Are Climates in Canada and the United States Suitable for the European Spruce Bark Beetle, Ips typographus, and Its Fungal Associate, Endoconidiophora polonica?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Distribution Data
2.2. Climate Data
2.3. Modelling
3. Results
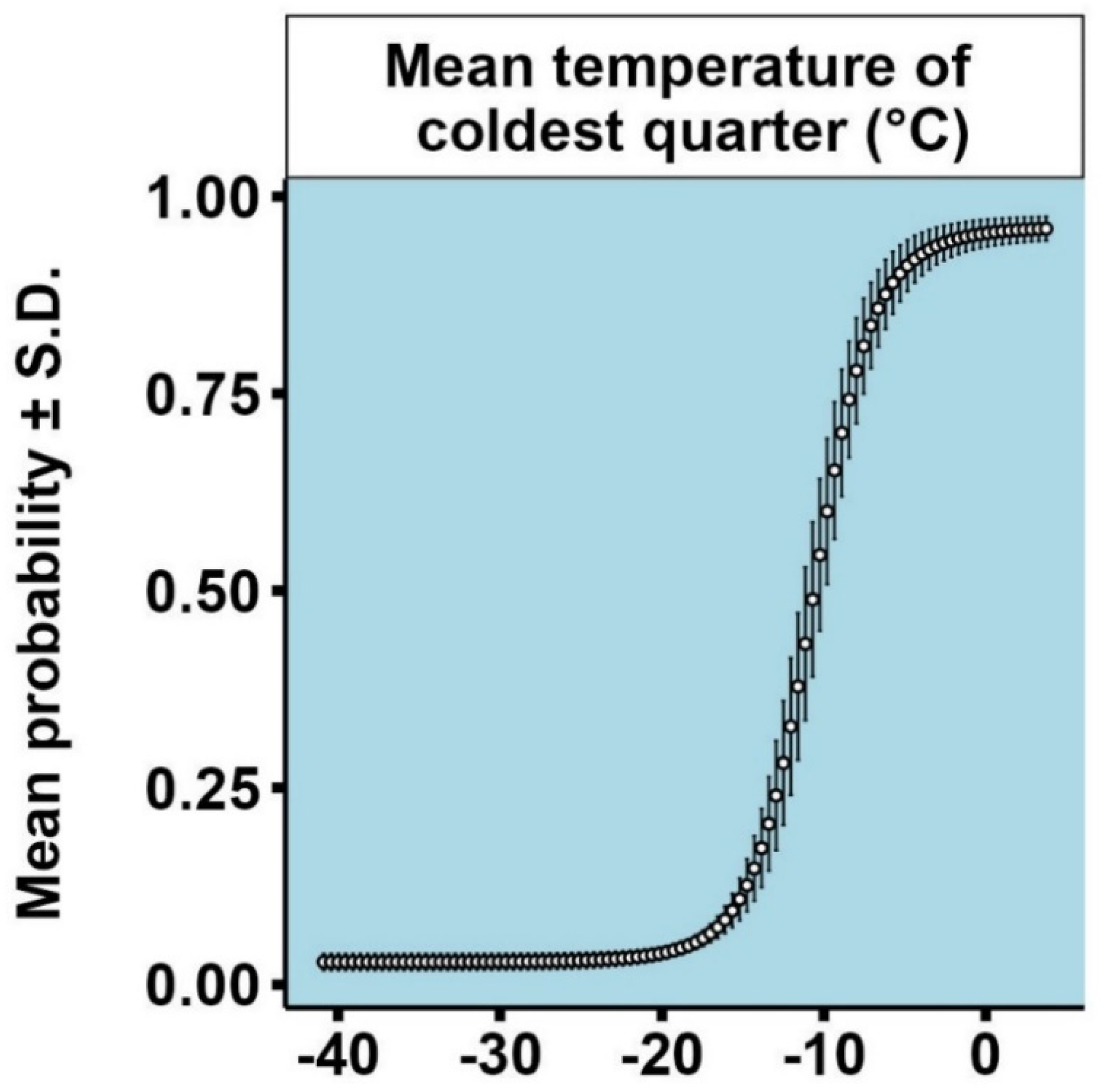
3.1. Models for Ips typographus
3.2. Models for Endoconidiophora polonica
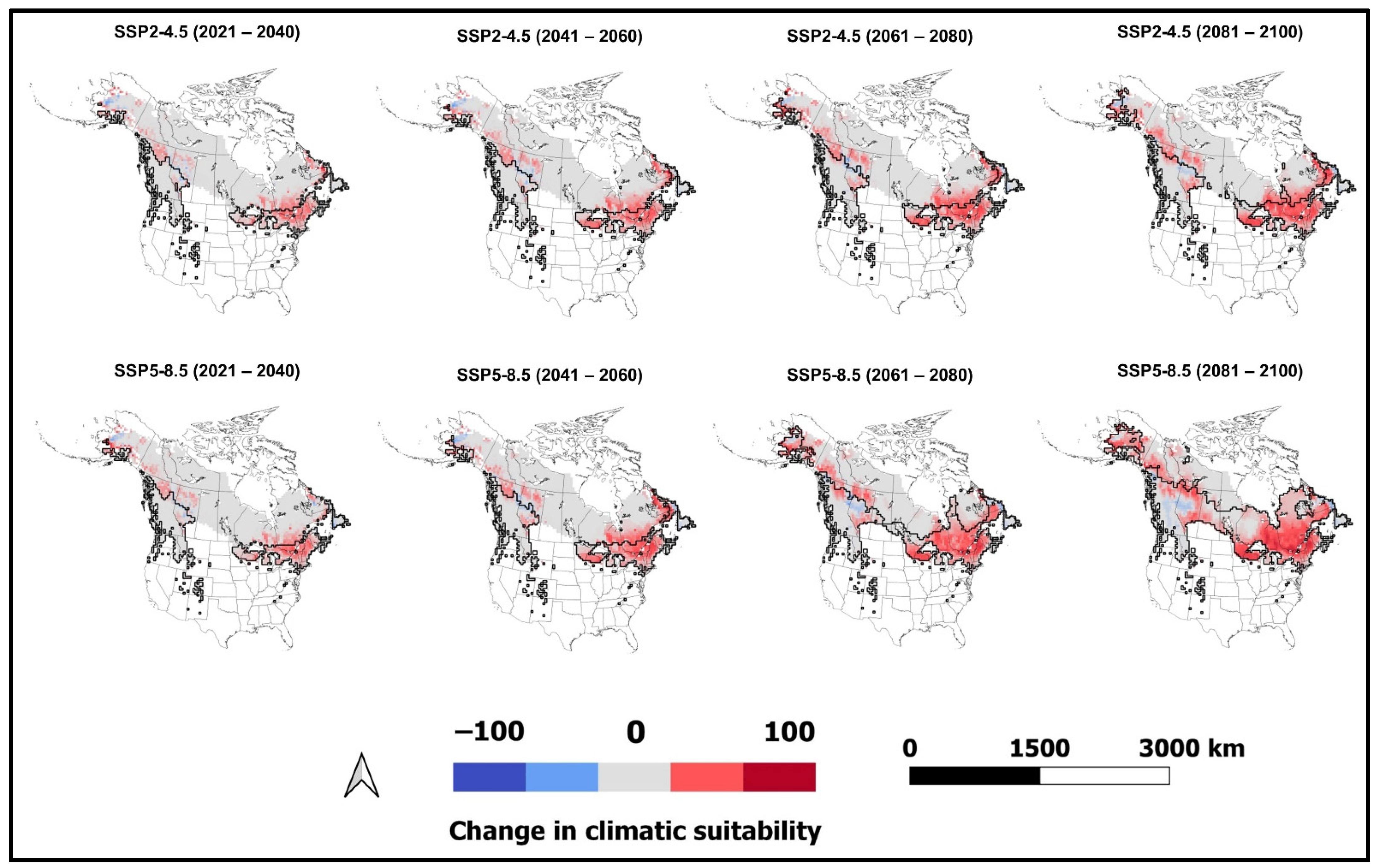
3.3. Climatically Suitable Regions for I. typographus and E. polonica in Canada and the United States
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orwig, D.A. Ecosystem to regional impacts of introduced pests and pathogens: Historical context, questions and issues. J. Biogeogr. 2002, 29, 1471–1474. [Google Scholar] [CrossRef]
- Lovett, G.M.; Canham, C.D.; Arthur, M.A.; Weathers, K.C.; Fitzhugh, R.D. Forest ecosystem responses to exotic pests and pathogens in eastern North America. Bioscience 2006, 56, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Allen, E.A.; Humble, L.M. Nonindigenous species introductions: A threat to Canada’s forests and forest economy. Can. J. Plant Pathol. 2002, 24, 103–110. [Google Scholar] [CrossRef]
- Aukema, J.E.; Leung, B.; Kovacs, K.; Chivers, C.; Britton, K.O.; Englin, J.; Frankel, S.J.; Haight, R.G.; Holmes, T.P.; Liebhold, A.M.; et al. Economic impacts of non-native forest insects in the continental United States. PLoS ONE 2011, 6, e24587. [Google Scholar] [CrossRef]
- Haack, R.A. Intercepted Scolytidae (Coleoptera) at U.S. ports of entry: 1985–2000. Integr. Pest Manag. Rev. 2001, 6, 253–282. [Google Scholar] [CrossRef]
- Haack, R.A. Exotic bark-and wood-boring Coleoptera in the United States: Recent establishments and interceptions. Can. J. For. Res. 2006, 36, 269–288. [Google Scholar] [CrossRef]
- Humble, L.; Allen, E. Forest biosecurity: Alien invasive species and vectored organisms. Can. J. Plant Pathol. 2006, 28, S256–S269. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Brockerhoff, E.G.; Garrett, L.J.; Parke, J.L.; Britton, K.O. Live plant imports: The major pathway for forest insect and pathogen invasions of the US. Front. Ecol. Environ. 2012, 10, 135–143. [Google Scholar] [CrossRef]
- Colautti, R.I.; Bailey, S.A.; Van Overdijk, C.D.A.; Amundsen, K.; MacIsaac, H.J. Characterised and projected costs of nonindigenous species in Canada. Biol. Invasions 2006, 8, 45–59. [Google Scholar] [CrossRef]
- Kovacs, K.; Václavík, T.; Haight, R.G.; Pang, A.; Cunniffe, N.J.; Gilligan, C.A.; Meentemeyer, R.K. Predicting the economic costs and property value losses attributed to sudden oak death damage in California (2010–2020). J. Environ. Manag. 2011, 92, 1292–1302. [Google Scholar] [CrossRef]
- Haight, R.G.; Homans, F.R.; Horie, T.; Mehta, S.V.; Smith, D.J.; Venette, R.C. Assessing the cost of an invasive forest pathogen: A case study with oak wilt. Environ. Manag. 2011, 47, 506–517. [Google Scholar] [CrossRef]
- Anagnostakis, S.L. Chestnut blight: The classical problem of an introduced pathogen. Mycologia 1987, 79, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Evans, A.M. The speed of invasion: Rates of spread for thirteen exotic forest insects and diseases. Forests 2016, 7, 99. [Google Scholar] [CrossRef] [Green Version]
- Prospero, S.; Cleary, M. Effects of host variability on the spread of invasive forest diseases. Forests 2017, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Lovett, G.M.; Weiss, M.; Liebhold, A.M.; Holmes, T.P.; Leung, B.; Lambert, K.F.; Orwig, D.A.; Campbell, F.T.; Rosenthal, J.; McCullough, D.J.; et al. Nonnative forest insects and pathogens in the United States: Impacts and policy options. Ecol. Appl. 2016, 26, 1437–1455. [Google Scholar] [CrossRef] [PubMed]
- Nealis, V.G.; Demerchant, I.; Langor, D.; Noseworthy, M.K.; Pohl, G.; Porter, K.; Shanks, E.; Turnquist, R.; Waring, V. Historical occurrence of alien arthropods and pathogens on trees in Canada. Can. J. For. Res. 2015, 46, 172–180. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Turner, R.M.; Blake, R.E.; Bertelsmeier, C.; Brockerhoff, E.G.; Nahrung, H.F.; Pureswaran, D.S.; Roques, A.; Seebens, H.; Yamanaka, T. Invasion disharmony in the global biogeography of native and non-native beetle species. Divers. Distrib. 2021, 27, 2050–2062. [Google Scholar] [CrossRef]
- Poland, T.M.; McCullough, D.G. Emerald ash borer: Invasion of the urban forest and the threat to North America’s ash resource. J. For. 2006, 104, 118–124. [Google Scholar]
- Valenta, V.; Moser, D.; Kapeller, S.; Essl, F. A new forest pest in Europe: A review of emerald ash borer (Agrilus planipennis) invasion. J. Appl. Entomol. 2017, 141, 507–526. [Google Scholar] [CrossRef]
- Rizzo, D.M.; Garbelotto, M.; Davidson, J.M.; Slaughter, G.W.; Koike, S.T. Phytophthora ramorum as the cause of extensive mortality of Quercus spp. and Lithocarpus densiflorus in California. Plant Dis. 2002, 86, 205–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dale, V.H.; Joyce, L.A.; McNulty, S.; Neilson, R.P.; Ayres, M.P.; Flannigan, M.D.; Hanson, P.J.; Irland, L.C.; Lugo, A.E.; Peterson, C.J.; et al. Climate change and forest disturbances. Bioscience 2001, 51, 723–734. [Google Scholar] [CrossRef] [Green Version]
- Økland, B.; Erbilgin, N.; Skarpaas, O.; Christiansen, E.; Långström, B. Inter-species interactions and ecosystem effects of non-indigenous invasive and native tree-killing bark beetles. Biol. Invasions 2011, 13, 1151–1164. [Google Scholar] [CrossRef]
- Flø, D.; Norli, H.R.; Økland, B.; Krokene, P. Successful reproduction and pheromone production by the spruce bark beetle in evolutionary naïve spruce hosts with familiar terpenoid defences. Agric. For. Entomol. 2018, 20, 476–486. [Google Scholar] [CrossRef] [Green Version]
- de Beer, Z.W.; Duong, T.A.; Barnes, I.; Wingfield, B.D.; Wingfield, M.J. Redefining Ceratocystis and allied genera. Stud. Mycol. 2014, 79, 187–219. [Google Scholar] [CrossRef] [Green Version]
- Kirisits, T. Fungal associates of European bark beetles with special emphasis on the ophiostomatoid fungi. In Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis; Springer: Dordrecht, The Netherlands, 2007; pp. 181–236. [Google Scholar]
- Krokene, P.; Solheim, H. Fungal associates of five bark-beetle species colonizing Norway spruce. Can. J. For. Res. 1996, 26, 2115–2122. [Google Scholar] [CrossRef]
- Kirisits, T. Pathogenocity of three blue-stain fungi associated with the bark beetle lps typographus to Norway spruce in Austria. Österr. Z. Pilzk. 1998, 7, 191–201. [Google Scholar]
- Krokene, P.; Solheim, H. Pathogenicity of four blue-stain fungi associated with aggressive and nonagressive bark beetles. Phytopathology 1998, 88, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Tanin, S.M.; Kandasamy, D.; Krokene, P. Fungal interactions and host tree preferences in the spruce bark beetle Ips typographus. Front. Microbiol. 2021, 12, 1375. [Google Scholar] [CrossRef]
- Christiansen, E.; Solheim, H. The bark beetle-associated blue-stain fungus Ophiostoma polonicum can kill various spruces and Douglas fir. Eur. J. For. Pathol. 1990, 20, 436–446. [Google Scholar] [CrossRef]
- Jankowiak, R.; Hilszczanski, J. Ophiostomatoid fungi associated with Ips typographus (L.) on Picea abies [(L.) H. Karst.] and Pinus sylvestris L. in north-eastern Poland. Acta Soc. Bot. Pol. 2005, 74, 345–350. [Google Scholar] [CrossRef]
- Schroeder, M.; Cocoş, D. Performance of the tree-killing bark beetles Ips typographus and Pityogenes chalcographus in non-indigenous lodgepole pine and their historical host Norway spruce. Agric. For. Entomol. 2018, 20, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Bentz, B.J.; Jonsson, A.M.; Schroeder, M.; Weed, A.; Wilcke, R.A.I.; Larsson, K. Ips typographus and Dendroctonus ponderosae models project thermal suitability for intra- and inter-continental establishment in a changing climate. Front. For. Glob. Chang. 2019, 2, 1–17. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Herpin-Saunier, N.Y.H.; Sambaraju, K.R.; Yin, X.; Feau, N.; Zeglen, S.; Ritóková, G.; Omdal, D.; Côté, C.; Hamelin, R.C. Genetic lineage distribution modelling to predict epidemics of a conifer disease. Front. For. Glob. Change 2021. In press. [Google Scholar]
- Briscoe, N.J.; Elith, J.; Salguero-Gómez, R.; Lahoz-Monfort, J.J.; Camac, J.S.; Giljohann, K.M.; Holden, M.H.; Hradsky, B.A.; Kearney, M.R.; McMahon, S.M.; et al. Forecasting species range dynamics with process-explicit models: Matching methods to applications. Ecol. Lett. 2019, 22, 1940–1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ireland, K.B.; Kriticos, D.J. Why are plant pathogens under-represented in eco-climatic niche modelling? Int. J. Pest Manag. 2019, 65, 207–216. [Google Scholar] [CrossRef]
- Brown, J.L. SDMtoolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Reddy, S.; Dávalos, L.M. Geographical sampling bias and its implications for conservation priorities in Africa. J. Biogeogr. 2003, 30, 1719–1727. [Google Scholar] [CrossRef]
- Wichmann, L.; Ravn, H.P. The spread of Ips typographus (L.) (Coleoptera, Scolytidae) attacks following heavy windthrow in Denmark analysed using GIS. For. Ecol. Manag. 2001, 148, 31–39. [Google Scholar] [CrossRef]
- Zumr, V. Dispersal of the spruce bark beetle Ips typographus (L.) (Col., Scolytidae) in spruce woods. J. Appl. Entomol. 1992, 114, 348–352. [Google Scholar] [CrossRef]
- Botterweg, P.F. Dispersal and flight behaviour of the spruce bark beetle Ips typographus in relation to sex, size and fat content. J. Appl. Entomol. 1982, 94, 466–489. [Google Scholar] [CrossRef]
- Forsse, E.; Solbreck, C.H. Migration in the bark beetle Ips typographus L.: Duration, timing and height of flight. Z. Angew. Entomol. 1985, 100, 47–57. [Google Scholar] [CrossRef]
- Nilssen, A. Long-range aerial dispersal of bark beetles and bark weevils (Coleoptera, Scolytidae and Curculionidae) in northern Finland [Ips typographus, Hylastes cunicularius, Dryocoetes autographus, Hylobius abietis]. Ann. Entomol. Fenn. 1984, 50, 37–42. [Google Scholar]
- Weslien, J.; Lindelöw, Å. Recapture of marked spruce bark beetles (Ips typographus) in pheromone traps using area-wide mass trapping. Can. J. For. Res. 1990, 20, 1786–1790. [Google Scholar] [CrossRef]
- Schmidt-Vogt, H. Monographie der Picea abies (L.) Karst. unter Berücksichtigung genetischer und züchterischer Aspekte. Forstwiss. Centralbl. 1978, 97, 281–302. [Google Scholar] [CrossRef]
- Kirisits, T. Fungi isolated from Picea abies infested by the bark beetle Ips typographus in the Białowieża forest in north-eastern Poland. For. Pathol. 2010, 40, 100–110. [Google Scholar] [CrossRef]
- Solheim, H. Fungal succession in sapwood of Norway spruce infested by the bark beetle Ips typographus. Eur. J. For. Path 1992, 22, 136–148. [Google Scholar] [CrossRef]
- Jankowiak, R.; Kacprzyk, M.; Młynarczyk, M. Diversity of ophiostomatoid fungi associated with bark beetles (Coleoptera: Scolytidae) colonizing branches of Norway spruce (Picea abies) in southern Poland. Biologia 2009, 64, 1170–1177. [Google Scholar] [CrossRef] [Green Version]
- Kirschner, R. Diversity of filamentous fungi in bark beetle galleries in central Europe. In Trichomycetes and Other Fungal Groups; Misra, J.K., Horn, B.W., Eds.; Science Publishers, Inc.: Plymouth, UK, 2001; pp. 175–196. [Google Scholar]
- Sallé, A.; Monclus, R.; Yart, A.; Garcia, J.; Romary, P.; Lieutier, F. Fungal flora associated with Ips typographus: Frequency, virulence, and ability to stimulate the host defence reaction in relation to insect population levels. Can. J. For. Res. 2005, 35, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Viiri, H. Fungal associates of the spruce bark beetle Ips typographus L. (Col. scolytidae) in relation to different trapping methods. J. Appl. Entomol. 1997, 121, 529–533. [Google Scholar] [CrossRef]
- Kotýnková-Sychrová, E. Mykoflóra chodeb kůrovců v Československu [The mycoflora of bark beetle galleries in Czechoslovakia.]. Česká Mykol. 1966, 20, 45–53. [Google Scholar]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Doležal, P.; Sehnal, F. Effects of photoperiod and temperature on the development and diapause of the bark beetle Ips typographus. J. Appl. Entomol. 2007, 131, 165–173. [Google Scholar] [CrossRef]
- Wermelinger, B.; Seifert, M. Analysis of the temperature dependant development of the spruce bark beetle Ips typographus (L.) (Col., Scolytidae). J. Appl. Entomol. 1998, 122, 185–191. [Google Scholar] [CrossRef]
- Wermelinger, B. Ecology and management of the spruce bark beetle Ips typographus—a review of recent research. For. Ecol. Manag. 2004, 202, 67–82. [Google Scholar] [CrossRef]
- Jönsson, A.M.; Harding, S.; Bärring, L.; Ravn, H.P. Impact of climate change on the population dynamics of Ips typographus in southern Sweden. Agric. For. Meteorol. 2007, 146, 70–81. [Google Scholar] [CrossRef]
- Faccoli, M. Effect of weather on Ips typographus (Coleoptera Curculionidae) phenology, voltinism, and associated spruce mortality in the Southeastern Alps. Environ. Entomol. 2009, 38, 307–316. [Google Scholar] [CrossRef]
- Netherer, S.; Panassiti, B.; Pennerstorfer, J.; Matthews, B. Acute drought is an important driver of bark beetle infestation in Austrian Norway spruce stands. Front. For. Glob. Chang. 2019, 2, 39. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, E.; Bakke, A. Does drought really enhance lps typographus epidemics? A scandinavian perspective. In Proceedings of the Integrating Cultural Tactics into the Management of Bark Beetle and Reforestation Pests, Vallombrosa, Italy, 1–3 September 1996; Grégoire, J.C., Liebhold, A.M., Stephen, F.M., Day, K.R., Salom, S.M., Eds.; USDA Forest Service General Technical Report NE-236. United States Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Radnor, PA, USA, 1997; pp. 163–171. [Google Scholar]
- Swart, N.C.; Cole, J.N.; Kharin, V.V.; Lazare, M.; Scinocca, J.F.; Gillett, N.P.; Anstey, J.; Arora, V.; Christian, J.R.; Hanna, S. The Canadian earth system model version 5 (CanESM5. 0.3). Geosci. Model Dev. 2019, 12, 4823–4873. [Google Scholar] [CrossRef] [Green Version]
- Séférian, R.; Nabat, P.; Michou, M.; Saint-Martin, D.; Voldoire, A.; Colin, J.; Decharme, B.; Delire, C.; Berthet, S.; Chevallier, M.; et al. Evaluation of CNRM earth system model, CNRM-ESM2-1: Role of earth system processes in present-day and future climate. J. Adv. Model. Earth Syst. 2019, 11, 4182–4227. [Google Scholar] [CrossRef] [Green Version]
- Hajima, T.; Watanabe, M.; Yamamoto, A.; Tatebe, H.; Noguchi, M.A.; Abe, M.; Ohgaito, R.; Ito, A.; Yamazaki, D.; Okajima, H.; et al. Development of the MIROC-ES2L earth system model and the evaluation of biogeochemical processes and feedbacks. Geosci. Model Dev. 2020, 13, 2197–2244. [Google Scholar] [CrossRef]
- Meinshausen, M.; Nicholls, Z.R.J.; Lewis, J.; Gidden, M.J.; Vogel, E.; Freund, M.; Beyerle, U.; Gessner, C.; Nauels, A.; Bauer, N.; et al. The shared socio-economic pathway (SSP) greenhouse gas concentrations and their extensions to 2500. Geosci. Model Dev. 2020, 13, 3571–3605. [Google Scholar] [CrossRef]
- O’Donnell, M.S.; Ignizio, D.A. Bioclimatic Predictors for Supporting Ecological Applications in the Conterminous United States; U.S. Geological Survey Data Series 691; U.S. Geological Survey: Reston, VA, USA, 2012; p. 10.
- Marin, M.; Preisig, O.; Wingfield, B.D.; Kirisits, T.; Yamaoka, Y.; Wingfield, M.J. Phenotypic and DNA sequence data comparisons reveal three discrete species in the Ceratocystis polonica species complex. Mycol. Res. 2005, 109, 1137–1148. [Google Scholar] [CrossRef] [Green Version]
- Ojeda Alayon, D.I.; Tsui, C.K.; Feau, N.; Capron, A.; Dhillon, B.; Zhang, Y.; Massoumi Alamouti, S.; Boone, C.K.; Carroll, A.L.; Cooke, J.E. Genetic and genomic evidence of niche partitioning and adaptive radiation in mountain pine beetle fungal symbionts. Mol. Ecol. 2017, 26, 2077–2091. [Google Scholar] [CrossRef]
- Rice, A.V.; Thormann, M.N.; Langor, D.W. Mountain pine beetle-associated blue-stain fungi are differentially adapted to boreal temperatures. For. Pathol. 2008, 38, 113–123. [Google Scholar] [CrossRef]
- Lieutier, F.; Yart, A.; Ye, H.; Sauvard, D.; Gallois, V. Variations in growth and virulence of Leptographium wingfieldii Morelet, a fungus associated with the bark beetle Tomicus piniperda L. Ann. For. Sci. 2004, 61, 45–53. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 2 December 2021).
- Thuiller, W.; Georges, D.; Engler, R.; Breiner, F. Biomod2: Ensemble Platform for Species Distribution Modeling. R Package Version 3.4.6. Available online: https://cran.r-project.org/web/packages/biomod2/biomod2.pdf (accessed on 24 November 2021).
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENM eval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P.; Heiberger, R.M.; Schuetzenmeister, A.; Scheibe, S. Package ‘Multcomp’. Simultaneous Inference in General Parametric Models. Project for Statistical Computing, Vienna, Austria. Available online: https://cran.r-project.org/web/packages/multcomp/multcomp.pdf (accessed on 24 November 2021).
- Lenth, R.V.; Buerkner, P.; Herve, M.; Love, J.; Riebl, H.; Singmann, H. Package ‘Emmeans’. Available online: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (accessed on 24 November 2021).
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Digital representation of “Atlas of United States Trees” by Elbert L. Little, Jr. 1999. Available online: http://esp.cr.usgs.gov/data/atlas/little/ (accessed on 24 November 2021).
- Brockerhoff, E.G.; Liebhold, A.M. Ecology of forest insect invasions. Biol. Invasions 2017, 19, 3141–3159. [Google Scholar] [CrossRef]
- Marini, L.; Haack, R.A.; Rabaglia, R.J.; Toffolo, E.P.; Battisti, A.; Faccoli, M. Exploring associations between international trade and environmental factors with establishment patterns of exotic Scolytinae. Biol. Invasions 2011, 13, 2275–2288. [Google Scholar] [CrossRef]
- Lockwood, J.L.; Cassey, P.; Blackburn, T. The role of propagule pressure in explaining species invasions. Trends Ecol. Evol. 2005, 20, 223–228. [Google Scholar] [CrossRef]
- Gauthier, S.; Bernier, P.; Kuuluvainen, T.; Shvidenko, A.Z.; Schepaschenko, D.G. Boreal forest health and global change. Science 2015, 349, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Millar, C.I.; Stephenson, N.L. Temperate forest health in an era of emerging megadisturbance. Science 2015, 349, 823–826. [Google Scholar] [CrossRef]
- Chen, I.C.; Hill, J.K.; Ohlemuller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Weed, A.S.; Ayres, M.P.; Hicke, J.A. Consequences of climate change for biotic disturbances in North American forests. Ecol. Monogr. 2013, 83, 441–470. [Google Scholar] [CrossRef]
- Ayres, M.P.; Lombardero, M.J. Assessing the consequences of global change for forest disturbance from herbivores and pathogens. Sci. Total Environ. 2000, 262, 263–286. [Google Scholar] [CrossRef]
- Dukes, J.S.; Pontius, J.; Orwig, D.; Garnas, J.R.; Rodgers, V.L.; Brazee, N.; Cooke, B.; Theoharides, K.A.; Stange, E.E.; Harrington, R.; et al. Responses of insect pests, pathogens, and invasive plant species to climate change in the forests of northeastern North America: What can we predict? Can. J. For. Res. 2009, 39, 231–248. [Google Scholar] [CrossRef]
- Sambaraju, K.; Goodsman, D. Mountain pine beetle: An example of a climate-driven eruptive insect impacting conifer forest ecosystems. CAB Rev. 2021, 16, 1–18. [Google Scholar] [CrossRef]
- Sturrock, R.; Frankel, S.; Brown, A.; Hennon, P.; Kliejunas, J.; Lewis, K.; Worrall, J.; Woods, A. Climate change and forest diseases. Plant Pathol. 2011, 60, 133–149. [Google Scholar] [CrossRef]
- Marini, L.; Ayres, M.P.; Battisti, A.; Faccoli, M. Climate affects severity and altitudinal distribution of outbreaks in an eruptive bark beetle. Clim. Chang. 2012, 115, 327–341. [Google Scholar] [CrossRef]
- Linnakoski, R.; Mahilainen, S.; Harrington, A.; Vanhanen, H.; Eriksson, M.; Mehtätalo, L.; Pappinen, A.; Wingfield, M.J. Seasonal succession of fungi associated with Ips typographus beetles and their phoretic mites in an outbreak region of Finland. PLoS ONE 2016, 11, e0155622. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sambaraju, K.R.; Côté, C. Are Climates in Canada and the United States Suitable for the European Spruce Bark Beetle, Ips typographus, and Its Fungal Associate, Endoconidiophora polonica? Forests 2021, 12, 1725. https://doi.org/10.3390/f12121725
Sambaraju KR, Côté C. Are Climates in Canada and the United States Suitable for the European Spruce Bark Beetle, Ips typographus, and Its Fungal Associate, Endoconidiophora polonica? Forests. 2021; 12(12):1725. https://doi.org/10.3390/f12121725
Chicago/Turabian StyleSambaraju, Kishan R., and Chantal Côté. 2021. "Are Climates in Canada and the United States Suitable for the European Spruce Bark Beetle, Ips typographus, and Its Fungal Associate, Endoconidiophora polonica?" Forests 12, no. 12: 1725. https://doi.org/10.3390/f12121725
APA StyleSambaraju, K. R., & Côté, C. (2021). Are Climates in Canada and the United States Suitable for the European Spruce Bark Beetle, Ips typographus, and Its Fungal Associate, Endoconidiophora polonica? Forests, 12(12), 1725. https://doi.org/10.3390/f12121725




