Spider Assemblages of Tree Branches in Managed and Primeval Deciduous Stands of the Białowieża Forest
Abstract
1. Introduction
2. Materials and Methods
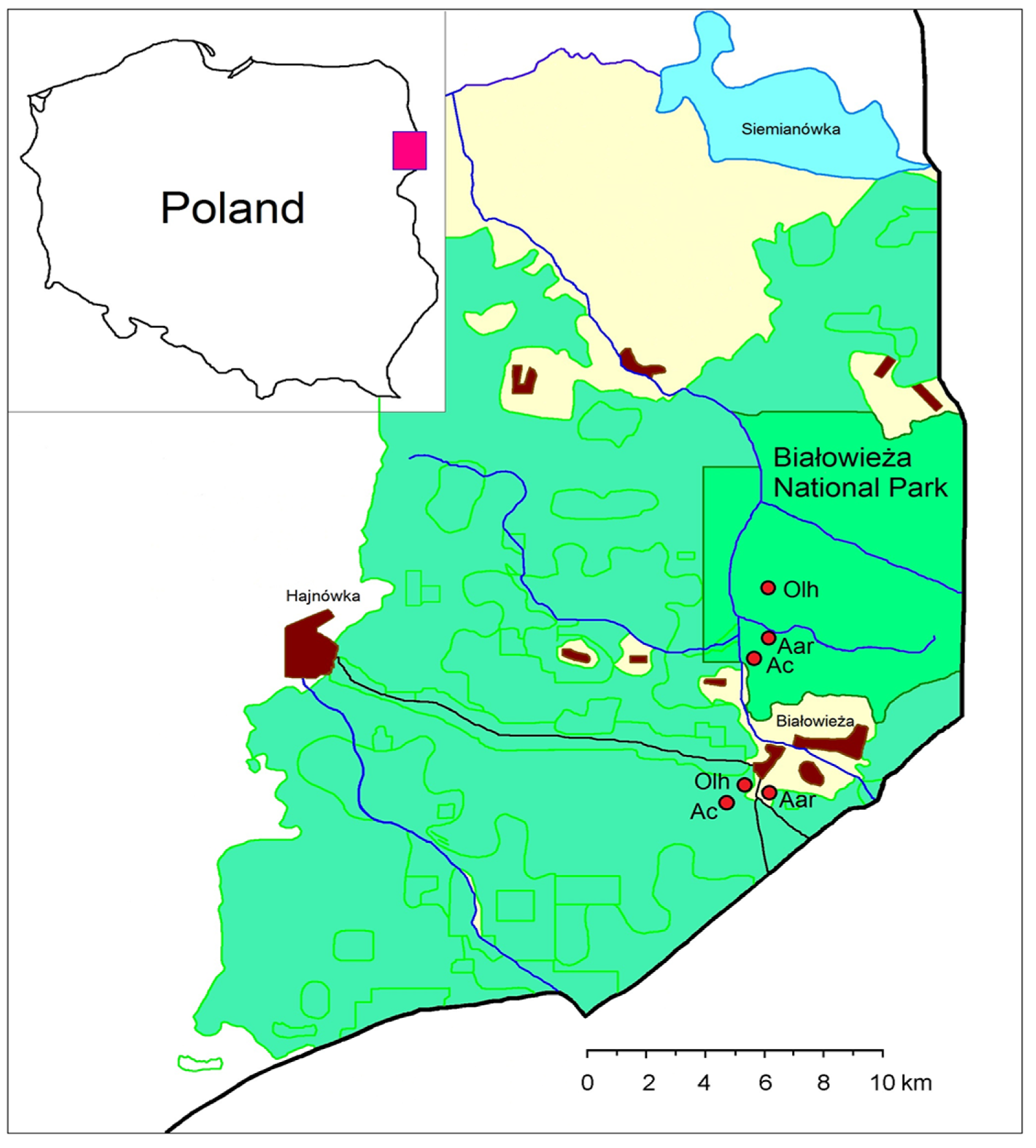
2.1. Study Area
2.2. Spider Sampling
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCaffrey, J.P.; Parrella, M.P.; Horsburgh, R.L. Evaluation of the limb-beating sampling method for estimating spider (Araneae) populations on apple trees. J. Arachnol. 1984, 11, 363–368. [Google Scholar]
- Herrmann, J.D.; Bailey, D.; Hofer, G.; Herzog, F.; Schmidt-Entling, M.H. Spiders associated with the meadow and tree canopies of orchard respond differently to habitat fragmentation. Landscape Ecol. 2010, 25, 1375–1384. [Google Scholar] [CrossRef]
- Russell-Smith, A.; Stork, N.E. Abundance and diversity of spider from the canopy of tropical rainforests with particular reference to Sulawesi, Indonesia. J. Trop. Ecol. 1994, 10, 545–558. [Google Scholar] [CrossRef]
- Faria, R.R.; Lima, T.N. Spiers associated with Psychotria carthagenensis Jacquin. (Rubiceae): Vegetative branches versus inflorescences, and the influence of Crematogaster sp. (Hymenoptera, Formicida), in South Pantanal, Brazil. Braz. J. Biol. 2008, 68, 229–232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodrigues, E.N.L.; Rodrigues, P.E.S.; de Mendonça, M., Jr. Spider species composition in the tree-shrub strata of riparian forests and its microhabitats in southern Brazil. Zoologia 2016, 33, e20150102. [Google Scholar] [CrossRef]
- Norberg, A. Energy content of some spiders and insects on branches of spruce (Picea abies) in winter; prey of certain passerine birds. Oikos 1978, 31, 222–229. [Google Scholar] [CrossRef]
- Blick, T.; Goßner, M. Spiders from branch-beating samples in tree crowns (Arachnida: Araneae), with remarks on Cinetata gradata (Linyphiidae) and Theridion boesenbergi (Theridiidae). Arachnol. Mitt. 2006, 31, 23–29. [Google Scholar]
- Korenko, S.; Kula, E.; Šimon, V.; Michalková, V.; Pekár, S. Are arboreal spiders associated with particular tree canopies? North-West. J. Zool. 2011, 7, 261–269. [Google Scholar]
- Otto, S.; Floren, A. The spider fauna (Araneae) of tree canopies in the Białowieża Forest. Fragm. Faun. 2007, 50, 57–70. [Google Scholar] [CrossRef]
- Mupepele, A.-C.; Müller, T.; Dittrich, M.; Floren, A. Are Temperate Canopy Spiders Tree-Species Specific? PLoS ONE 2014, 9, e86571. [Google Scholar] [CrossRef]
- Zhang, Q.; He, D.; Wu, H.; Shi, W.; Chen, C. Local-scale determinants of arboreal spider beta diversity in a temperate forest: Roles of tree architecture, spatial distance, and dispersal capacity. PeerJ 2018, 6, e5596. [Google Scholar] [CrossRef] [PubMed]
- Lapinski, W.; Tschapka, M. Desiccation resistance reflects patterns of microhabitat choice in a Central American assemblage of wandering spiders. J. Exp. Biol. 2014, 217, 2789–2795. [Google Scholar] [CrossRef]
- Stańska, M.; Stański, T.; Hawryluk, J. Spider assemblages on tree trunks in primeval deciduous forests of the Białowieża National Park in eastern Poland. Ent. Fenn. 2018, 29, 75–85. [Google Scholar] [CrossRef]
- Tomiałojć, L. Characteristics of old growth in the Białowieża Forest, Poland. Nat. Area. J. 1991, 11, 7–18. [Google Scholar]
- Bobiec, A. Living stands and dead wood in the Białowieża forest: Suggestions for restoration management. Forest Ecol. Manag. 2002, 165, 125–140. [Google Scholar] [CrossRef]
- Mikusiński, G.; Bubnicki, J.W.; Churski, M.; Czeszczewik, D.; Walankiewicz, W.; Kuijper, D.P.J. Is the impact of loggings in the last primeval lowland forest in Europe underestimated? The conservation issues of Białowieża Forest. Biol. Conserv. 2018, 227, 266–274. [Google Scholar] [CrossRef]
- Walankiewicz, W.; Czeszczewik, D.; Mitrus, C.; Bida, E. Znaczenie martwych drzew dla zespołu dzięciołów w lasach liściastych Puszczy Białowieskiej [Snag importance for woodpeckers in deciduous stands of the Białowieża Forest]. Not. Orn. 2002, 43, 61–71. [Google Scholar]
- Wesołowski, T. Virtual Conservation: How the European Union is Turning a Blind Eye to its Vanishing Primeval Forests. Conserv. Biol. 2005, 19, 1349–1358. [Google Scholar] [CrossRef]
- Walankiewicz, W.; Czeszczewik, D.; Tumiel, T.; Stański, T. Woodpeckers abundance in the Białowieża Forest—A comparison between deciduous, strictly protected and managed stands. Ornis Polon. 2011, 52, 161–168. [Google Scholar]
- Czeszczewik, D.; Zub, K.; Stanski, T.; Sahel, M.; Kapusta, A.; Walankiewicz, W. Effects of forest management on bird assemblages in the Bialowieza Forest, Poland. iForest 2014, 8, 377. [Google Scholar] [CrossRef]
- Skłodowski, J. Consequence of the transformation of a primeval forest into a managed forest for carabid beetles (Coleoptera: Carabidae)—A case study from Białowieża (Poland). Eur. J. Entomol. 2014, 111, 639–648. [Google Scholar] [CrossRef]
- Sławski, M.; Sławska, M. Seven Decades of Spontaneous Forest Regeneration after Large-Scale Clear-Cutting in Białowieża Forest do not Ensure the Complete Recovery of Collembolan Assemblages. Forests 2019, 10, 948. [Google Scholar] [CrossRef]
- Buchar, J.; Ruzicka, V. Catalogue of Spiders of the Czech Republic, 1st ed.; Peres Publ.: Praha, Czech Republic, 2002; pp. 1–351. [Google Scholar]
- Stańska, M. Rare and threatened spider species (Araneae) in selected types of deciduous forests in the Białowieża Forest. Nat. Conserv. 2007, 64, 13–29. [Google Scholar]
- Stańska, M.; Stański, T.; Gładzka, A.; Bartos, M. Spider assemblages of hummocks and hollows in a primeval alder carr in the Białowieża National Park—Effect of vegetation structure and soil humidity. Pol. J. Ecol. 2016, 64, 564–577. [Google Scholar] [CrossRef]
- Lafage, D.; Djoudi, A.; Perrin, G.; Gallet, S.; Pétillon, J. Responses of ground-dwelling spider assemblages to changes vegetation from wet oligotrophic habitats of Western France. Arthropod-Plant Interact. 2019, 13, 653–662. [Google Scholar] [CrossRef]
- Ramberg, E.; Burdon, F.J.; Sargac, J.; Kupilas, B.; Rȋşnoveanu, G.; Lau, D.C.P.; Johnson, R.K.; McKie, B.G. The structure of riparian vegetation in agricultural landscapes influences spider communities and aquatic-terrestrial linkages. Water 2020, 12, 2855. [Google Scholar] [CrossRef]
- Gallé, R.; Gallé-Szpisjak, N.; Zsigmond, A.-R.; Könczey, B.; Urák, I. Tree species and microhabitat affect forest bog spider fauna. Eur. J. For. Res. 2021, 140, 691–702. [Google Scholar] [CrossRef]
- Esquivel-Gómez, L.; Abdala-Roberts, L.; Pinkus-Rendón, M.; Parra-Tabla, V. Effects of tree species diversity on a community of weaver spiders in a tropical forest plantation. Biotropica 2017, 49, 63–70. [Google Scholar] [CrossRef]
- Košulič, O.; Michalko, R.; Hula, V. Impact of canopy openness on spider communities: Implications for conservation management of formerly coppiced oak forests. PLoS ONE 2016, 11, e0148585. [Google Scholar]
- Ziesche, T.M.; Roth, M. Influence of environmental parameters on small-scale distribution of soil-dwelling spiders in forests: What makes the difference, tree species or microhabitat? Forest Ecol. Manag. 2008, 255, 738–752. [Google Scholar] [CrossRef]
- Tomiałojć, L.; Wesołowski, T. Diversity of the Białowieża Forest avifauna in space and time. J. Ornith. 2004, 145, 8–92. [Google Scholar] [CrossRef]
- Locket, G.H.; Millidge, A.F.; Merrett, P. British Spiders, 1st ed.; Ray Society: London, UK, 1974; p. 450. [Google Scholar]
- Roberts, M.J. The spiders of Graet Britain and Ireland, 1st ed.; Harley Books: Colchester, UK, 1985; Volume 1, p. 229, Volume 2, p. 204. [Google Scholar]
- Heimer, S.; Nentwig, W. Spinnen Mitteleuropas: Ein Bestimmungsbuch, 1st ed.; Paul Parey: Berlin, Germany, 1991; pp. 1–543. [Google Scholar]
- Roberts, M.J. Spiders of Britain and Northern Europe. Collins Field Guide, 1st ed.; Harper Collins: London, UK, 1995; pp. 1–383. [Google Scholar]
- Chao, A.; Jost, L. Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef]
- Colwell, R.K.; Mao, C.X.; Chang, J. Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 2004, 85, 2717–2727. [Google Scholar] [CrossRef]
- Colwell, R.K.; Chao, A.; Gotelli, N.J.; Lin, S.-Y.; Mao, C.X.; Chazdon, R.L.; Longino, J.T. Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J. Plant Ecol. 2012, 5, 3–21. [Google Scholar] [CrossRef]
- Cardoso, P.; Sharff, N.; Gaspar, C.; Henriques, S.S.; Carvalho, R.; Castro, P.H.; Schmidt, J.B.; Silva, I.; Sztüs, T.; de Castro, A.; et al. Rapid biodiversity assessment of spiders (Araneae) using semi-quantitative sampling: A case study in a Mediterranean forest. Insect Conserv. Divers. 2008, 1, 71–84. [Google Scholar] [CrossRef]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples; Version 9.1.0.; Robert K. Colwell: Boulder, CO, USA, 2019. [Google Scholar]
- Gutowski, J.M.; Jaroszewicz, B. Białowieża Primeval Forest as a refuge of the European entomofauna. Wiad. Entomol. 2004, 23, 67–87, (In Polish, English Summary). [Google Scholar]
- Okołów, C.; Karaś, M.; Bołbot, A. (Eds.) Białowieża National Park. Know It—Under-Stand It—Protect It; Białowieski Park Narodowy: Białowieża, Poland, 2009; pp. 37–58. [Google Scholar]
- Wesołowski, T.; Czeszczewik, D.; Rowiński, P. Effects of forest management on Three-toed Woodpecker Picoides tridactylus distribution in the Białowieża Forest (NE Poland): Conservation implications. Acta Ornithol. 2005, 40, 53–60. [Google Scholar] [CrossRef][Green Version]
- Skłodowski, J.J.W. Antropogenic transformation of ground beetle assemblages (Coleoptera: Carabidae) in Białowieża Forest, Poland: From primeval forests to managed woodlands of various ages. Entomol. Fenn. 2006, 17, 296–314. [Google Scholar] [CrossRef]
- Malumbres-Olarte, J.; Vink, C.J.; Ross, J.G.; Cruickshank, R.H.; Paterson, A.M. The role of habitat complexity on spider communities in native alpine grasslands of New Zealand. Insect Conserv. Diver. 2013, 6, 124–134. [Google Scholar] [CrossRef]
- St. Pierre, J.I.; Kovalenko, K.E. Effect of habitat complexity attributes on species richness. Ecosphere 2014, 5, 22. [Google Scholar] [CrossRef]
- Áviala, A.C.; Stenert, C.; Rodrigues, E.N.L.; Maltchik, L. Habitat structure determines spider diversity in highland ponds. Ecol. Res. 2017, 32, 359–367. [Google Scholar] [CrossRef]
- Hamřik, T.; Košulič, O. Impact of small-scale conservation management methods on spider assemblages in xeric grassland. Agr. Ecosyst. Environ. 2021, 307, 107225. [Google Scholar] [CrossRef]
- Stańska, M.; Stański, T. Plant-dwelling spider communities of three developmental phases in primeval oak-lime-hornbeam forest in the Białowieża National Park, Poland. Eur. Zool. J. 2021, 88, 706–717. [Google Scholar] [CrossRef]



| Family/Genus/Species | Man. Olh ad./juv. | BNP Olh ad./juv. | Man. Aar ad./juv. | BNP Aar ad./juv. | Man. Ac ad./juv. | BNP Ac ad./juv. |
|---|---|---|---|---|---|---|
| Family Amaurobiidae | ||||||
| Amaurobius fenestralis (Ström, 1768) | -/1 | |||||
| Family Anyphaeniidae | ||||||
| Anyphaena accentuata (Walckenaer, 1802) | (31) 3/107 | (10) 3/12 | (46) -/34 | (17) 1/21 | (8) 2/20 | (15) 1/23 |
| Family Araneidae | ||||||
| Araneidae un. Clerck, 1757 | -/1 | -/2 | ||||
| Araneus diadematus Clerck, 1757 | -/2 | -/1 | ||||
| Araneus marmoreus Clerck, 1757 | -/2 | |||||
| Araneus quadratus Clerck, 1757 | -/1 | |||||
| Araneus sp. Clerck, 1757 | -/5 | |||||
| Araniella cucurbitina (Clerck, 1757) | 1/- | 1/- | ||||
| Araniella proxima (Kulczyński, 1885) | 2/- | |||||
| Araniella sp. Chamberlin & Ivie, 1942 | -/6 | -/4 | -/4 | -/2 | -3 | -/11 |
| Cyclosa conica (Pallas, 1772) | -/4 | (6) 3/6 | -/1 | -/1 | ||
| Gibbaranea bituberculata (Walckenaer, 1802) | -/1 | |||||
| Family Clubionidae | ||||||
| Clubiona lutescens Westring, 1851 | 3/- | |||||
| Clubiona sp. Latreille, 1804 | -/3 | -/1 | -/5 | -/5 | -/2 | -/1 |
| Family Dictynidae | ||||||
| Dictyna arundinacea (Linnaeus, 1758) | 1/- | |||||
| Dictyna sp. Sundevall, 1833 | -/4 | -/4 | -/5 | |||
| Family Linyphiidae | ||||||
| Bathyphantes sp. Menge, 1866 | -/2 | |||||
| Ceratinella brevis (Wider, 1834) | 1/- | |||||
| Drapetisca socialis (Sundevall, 1833) | -/3 | 1/- | ||||
| Entelecara acuminata (Wider, 1834) | 1/- | |||||
| Gongylidium rufipes (Linnaeus, 1758) | 6/- | 2/- | 1/- | |||
| Helophora insignis (Blackwall, 1841) | 1/- | (13) -/16 | -/8 | -/5 | ||
| Hypomma cornutum (Blackwall, 1833) | 1/- | |||||
| Tenuiphantes sp. Saaristo & Tanasevitch, 1996 | -/5 | -/18 | -/5 | -/2 | ||
| Linyphia sp. Latreille, 1804 | -/1 | |||||
| Linyphia triangularis (Clerck, 1757) | 1/2 | 2/2 | ||||
| Linyphiidae un. Blackwall, 1859 | -/19 | -/18 | -/18 | -/17 | -/17 | |
| Neriene emphana (Walckenaer, 1841) | 1/3 | 2/1 | 2/1 | |||
| Neriene montana (Clerck, 1757) | -/1 | -/5 | -/6 | (6) -/10 | ||
| Neriene peltata (Wider, 1834) | 1/- | (6) 7/3 | (22) 2/26 | 1/4 | (9) 1/13 | |
| Neriene sp. Blackwall, 1833 | -/51 | -/25 | -/2 | -/32 | -/50 | |
| Pityohyphantes phrygianus (C. L. Koch, 1836) | -/2 | |||||
| Porrhomma oblitum (O. Pickard- Cambridge, 1871) | 6/- | |||||
| Porrhomma pygmaeum (Blackwall, 1834) | 1/- | (50) 131/- | (28) 44/- | |||
| Trematocephalus cristatus (Wider, 1834) | (50) -/178 | (19) -/29 | (32) -/24 | (6) -/8 | (23) -/61 | 1/5 |
| Family Lycosidae | ||||||
| Pardosa sp. C. L. Koch, 1847 | -/12 | |||||
| Family Mimetidae | ||||||
| Ero sp. C. L. Koch, 1836 | -/1 | |||||
| Family Philodromidae | ||||||
| Philodromus rufus Walckenaer, 1826 | 1/- | |||||
| Philodromus sp. Walckenaer, 1826 | -/7 | -/10 | -/4 | -/2 | -/11 | |
| Family Pisauridae | ||||||
| Pisaura mirabilis (Clerck, 1757) | -/2 | |||||
| Family Salticidae | ||||||
| Salticidae un. Blackwall, 1841 | -/1 | |||||
| Family Tetragnathidae | ||||||
| Metellina mengei (Blackwall, 1869) | 5/- | |||||
| Metellina segmentata (Clerck, 1757) | 2/- | 4/- | ||||
| Metellina sp. Chamberlin & Ivie, 1941 | -/1 | -/9 | -/4 | -/4 | ||
| Pachygnatha listeri Sundevall, 1830 | 1/- | |||||
| Pachygnatha sp. Sundevall, 1823 | -/1 | |||||
| Tetragnatha montana Simon, 1874 | 2/- | |||||
| Tetragnatha sp. Menge, 1866 | -/16 | -/39 | -/27 | -/28 | -/32 | |
| Family Theridiidae | ||||||
| Parasteatoda sp. Archer, 1946 | -/1 | -/1 | ||||
| Enoplognatha ovata (Clerck, 1757) | (7) 9/17 | (23) 6/30 | (17) 12/10 | 2/3 | 1/2 | |
| Robertus arundineti (O. Pickard-Cambridge, 1871) | 1/- | |||||
| Rugathodes instabilis (O. Pickard-Cambridge, 1871) | 3/- | |||||
| Paidiscura pallens (Blackwall, 1834) | 1/- | |||||
| Theridiidae un. Sundevall, 1833 | -/26 | -/9 | -/6 | -/2 | -/3 | -/18 |
| Platnickina tincta (Walckenaer, 1802) | 1/- | 1/- | ||||
| Theridion varians Hahn, 1833 | 2/- | 1/1 | 1/0 | 2/1 | 1/0 | |
| Family Thomisidae | ||||||
| Diaea dorsata (Fabricius, 1777) | (8) 3/24 | (24) -/37 | 1/1 | (11) 1/13 | -/5 | (15) 2/21 |
| Misumena vatia (Clerck, 1757) | 1/- | |||||
| Ozyptila sp. Simon, 1864 | -/2 | -/2 | -/1 | -/1 | -/1 | |
| Xysticus cristatus (Clerck, 1757) | 1/- | |||||
| Xysticus sp. C. L. Koch, 1835 | -2 | |||||
| Xysticus ulmi (Hahn, 1831) | 1/- | |||||
| Total no. of individuals | 21/471 | 29/189 | 8/170 | 28/180 | 151/208 | 70/236 |
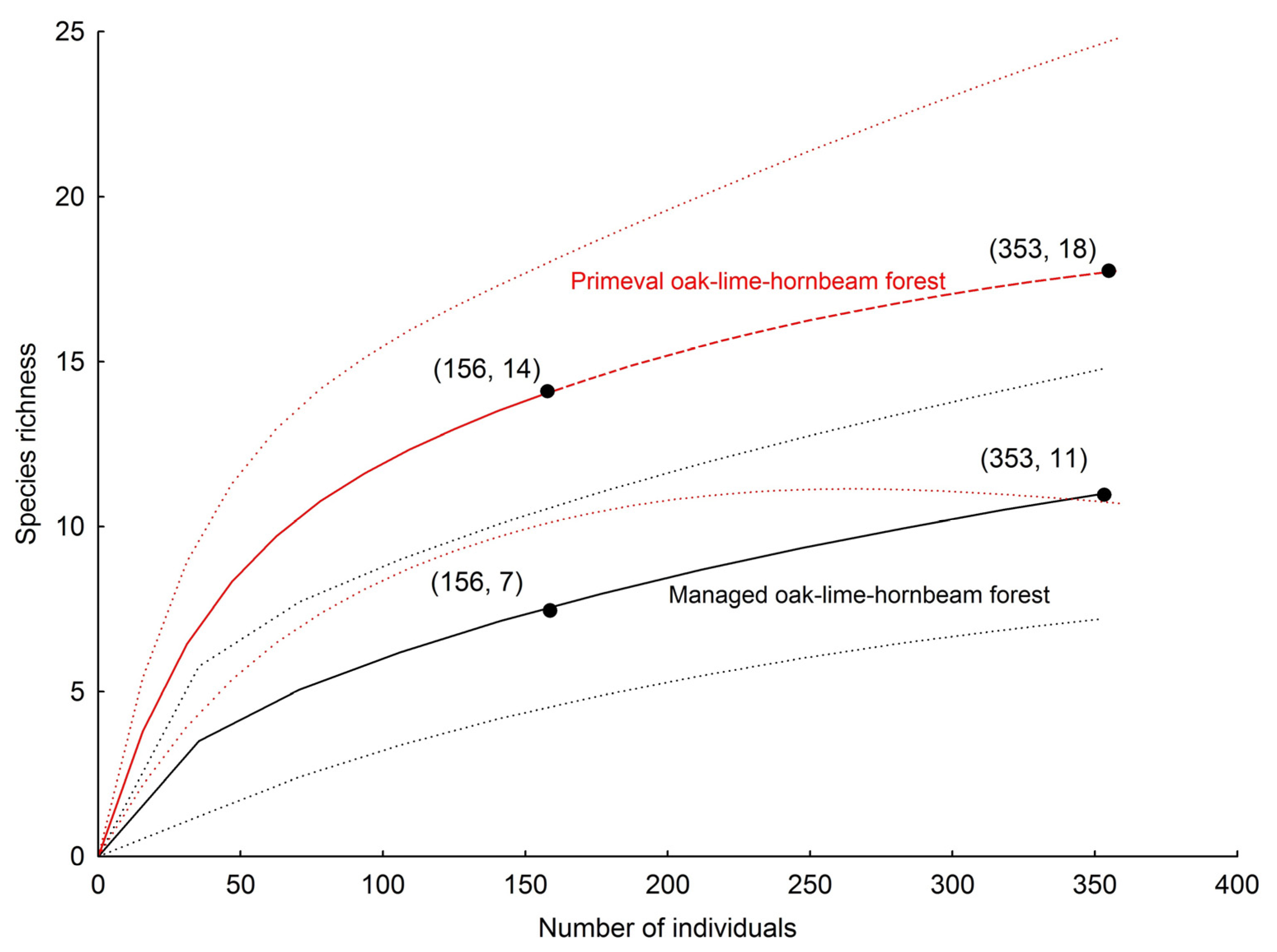
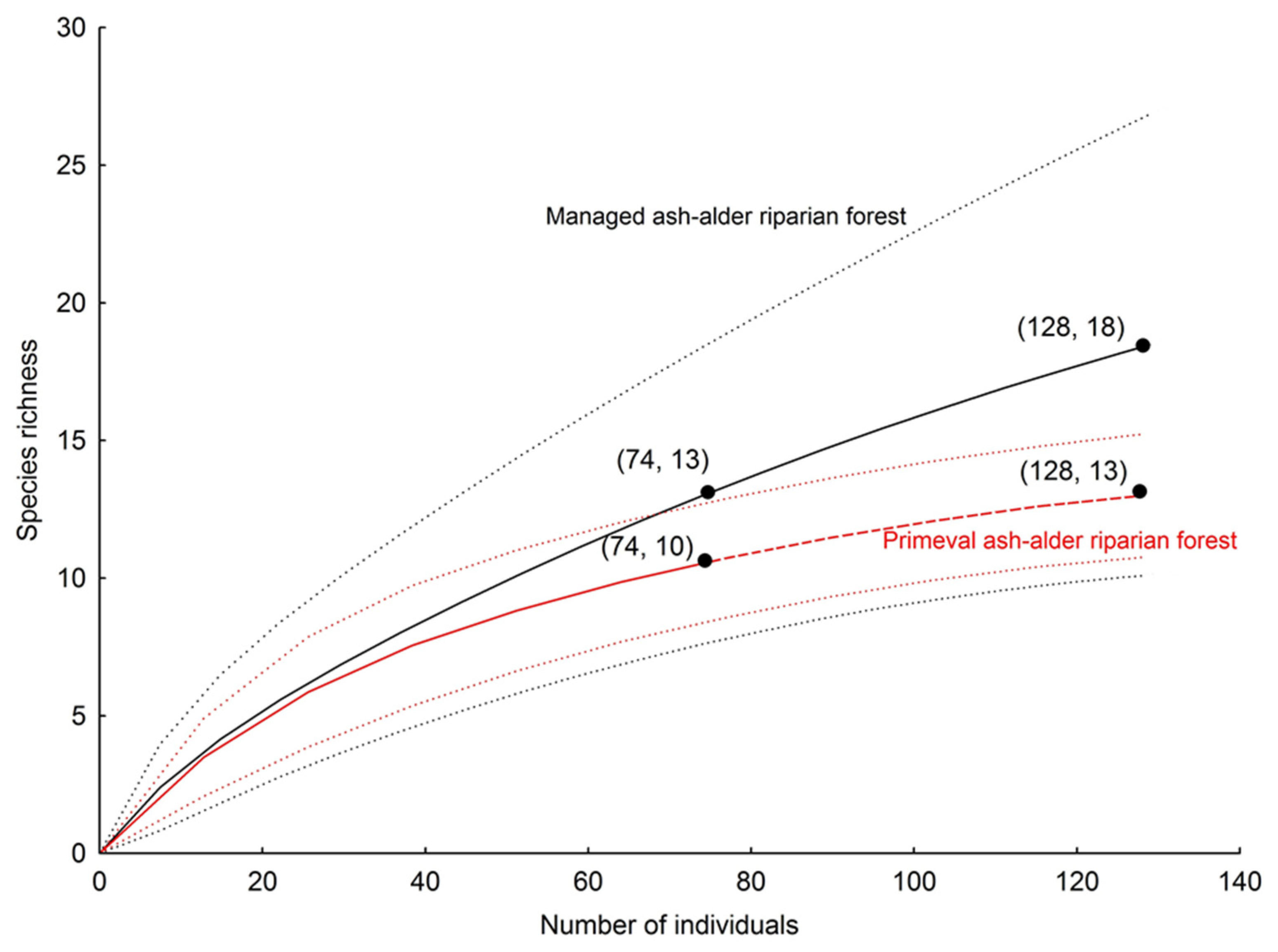
| Total no. of species | 11 | 14 | 13 | 13 | 15 | 23 |
| No. of common species | 6 | 5 | 11 | |||
| Man Olh | BNP Olh | Man Aar | BNP Aar | Man Ac | BNP Ac | |
|---|---|---|---|---|---|---|
| Observed richness | 11 | 14 | 13 | 13 | 15 | 23 |
| Estimates | ||||||
| Chao 1 ± SD | 15 ± 5 | 18 ± 7 | 17 ± 4 | 19 ± 7 | 19 ± 7 | 43 ± 20 |
| Chao 2 ± SD | 17 ± 7 | 20 ± 7 | 31 ± 18 | 14 ± 2 | 19 ± 4 | 48 ± 21 |
| Jackknife 1 ± SD | 16 ± 3 | 19 ± 3 | 21 ± 2 | 17 ± 2 | 20 ± 2 | 35 ± 3 |
| Jackknife 2 | 18 | 21 | 27 | 16 | 21 | 43 |
| Sampling completeness | 73% | 78% | 76% | 68% | 79% | 53% |
| Variable | df1, df2 | F | p |
|---|---|---|---|
| Forest type | 2, 47 | 5.839 | 0.005 |
| Managed/Primeval | 1, 47 | 9.487 | 0.003 |
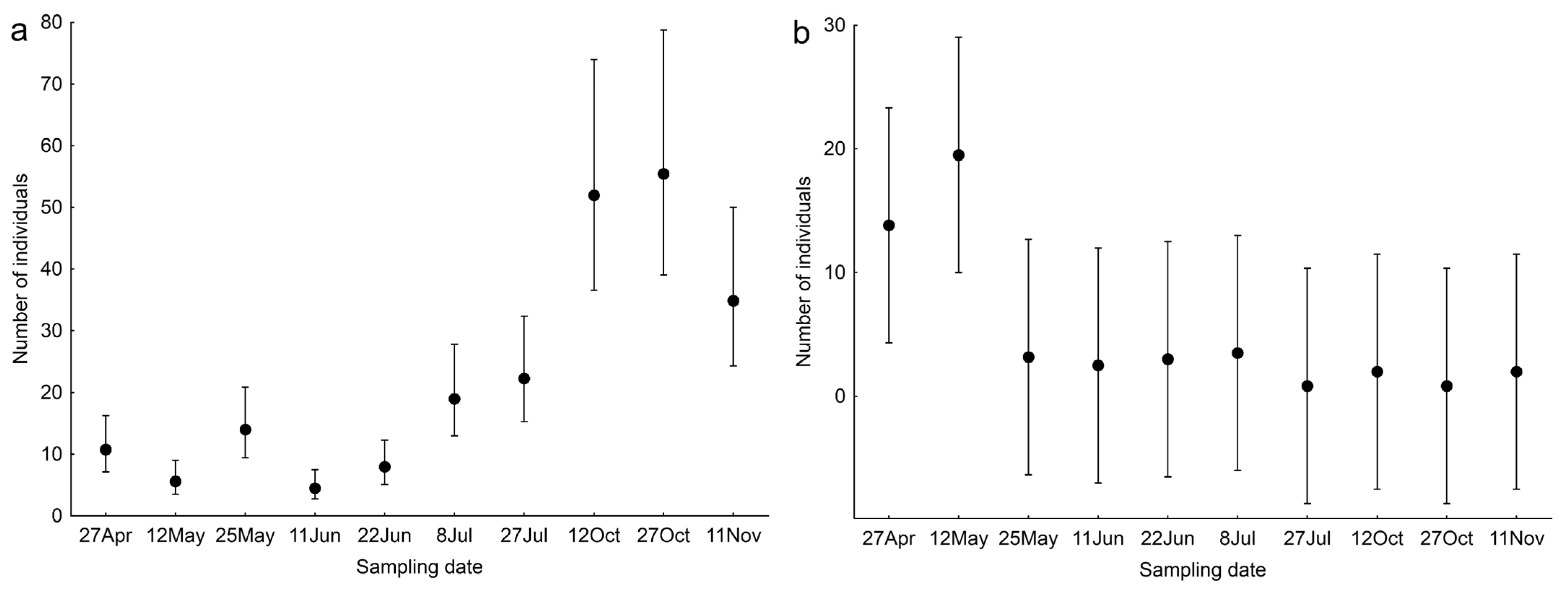
| Sampling date | 9, 47 | 1.931 | 0.070 |
| Random effect | Estimate ± SE | Z | p |
| Plot | 0.001 ± 0.014 | 0.077 | 0.939 |
| Variable | df1, df2 | F | p |
|---|---|---|---|
| Forest type | 2, 47 | 3.485 | 0.039 |
| Managed/Primeval | 1, 47 | 0.308 | 0.582 |
| Sampling date | 9, 47 | 1.802 | 0.093 |
| Random effect | Estimate ± SE | Z | p |
| Plot | 2.027 ± 15.474 | 0.131 | 0.896 |
| Variable | df1, df2 | F | p |
|---|---|---|---|
| Forest type | 2, 47 | 0.852 | 0.433 |
| Managed/Primeval | 1, 47 | 0.530 | 0.470 |
| Sampling date | 9, 47 | 77.632 | <0.001 |
| Random effect | Estimate ± SE | Z | p |
| Plot | 0.165 ± 0.169 | 0.976 | 0.329 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stańska, M.; Stański, T.; Bartos, M. Spider Assemblages of Tree Branches in Managed and Primeval Deciduous Stands of the Białowieża Forest. Forests 2022, 13, 5. https://doi.org/10.3390/f13010005
Stańska M, Stański T, Bartos M. Spider Assemblages of Tree Branches in Managed and Primeval Deciduous Stands of the Białowieża Forest. Forests. 2022; 13(1):5. https://doi.org/10.3390/f13010005
Chicago/Turabian StyleStańska, Marzena, Tomasz Stański, and Maciej Bartos. 2022. "Spider Assemblages of Tree Branches in Managed and Primeval Deciduous Stands of the Białowieża Forest" Forests 13, no. 1: 5. https://doi.org/10.3390/f13010005
APA StyleStańska, M., Stański, T., & Bartos, M. (2022). Spider Assemblages of Tree Branches in Managed and Primeval Deciduous Stands of the Białowieża Forest. Forests, 13(1), 5. https://doi.org/10.3390/f13010005






