Liu, L.Y.; Chen, H.; Chen, W.J. Effects of different coir culture media on growth and physiological characteristics of watermelon seedlings. Chin. J. Trop. Agri. 2011, 31, 6–11. [Google Scholar]
- Liu, W.J.; Li, Z.S.; Xin, F.M. Effects of different substrates on growth and root morphology of Sophora moorcroftiana seedlings. Southwest China J. Agric. Sci. 2021, 34, 1668–1674. [Google Scholar] [CrossRef]
- Bonifas, K.D.; Lindquist, J.L. Predicting biomass partitioning to root versus shoot in corn and velvetleaf (Abutilon theophrasti). Weed Sci. 2006, 54, 133–137. [Google Scholar] [CrossRef]
- Wang, S.S.; Cai, J.; Liu, J.P.; Wang, D.W.; Fan, X. Effect of soil substrate and shade on the seedling components and biomass allocation of Zoysia japonica in the winter. Acta Agrestia Sin. 2016, 24, 1296–1303. [Google Scholar] [CrossRef]
- Rebecca, M. Relative importance of photosynthetic physiology and biomass allocation for tree seedling growth across a broad light gradient. Tree Physiol. 2004, 24, 155–167. [Google Scholar] [CrossRef]
- Tilman, D. The resource-ratio hypothesis of plant succession. Am. Nat. 1985, 125, 827–852. [Google Scholar] [CrossRef]
- Cahill, J.F.; Casper, B.B. Investigating the relationship between neighbor root biomass and belowground competition: Field evidence for symmetric competition belowground. Oikos 2000, 90, 311–320. [Google Scholar] [CrossRef]
- Lin, X.; Zheng, J.; Liu, H.J.; Qian, R.J.; Wang, J.W. Effects of different media on growth and leaf physiological characteristics of Ficus concinna var. subsessilis container seedlings. Sci. Silvae Sin. 2010, 46, 62–70. [Google Scholar] [CrossRef]
- Sheehy, J.E.; Hardy, B.; Mitchell, P.L. Redesigning rice photosynthesis to increase yield. Crop Sci. 2002, 42, 2227–2228. [Google Scholar] [CrossRef]
- Cornic, G.; Briantais, J.M. Partitioning of photosynthetic electron flow between CO2 and O2 reduction in a C3 leaf (Phaseolus vulgaris L.) at different CO2 concentrations and during drought stress. Planta 1991, 183, 178–184. [Google Scholar] [CrossRef]
- Filella, I.; Penuelas, J. The red edge position and shape as indicators of plant chlorophyll content, biomass and hydric status. Int. J. Remote Sens. 1994, 15, 1459–1470. [Google Scholar] [CrossRef]



| Treatment | Composted Eucalyptus Bark | Coconut Bran | Carbonized Rice Husk | Perlite | Treatment | Composted Eucalyptus Bark | Coconut Bran | Carbonized Rice Husk | Perlite |
|---|---|---|---|---|---|---|---|---|---|
| T1 | 1 | 0 | 0 | 0 | T9 | 0.5 | 0 | 0.5 | 0 |
| T2 | 0 | 1 | 0 | 0 | T10 | 0.5 | 0.5 | 0 | 0 |
| T3 | 0 | 0 | 1 | 0 | T11 | 0 | 0.33 | 0.33 | 0.33 |
| T4 | 0 | 0 | 0 | 1 | T12 | 0.33 | 0 | 0.33 | 0.33 |
| T5 | 0 | 0 | 0.5 | 0.5 | T13 | 0.33 | 0.33 | 0 | 0.33 |
| T6 | 0 | 0.5 | 0 | 0.5 | T14 | 0.33 | 0.33 | 0.33 | 0 |
| T7 | 0 | 0.5 | 0.5 | 0 | T15 | 0.25 | 0.25 | 0.25 | 0.25 |
| T8 | 0.5 | 0 | 0 | 0.5 | CK | ||||
| Trait | Mean | SD | Min | Max | Skewness | Kurtosis | p |
|---|---|---|---|---|---|---|---|
| Bulk density | 0.18 | 0.12 | 0.08 | 0.57 | 1.95 | 4.13 | <0.000 |
| Total porosity | 68.97 | 6.35 | 56.10 | 82.00 | −0.01 | −0.51 | <0.000 |
| Aeration porosity | 37.97 | 12.16 | 8.30 | 61.25 | 0.00 | −0.38 | <0.000 |
| Water-holding porosity | 30.99 | 9.94 | 12.70 | 58.30 | 0.45 | 0.36 | <0.000 |
| Maximum water-holding capacity | 4.99 | 2.30 | 1.04 | 8.38 | −0.05 | −1.46 | <0.000 |
| Water–air ratio | 1.50 | 1.01 | 0.14 | 4.52 | 1.49 | 2.07 | <0.000 |
| Treatment | Bulk Density (g/cm−3) | Total Porosity (%) | Aeration Porosity (%) | Water-Holding Porosity (%) | Maximum Water -Holding Capacity | Water–Air Ratio |
|---|---|---|---|---|---|---|
| T1 | 0.11 ± 0.012 | 66.17 ± 1.531 | 43.80 ± 1.277 | 22.37 ± 0.757 | 5.81 ± 0.627 | 1.96 ± 0.085 |
| T2 | 0.56 ± 0.012 | 62.54 ± 3.685 | 23.79 ± 4.194 | 38.75 ± 0.546 | 1.11 ± 0.082 | 0.62 ± 0.115 |
| T3 | 0.09 ± 0.006 | 73.07 ± 2.57 | 59.37 ± 1.739 | 13.70 ± 0.917 | 7.70 ± 0.478 | 4.34 ± 0.191 |
| T4 | 0.11 ± 0.006 | 69.63 ± 3.525 | 15.77 ± 6.469 | 53.87 ± 4.609 | 6.63 ± 0.295 | 0.30 ± 0.140 |
| T5 | 0.08 ± 0.006 | 68.6 ± 1.386 | 26.03 ± 1.155 | 42.57 ± 0.231 | 8.27 ± 0.173 | 0.61 ± 0.029 |
| T6 | 0.10 ± 0.010 | 65.73 ± 4.196 | 26.47 ± 2.987 | 39.27 ± 2.558 | 6.58 ± 0.797 | 0.67 ± 0.086 |
| T7 | 0.09 ± 0.010 | 69.03 ± 2.139 | 40.73 ± 2.065 | 28.30 ± 0.361 | 7.67 ± 0.644 | 1.44 ± 0.075 |
| T8 | 0.11 ± 0.006 | 73.13 ± 5.187 | 42.27 ± 4.649 | 30.87 ± 0.723 | 6.90 ± 0.415 | 1.37 ± 0.127 |
| T9 | 0.22 ± 0.025 | 57.43 ± 1.350 | 30.10 ± 1.311 | 27.33 ± 2.401 | 2.67 ± 0.356 | 1.11 ± 0.137 |
| T10 | 0.29 ± 0.031 | 63.34 ± 1.752 | 40.33 ± 0.722 | 23.00 ± 2.415 | 2.21 ± 0.182 | 1.77 ± 0.208 |
| T11 | 0.27 ± 0.010 | 72.17 ± 1.010 | 53.71 ± 1.736 | 18.46 ± 0.730 | 2.68 ± 0.082 | 2.92 ± 0.215 |
| T12 | 0.10 ± 0.000 | 71.23 ± 3.362 | 36.37 ± 2.566 | 34.87 ± 1.002 | 7.22 ± 0.272 | 1.04 ± 0.056 |
| T13 | 0.22 ± 0.012 | 76.00 ± 3.803 | 40.75 ± 5.517 | 35.25 ± 2.379 | 3.54 ± 0.236 | 1.16 ± 0.221 |
| T14 | 0.23 ± 0.006 | 77.00 ± 5.130 | 43.29 ± 5.156 | 33.71 ± 1.279 | 3.41 ± 0.320 | 1.29 ± 0.171 |
| T15 | 0.22 ± 0.010 | 77.46 ± 3.741 | 54.67 ± 5.72 | 22.79 ± 2.197 | 3.52 ± 0.097 | 2.43 ± 0.493 |
| CK | 0.16 ± 0.015 | 60.93 ± 1.457 | 30.13 ± 2.011 | 30.80 ± 1.992 | 3.92 ± 0.429 | 0.98 ± 0.127 |
| Treatment | RL (cm) | PA (cm2) | SA (cm2) | AD (mm) | VS (cm/m3) | RV (cm3) | NTips |
|---|---|---|---|---|---|---|---|
| T1 | 4549.73 a | 371.26 a | 1166.33 a | 1.63 ab | 4549.73 a | 23.98 abc | 8574.00 ab |
| T2 | 477.30 c | 37.46 c | 117.69 c | 0.81 b | 477.30 c | 2.33 e | 1307.00 c |
| T3 | 3864.07 a | 375.42 a | 1179.42 a | 1.94 a | 3864.07 a | 28.90 ab | 9445.33 a |
| T4 | 409.86 c | 62.01 c | 194.81 c | 1.43 ab | 409.86 c | 7.96 de | 1888.00 c |
| T5 | 1633.65 bc | 162.80 bc | 511.44 bc | 1.01 ab | 1633.65 bc | 12.76 cde | 4572.67 bc |
| T6 | 833.46 c | 70.84 c | 222.56 c | 0.84 b | 833.46 c | 4.76 e | 2261.67 c |
| T7 | 1736.59 bc | 150.48 bc | 472.75 bc | 0.88 b | 1736.59 bc | 10.62 de | 4503.00 bc |
| T8 | 3001.88 ab | 330.54 a | 1038.41 a | 1.48 ab | 3001.88 ab | 28.62 ab | 7459.00 ab |
| T9 | 3494.05 ab | 328.40 a | 1031.71 a | 1.56 ab | 3494.05 ab | 24.48 abc | 8356.67 ab |
| T10 | 3835.65 a | 360.47 a | 1132.46 a | 1.57 ab | 3835.65 a | 27.10 ab | 9406.67 a |
| T11 | 1841.35 bc | 176.76 bc | 555.30 bc | 0.96 ab | 1841.35 bc | 13.49 cde | 5702.67 abc |
| T12 | 3356.07 ab | 318.97 a | 1002.09 a | 1.59 ab | 3356.07 ab | 24.59 abc | 8693.33 ab |
| T13 | 3188.09 ab | 270.94 ab | 851.18 ab | 1.13 ab | 3188.09 ab | 18.18 bcd | 9012.67 ab |
| T14 | 3554.03 ab | 301.01 a | 945.64 a | 1.11 ab | 3554.03 ab | 20.22 abcd | 8730.67 ab |
| T15 | 4261.76 a | 417.87 a | 1312.78 a | 1.64 ab | 4261.76 a | 32.45 a | 10475.67 a |
| CK | 751.04 c | 55.57 c | 174.59 c | 0.74 b | 751.04 c | 3.25 e | 2441.67 c |
| Treatment | PC1 | PC2 | PC3 | Comprehensive Assessment p | Sequence |
|---|---|---|---|---|---|
| T1 | 1.11737 | 1.13470 | −0.12826 | 0.82098 | 2 |
| T2 | −1.59405 | 0.36335 | 0.17058 | −1.17247 | 16 |
| T3 | 0.80811 | −0.47342 | 2.45883 | 0.58468 | 5 |
| T4 | −1.28365 | −0.87330 | 0.91704 | −0.95145 | 14 |
| T5 | −0.50753 | 0.26670 | 0.54267 | −0.37223 | 10 |
| T6 | −1.28408 | 0.08469 | −0.56264 | −0.94531 | 13 |
| T7 | −0.58905 | −0.54412 | −1.09420 | −0.42836 | 11 |
| T8 | 0.92561 | 0.07797 | −0.84752 | 0.68055 | 3 |
| T9 | 0.70705 | −1.69929 | −1.03993 | 0.53544 | 6 |
| T10 | 0.83394 | −0.74261 | −0.82626 | 0.61892 | 4 |
| T11 | −0.64423 | 0.24651 | 1.12319 | −0.47169 | 12 |
| T12 | 0.69211 | 0.53705 | −0.56217 | 0.50671 | 7 |
| T13 | 0.45335 | 2.65669 | 0.15759 | 0.33719 | 9 |
| T14 | 0.47484 | 0.20960 | −0.79075 | 0.34800 | 8 |
| T15 | 1.22437 | −1.14602 | 1.08740 | 0.89028 | 1 |
| CK | −1.33416 | −0.09848 | −0.60556 | −0.98124 | 15 |
| Source | Sum of Squares | Df | Mean Square | F Value | p-Value |
|---|---|---|---|---|---|
| Model | 6.6331 | 9 | 0.7370 | 8.1228 | 0.0164 |
| Error | 0.4537 | 5 | 0.0907 | ||
| Total | 7.0888 | 14 |
| Treatment | Observed Value | Fitting Value | Fitting Error | Treatment | Observed Value | Fitting Value | Fitting Error |
|---|---|---|---|---|---|---|---|
| T1 | 0.8210 | 0.7668 | 0.0542 | T9 | 0.5354 | 0.6473 | −0.1118 |
| T2 | −1.1725 | −1.2228 | 0.0503 | T10 | 0.6189 | 0.6843 | −0.0654 |
| T3 | 0.5847 | 0.5228 | 0.0619 | T11 | −0.4717 | −0.4411 | −0.0306 |
| T4 | −0.9515 | −1.0121 | 0.0606 | T12 | 0.5067 | 0.5024 | 0.0043 |
| T5 | −0.3722 | −0.2345 | −0.1377 | T13 | 0.3372 | 0.4374 | −0.1003 |
| T6 | −0.9453 | −0.8541 | −0.0912 | T14 | 0.3480 | 0.4368 | −0.0888 |
| T7 | −0.4284 | −0.3320 | −0.0964 | T15 | 0.8903 | 0.2927 | 0.5975 |
| T8 | 0.6806 | 0.7873 | −0.1067 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Zhang, G.; Shang, X. Effect of Different Peat Substitute Substrates on the Growth and Quality of Seedlings of Handroanthus chrysanthus (Jacq.) S.O. Grose. Forests 2022, 13, 1626. https://doi.org/10.3390/f13101626
Zhang P, Zhang G, Shang X. Effect of Different Peat Substitute Substrates on the Growth and Quality of Seedlings of Handroanthus chrysanthus (Jacq.) S.O. Grose. Forests. 2022; 13(10):1626. https://doi.org/10.3390/f13101626
Chicago/Turabian StyleZhang, Peijian, Guowu Zhang, and Xiuhua Shang. 2022. "Effect of Different Peat Substitute Substrates on the Growth and Quality of Seedlings of Handroanthus chrysanthus (Jacq.) S.O. Grose" Forests 13, no. 10: 1626. https://doi.org/10.3390/f13101626
APA StyleZhang, P., Zhang, G., & Shang, X. (2022). Effect of Different Peat Substitute Substrates on the Growth and Quality of Seedlings of Handroanthus chrysanthus (Jacq.) S.O. Grose. Forests, 13(10), 1626. https://doi.org/10.3390/f13101626






