Degradation of White Birch Shelterbelts by the Attack of White-Spotted Longicorn Beetles in Central Hokkaido, Northern Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Status
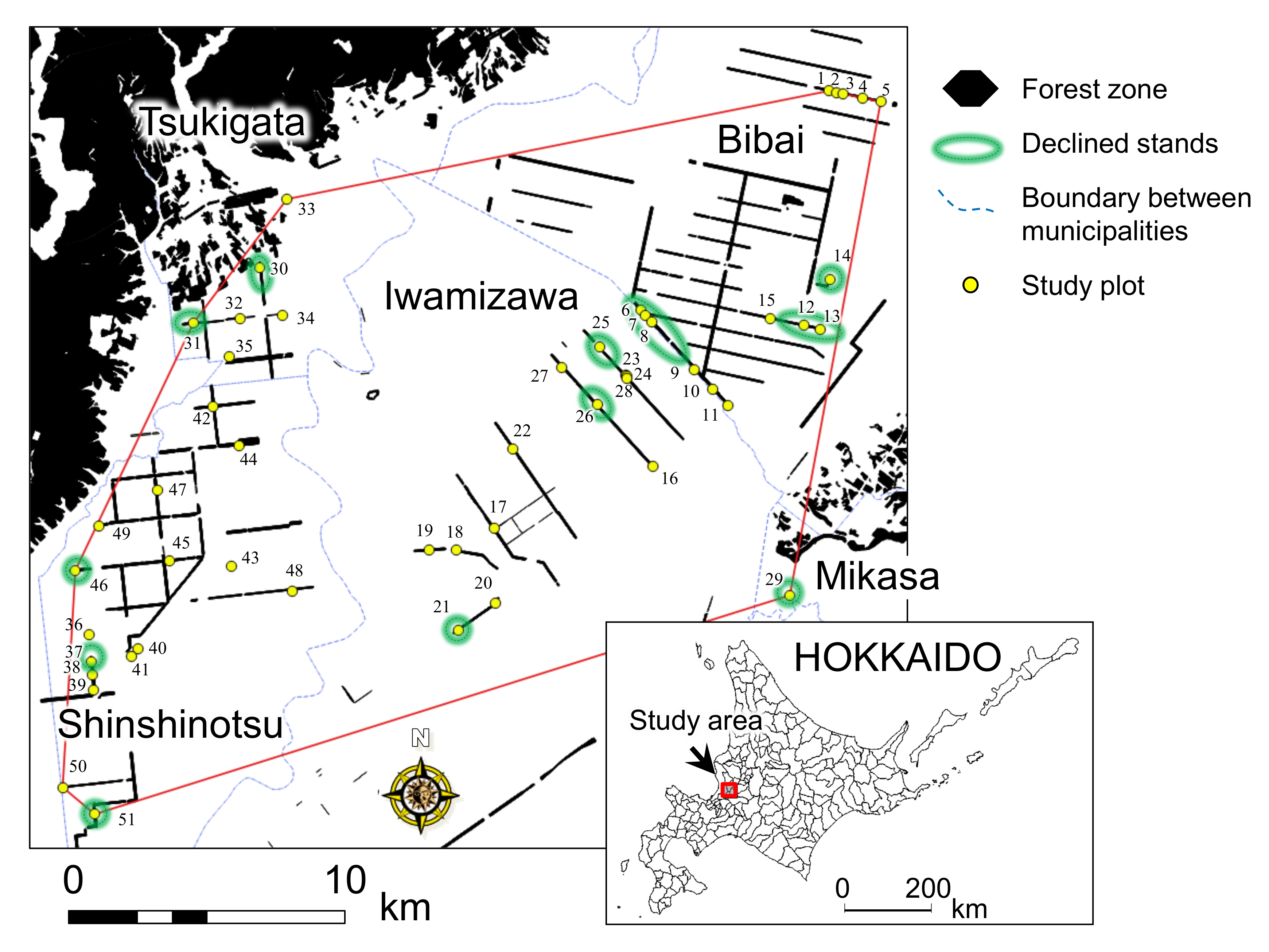
2.2. Study Area
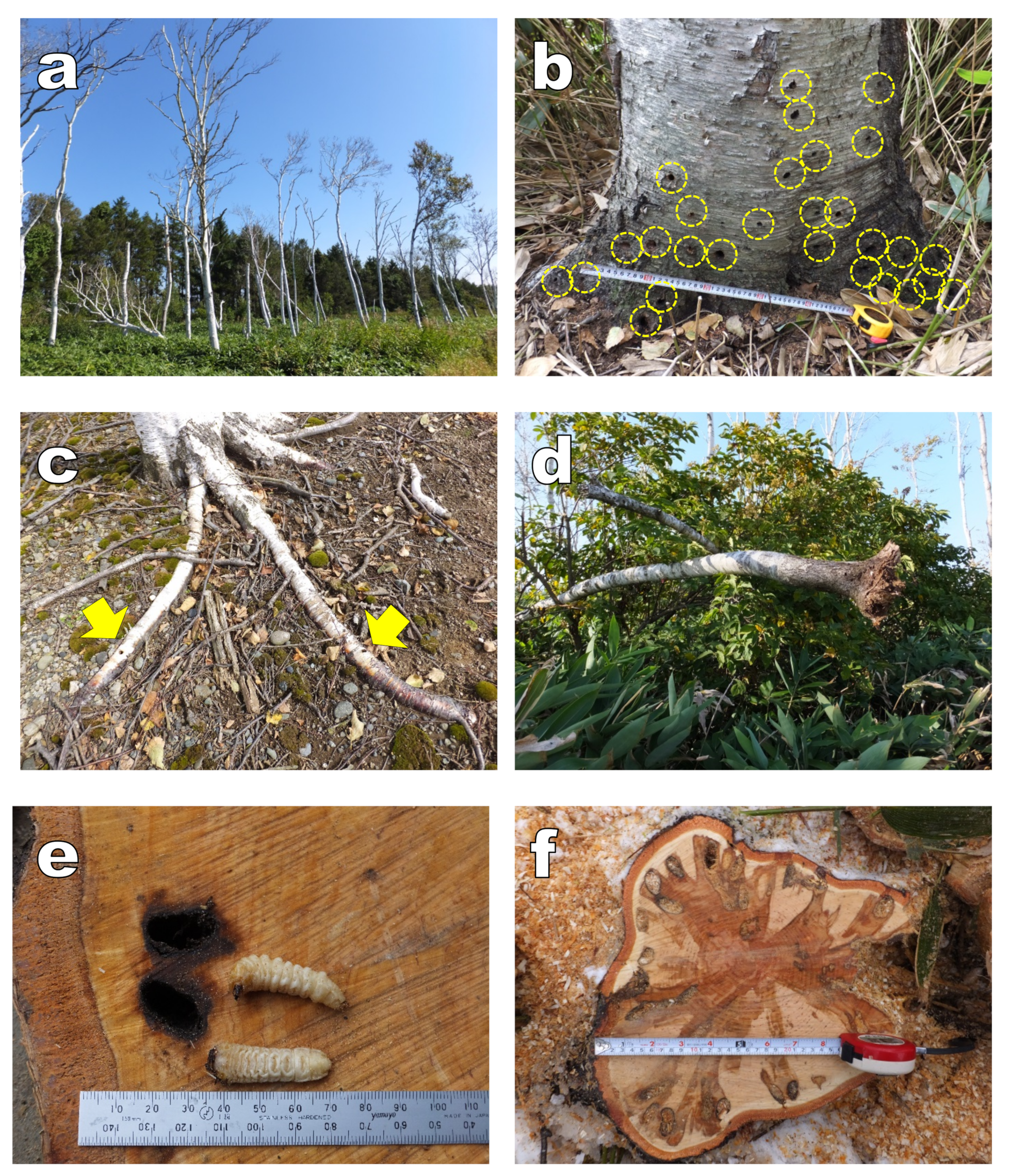
2.3. Relationship between the Number of Adult Exit Holes and Tree Vigor
μ = exp (a0j + a1j DBH)
2.4. Observation of Infested Wood Inside
2.5. Estimating the Value of Longicorn Severity of Attacks as an Indicator of Stand Degradation
2.6. Statistical Analyses
3. Results
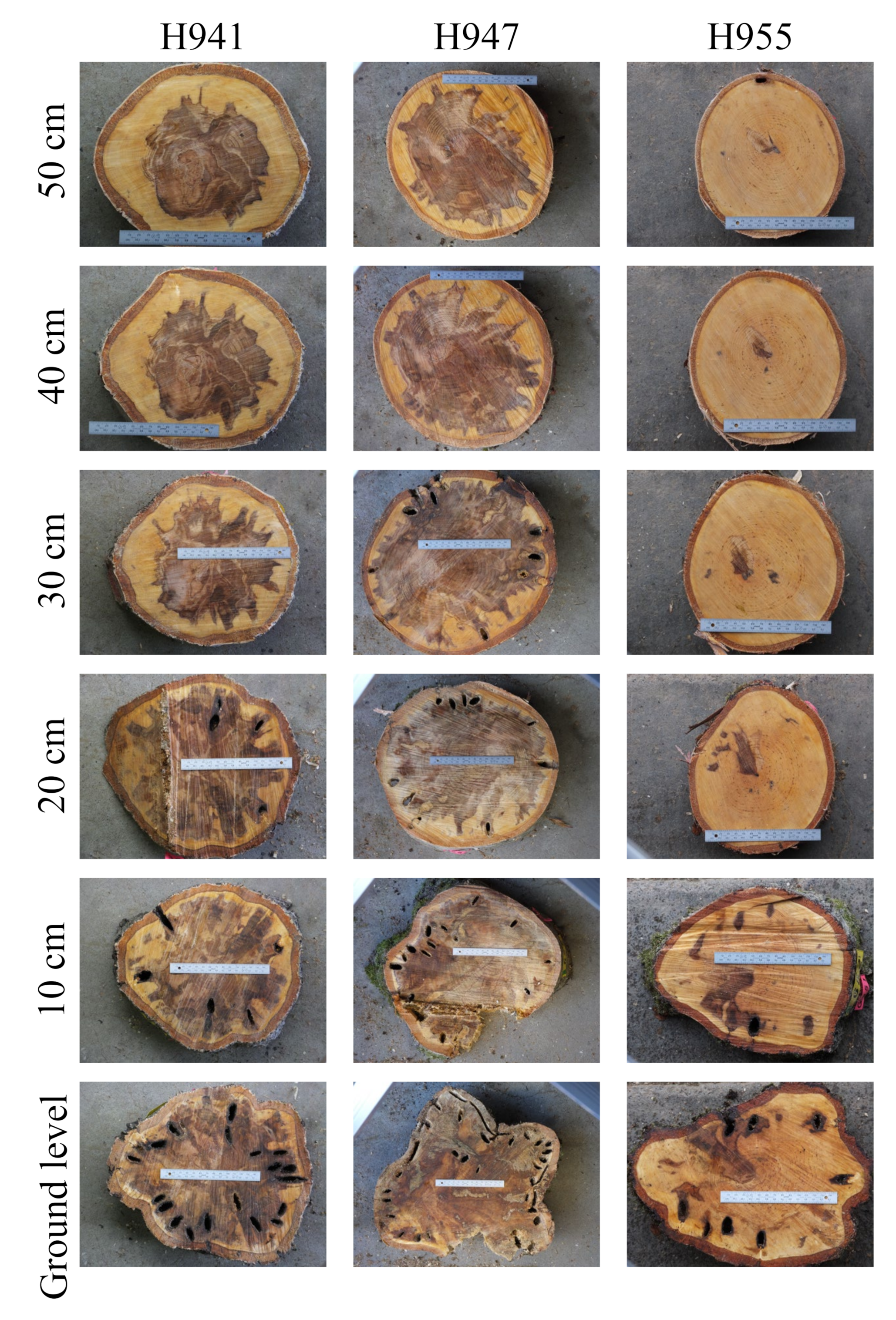
3.1. Infected Wood Inside
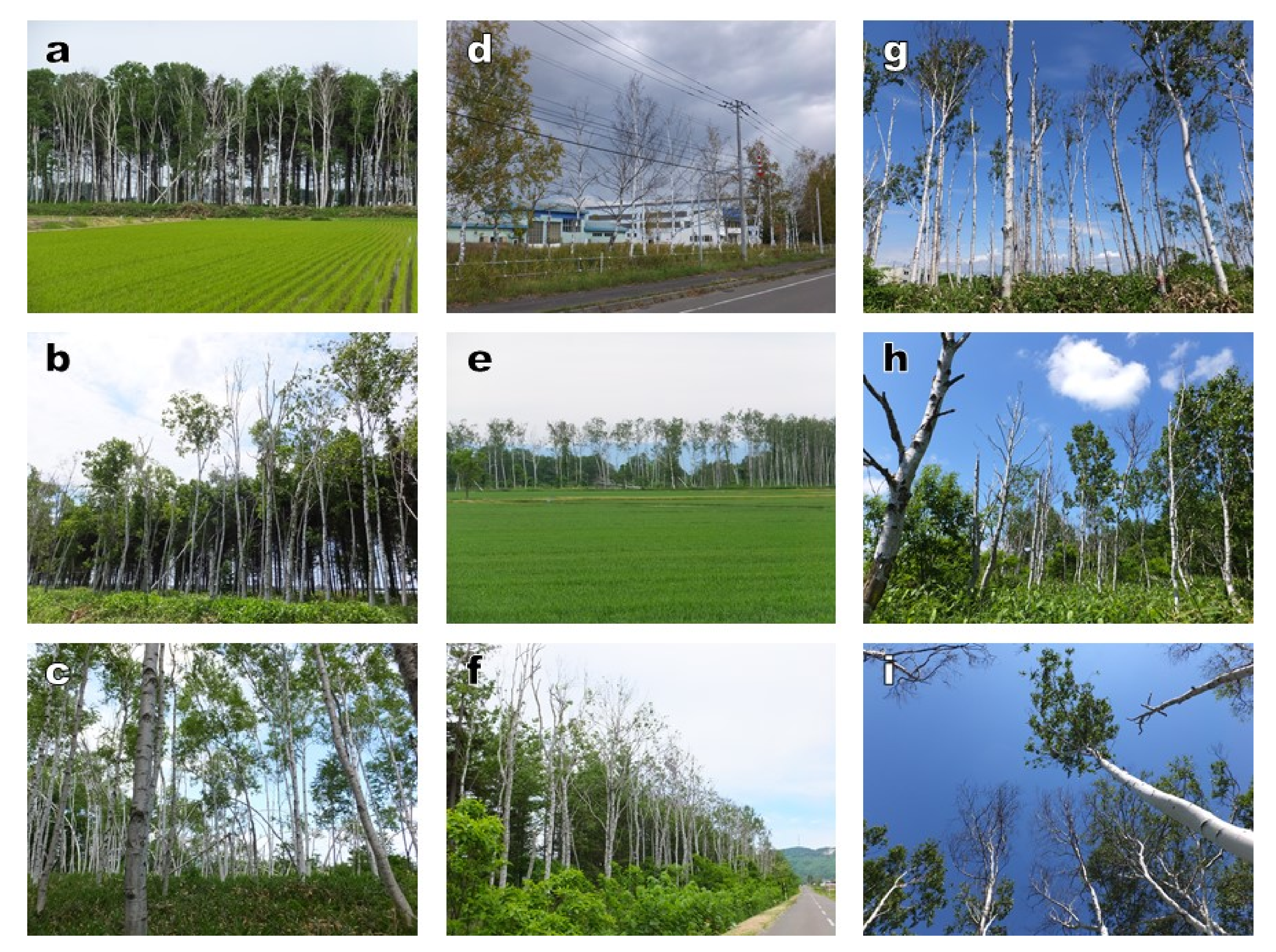
3.2. Relationship between the Decline of White Birch and Infestation by Longicorn Beetle
3.3. Estimating the Value of Longicorn Severity of Attacks as an Indicator of Stand Degradation
4. Discussion
4.1. Infected Wood Inside
4.2. Degradation of White Birch Shelterbelts
4.3. Cause of Massive Mortality
4.4. Pest Control for the Shelterbelts
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Haack, R.A.; Hérard, F.; Sun, J.; Turgeon, J.J. Managing invasive populations of Asian longhorned beetles and citrus longhorned beetle: A worldwide perspective. Ann. Rev. Entomol. 2010, 55, 521–546. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Angeli, S.; Schuetz, S.; Luo, Y.; Hajek, A.E. Ecology and management of exotic and endemic Asian longhorned beetle Anoplophora glabripennis. Agric. For. Entomol. 2009, 11, 359–375. [Google Scholar] [CrossRef]
- Meng, P.S.; Hoover, K.; Keena, M.A. Asian longhorned beetle (Coleoptera: Cerambycidae), an introduced pest of maple and other hardwood trees in North America and Europe. J. Integ. Pest. Manag. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Van der Gaag, D.J.; Loomans, A.J.M. Host plants of Anoplophora glabripennis, a review. Bull. OEPP/EPPO Bull. 2014, 44, 518–528. [Google Scholar] [CrossRef]
- Faccoli, M.; Favaro, R. Host preference and host colonization of the Asian long-horned beetle, Anoplophora glabripennis (Coleoptera Cerambycidae), in Southern Europe. Bull. Entomol. Res. 2016, 106, 359–367. [Google Scholar] [CrossRef]
- Iwaizumi, R.; Arimoto, M.; Kurauchi, T. A study on the occurrence and fecundity of white spotted longicorn, Anoplophora malasiaca (Coleoptera: Cerambycidae). Res. Bull. Plant Prot. Jpn. 2014, 50, 9–15. [Google Scholar]
- Sjörman, H.; Östberg, J.; Nilsson, J. Review of host trees for wood-boring pests Anoplophora glabripennis and Anoplophora chinensis: An urban forest perspective. Arboric. Urban For. 2014, 40, 143–164. [Google Scholar] [CrossRef]
- Adachi, I. Development and life cycle of Anoplophora malasiaca (Thomson) (Coleoptera: Cerambycidae) on citrus trees under fluctuating and constant temperature regimes. Appl. Entomol. Zool. 1994, 29, 485–497. [Google Scholar] [CrossRef]
- Bancroft, J.S.; Smith, M.T. Dispersal and influences on movement for Anoplophora glabripennis calculated from individual mark-recapture. Entomol. Exp. Appl. 2005, 116, 83–92. [Google Scholar] [CrossRef]
- Favaro, R.; Wichmann, L.; Ravn, H.P.; Faccoli, M. Spatial spread and infestation risk assessment in the Asian longhorned beetle, Anoplophora glabripennis. Entomol. Exp. Appl. 2015, 155, 1–7. [Google Scholar] [CrossRef]
- Hull-Sanders, H.; Pepper, E.; Davis, K.; Trotter, R.T., III. Description of an establishment event by the invasive Asian longhorned beetle (Anoplophora glabripennis) in a suburban landscape in the north eastern United States. PLoS ONE 2017, 12, e0181655. [Google Scholar] [CrossRef]
- Smith, M.T.; Tobin, P.C.; Bancroft, J.; Li, G.; Gao, R. Dispersal and spatiotemporal dynamics of Asian Longhorned Beetle (Coleoptera: Cerambycidae) in China. Environ. Entomol. 2004, 33, 435–442. [Google Scholar] [CrossRef]
- Williams, D.W.; Li, G.; Gao, R. Tracking movements of individual Anoplophora glabripennis (Coleoptera: Cerambycidae) adults: Application of harmonic radar. Environ. Entomol. 2004, 33, 644–649. [Google Scholar] [CrossRef]
- Fujiwara-Tujii, N.; Yasui, H.; Tanaka, S. Comparison of fecundity and longevity of Anoplophora malasiaca (Coleoptera: Cerambycidae) adults fed on three different host-plants. Entomol. Sci. 2016, 19, 1–6. [Google Scholar] [CrossRef]
- Yasui, H.; Fujiwara-Tsujii, N. Host plant affects the sexual attractiveness of the female white-spotted longicorn beetle, Anoplophora malasiaca. Sci. Rep. 2016, 6, 29526. [Google Scholar] [CrossRef]
- Javal, M.; Roques, A.; Haran, J.; Hérard, F.; Keena, M.; Roux, G. Complex invasion history of the Asian long-horned beetle: Fifteen years after first detection in Europe. J. Pest Sci. 2019, 92, 173–187. [Google Scholar] [CrossRef]
- Javal, M.; Lombaert, E.; Tsykun, T.; Courtin, C.; Kerdelhué, C.; Prospero, S.; Roques, A.; Roux, G. Deciphering the worldwide invasion of the Asian long-horned beetle: A recurrent invasion process from the native area together with a bridgehead effect. Mol. Ecol. 2019, 28, 951–967. [Google Scholar] [CrossRef]
- Tsykun, T.; Javal, M.; Hölling, D.; Roux, G.; Prospero, S. Fine-scale invasion genetics of the quarantine pest, Anoplophora glabripennis, reconstructed in single outbreaks. Sci. Rep. 2019, 9, 19436. [Google Scholar] [CrossRef]
- Kojima, K.; Nakamura, S. Food Plants of Cerambycid Beetles (Cerambycidae, Coleoptera) in Japan; Hiba Society of Natural History: Shohara, Japan, 2011; 336p, (Original in Japanese, translated in English). [Google Scholar]
- Kobayashi, K.; Okuda, M. Damage of a young cryptomeria plantation by the white spotted longicorn, Anoplophora malasiaca Thomson. Trans. Jpn. For. Soc. 1981, 92, 357–358, (Original in Japanese, translated in English). [Google Scholar]
- Taniguchi, A.; Takemura, K.; Aoki, H. Damage of Cryptomeria japonica plantations by Anoplophora malasiaca in Tanegashima, Kagoshima Pref. For. Pests 1982, 31, 85–89, (Original in Japanese, translated in English). [Google Scholar]
- Masaka, K. Decline of wind-shelter belts composed of white birch by white-spotted longicorn beetle in southern Sorachi, Hokkaido. Hoppo-Ringyo 2017, 68, 67–70, (Original in Japanese, translated in English). [Google Scholar]
- Fukaya, M.; Yasuda, T.; Akino, T.; Yasui, H.; Wakamura, S.; Fukuda, T.; Ogawa, Y. Effects of male body size on mating behavior and female mate refusal in the white-spotted longicorn beetle, Anoplophora malasiaca (Thomson) (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 2004, 39, 731–737. [Google Scholar] [CrossRef][Green Version]
- Hara, H. Major insects injurious to the trees of Betula spp. and Fraxinus mandschurica var. japonica in Hokkaido and their managements. Bull. Hok. For. Res. Inst. 2000, 37, 67–74. (In Japanese) [Google Scholar]
- Hara, H. Scientific and Japanese names of a buprestid beetle, Agrilus sp. called as “Shirakaba-naga-tamamushi”, infesting Japanese white birch. Bull. Hok. For. Res. Inst. 2018, 55, 21–22. (In Japanese) [Google Scholar]
- Gao, R.; Baode, W.; Mastro, V.C.; Li, Y.; Wang, Y.; Yang, X. Infestation of Betula platyphylla by Anoplophora glabripennis and its control using insecticides. Sci. Silvae Sini. 2009, 45, 163–164, (In Chinese with English summary). [Google Scholar]
- Makihara, H.; Igarashi, Y.; Funakoshi, H. Host species and damage condition of Anoplophora malasiaca at Tohoku Research Centre, Forestry and Forest Products Research Institute. Tohoku Soc. For. Sci. 1989, 41, 182–183, (Original in Japanese, translated in English). [Google Scholar]
- Onodera, H.; Nishida, I.; Oota, I.; Touhachi, M. Damage of Betula platyphylla var. japonica plantation by Anoplophora malasiaca in Assabu. In Procceeding of Technical Report of Silviculture; Hokkaido Ringyo Kairyo Fukyu Kyokai: Sapporo, Japan, 1995; pp. 136–137, (Original in Japanese, translated in English). [Google Scholar]
- Onodera, H.; Nishida, I.; Chiba, H.; Touhachi, M. Damage of Betula platyphylla var. japonica plantation by Anoplophora malasiaca in southern Hiyama. In Procceeding of Technical Report of Silviculture; Hokkaido Ringyo Kairyo Fukyu Kyokai: Sapporo, Japan, 1997; pp. 118–119, (Original in Japanese, translated in English). [Google Scholar]
- Takahashi, Y.; Asai, T.; Kikuzawa, K. On biomass estimation of Betula platyphylla var. japonica forest stand in Nayoro. Bull. Hok. For. Exp. Stat. 1974, 12, 29–38, (In Japanese with English summary). [Google Scholar]
- Sato, H.; Torita, H.; Masaka, K.; Kon, H.; Shibuya, M. Analysis of winthrow factors in windbreaks: In the case of Bibai, Hokkaido by Tyhoon no. 18 in 2004. J. Jpn. For. Soc. 2009, 91, 307–312, (In Japanese with English summary). [Google Scholar] [CrossRef][Green Version]
- Makihara, H. Longicorn beetles in tropical forest (5). Longicorn beetles in Asia (3). Asian longhorned beetle, Tribe laminii (2). Jpn. J. Inter. For. For. 2009, 74, 59–64. (In Japanese) [Google Scholar]
- Muraji, M.; Wakamura, S.; Yasui, H.; Arakaki, N.; Sadoyama, Y.; Ohno, S.; Matsuhira, K. Genetic variation of the white-spotted longicorn beetle Anoplophora spp. (Coleoptera: Cerambycidae) in Japan detected by mitochondrial DNA sequence. Appl. Entomol. Zool. 2011, 46, 363–373. [Google Scholar] [CrossRef]
- Ohbayashi, N.; Ogawa, J.; Su, Z.-H. Phylogenetic analysis of the lamiine genus Anoplophora and its relatives (Coleoptera, Cerambycidae) based on the mitochondrial COI gene. Spec. Bull. Jpn. Soc. Coleopterol. 2009, 7, 309–324. [Google Scholar]
- Institute of Bibai City History. Bibai City History; Bibai City Office: Bibai, Japan, 1970; 997p. (In Japanese) [Google Scholar]
- Iwasaki, K.; Torita, H.; Abe, T.; Uraike, T.; Touze, M.; Fukuchi, M.; Sato, H.; Iijima, T.; Imaoka, K.; Igawa, H. Spatial pattern of windbreak effects on maize growth evaluated by an unmanned aerial vehicle in Hokkaido, northern Japan. Agrofor. Syst. 2019, 93, 1133–1145. [Google Scholar] [CrossRef]
- Masaka, K.; Ohno, Y.; Yamada, K. Fire tolerance and the fire-related sprouting characteristics of two cool-temperate broad-leaved tree species. Ann. Bot. 2000, 85, 137–142. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.r-project.org/index.html (accessed on 10 January 2020).
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; 488p. [Google Scholar]
- Crawley, M.J. Statistics: An Introduction Using R.; John Wiley & Sons Ltd.: Chichester, UK, 2005; 342p, ISBN 978-0470022986. [Google Scholar]
- McCarthy, M.A. Bayesian Methods for Ecology; Cambridge University Press: New York, NY, USA, 2007; 312p, ISBN 978-0521615594. [Google Scholar]
- Hérard, F.; Maspero, M.; Ramualde, N.; Jucker, C.; Colombo, M.; Ciampitti, M.; Cavagna, B. Anoplophora glabripennis infestation (col.: Cerambycidae) in Italy. Bull. OEPP/EPPO Bull. 2009, 39, 146–152. [Google Scholar] [CrossRef]
- Wagener, W.W.; Davidson, R.W. Heart rots in living trees. Bot. Rev. 1954, 20, 61–134. [Google Scholar] [CrossRef]
- Sakaue, T. Non-destructive diagnosis of stem rots in living Betula platyphylla var. japonica trees by the lateral impact vibration method. Bor. For. Res. 2016, 64, 31–33. (In Japanese) [Google Scholar]
- Yamaguchi, T. Incidence of decay and discoloration on overmatured birch (Betula platyphylla var. japonica Hara) stands. Trans. Meet. Hok. Br. Jpn. For. Soc. 2001, 49, 93–95. (In Japanese) [Google Scholar]
- Adachi, I. Spatial distribution and mortality process of Anoplophora malasiaca (Coleoptera: Cerambycidae) eggs in citrus groves. Res. Pop. Eco. 1989, 31, 343–352. [Google Scholar] [CrossRef]
- Arango-Velez, A.; González, L.M.G.; Meents, M.J.; Kayal, W.E.; Cooke, B.J.; Linsky, J.; Lusebrink, I.; Cooke, J.E.K. Influence of water deficit on the molecular responses of Pinus contorta × Pinus banksiana mature trees to infection by the mountain pine beetle fungal associate, Grosmannia clavigera. Tree Physiol. 2013, 34, 1220–1239. [Google Scholar] [CrossRef]
- Terazawa, K.; Kikuzawa, K. Effects of flooding on leaf dynamics and other seedling responses in flood-tolerant Alnus japonica and flood-intolerant Betula platyphylla var. japonica. Tree Physiol. 1994, 14, 251–261. [Google Scholar] [CrossRef]
- Iwasaki, K.; Tamura, M.; Sato, H.; Masaka, K.; Oka, D.; Yamakawa, Y.; Kosugi, K. Application of ground-penetrating radar and a combined penetrometer–moisture probe for evaluating spatial distribution of soil moisture and soil hardness in coastal and inland windbreaks. Geoscience 2020, 10, 238. [Google Scholar] [CrossRef]
- Liu, C.L.C.; Kuchma, O.; Krutovsky, K.V. Mixed-species versus monocultures in plantation forestry: Development, benefits, ecosystem services and perspectives for the future. Global Ecol. Conserv. 2018, 15, e00419. [Google Scholar] [CrossRef]
- Futai, K. Pine wilt in Japan: From first incidence to the present. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: New York, NY, USA, 2008; pp. 5–12. [Google Scholar] [CrossRef]
- Kuroda, K. Responses of Quercus sapwood to infection with the pathogenic fungus of a new wilt disease vectored by the ambrosia beetle Platypus quercivorus. J. Wood Sci. 2001, 47, 425–429. [Google Scholar] [CrossRef]
- Tateishi, T. The afforestations of coastal sand dune forests on the Japan Sea. Proc. Inst. Nat. Sci. Nihon Univ. 1974, 9, 15–44, (In Japanese with English summary). [Google Scholar]
- Yoshioka, K. Ecological Study of The Pine Forests in Japan; Japan Forest Technology Association: Tokyo, Japan, 1958; 198p. [Google Scholar]
- Saito, O.; Hoshino, Y.; Tsuji, S.; Kanno, A. Changes in species richness and species composition of Quercus serrata secondary forests after more than 20 years in Kanto region, Japan. Veget. Sci. 2003, 20, 83–96, (In Japanese with English summary). [Google Scholar]
- Shinozaki, K.; Kira, T. Intraspecific competition among higher plants. VII. Logistic theory of the C-D effect. J. Inst. Polytech. Osaka City Univ. 1956, 7, 35–72. [Google Scholar]
- Shibuya, M.; Yajima, T.; Matsuda, K. A modified stand density control diagram for Japanese white birch based on a trend of mean volume-density relationships with stand growth. Res. Bull. Hok. Univ. For. 1997, 54, 202–211, (In Japanese with English summary). [Google Scholar]
- Five Regional Forest Offices in Hokkaido; Forest and Forest Products Research Institute, Hokkaido Research Center. Density Control Diagram for White Birch Stand (Preliminary Version). 1992. Available online: https://www.ffpri.affrc.go.jp/hkd/research/documents/shirakanba.pdf (accessed on 11 November 2020).
- Samejima, R.; Ishikawa, K.; Okada, K.; Kutomi, Y. Influence of forest windbreaks on paddy field environments and rice growth in Sorachi, Hokkaido. Clim. Biosph. 2018, 18, 39–47, (In Japanese with English summary). [Google Scholar] [CrossRef]
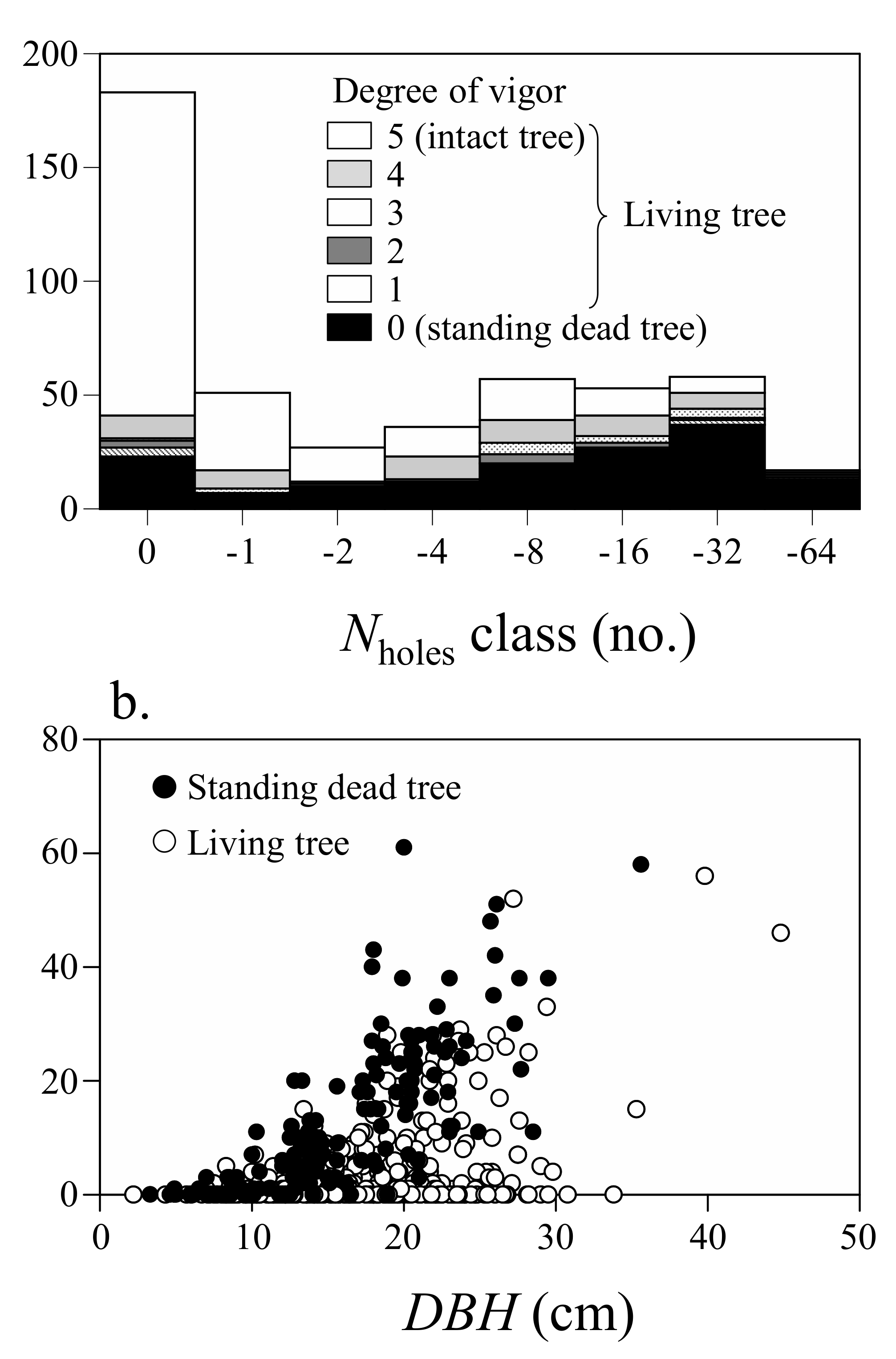
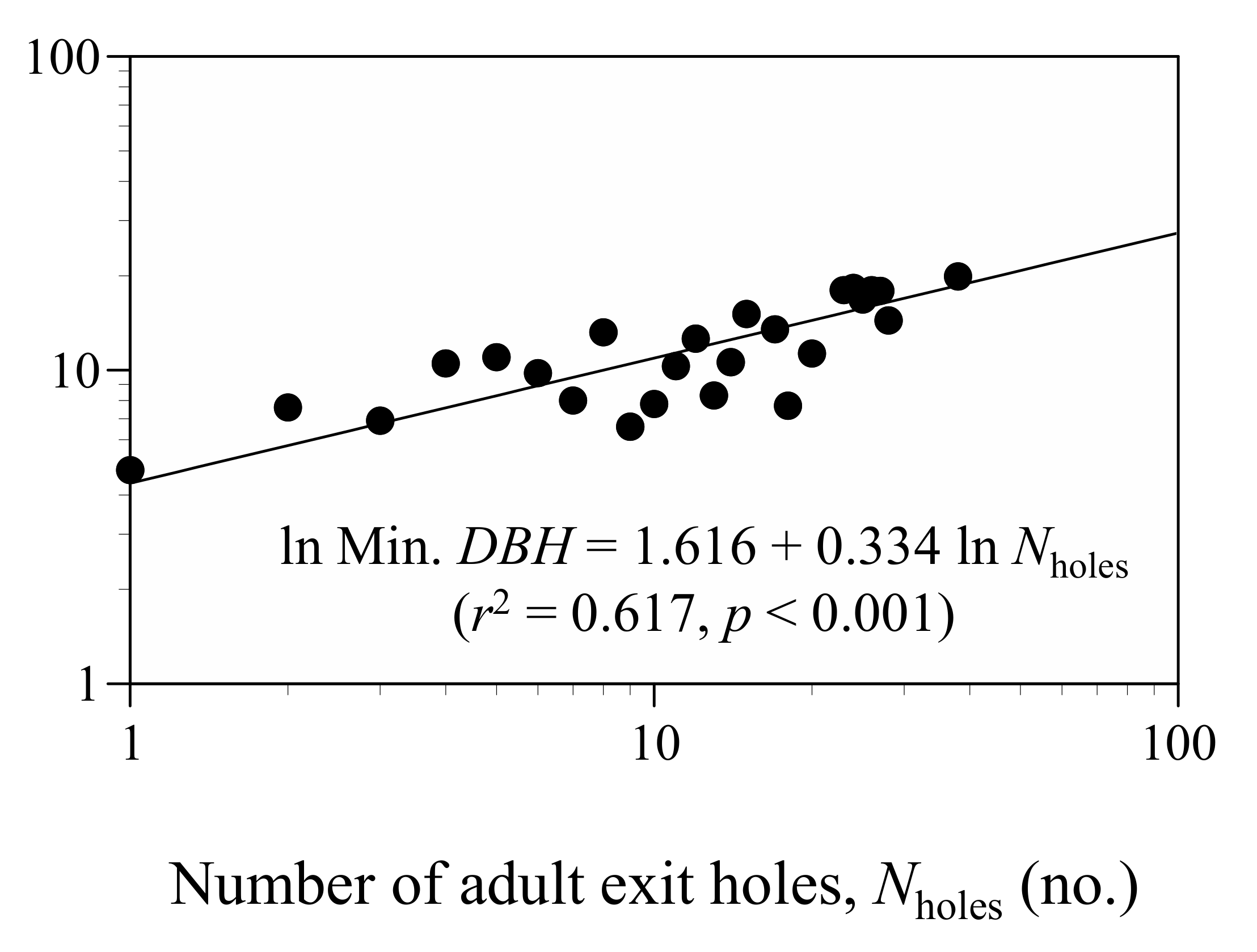

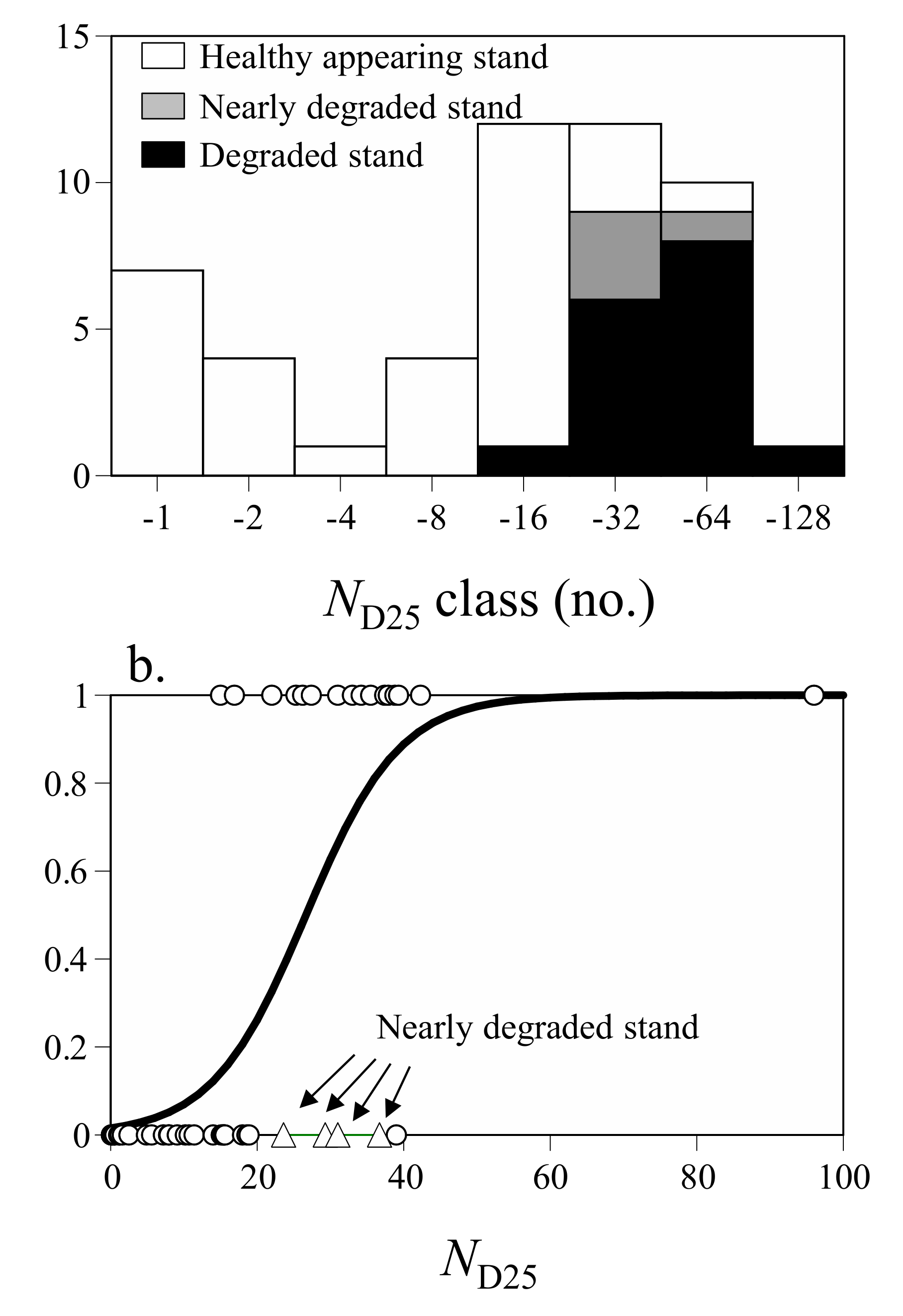
| Variables | Coeff. | SE | z |
|---|---|---|---|
| Intercept | −0.506 | 0.527 | −0.960 |
| DBH | 0.094 | 0.016 | 5.969 |
| Vigor | −0.155 | 0.011 | −14.304 |
| Variables | Coeff. | SE | z |
|---|---|---|---|
| Intercept | −0.831 | 0.288 | −2.890 |
| Survival_dead | 0.395 | 0.102 | 3.891 |
| DBH × Survival_alive | 0.088 | 0.008 | 10.816 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masaka, K.; Wakita, Y.; Iwasaki, K.; Hayamizu, M. Degradation of White Birch Shelterbelts by the Attack of White-Spotted Longicorn Beetles in Central Hokkaido, Northern Japan. Forests 2022, 13, 34. https://doi.org/10.3390/f13010034
Masaka K, Wakita Y, Iwasaki K, Hayamizu M. Degradation of White Birch Shelterbelts by the Attack of White-Spotted Longicorn Beetles in Central Hokkaido, Northern Japan. Forests. 2022; 13(1):34. https://doi.org/10.3390/f13010034
Chicago/Turabian StyleMasaka, Kazuhiko, Yohichi Wakita, Kenta Iwasaki, and Masato Hayamizu. 2022. "Degradation of White Birch Shelterbelts by the Attack of White-Spotted Longicorn Beetles in Central Hokkaido, Northern Japan" Forests 13, no. 1: 34. https://doi.org/10.3390/f13010034
APA StyleMasaka, K., Wakita, Y., Iwasaki, K., & Hayamizu, M. (2022). Degradation of White Birch Shelterbelts by the Attack of White-Spotted Longicorn Beetles in Central Hokkaido, Northern Japan. Forests, 13(1), 34. https://doi.org/10.3390/f13010034






