Population Dynamics of Juniperus macropoda Bossier Forest Ecosystem in Relation to Soil Physico-Chemical Characteristics in the Cold Desert of North-Western Himalaya
Abstract
1. Introduction
2. Materials and Methods
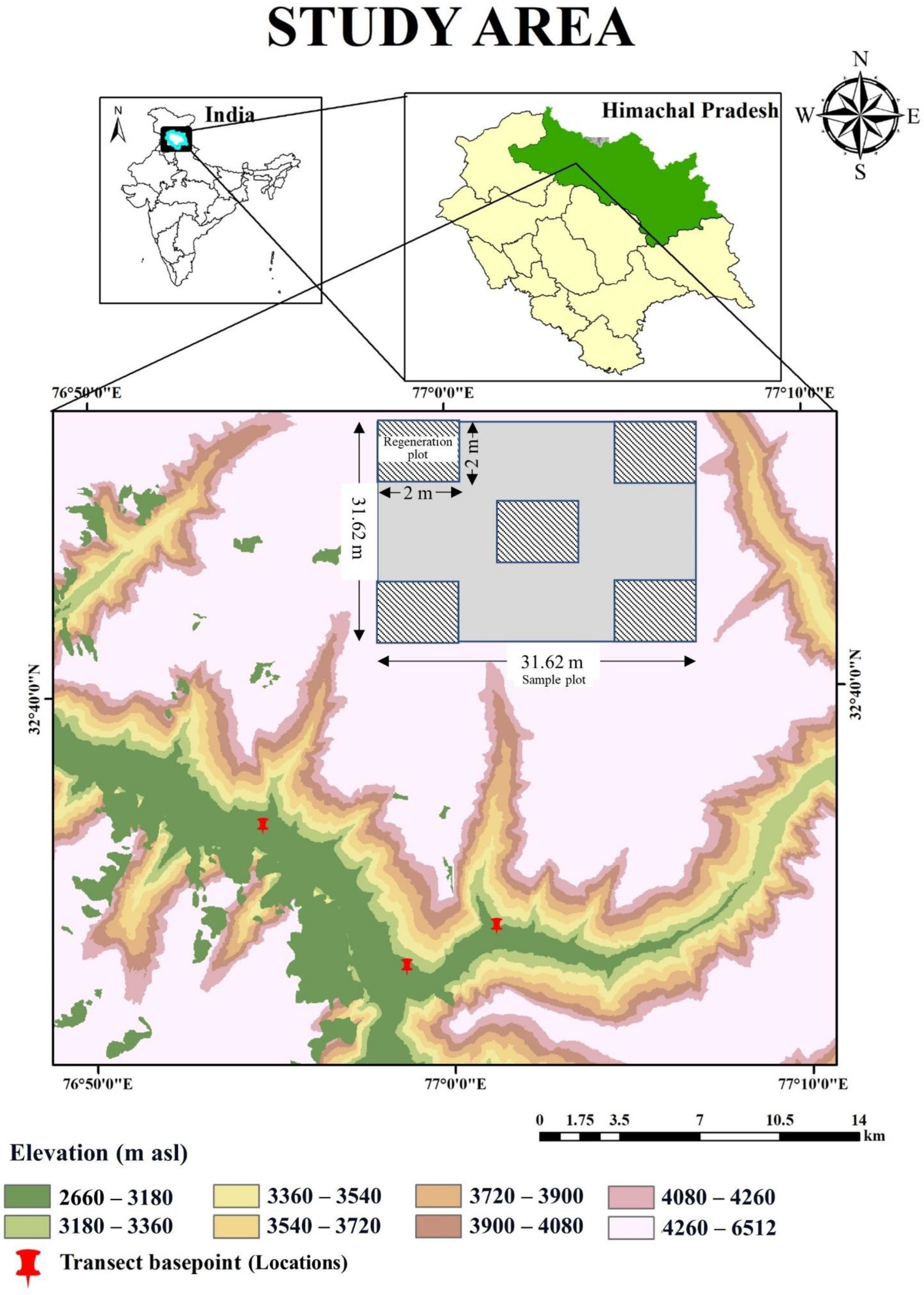
2.1. Study Area and Sampling Procedure
2.2. Soil Physico-Chemical Analysis
2.3. Statistical Analysis
3. Results
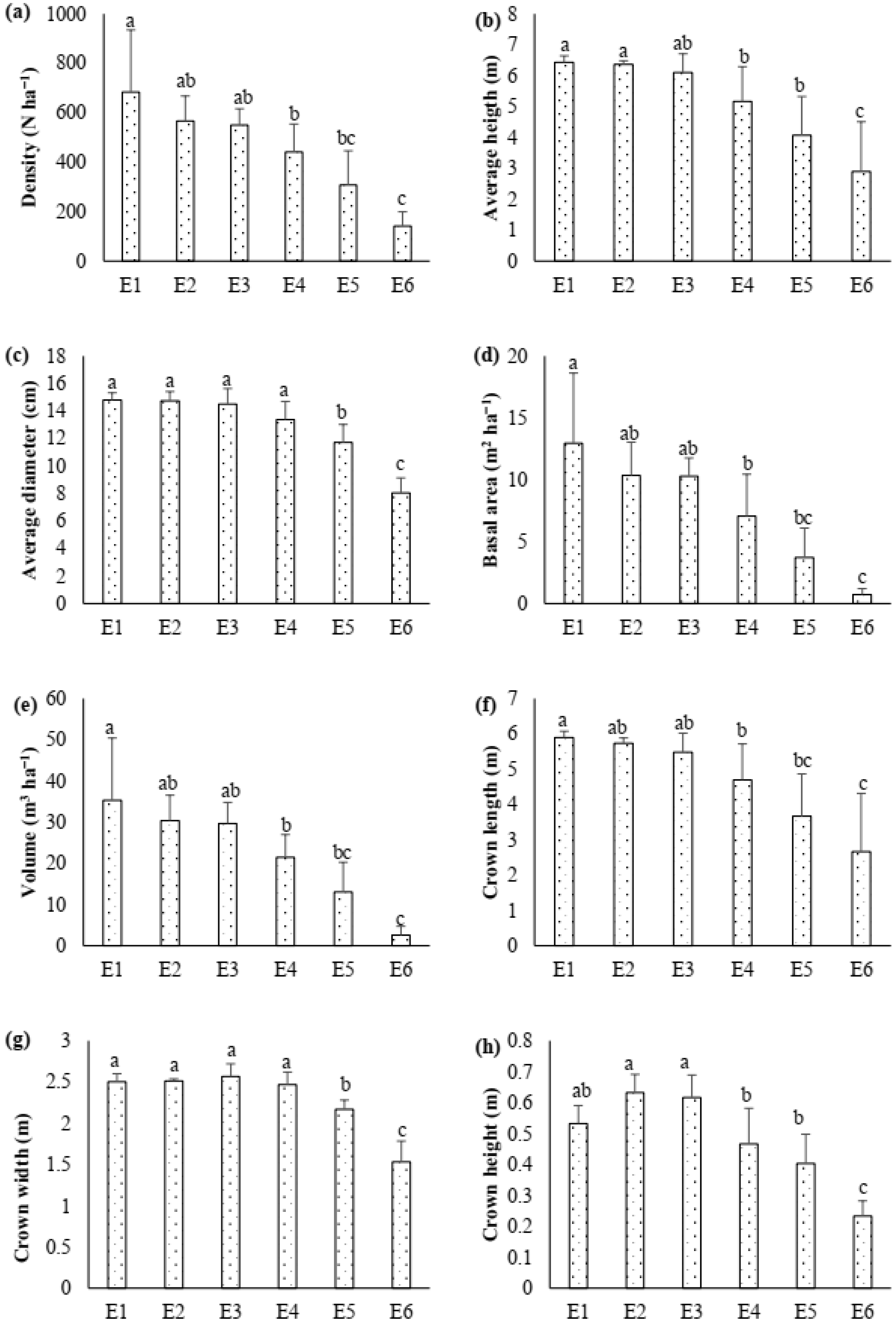
3.1. Stand Characteristics and Population Dynamics at Tree Line
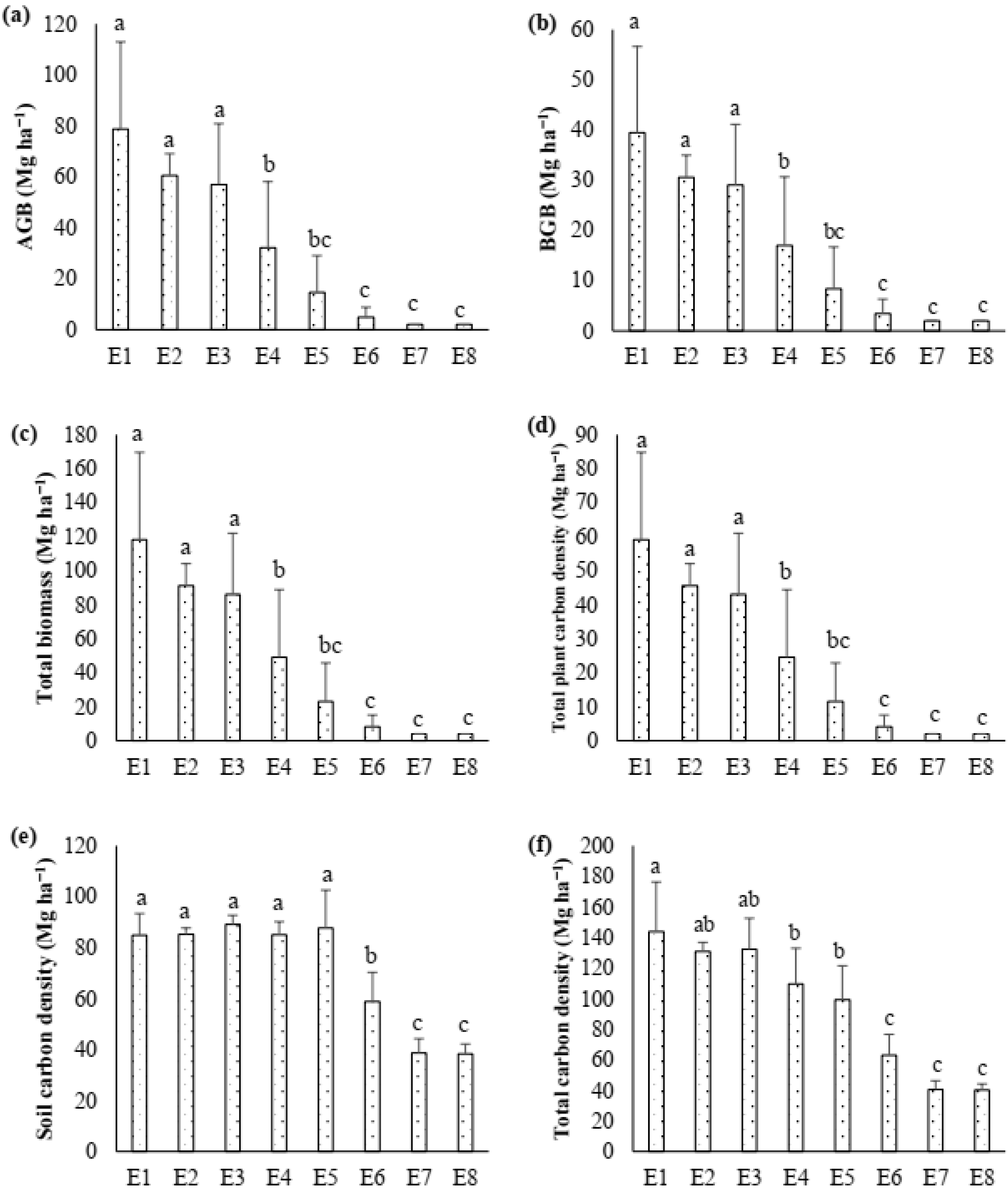
3.2. Biomass and Carbon Density (Mg ha−1)
3.3. Soil Physico-Chemical Properties
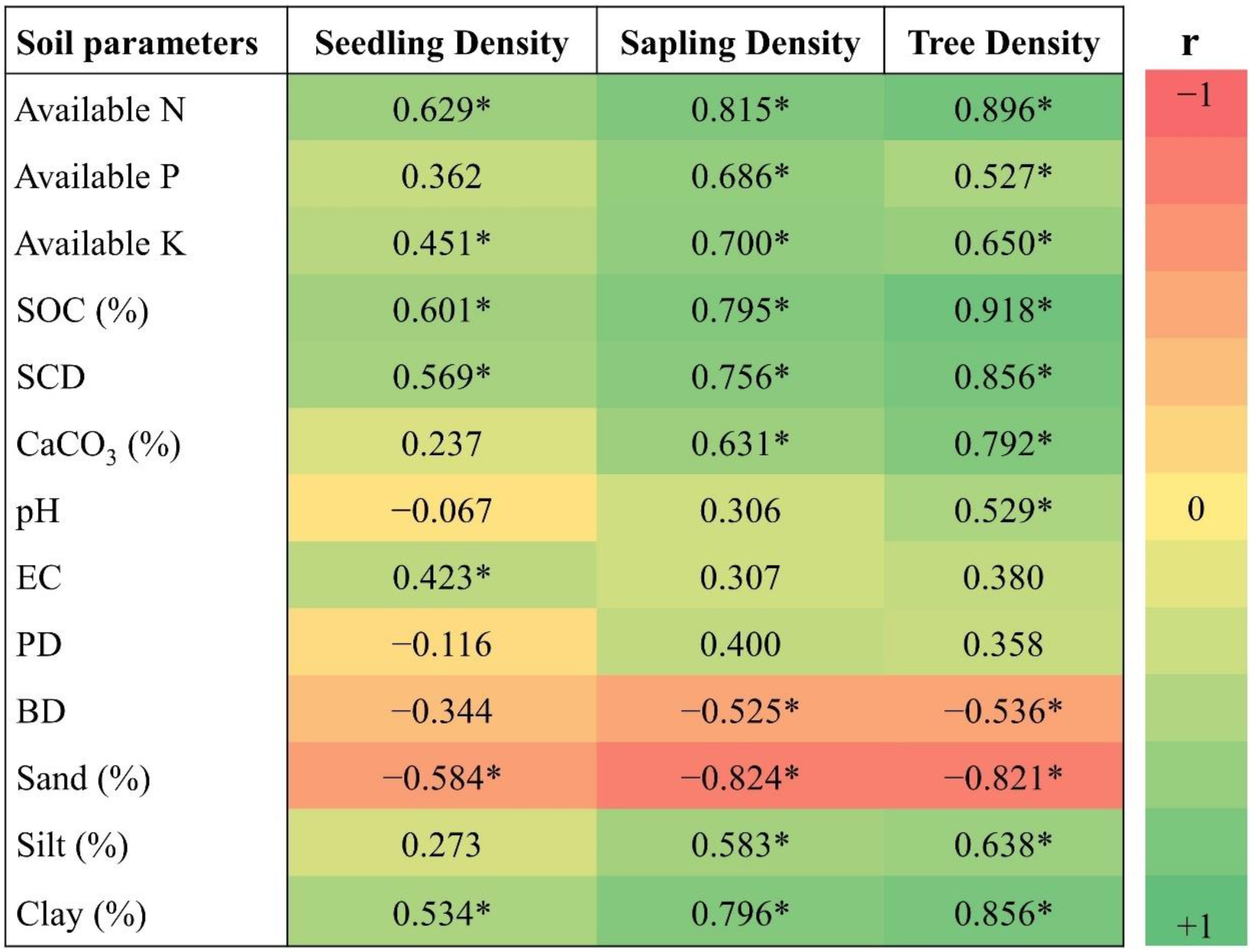
3.4. Correlation and Regression Studies
4. Discussion
4.1. Population Dynamics, Biomass and Carbon Density
4.2. Soil Physico-Chemical Properties and Correlation Studies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barthlott, W.; Lauer, W.P.A.; Placke, A. Global Distribution of Species Diversity in Vascular Plants: Towards a World Map of Phylodiversity. Erdkunde 1996, 50, 317–327. [Google Scholar] [CrossRef]
- Tiwari, R.M. Community Structure and Regeneration of Sub-Alpine Abies spectabilis (D.Don) Mirb. Forest in Langtang National Park, Central Nepal. Ph.D. Thesis, Tribhuvan University, Kirtipur, Nepal, 2010. [Google Scholar]
- Palombo, C.; Chirici, G.; Marchetti, M.; Tognetti, R. Island abandonment affecting forest dynamics at high elevation in Mediterranean mountains more than climate change? Plant Biosyst. 2013, 147, 1–11. [Google Scholar] [CrossRef]
- Tietjen, B.; Schlaepfer, D.R.; Bradford, J.B.; Lauenroth, W.K.; Hall, S.A.; Duniway, M.C.; Hochstrasser, T.; Jia, G.; Munson, S.M.; Pyke, D.A.; et al. Climate change-induced vegetation shifts lead to more ecological droughts despite projected rainfall increases in many global temperate drylands. Glob. Chang. Biol. 2017, 23, 2743–2754. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ruiz, E.P.; Lacher, T.E. Climate change, range shifts, and the disruption of a pollinator-plant complex. Sci. Rep. 2019, 9, 14048. [Google Scholar] [CrossRef]
- Trindade, W.C.F.; Santos, M.H.; Artoni, R.F. Climate change shifts the distribution of vegetation types in South Brazilian hotspots. Reg. Environ. Chang. 2020, 20, 90. [Google Scholar] [CrossRef]
- James, T.M. Temperature sensitivity and recruitment dynamics of Siberian larch (Larix sibirica) and Siberian spruce (Picea obovata) in northern Mongolia’s boreal forest. For. Ecol. Manag. 2011, 262, 629–636. [Google Scholar] [CrossRef]
- Liang, E.; Wang, Y.; Eckstein, D.; Luo, T. Little change in the fir tree-line position on the southeastern Tibetan Plateau after 200 years of warming. New Phytol. 2011, 190, 760–769. [Google Scholar] [CrossRef]
- He, Z.; Zhao, W.; Zhang, L.; Liu, H. Response of tree recruitment to climatic variability in the Alpine treeline ecotone of the Qilian Mountains, northwestern China. For. Sci. 2013, 59, 118–126. [Google Scholar] [CrossRef]
- Wang, B.; Chen, T.; Xu, G.; Liu, X.; Wang, W.; Wu, G.; Zhang, Y. Alpine timberline population dynamics under climate change: A comparison between Qilian juniper and Qinghai spruce tree species in the middle Qilian Mountains of northeast Tibetan Plateau. Boreas 2016, 45, 411–422. [Google Scholar] [CrossRef]
- Urbanov, A.M.; Snajdr, J.; Baldrian, P. Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biol. Biochem. 2015, 84, 53–64. [Google Scholar] [CrossRef]
- Hume, A.; Chen, H.Y.; Taylor, A.R.; Kayahara, G.J.; Man, R. Soil C: N: P dynamics during secondary succession following fire in the boreal forest of central Canada. For. Ecol. Manag. 2016, 369, 1–9. [Google Scholar] [CrossRef]
- James, J.; Harrison, R. The effect of harvest on forest soil carbon: A meta-analysis. Forests 2016, 7, 308. [Google Scholar] [CrossRef]
- Widenfalk, L.A.; Malmstrom, A.; Berg, M.P.; Bengtsson, J. Small-scale Collembola community composition in a pine forest soileOverdispersion in functional traits indicates the importance of species interactions. Soil Biol. Biochem. 2016, 103, 52–62. [Google Scholar] [CrossRef]
- Potter, C.; Klooster, S.; Myneni, R.; Genovese, V.; Tan, P.N.; Kumar, V. Continental-scale comparisons of terrestrial carbon sinks estimated from satellite data and ecosystem modeling. Glob. Planet. Chang. 2003, 39, 201–213. [Google Scholar] [CrossRef]
- Ter Steege, H.; Pitman, N.C.; Phillips, O.L.; Chave, J.; Sabatier, D.; Duque, A.; Molino, J.F.; Pr_evost, M.F.; Spichiger, R.; Castellanos, H.; et al. Continental-scale patterns of canopy tree composition and function across Amazonia. Nature 2006, 443, 444. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.Z.; Li, Q.F.; Li, N.; Si, J.H.; Zhang, Z.X.; Wang, C.J.; Li, X.L.; Li, Z.R. Soil indicators of plant diversity for global ecoregions: Implications for management practices. Glob. Ecol. Conserv. 2018, 14, 404. [Google Scholar] [CrossRef]
- Holtmeier, F.K. Mountain Timberline: Ecology, Patchiness and Dynamics; Springer Science and Business Media, B.V.: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Fisher, J.B.; Malhi, Y.; Torres, I.C.; Metcalfe, D.B.; vandeWeg, M.J.; Meir, P.; Silva-Espejo, J.E.; Huasco, W.H. Nutrient limitation in rainforests and cloud forests along a 3000-m elevation gradient in the Peruvian Andes. Oecologia 2013, 172, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.; Anand, M. Historical links and new frontiers in the study of forest-atmosphere interactions. Community Ecol. 2013, 14, 208–218. [Google Scholar] [CrossRef]
- Fernandez-Martinez, M.; Vicca, S.; Janssens, I.A.; Sardans, J.; Luyssaert, S.; Campioli, M.; Chapin III, F.S.; Ciais, P.; Malhi, Y.; Obersteiner, M.; et al. Nutrient availability as the key regulator of global forest carbon balance. Nat. Clim. Chang. 2014, 4, 471–476. [Google Scholar] [CrossRef]
- Silva, L.C.; Sun, G.; Zhu-Barker, X.; Liang, Q.; Wu, N.; Horwath, W.R. Tree growth acceleration and expansion of alpine forests: The synergistic effect of atmospheric and edaphic change. Sci. Adv. 2016, 2, 1501. [Google Scholar] [CrossRef]
- Hayati, E.; Abdi, E.; Saravi, M.M.; Nieber, J.L.; Majnounian, B.; Chirico, G.B.; Wilson, B.; Nazarirad, M. Soil water dynamics under different forest vegetation cover: Implications for hillslope stability. Earth Surf. Process. Landforms 2018, 43, 2106–2120. [Google Scholar] [CrossRef]
- Molina, A.J.; Llorens, P.; Garcia-Estringana, P.; de las Heras, M.M.; Cayuela, C.; Gallart, F.; Latron, J. Contributions of throughfall, forest and soil characteristics to near-surface soil water-content variability at the plot scale in a mountainous Mediterranean area. Sci. Total Environ. 2019, 647, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Wu, N. The Timberline Ecotone in the Himalayan Region: An Ecological Review. In High-Elevation Rangelands and their Interfaces in the Hindu Kush Himalayas; International Center for Integrated Mountain Development (ICIMOD): Kathmandu, Nepal, 2013; pp. 108–116. [Google Scholar]
- Li, W.H.; Zhou, X.M. Ecosystems of Qinghai-Xizang (Tibetan) Plateau and their Influence on Environments; Guangdong Science and Technology Press: Guangzhou, China, 1998. [Google Scholar]
- Oechel, W.C.; Vourlitis, G.L.; Hastings, S.J.; Zulueta, R.C.; Hinzman, L.; Kane, D. Acclimation of ecosystem CO2 exchange in the Alaskan Arctic in response to decadal climate warming. Nature 2000, 406, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Charan, G.; Bharti, V.K.; Jadhav, S.E.; Kumar, S.; Acharya, S.; Kumar, P.; Gogoi, D.; Srivastava, R.B. Elevational variations in soil physico-chemical properties at cold desert high elevation. J. Plant. Nutr. Soil Sci. 2013, 13, 267–277. [Google Scholar]
- Wan, J.Z.; Yum, J.H.; Yin, G.J.; Song, Z.M.; Wei, D.X.; Wang, C.J. Effects of soil properties on the spatial distribution of forest vegetation across China. Glob. Ecol. Conserv. 2019, 1, 635. [Google Scholar] [CrossRef]
- Chauvier, Y.; Thuiller, W.; Brun, P.; Lavergne, S.; Descombes, P.; Karger, D.N.; Renaud, J.; Zimmermann, N.E. Influence of climate, soil, and land cover on plant species distribution in the European Alps. Ecol. Monogr. 2021, 91, 1433. [Google Scholar] [CrossRef]
- Kuniyal, J.C. Environmental pollution, their impact on climate change and implications on Himalayan mountain society. ENVIS Bull. Hima. Ecol. 2016, 24, 133–143. [Google Scholar]
- Rawat, Y.S.; Vishvakarma, S.C.; Oinam, S.S.; Kuniyal, J.C. Diversity, distribution and vegetation assessment in the Jahlmanal watershed in cold desert of the Lahaul valley, north-western Himalaya, India. iForest-Biog. For. 2010, 3, 65–71. [Google Scholar] [CrossRef]
- Rawat, Y.S.; Everson, C.S. Ecological status and uses of juniper species in the cold desert environment of the Lahaul valley, North-western Himalaya, India. J. Moun. Sci. 2012, 9, 676–686. [Google Scholar] [CrossRef]
- Gottfried, M.; Pauli, H.; Futschik, A.; Akhalkatsi, M.; Barancok, P.; Alonso, J.L.B.; Grabherr, G. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Chan. 2012, 2, 111–115. [Google Scholar] [CrossRef]
- Nowak, R.S.; Nowak, C.L.; Tausch, R.J. Vegetation dynamics during last 35,000 years at a cold desert locale: Preferential loss of forbs with increased aridity. Ecosphere 2017, 8, 1873. [Google Scholar] [CrossRef]
- Garnett, M.H.; Ineson, P.; Stevenson, A.C.; Howard, D.C. Terrestrial organic carbon storage in a British moorland. Glob. Chan. Biol. 2001, 7, 375–388. [Google Scholar] [CrossRef]
- Liu, O.H.; Shi, X.Z.; Weindorf, D.C.; Yu, D.S.; Zhao, Y.C.; Sun, W.X.; Wang, H.J. Soil organic carbon storage of paggy soil in China using the 1:1,000,000 soil database and their implications for C sequestration. Glob. Biogeo. Cycle. 2006, 20, GB3024. [Google Scholar] [CrossRef]
- Yang, Y.H.; Mohammat, A.; Feng, J.M.; Zhou, R.; Fang, J.Y. Storage, patterns and environmental controls of soil organic carbon in China. Biogeochemistry 2007, 6, 84–95. [Google Scholar] [CrossRef]
- Devetter, M.; Hanel, L.; Rehakova, K.; Dolezal, J. Diversity and feeding strategies of soil microfauna along elevation gradients in Himalayan cold deserts. PLoS ONE 2017, 12, e0187646. [Google Scholar] [CrossRef]
- Tong, F.; Xiao, Y.; Wang, Q. Soil nematode community structure on the northern slope of Changbai Mountain, Northeast China. J. For. Res. 2010, 21, 93–98. [Google Scholar] [CrossRef]
- Yang, Y.; Li, T.; Wang, Y.; Cheng, H.; Chang, S.X.; Liang, C.; An, S. Negative effects of multiple global change factors on soil microbial diversity. Soil Biol. Biochem. 2021, 156, 108229. [Google Scholar] [CrossRef]
- Petz, W. Ecology of the active soil microfauna (Protozoa, Metazoa) of Wilkes Land, East Antarctica. Polar Biol. 1997, 18, 33–44. [Google Scholar] [CrossRef]
- Gupta, P.; Sangwan, N.; Lal, R.; Vakhlu, J. Bacterial diversity of Drass, cold desert in Western Himalaya, and its comparison with Antarctic and Arctic. Arch. Microb. 2015, 197, 851–860. [Google Scholar] [CrossRef]
- Darby, B.J.; Neher, D.A.; Housman, D.C.; Belnap, J. Few apparent short-term effects of elevated soil temperature and increased frequency of summer precipitation on the abundance and taxonomic diversity of desert soil micro- and meso-fauna. Soil Biol. Biochem. 2011, 43, 1474–1481. [Google Scholar] [CrossRef]
- Weicht, T.R.; Barbercheck, M.E. Linking invertebrate communities to decomposition rate and nitrogen availability in pine forest soils. Appl. Soil Ecol. 2012, 54, 14–23. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setala, H.; van der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Bowman, W.D.; Kaufmann, R.; Schmidt, S.K. A temporal approach to linking aboveground and belowground ecology. Trends Ecol. Evol. 2005, 20, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Korner, C. Climatic treelines: Conventions, global patterns, causes. Erdkunde 2007, 61, 316–324. [Google Scholar] [CrossRef]
- Andrassy, I. Free-living Nematodes of Hungary (Nematoda Errantia) Volume III; Hungarian Natural History Museum and Systematic Zoology Research Group of the Hungarian Academy of Sciences: Budapest, Hungary, 2009. [Google Scholar]
- Chimani, B.; Matulla, C.; Bohm, R.; Hofstatter, M. A new high resolution absolute temperature grid for the Greater Alpine Region back to 1780. Int. J. Climatol. 2013, 33, 2129–2141. [Google Scholar] [CrossRef]
- Moran-Tejeda, E.; Lopez-Moreno, J.I.; Beniston, M. The changing roles of temperature and precipitation on snowpack variability in Switzerland as a function of elevation. Geophys. Res. Lett. 2013, 40, 2131–2136. [Google Scholar] [CrossRef]
- Maharjan, S.K.; Sterck, F.J.; Raes, N.; Poorter, L. Temperature and soils predict the distribution of plant species along the Himalayan elevational gradient. J. Trop. Ecol. 2022, 38, 58–70. [Google Scholar] [CrossRef]
- Antony, V.; Erskine, P.; Mulligan, D.; Repin, R.; Karim, R. Vegetation on ultramafic edaphic ‘islands’ in kinabalu park (sabah, malaysia) in relation to soil chemistry and elevation. Plant Soil 2016, 403, 77–101. [Google Scholar] [CrossRef]
- Tesfaye, M.A.; Bravo, F.; Ruiz-Peinado, R.; Pando, V.; Bravo-Oviedo, A. Impact of changes in land use, species and elevation on soil organic carbon and total nitrogen in ethiopian central highlands. Geoderma 2016, 261, 70–79. [Google Scholar] [CrossRef]
- Han, W.; Chen, L.; Su, X.; Liu, D.; Jin, T.; Shi, S.; Li, T.; Liu, G. Effects of Soil Physico-Chemical Properties on Plant Species Diversity Along an Elevation Gradient Over Alpine Grassland on the Qinghai-Tibetan Plateau, China. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef]
- Scherrer, D.; Guisan, A. Ecological indicator values reveal missing predictors of species distributions. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Buri, A.S.; Grand, E.; Yashiro, T.; Adatte, J.E.; Spangenberg, E.; Pinto-figueroa, E.; Verrecchia, A.; Guisan. What are the most crucial soil variables for predicting the distribution of mountain plant species? A comprehensive study in the Swiss Alps. J. Biogeogr. 2022, 47, 1143–1153. [Google Scholar] [CrossRef]
- Jobbagy, E.G.; Jackson, R.B. The Vertical Distribution of Soil Organic Carbon and its Relation to Climate and Vegetation. Ecol. Appl. 2020, 10, 423–436. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Sharma, V.K.; Bharadwaj, V. Status of available nutrients in soil of cold arid region of Ladakh. J. Indian Soc. Soil Sci. 2005, 53, 421–423. [Google Scholar]
- Sharma, V.K.; Dwivedi, K.S.; Tripathi, D.; Ahmed, Z. Status of available major and micro-nutrients in the soils of different blocks of Leh district of cold arid region of Ladakh in relation to soil characteristics. J. Indian Soc. Soil Sci. 2006, 54, 248–250. [Google Scholar]
- Dorjey, K.; Maurya, A.K. Ethnobotany of Juniperus polycarpos C. Koch (Cupressaceae) in the Himalayan cold desert of Union Territory of Ladakh, India. Indian J. Trad. Know. 2021, 20, 83–90. [Google Scholar]
- Hall, J.B. Juniperus excelsa in Africa: A biogeographical study of an Afromontane tree. J. Biogeogr. 1984, 11, 47–61. [Google Scholar] [CrossRef]
- Ahmed, M.; Ahmed, I.; Anjum, P.I. A study of natural regeneration of Juniperus excelsa M. Bieb in Balochistan. Pak. J. Bot. 1989, 21, 118–127. [Google Scholar]
- Kumar, R.R.; Paliyal, S.S.; Sharma, K.S. Soil physical properties of cold desert region of different land uses in north-western Himalayas, H.P-India. Int. J. Adv. Res. 2017, 5, 232–235. [Google Scholar] [CrossRef]
- Fisher, M.; Gardner, A.S. The status and ecology of Juniperus excelsa sub-species polyarpus woodlands in the northern mountains of Oman. Vegetation 1995, 119, 33–51. [Google Scholar] [CrossRef]
- Korouri, S.A.A.; Khoshnavis, M. Ecological and Environmental Studies of Juniper Habitats in Iran; Research Institute of Forest and Rangelands: Tehran, Iran, 2000; pp. 122–178. [Google Scholar]
- Carus, S. Increment and growth in Crimean Juniper (Juniperus excelsa Bieb.) stands in Isparta-Sutculer region of Turkey. J. Biol. Sci. 2004, 4, 173–179. [Google Scholar]
- Vaneet, J.; Rupam, K.; Joginder, S.; Lakhanpal, T.N. Arbuscular Mycorrhizal (AM) Diversity in some Threatened North West Himalayan Flora of Kinnaur. Kavaka 2019, 52, 42–51. Available online: http://www.fungiindia.co.in/images/kavaka/52/5.pdf (accessed on 28 June 2022).
- Giri, B.; Kapoor, R.; Mukerji, K.G. Influence of arbuscular mycorrhizal fungi and salinity on growth, biomass and mineral nutrition of Acacia auriculiformis. Biol. Fert. Soils 2003, 38, 176–180. [Google Scholar] [CrossRef]
- Cavagnaro, T.R.; Bender, S.F.; Asghari, H.R.; van der Heijden, M.G.A. The role of arbuscular mycorrhizas in reducing soil nutrient loss. Trends Plant Sci. 2015, 20, 283290. [Google Scholar] [CrossRef]
- Giri, B.; Kapoor, R.; Mukerji, K.G. Improved Tolerance of Acacia Nilotica to Salt Stress by Arbuscular Mycorrhiza, Glomus Fasciculatum May Be Partly Related to Elevated K/Na Ratios in Root and Shoot Tissues. Microb. Ecol. 2007, 54, 753–760. Available online: https://www.jstor.org/stable/25256247 (accessed on 13 August 2022). [CrossRef]
- Li, W.; Shi, Y.; Zhu, D.; Wang, W.; Liu, H.; Li, J.; Fu, S. Fine root biomass and morphology in a temperate forest are influenced more by the nitrogen treatment approach than the rate. Ecol. Indic. 2021, 130, 108031. [Google Scholar] [CrossRef]
- Stampoulidis, A.; Milios, E.; Kitikidou, K. The regeneration of pure Juniperus excelsa stands in Prespa National Park` in Greece. Sumar. List 2013, 137, 163–172. [Google Scholar]
- Ciesla, W.M. Juniper forests—A special challenge for sustainable forestry. For. Trees Livelihoods 2002, 12, 195–207. [Google Scholar] [CrossRef]
- Korouri, S.A.A.; Khoshnevis, M.; Matinizadeh, M. Comprehensive Studies of Juniper Species in Iran; Forest Range and Watershed Management Organization: Tajrish, Iran, 2012; pp. 144–193. [Google Scholar]
- IUCN 2022. The IUCN Red List of Threatened Species. Version 2022-1. Available online: https://www.iucnredlist.org (accessed on 22 September 2022).
- Ahani, H.; Jalilv, H.; Hosseini, N.S.M.; Soltani, K.H.; Ghazi, M.R.; Mohammad, Z.H. Reproduction of Juniperus polycarpusin Khorasan Razavi, Iran. For. Sci. Prac. 2013, 15, 231–237. [Google Scholar] [CrossRef]
- Ravanbakhsh, H.; Marvi, M.R.; Nourzad, M.M. Qualitative and quantitative investigation of Juniper -cotoneaster forest reserve in Ooshan (Central Alborz, Iran). Iran J. For. Poplar Res. 2010, 18, 253–264. [Google Scholar]
- Shirzad, M.A.; Tabari, M. Effect of some environmental factors on diversity of woody plants in Juniperus excelsa habitat of Hezarmasjed mountains. Iran J. Biol. 2013, 24, 800–808. [Google Scholar]
- Ramin, M.; Shataei, S.H.; Habashi, H.; Khoshnevis, M. Investigation on some quantitative and qualitative characteristics of juniper stands in Aminabad of Firouzkoh. J. Wood Sci. 2012, 19, 21–40. [Google Scholar]
- Taheri, A.K.; Khosh, F.F.; Jahdi, R.; Foumani, B.S. Structure and regeneration patterns of Juniperus polycarpusin Alborz mountains, Iran. JBASR 2012, 2, 5993–5996. [Google Scholar]
- Sarangzai, A.M.; Siddiqui, M.F.; Ahmed, M.; Hussain, M.I.; Laghari, S.K.; Ahmed, A. Relationship between soil properties and natural regeneration pattern of Juniperus excelsaforest in Ziarat, Baluchistan. Pak. J. Bot. 2015, 47, 905–910. [Google Scholar]
- Ahmed, M.; Naqui, E.E.; Wang, E.L.M. Present state of juniper in Roadhmullazi forest of Baluchistan. Pak. J. For. 1990, 40, 227–236. [Google Scholar]
- Kumar, D.; Thakur, C.L.; Bhardwaj, D.R.; Sharma, N.; Sharma, P.; Sankhyan, N. Biodiversity conservation and carbon storage of Acacia catechu Willd. dominated northern tropical dry deciduous forest ecosystems in north-western Himalaya: Implications of different forest management regimes. Front. Environ. Sci. 2022, 1–16. [Google Scholar] [CrossRef]
- Shen, Y.X.; Liu, W.L.; Li, Y.H.; Guan, H.L. Large sample area and size are needed for forest soil seed bank studies to ensure low discrepancy with standing vegetation. PLoS ONE 2014, 9, e105235. [Google Scholar] [CrossRef][Green Version]
- Sheikh, M.I. Trees of Pakistan; Pictorial Printers: Islamabad, Pakistan, 1993; pp. 100–151. [Google Scholar]
- Kramer, S. Development and Morphology of Juvenile Western Juniper (Juniperus Occidentalis Hook.). Master’s Thesis, Oregon State University, Corvallis, OR, USA, 1990. [Google Scholar]
- Bhardwaj, D.R.; Kumar, A.; Pala, N.A.; Sharma, P.; Kumar, D.; Kumar, A.; Zahoor, S. Carbon density and C-sequestration by tree plantation ecosystems in mid-hill of NW-Himalayas: Implications for climate change mitigation. Land Degrad. Dev. 2022, 33, 2115–2126. [Google Scholar] [CrossRef]
- Bhardwaj, D.R.; Tahiry, H.; Sharma, P.; Pala, N.A.; Kumar, D.; Kumar, A. Influence of aspect and elevational gradient on vegetation pattern, tree characteristics and ecosystem carbon density in Northwestern Himalayas. Land 2021, 10, 1109. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice hall of India Private Limited: New Delhi, India, 1973; p. 498. [Google Scholar]
- Walkley, A.; Black, J.A. Estimation of soil organic carbon by chromic acid filtration method. Soil Sci. 1934, 37, 38–39. [Google Scholar]
- Subbiah, B.V.; Asija, G.S. Rapid procedure for estimation of available nitrogen in soil. Curr. Sci. 1956, 25, 259–260. [Google Scholar]
- Olsen, S.R.; Cole, W.; Watanable, F.S.; Dean, L.A. Estimation of available phosphorus I soils by extraction with sodium bicarbonate. In Methods of Soil Analysis; Black, C.A., Ed.; US Department of Agriculture: Washington, DC, USA, 1954; pp. 1044–1046. [Google Scholar]
- Merwin, H.D.; Peach, P.M. Exchangeability of soil potassium in the sand, silt and clay fractions as influenced by nature of complementary exchangeable cation. Soil Sci. Soc. Am. J. 1951, 15, 125–128. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Matter. In Method of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar] [CrossRef]
- Fisher, R.A. Statistical Methods for Research Workers; Oliver and Boyd.: Edinburgh, UK, 1995. [Google Scholar]
- Marquardt, D.W. Generalized inverses, ridge regression, biased linear estimation, and nonlinear estimation. J. Soc. Ind. Appl. Math. 1970, 2, 431–441. [Google Scholar] [CrossRef]
- Mongomery, D.C.; Elizabeth, A.P.; Geoffrey, V. Introduction to Linear Regression Analysis, 3rd ed.; Wiley Publication: Hoboken, NJ, USA, 2003; p. 531. [Google Scholar]
- Bojko, O.; Kabala, C. Organic carbon pools in mountain soils—Sources of variability and predicted changes in relation to climate and land use changes. Catena 2017, 149, 209–220. [Google Scholar] [CrossRef]
- Miao, R.; Liu, Y.; Wu, L.; Wang, D.; Liu, Y.; Miao, Y.; Ma, J. Effects of long-term grazing exclusion on plant and soil properties vary with position in dune systems in the Horqin Sandy Land. CATENA. 2022, 209. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gutierrez, E.; Fortin, M. Spatial patterns of plant richness across treeline ecotones in the Pyrenees reveal different locations for richness and tree cover boundaries. Global Ecol. Biogeogr. 2006, 15, 182–191. Available online: https://www.jstor.org/stable/3697615 (accessed on 6 March 2022). [CrossRef]
- Elumeeva, T.G.; Tekeev, D.K.; Wu, Y.; Wang, Q.; Onipchenko, V.G. Life-form composition of alpine plant communities at the Eastern Qinghai-Tibetan plateau. Plan. Biosyst. Int. J. Deal. Asp. Plan. Biol. 2013, 148, 988–994. [Google Scholar] [CrossRef]
- Walker, D.A.; Molenaar, J.G.; Billings, W.D. Snow-Vegetation Interactions in Tundra Environments. In Snow Ecology; Jones, H.G., Pomeroy, J., Walker, D.A., Wharton, R., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 264–322. [Google Scholar]
- Zhang, K.; Ali, A.; Antonarakis, A.; Moghaddam, M.; Saatchi, S.; Tabatabaeenejad, A.; Moorcroft, P. The Sensitivity of North American Terrestrial Carbon Fluxes to Spatial and Temporal Variation in Soil Moisture: An Analysis Using Radar-Derived Estimates of Root-Zone Soil Moisture. Journal of geophysical research. J. Geophys. Res. Biogeoscience 2019, 124, 3208–3231. [Google Scholar] [CrossRef]
- Sarangzai, A.M.; Ahmed, M.; Ahmed, A.; Tareen, L.; Jan, S.U. The ecology and dynamics of Juniperus excelsa forest in Balochistan-Pakistan. Pak. J. Bot. 2012, 44, 1617–1625. [Google Scholar]
- Atta, M.S. Population Structure and Regeneration Potential of Juniper forests in Baluchistan Province, Pakistan. Ph.D. Thesis, University of Baluchistan, Quetta, Pakistan, 2000. [Google Scholar]
- Sharma, B.M.; Anup, R. Status of natural regeneration of Juniperus macropoda Boisser in Ladakh, the cold arid region of Western Trans- Himalayas. Iran J. For. 2004, 27, 237–240. [Google Scholar]
- Momeni, M.T.; Sagheb, T.; Akbarinia, M.; Akhavank, R.; Hosseini, S.M. Impact of some physiographic and edaphic factor on quantitative and qualitative characteristics of juniper forest. Iran J. For. 2012, 4, 143–156. [Google Scholar]
- Machler, F.; Noseberger, J. Effect of Light Intensity and Temperature on Apparent Photosynthesis of Elevational Ecotypes of Trifolium Repens L. Oecologia 1977, 31, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ma, K.; Chen, L. Response of photosynthetic plasticity of Paeonia suffruticosa to changed light environments. Environ. Exp. Bot. 2003, 49, 121–133. [Google Scholar] [CrossRef]
- An, H.; Shangguan, Z.P. Effects of light intensity and nitrogen application on the growth and photosynthetic characteristics of Trifolium repens L. Shengtai Xuebao. Acta Ecol. Sinica 2009, 29, 6017–6024. [Google Scholar]
- Wang, Y.; Guo, Q.; Jin, M. Effects of light intensity on growth and photosynthetic characteristics of Chrysanthemum morifolium. Zhongguo Zhongyao Zazhi 2009, 34, 1633–1635. [Google Scholar]
- Mielke, M.S.; Schaffer, B. Leaf gas exchange, chlorophyll fluorescence and pigment indexes of Eugenia uniflora L. inresponse to changes in light intensity and soil flooding. Tree Physiol. 2010, 30, 45–55. [Google Scholar] [CrossRef]
- Yang, X.Y.; Liu, X.; Xu, Z.; Jiao, X. Effects of light intensity on leaf microstructure and growth of rape seedlings cultivated under a combination of red and blue LEDs. J. Integr. Agric. 2017, 16, 97–105. [Google Scholar]
- Briceno, B.; Azocar, A.; Farinas, M.; Rada, F. Características anatomicas de dos especies Lupinus L. de los Andes venezolanos. (Anatomical characteristics of two species Lupinus L. of the Venezuelan Andes). Pittieria 2000, 29, 21–31. [Google Scholar]
- Cavieres, L.A. Variacion morfologica de Phacelia secunda J.F.Gmel. (Hydrophyllaceae) a lo largo de un gradient elevationalen Chile central. (In Spanish) (Morphological variation of Phacelia secunda J.F. Gmel. (Hydrophyllaceae) along an elevational gradient in central Chile). Gaya Bota. 2000, 57, 89–96. [Google Scholar]
- Abido, M.S.; Kurbaisa, M.S. The present status of the Syrian juniper forests on the East Lebanon Mountain chain. Arab Gulf J. Sci. Res. 2003, 21, 64–70. [Google Scholar]
- Hoch, G.; Korner, C. Growth, demography and carbon relations of Polylepis trees at the world’s highest treeline. Funct. Ecol. 2005, 19, 941–951. [Google Scholar] [CrossRef]
- Miehe, G.; Miehe, S.; Schlutz, F. Early human impact in the forest ecotone of southern High Asia (Hindu Kush, Himalaya). Quat. Res. 2009, 71, 255–265. [Google Scholar] [CrossRef]
- Binkley, D.; Sollins, P. Acidification of soils in mixtures of conifers and red alder. Soil Sci. Soc. Am. J. 1990, 54, 1427–1433. [Google Scholar] [CrossRef]
- Norris, M.D.; Blair, J.M.; Johnson, L.C.; Robert, M.B. Assessing changes in biomass, productivity, and C and N stores following Juniperus virginiana forest expansion into tall grass prairie. Can. J. For. Res. 2001, 31, 1940–1946. [Google Scholar] [CrossRef]
- Rasteller, E.B.; Kwiatkowski, B.L.; Deizes, S.L.; Hobbie, J.E. The role of down slope water and nutrient fluxes in the response of Arctic hill slope to climate change. Biogeochemistry 2004, 69, 37–62. [Google Scholar] [CrossRef]
- Schinner, F. Soil microbial activities and litter decomposition related to elevation. Plant Soil. 1982, 65, 87–94. [Google Scholar] [CrossRef]
- Jacot, K.A.; Luscher, A.; Nosberger, J.; Hartwig, U.A. Symbiotic N2 fixation of various legume species along an elevational gradient in the Swiss Alps. Soil Biol. Biochem. 2000, 32, 1043–1052. [Google Scholar] [CrossRef]
- Bhattacharyya, T.; Pal, D.K.; Chandran, P.; Ray, S.K.; Mandal, C.; Telpande, B. Soil carbon storage capacity as a tool to prioritize areas for carbon sequestration. Curr. Sci. 2008, 95, 482–492. [Google Scholar]
- Velmuurugan, A.; Kumar, S.; Dadhwal, V.K.; Gupta, M.K. Soil organic carbon status of Indian forests. Indian For. 2014, 140, 468–477. [Google Scholar]
- Jenny, H. Factors of Soil Formation: A System of Quantitative Pedology; Dover Publica- tions, Inc.: Mineola, NY, USA, 1994. [Google Scholar]
- Alexandrovskiy, A.L. Rates of soil-forming processes in three main models of pedogenesis. Rev. Mex. Cienc. Geol. 2007, 24, 283–292. [Google Scholar]
- Rubinic, V.; Galovic, L.; Husnjak, S.; Durn, G. Climate vs. parent material—Which is the key of Stagnosol diversity in Croatia? Geoderma 2015, 241, 250–261. [Google Scholar] [CrossRef]
- Dahlgren, R.A.; Boettinger, J.L.; Huntington, G.L.; Amundson, R.G. Soil development along an elevational transect in the western Sierra Nevada, California. Geoderma 1997, 78, 207–236. [Google Scholar] [CrossRef]
- Mani, M.S. Fundamentals of High Elevation Biology, 2nd ed.; Oxford and IBM Publishing Co. Pvt. Ltd.: New Delhi, India, 1990. [Google Scholar]
- Bowman, W.D.; Cairns, D.M.; Baron, J.S.; Seastedt, T.R. Islands in the sky: Alpine and treeline ecosystems of the Rockies. In Rocky Mountain Futures: An Ecological Perspective; Baron, J.S., Ed.; Island Press: Washington, DC, USA, 2002; pp. 183–202. [Google Scholar]
- Unger, P.W. Overwinter changes in physical properties of no tillage soil. Soil Sci. Soc. Am. J. 1991, 55, 778–782. [Google Scholar] [CrossRef]
- Chen, Y.; Tessier, S.; Rouffignat, J. Soil bulk density estimation for soil tillage system and soil texture. Trans ASAE 1998, 41, 1601–1610. [Google Scholar] [CrossRef]
- Mati, B.M. Overview of Water and Soil Nutrient Management under Smallholder Rain-Fed Agriculture in East Africa; IWMI: Colombo, Sri Lanka, 2005; p. 105. [Google Scholar]
- Asadi, H.; Raeisvandi, A.; Rabiei, B.; Ghadiri, H. Effect of land use and topography on soil properties and agronomic productivity on calcareous soils of a semi-arid region, Iran. Land Degrad. Dev. 2011, 23, 496–504. [Google Scholar] [CrossRef]
- Charley, J.L.; Cowling, S.W. Changes in soil nutrient status resulting from overgrazing and their consequences in plant communities of semi-arid areas. Proc. Ecol. Soc. Aust. 1968, 3, 28–38. [Google Scholar]
- Mengele, K.; Kirby, E.A. Principles of Plant Nutrition; Panima Publishers Corporation: New Delhi, India, 1987. [Google Scholar]
- Tisdale, S.L.; Nelson, W.L.; Beaton, J.D.; Havlin, J.L. Soil Fertility and Fertilizer, 5th ed.; Prentice-Hall of India: New Delhi, India, 1995; p. 684. [Google Scholar]
- Yang, Y.H.; Fang, J.Y.; Ji, C.J.; Han, W.X. Above and belowground biomass allocation in Tibetan grasslands. J. Veg. Sci. 2009, 20, 177–184. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. Nature and Properties of Soil, 12th ed.; Prentice-Hall, Inc. Pearson Education: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
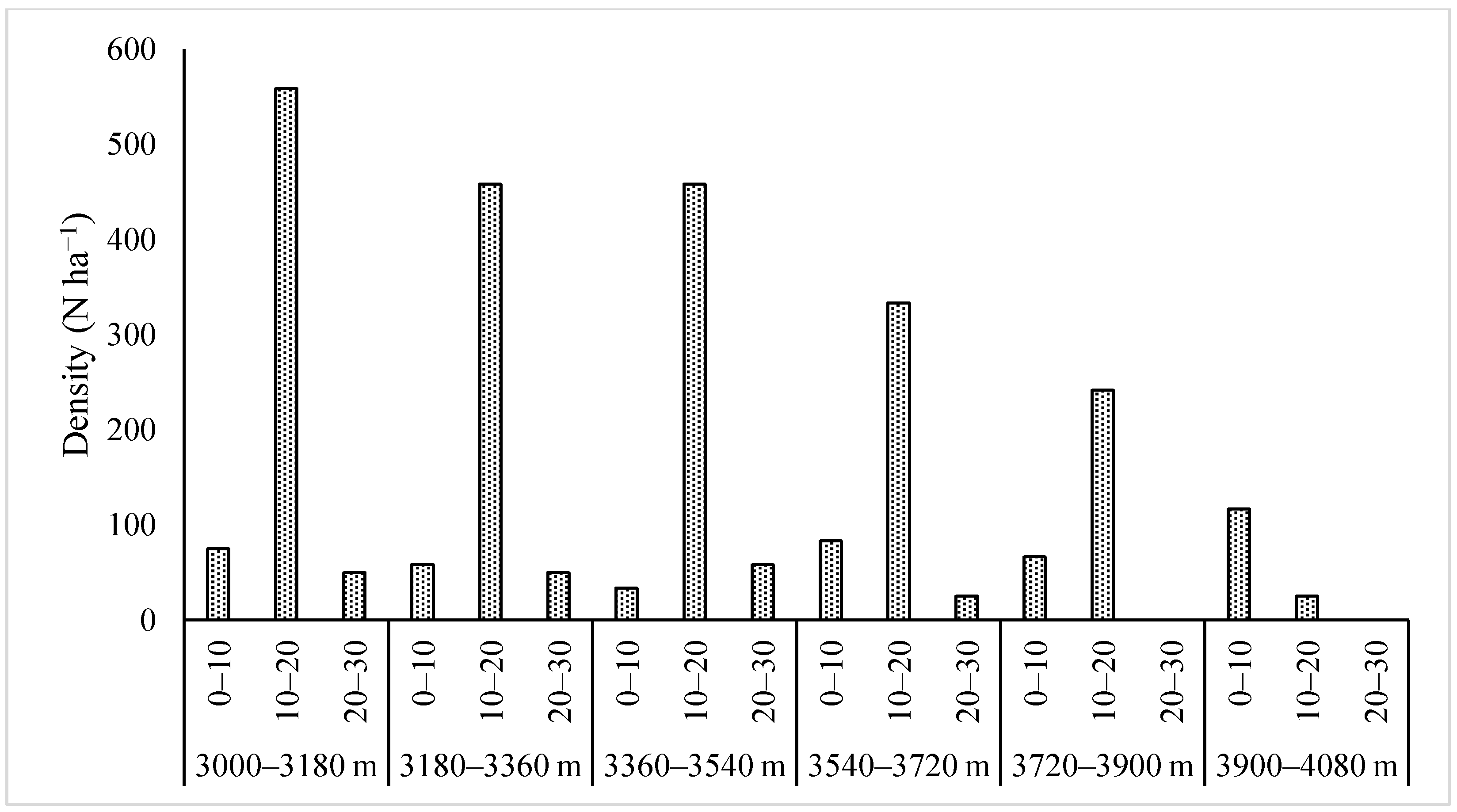
| Elevation (m asl) | Seedling (<0.5 m) | Sapling (0.5–2 m) | Seed Bank | |||||
|---|---|---|---|---|---|---|---|---|
| Density (N ha−1) | Height (m) | Diameter (cm) | Density (N ha−1) | Height (m) | Diameter (cm) | Number of Seeds m−2 | Seed Weight (g m−2) | |
| E1 (3000–3180 m) | 166.67 bcd | 0.33 a | 0.52 a | 333.33 b | 1.57 a | 3.39 a | 58.67 ab | 2.78 a |
| E2 (3180–3360 m) | 233.33 bc | 0.40 a | 0.77 a | 500.00 ab | 1.62 a | 3.38 a | 44.67 b | 2.13 ab |
| E3 (3360–3540 m) | 266.67 b | 0.34 a | 0.59 a | 533.33 a | 1.55 a | 3.41 a | 64.00 a | 3.00 a |
| E4 (3540–3720 m) | 433.33 a | 0.37 a | 0.53 a | 300.00 b | 1.51 a | 3.06 a | 51.33 ab | 2.47 ab |
| E5 (3720–3900 m) | 200.00 bcd | 0.36 a | 0.55 a | 366.67 b | 1.48 a | 3.14 a | 35.67 bc | 1.6 b |
| E6 (3900–4080 m) | 133.33 cd | 0.35 a | 0.64 a | 266.67 b | 1.45 a | 3.05 a | 19.00 c | 0.9 bc |
| E7 (4080–4260 m) | 100.00 cd | 0.22 b | 0.21 b | 133.33 c | 0.48 b | 2.00 b | 3.67 c | 0.2 c |
| E8 (>4260 m) | - | - | - | - | - | - | 0.2 d | 0.003 |
| SE (m) | 42.10 | 0.043 | 0.09 | 52.20 | 0.13 | 0.39 | 5.51 | 0.28 |
| Lsd | 131.16 | 0.13 | 0.30 | 162.63 | 0.44 | 1.13 | 18.44 | 0.94 |
| p-value | 0.00240 | 0.01600 | 0.04469 | 0.00233 | 0.00121 | 0.023185 | 0.00010 | 0.00021 |
| Elevation (m asl) | Soil pH | EC (dS m−2) | Bulk Density (g cm−3) | SOC (%) | CaCO3 (%) | Soil Texture | Available N (kg ha−1) | Available P (kg ha−1) | Available K (kg ha−1) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand (%) | Silt (%) | Clay (%) | |||||||||
| E1(3000–3180 m) | 6.70 a | 0.67 a | 1.01 b | 2.80 a | 5.17 ab | 33.97 c | 31.23 ab | 30.37 ab | 342.30 a | 6.90 ab | 235.94 a |
| E2 (3180–3360 m) | 6.55 ab | 0.65 ab | 1.03 b | 2.77 a | 5.42 a | 32.70 cd | 32.07 a | 29.57 b | 338.41 a | 7.48 a | 283.87 a |
| E3 (3360–3540 m) | 6.55 ab | 0.60 abc | 1.04 b | 2.86 a | 5.21 ab | 31.57 d | 31.97 a | 30.90 a | 348.18 a | 7.69 a | 276.69 a |
| E4 (3540–3720 m) | 6.33 b | 0.69 a | 1.06 b | 2.69 a | 4.58 bc | 33.13 c | 31.07 ab | 29.00 bc | 334.63 a | 8.67 a | 278.37 a |
| E5 (3720–3900 m) | 6.54 ab | 0.69 a | 1.27 ab | 2.30 b | 4.05 c | 33.20 c | 30.73 b | 27.87 c | 277.03 b | 5.73 ab | 173.79 b |
| E6 (3900–4080 m) | 6.60 a | 0.69 a | 1.06 b | 1.84 c | 3.72 c | 37.63 b | 30.37 b | 27.57 c | 218.51 c | 5.68 ab | 169.88 b |
| E7 (4080–4260 m) | 6.35 b | 0.56 b | 1.21 ab | 1.08 d | 3.75 c | 38.23 b | 30.57 b | 24.97 d | 189.32 d | 4.14 b | 147.19 b |
| E8 (>4260 m) | 6.33 b | 0.53 b | 1.33 a | 0.96 d | 3.79 c | 40.97 a | 30.23 b | 23.07 e | 136.08 e | 3.66 b | 127.53 b |
| SE (m) | 0.08 | 0.03 | 0.07 | 0.08 | 0.22 | 0.45 | 0.37 | 0.42 | 7.37 | 0.99 | 29.42 |
| Lsd | 0.24 | 0.10 | 0.21 | 0.23 | 0.67 | 1.38 | 1.12 | 1.27 | 22.56 | 3.03 | 90.09 |
| p-value | 0.02897 | 0.01685 | 0.02358 | 0.000 | 0.00010 | 0.0000 | 0.01977 | 0.0000 | 0.0000 | 0.03159 | 0.00981 |
| Dependent Variable | Equation | Adj R2 | p-Values of Estimates | VIF |
|---|---|---|---|---|
| Seedling density | 480.414 + 2.412 Available N—158.297 CaCO3—273.412 pH—15.255 Available P + 52.915 Silt | 0.686 | <0.05, 0.091, 0.012, 0.001, 0.099 | 4.01, 4.18, 1.46, 2.01, 3.02 |
| Sapling density | 1241.549 + 21.853 Available P—30.072 Sand | 0.614 | 0.04, 0.004 | 1.51,1.51 |
| Tree density | −2478.323 + 439.175 SOC + 276.616 Ph | 0.776 | <0.05, 0.04 | 1.01, 1.01 |
| Above-ground biomass | −103.536 + 22.637CaCO3 + 15.749 SOC | 0.709 | 0.04, 0.01 | 2.30,2.30 |
| Soil carbon density | −47.280 + 32.460 SOC + 42.610 BD | 0.988 | <0.05, <0.05 | 1.59,1.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, D.; Bhardwaj, D.R.; Sharma, P.; Bharti; Sankhyan, N.; Al-Ansari, N.; Linh, N.T.T. Population Dynamics of Juniperus macropoda Bossier Forest Ecosystem in Relation to Soil Physico-Chemical Characteristics in the Cold Desert of North-Western Himalaya. Forests 2022, 13, 1624. https://doi.org/10.3390/f13101624
Kumar D, Bhardwaj DR, Sharma P, Bharti, Sankhyan N, Al-Ansari N, Linh NTT. Population Dynamics of Juniperus macropoda Bossier Forest Ecosystem in Relation to Soil Physico-Chemical Characteristics in the Cold Desert of North-Western Himalaya. Forests. 2022; 13(10):1624. https://doi.org/10.3390/f13101624
Chicago/Turabian StyleKumar, Dhirender, Daulat Ram Bhardwaj, Prashant Sharma, Bharti, Neeraj Sankhyan, Nadhir Al-Ansari, and Nguyen Thi Thuy Linh. 2022. "Population Dynamics of Juniperus macropoda Bossier Forest Ecosystem in Relation to Soil Physico-Chemical Characteristics in the Cold Desert of North-Western Himalaya" Forests 13, no. 10: 1624. https://doi.org/10.3390/f13101624
APA StyleKumar, D., Bhardwaj, D. R., Sharma, P., Bharti, Sankhyan, N., Al-Ansari, N., & Linh, N. T. T. (2022). Population Dynamics of Juniperus macropoda Bossier Forest Ecosystem in Relation to Soil Physico-Chemical Characteristics in the Cold Desert of North-Western Himalaya. Forests, 13(10), 1624. https://doi.org/10.3390/f13101624








