Influence of Weather Conditions and Climate Oscillations on the Pine Looper Bupalus piniaria (L.) Outbreaks in the Forest-Steppe of the West Siberian Plain
Abstract
1. Introduction
2. Materials and Methods
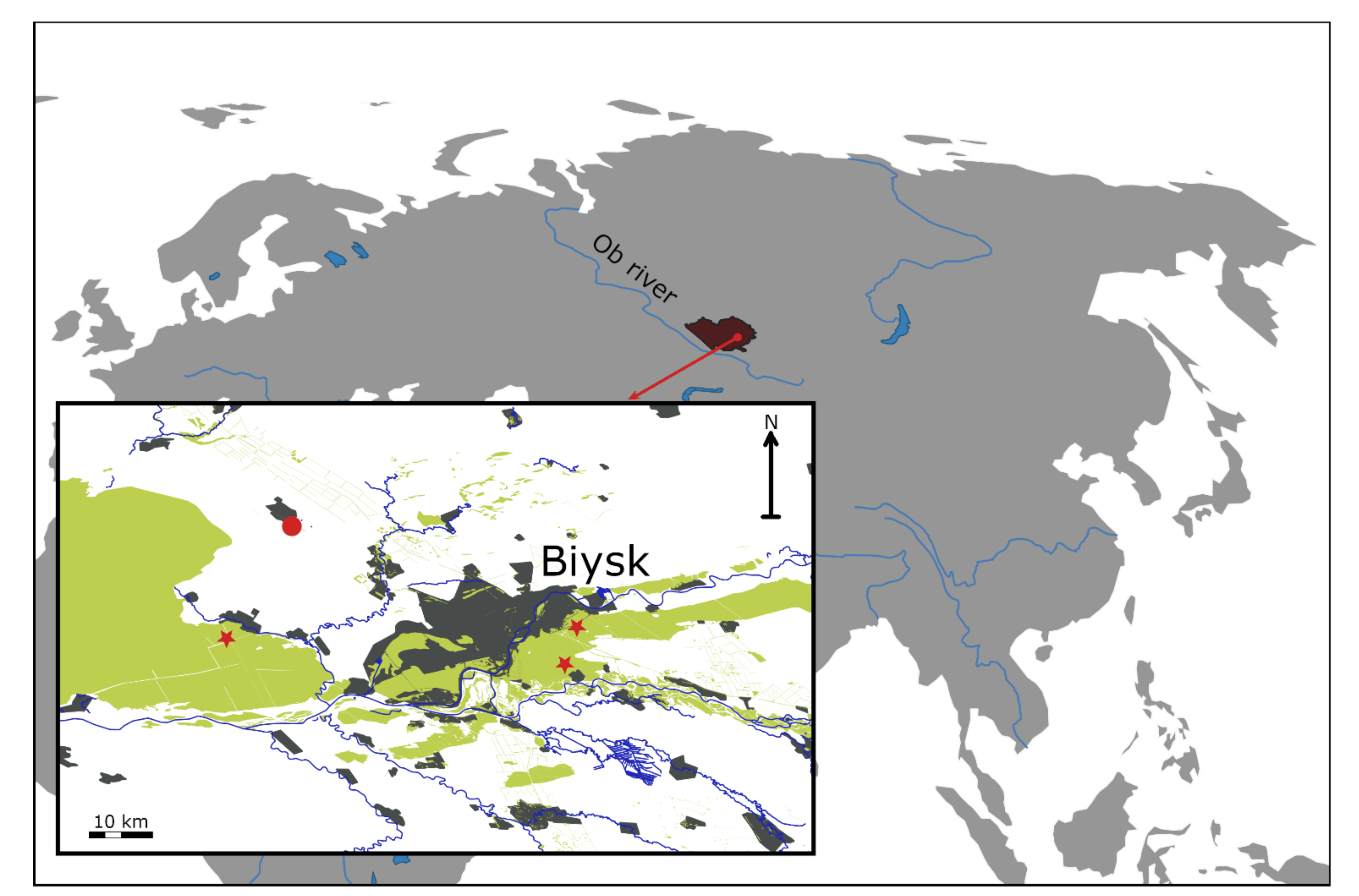
2.1. Study Region
2.2. Study Objects
2.3. Weather and Climate Data
2.4. Statistical Analysis
3. Results
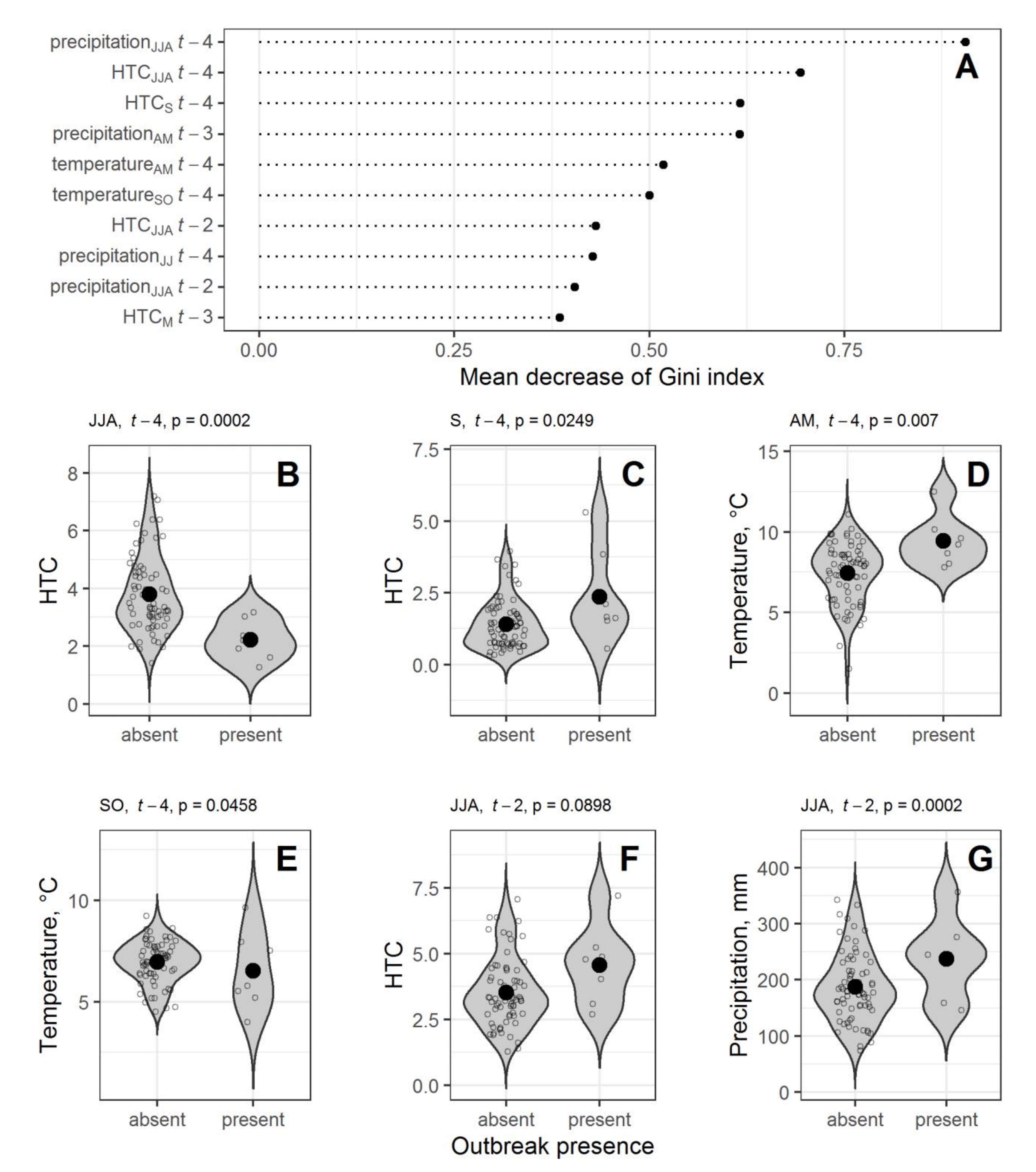
3.1. Weather Conditions as Predictors of Outbreaks
3.2. Frequency Characteristics of Outbreak Series
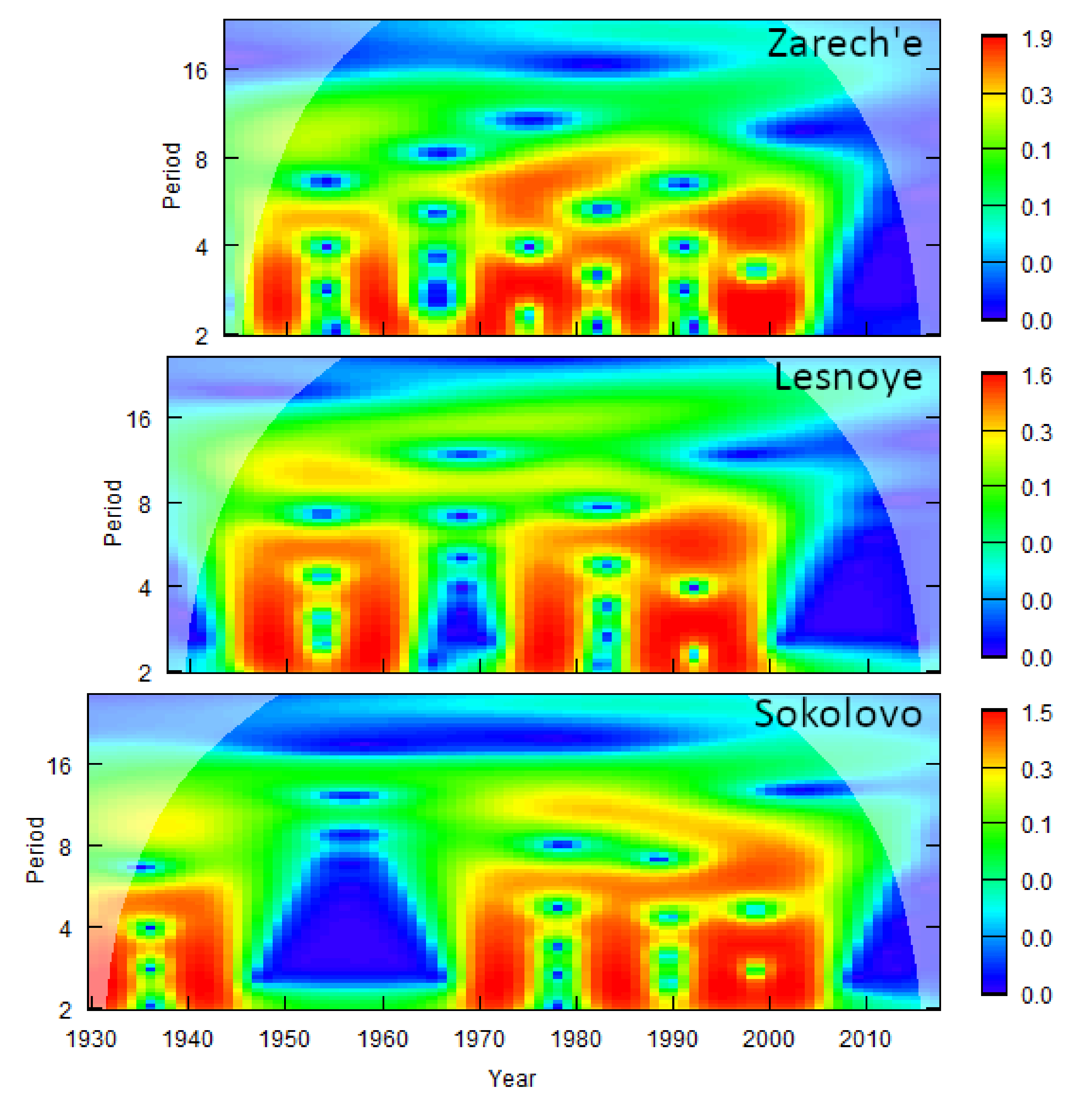
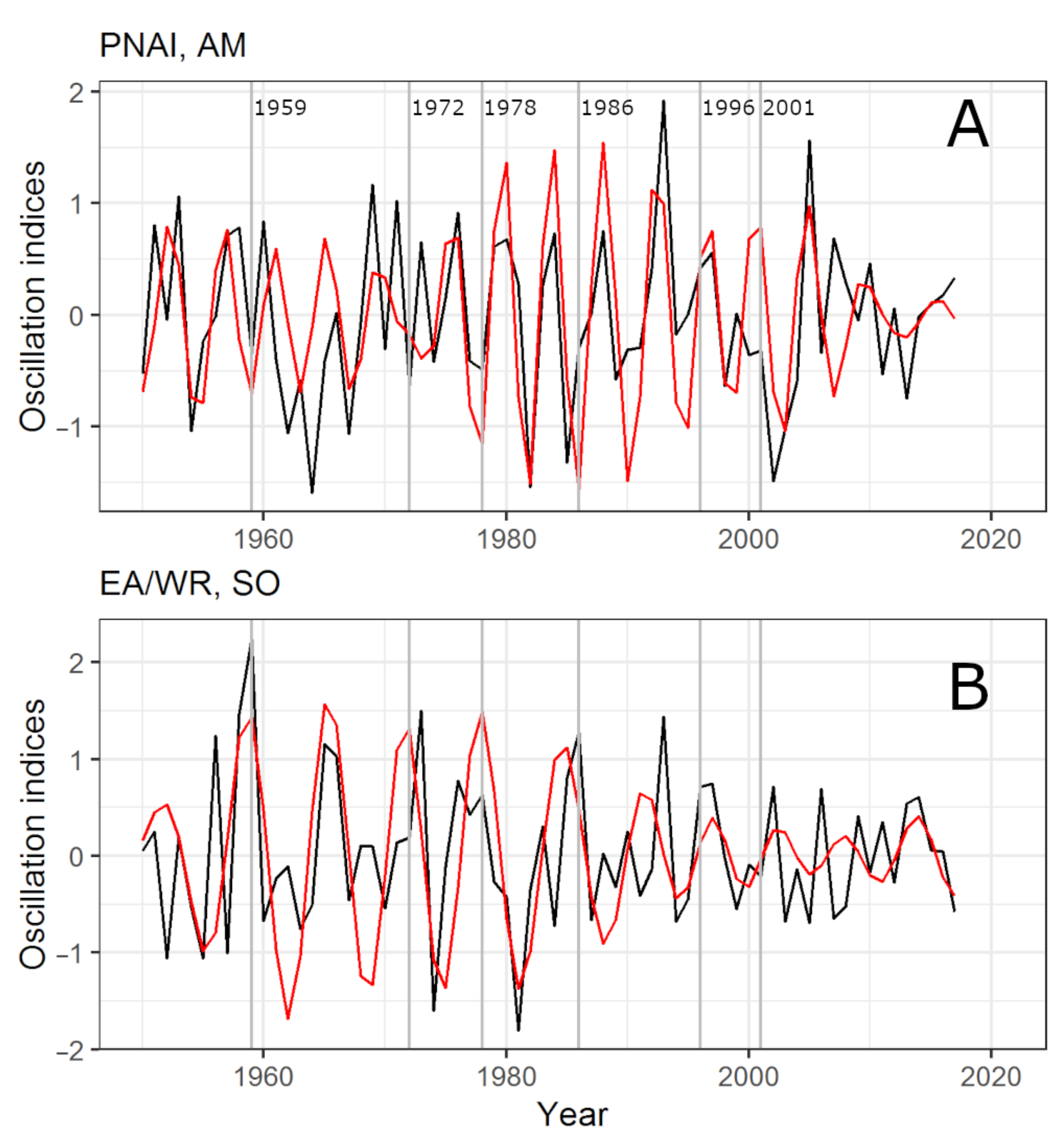
3.3. Links between Climate Oscillations, Outbreaks, and Weather
4. Discussion
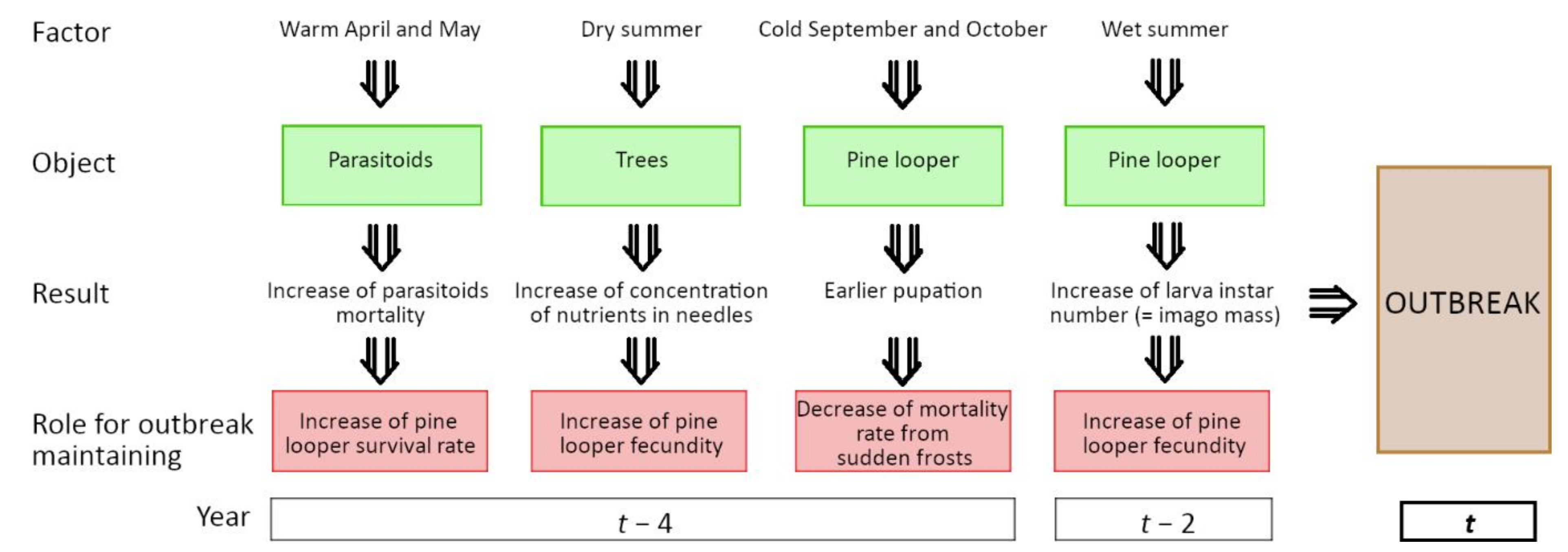
4.1. Weather Conditions Preceding Pine Looper Outbreaks
4.2. Influence of Oscillations on the “Weather–Pine Looper” System
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kondakov, Y.P. The outbreaks patterns of Siberian silkmoth. In The Population Ecology of Siberian Forest Animals; Nauka: Novosibirsk, Russia, 1974; pp. 206–265. [Google Scholar]
- Kharuk, V.I.; Im, S.T.; Ranson, K.J.; Yagunov, M.N. Climate-induced northerly expansion of Siberian Silkmoth Range. Forests 2017, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Navarro, L.; Morin, H.; Bergeron, Y.; Girona, M.M. Changes in spatiotemporal patterns of 20th century spruce budworm outbreaks in eastern Canadian boreal forests. Front. Plant Sci. 2018, 9, 1095. [Google Scholar] [CrossRef]
- Mattson, W.J.; Simmons, G.A.; Witter, J.A. The Spruce Budworm in Eastern North America. In Dynamics of Forest Insect Populations. Patterns, Causes, Implications; Berryman, A.A., Ed.; Springer: New York, NY, USA, 1988; pp. 309–330. [Google Scholar]
- Büntgen, U.; Liebhold, A.; Nievergelt, D.; Wermelinger, B.; Roques, A.; Reinig, F.; Krusic, P.J.; Piermattei, A.; Egli, S.; Cherubini, P.; et al. Return of the moth: Rethinking the effect of climate on insect outbreaks. Oecologia 2020, 192, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Lyytikäinen-Saarenmaa, P.; Tomppo, E. Impact of sawfly defoliation on growth of Scots pine Pinus sylvestris (Pinaceae) and associated economic losses. Bull. Entomol. Res. 2002, 92, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Notaro, S.; Paletto, A.; Raffaelli, R. Economic impact of forest damage in an alpine environment. Acta Silv. Lignaria Hungarica 2009, 5, 131–143. [Google Scholar]
- Jepsen, J.U.; Biuw, M.; Ims, R.A.; Kapari, L.; Schott, T.; Vindstad, O.P.L.; Hagen, S.B. Ecosystem Impacts of a Range Expanding Forest Defoliator an the Fores-Tundra Ecotone. Ecosystems 2013, 16, 561–575. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Palnikova, E.N.; Sviderskaya, I.V.; Soukhovolsky, V.G. The Pine Looper in Siberian Forests. Ecology, Population Dynamics, Impact on Forests; Nauka: Novosibirsk, Russia, 2002; ISBN 5-02-032018-8. [Google Scholar]
- Barbour, D.A. Pine Looper Moth in Britain and Europe. In Dynamics of Forest Insect Populations. Patterns, Causes, Implications; Berryman, A.A., Ed.; Springer: New York, NY, USA, 1988; pp. 291–330. ISBN 978-1-4899-0789-9. [Google Scholar]
- Cedervind, J.; Långström, B. Tree Mortality, Foliage Recovery and Top-kill in Stands of Scots Pine (Pinus sylvestris) Subsequent to Defoliation by the Pine Looper (Bupalus piniaria). Scand. J. For. Res. 2003, 18, 505–513. [Google Scholar] [CrossRef]
- Straw, N.A.; Armour, H.L.; Day, K.R. The financial costs of defoliation of Scots pine (Pinus sylvestris) by pine looper moth (Bupalus piniaria). Forestry 2002, 75, 525–536. [Google Scholar] [CrossRef]
- Prozorov, S.S. Pine looper Bupalus piniarius L. in the forests of West Siberia. In Proceedings of Siberian Institute of Forest Technology. V. XII, Iss. II; Siberian Forest Engineering Institute: Krasnoyarsk, Russia, 1956; pp. 13–84. [Google Scholar]
- Ovchinnikova, T.M.; Fomina, V.A.; Andreeva, E.B.; Dolzhkovaya, N.P.; Soukhovolsky, V.G. Analysis of seasonal development of woody plants in Stolby Natural Reserve with regard to climatic factors. Conifers Boreal Zo. 2011, XXVIII, 54–59. [Google Scholar]
- Klomp, H. The Dynamics of a Field Population of the Pine Looper, Bupalus piniarius L. (Lep., Geom.). Adv. Ecol. Res. 1966, 3, 207–305. [Google Scholar] [CrossRef]
- Gruys, P. Growth of Bupalus piniarius (Lepidoptera: Geometridae) in Relation to Larval Population Density; Centre for Agricultural Publishing and Documentation: Wageningen, The Netherlands, 1970; ISBN 90 220 0300 0. [Google Scholar]
- RIHMI-WDC. Available online: http://aisori.meteo.ru/ClspR (accessed on 15 October 2020).
- Bao, Y.; Na, L.; Han, A.; Guna, A.; Wang, F.; Liu, X.; Zhang, J.; Wang, C.; Tong, S.; Bao, Y. Drought drives the pine caterpillars (Dendrolimus spp.) outbreaks and their prediction under different RCPs scenarios: A case study of Shandong Province, China. For. Ecol. Manag. 2020, 475, 118446. [Google Scholar] [CrossRef]
- Bao, Y.; Wang, F.; Tong, S.; Na, L.; Han, A.; Zhang, J.; Bao, Y.; Han, Y.; Zhang, Q. Effect of drought on outbreaks of major forest pests, pine caterpillars (Dendrolimus spp.), in Shandong Province, China. Forests 2019, 10, 264. [Google Scholar] [CrossRef]
- Haynes, K.J.; Allstadt, A.J.; Klimetzek, D. Forest defoliator outbreaks under climate change: Effects on the frequency and severity of outbreaks of five pine insect pests. Glob. Chang. Biol. 2014, 20, 2004–2018. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.M.; Büntgen, U.; Frank, D.C.; Kausrud, K.; Haynes, K.J.; Liebhold, A.M.; Esper, J.; Stenseth, N.C. Climatic warming disrupts recurrent Alpine insect outbreaks. Proc. Natl. Acad. Sci. USA 2010, 107, 20576–20581. [Google Scholar] [CrossRef]
- Ryerson, D.E.; Swetnam, T.W.; Lynch, A.M. A tree-ring reconstruction of western spruce budworm outbreaks in the San Juan Mountains, Colorado, USA. Can. J. For. Res. 2003, 33, 1010–1028. [Google Scholar] [CrossRef]
- Broekhuizen, N.; Evans, H.F.; Hassell, M.P. Site Characteristics and the Population Dynamics of the Pine Looper Moth. J. Anim. Ecol. 1993, 62, 511–518. [Google Scholar] [CrossRef]
- Straw, N.A. The impact of pine looper moth, Bupalus piniaria L. (Lepidoptera; Geometridae) on the growth of Scots pine in Tentsmuir Forest, Scotland. For. Ecol. Manag. 1996, 87, 209–232. [Google Scholar] [CrossRef]
- Barnston, A.G.; Livezey, R.E. Classification, Seasonality and Persistence of Low-Frequency Atmospheric Circulation Patterns. Mon. Weather Rev. 1987, 115, 1083–1126. [Google Scholar] [CrossRef]
- Salugashvili, R.S. The role of long-distant relationships in regional climatic fluctuations in West Siberia. In Proceedings of RIHMI-WDC. Iss. 176; Sherstukov, B.G., Ed.; RIHMI-WDC: Obninsk, Russia, 2012; pp. 186–193. [Google Scholar]
- Woli, P.; Ortiz, B.V.; Buntin, D.; Flanders, K. El Niño-Southern Oscillation (ENSO) effects on Hessian fly (Diptera: Cecidomyiidae) infestation in the Southeastern United States. Environ. Entomol. 2014, 43, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Yao, Q.; Camarero, J.J.; Hu, H.; Dong, Z.; Li, Y.; Zhou, F.; Chen, X.; Guo, G.; Cao, X.; et al. El Niño-Southern Oscillation modulates insect outbreaks in humid subtropical China: Evidences from tree rings and carbon isotopes. Dendrochronologia 2021, 66, 125815. [Google Scholar] [CrossRef]
- Ray, D.; Peace, A.; Moore, R.; Petr, M.; Grieve, Y.; Convery, C.; Ziesche, T. Improved prediction of the climate-driven outbreaks of Dendrolimus pini in Pinus sylvestris forests. Forestry 2016, 89, 230–244. [Google Scholar] [CrossRef]
- Egorov, N.N. Some harmful insects population dynamics in the protective forests of the Altai Krai for the last 20 years (1930–1949 incl.). In Proceedings of the Second Ecological Conference on the Problem: Animals Outbreaks and Their Forecasts; Part 3; Kiev State University Publishing House: Kiev, Ukraine, 1951; pp. 77–88. [Google Scholar]
- Gorbatenko, V.P.; Ippolitov, I.I.; Kabanov, M.V.; Loginov, S.V.; Podnebesnych, N.V.; Kharyutkina, E.V. Effect of atmospheric circulation on temperature variations in Siberia. Atmos. Ocean. Opt. 2011, 24, 15–21. [Google Scholar]
- Demidko, D.A.; Trefilova, O.V.; Kulakov, S.S.; Mikhaylov, P. V Pine Looper Bupalus piniaria (L.) Outbreaks Reconstruction: A Case Study for Southern Siberia. Insects 2021, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Kuminova, A.V.; Vagina, T.A.; Lapshina, E.I. Geobotanical Regional Assignment of the South-Eastern Part of the West-Siberian Lowlands. In Vegetation of the Steppe and Wood Steppe Zones of Western Siberia (The Novosibirsk Region and Altai Territory). Transactions of the CSBG. Issue 6; Kuminova, A.V., Ed.; Siberian Branch of the Academy of Sciences of the USSR: Novosibirsk, Russia, 1963; pp. 35–62. [Google Scholar]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Koppen-Geiger climate classification. Hydrol. Earth Syst. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Cook, E.R.; Kairiukstis, L.A. (Eds.) Methods of Dendrochronology. Applications in the Environmental Sciences; Springer-Science + Business Media: Dordrecht, The Netherlandes, 1992; ISBN 978-94-015-7879-0. [Google Scholar]
- Cybis Dendrochronology. Home of CDendro & CooRecorder. Available online: http://cybis.se/forfun/dendro/index.php (accessed on 15 October 2020).
- Swetnam, T.W.; Wickman, B.E.; Gene, P.H.; Baisan, C.H. Historical Patterns of Western Spruce Budworm and Douglas-Fir Tussock Moth Outbreaks in the Northern Blue Mountains, Oregon, since AD 1700; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 1995.
- Fan, Z.X.; Bräuning, A. Tree-ring evidence for the historical cyclic defoliator outbreaks on Larix potaninii in the central Hengduan Mountains, SW China. Ecol. Indic. 2017, 74, 160–171. [Google Scholar] [CrossRef]
- Humbert, L.; Kneeshaw, D. Identifying insect outbreaks: A comparison of a blind-source separation method with host vs non-host analyses. Forestry 2011, 84, 453–462. [Google Scholar] [CrossRef][Green Version]
- Il’inskiy, A.I.; Tropin, I.V. (Eds.) Monitoring, Field Surveys and Prognosis of Phyllophagous Insects Outbreaks in the Forests of USSR; Lesnaya Promyshlennost: Moscow, Russia, 1965. [Google Scholar]
- Vlăduţ, A.Ş.; Nikolova, N.; Licurici, M. Aridity assessment within southern Romania and northern Bulgaria. Hrvat. Geogr. Glas. 2017, 79, 5–26. [Google Scholar] [CrossRef]
- Teleconnections. National Centers for Environmental Information (NCEI). Available online: https://www.ncdc.noaa.gov/teleconnections/ (accessed on 13 February 2020).
- Climate Explorer: Climate Indices. Available online: https://climexp.knmi.nl/selectindex.cgi (accessed on 13 February 2020).
- Isaev, A.S.; Khlebopros, R.G.; Nedorezov, L.V.; Kondakov, Y.P.; Kiselev, V.V.; Soukhovolsky, V.G. Population Dynamics of Forest Insects; Isaev, A.S., Ed.; Nauka: Moscow, Russia, 2001; ISBN 5-02-004270-6. [Google Scholar]
- Baltensweiler, W.; Fischlin, A. The Larch Budmoth in the Alps. In Dynamics of Forest Insect Populations. Patterns, Causes, Implications; Berryman, A.A., Ed.; Springer: Boston, MA, USA, 1988; pp. 331–351. ISBN 978-1-4899-0789-9. [Google Scholar]
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Kuhn, M. Caret: Classification and Regression Training. R Package Version 6.0-84. Available online: https://cran.r-project.org/package=caret (accessed on 26 October 2020).
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Canty, A.; Ripley, B. Boot: Bootstrap R (S-Plus) Functions. R Package Version 1.3-27. Available online: https://cran.r-project.org/web/packages/boot/ (accessed on 29 October 2020).
- Koronovskiy, A.A.; Khramov, A.E. Continuous Wavelet Analysis and Its Applications; Phizmatlit: Moscow, Russia, 2003; ISBN 5-9221-0389-X. [Google Scholar]
- Roesch, A.; Schmidbaue, H. WaveletComp: Computational Wavelet Analysis. Available online: https://cran.r-project.org/package=WaveletComp (accessed on 25 October 2020).
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Falk, R.F.; Miller, N.B. A Primer for Soft Modeling, 1st ed.; The University of Akron Press: Akron, OH, USA, 1992; ISBN 0962262846, 9780962262845. [Google Scholar]
- Schwerdtfeger, F. Untersuchungen über den “Eisernen Bestand” von Kiefernspanner (Bupalus piniarius L.), Forleule (Panolis flammea Schiff.) und Kiefernschwärmer (Hyloicus pinastri L.). Zeitschrift für Angew. Entomol. 1952, 34, 216–283. [Google Scholar] [CrossRef]
- Õunap, H. On the pupal parasitoids of the pine looper, Bupalus piniarius (L.) in Estonia. Balt. For. 1996, 2, 2–5. [Google Scholar]
- Kolomiets, N.G.; Artamonov, S.D. Diptera as Entomophags of Forest Silkworm Moth; Nauka: Novosibirsk, Russia, 1994; ISBN 5-02-030833-1. [Google Scholar]
- Ambadan, J.T.; Berg, A.A.; Merryfield, W.J.; Lee, W.-S. Influence of snowmelt on soil moisture and on near surface air temperature during winter–spring transition season. Clim. Dyn. 2017, 51, 1295–1309. [Google Scholar] [CrossRef]
- Hasebe, M.; Kumekawa, T. Estimation of snowmelt volume using air temperature and wind speed. Environ. Int. 1995, 21, 497–500. [Google Scholar] [CrossRef]
- Heron, R.J. The Relative Effects of Cocoon Submergence on the Mortality of the Larch Sawfly, Pristiphora erichsonii (Hymenoptera: Tenthredinidae), and Its Parasite Bessa harveyi (Diptera: Tachinidae). Ann. Entomol. Soc. Am. 1960, 53, 476–481. [Google Scholar] [CrossRef]
- Viktorov, G.A. The Ecology of Parasitic Entomophages; Nauka: Moscow, Russia, 1976. [Google Scholar]
- Baltensweiler, W.; Weber, U.M.; Cherubini, P. Tracing the influence of larch-bud-moth insect outbreaks and weather conditions on larch tree-ring growth in Engadine (Switzerland). Oikos 2008, 117, 161–172. [Google Scholar] [CrossRef]
- Mattson, W.J.; Haack, R.A. The Role of Drought in Outbreaks of Plant-Eating Insects. Bioscience 1987, 37, 110–118. [Google Scholar] [CrossRef]
- Rouault, G.; Candau, J.N.; Lieutier, F.; Nageleisen, L.M.; Martin, J.C.; Warzée, N. Effects of drought and heat on forest insect populations in relation to the 2003 drought in Western Europe. Ann. For. Sci. 2006, 63, 613–624. [Google Scholar] [CrossRef]
- Teshome, D.T.; Zharare, G.E.; Naidoo, S. The Threat of the Combined Effect of Biotic and Abiotic Stress Factors in Forestry Under a Changing Climate. Front. Plant Sci. 2020, 11, 601009. [Google Scholar] [CrossRef]
- Carvalho, A.; Pavia, I.; Fernandes, C.; Pires, J.; Correia, C.; Bacelar, E.; Moutinho-Pereira, J.; Gaspar, M.J.; Bento, J.; Silva, M.E.; et al. Differential physiological and genetic responses of five European Scots pine provenances to induced water stress. J. Plant Physiol. 2017, 215, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, Y.V.; Kartashov, A.V.; Zlobin, I.E.; Sarvin, B.; Stavrianidi, A.N.; Kuznetsov, V.V. Water deficit-dependent changes in non-structural carbohydrate profiles, growth and mortality of pine and spruce seedlings in hydroculture. Environ. Exp. Bot. 2019, 157, 151–160. [Google Scholar] [CrossRef]
- Xiao, X.; Xu, X.; Yang, F. Adaptive responses to progressive drought stress in two Populus cathayana populations. Silva Fenn. 2008, 42, 705–719. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Guo, K.; Fan, D.; Li, G.; Zheng, Y.; Yu, L.; Yang, R. Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China. Environ. Exp. Bot. 2011, 71, 174–183. [Google Scholar] [CrossRef]
- Leuschner, C. Drought response of European beech (Fagus sylvatica L.)—A review. Perspect. Plant Ecol. Evol. Syst. 2020, 47, 125576. [Google Scholar] [CrossRef]
- Guo, X.; Peng, C.; Li, T.; Huang, J.; Song, H.; Zhu, Q.; Wang, M. The Effects of Drought and Re-Watering on Non-Structural Carbohydrates of Pinus tabulaeformis Seedlings. Biology 2021, 10, 281. [Google Scholar] [CrossRef]
- Roitto, M.; Rautio, P.; Markkola, A.; Julkunen-Tiitto, R.; Varama, M.; Saravesi, K.; Tuomi, J. Induced accumulation of phenolics and sawfly performance in Scots pine in response to previous defoliation. Tree Physiol. 2009, 29, 207–216. [Google Scholar] [CrossRef]
- Honěk, A. Intraspecific Variation in Body Size and Fecundity in Insects: A General Relationship. Oikos 1993, 66, 483. [Google Scholar] [CrossRef]
- Broekhuizen, N.; Hassell, M.P.; Evans, H.F. Common Mechanisms Underlying Contrasting Dynamics in Two Populations of the Pine Looper Moth. J. Anim. Ecol. 1994, 63, 511–518. [Google Scholar] [CrossRef]
- Klimetzek, D.; Yue, C. Climate and forest insect outbreaks. Biologia 1997, 52, 153–157. [Google Scholar]
- Li, M.; MacLean, D.A.; Hennigar, C.R.; Ogilvie, J. Previous year outbreak conditions and spring climate predict spruce budworm population changes in the following year. For. Ecol. Manag. 2020, 458, 117737. [Google Scholar] [CrossRef]
- Leśniak, A. Climatic and Meteorological Conditions of the Pine Moth (Dendrolimus pini L.) Outbreaks. Ekol. Pol. 1976, 24, 515–547. [Google Scholar]
- Raspopov, P.M. Outbreaks of forest phyllophagous insects in Chelyabinsk Oblast from 1949 to 1973 and control of their populations. In Proceedings of Ilmenskiy Natural Reserve. Iss. X; USSR Academy of Science, Ural Branch: Chelyabinsk, Russia, 1973; pp. 83–97. [Google Scholar]
- Ims, R.A.; Henden, J.A.; Killengreen, S.T. Collapsing population cycles. Trends Ecol. Evol. 2008, 23, 79–86. [Google Scholar] [CrossRef]
- Clark, P.W.; Speer, J.H.; Winship, L.J. Identifying and Separating Pandora Moth Outbreaks and Climate from A 1500-Year Ponderosa Pine Chronology from Central Oregon. Tree-Ring Res. 2017, 73, 113–125. [Google Scholar] [CrossRef]
- Netherer, S.; Schopf, A. Potential effects of climate change on insect herbivores in European forests-General aspects and the pine processionary moth as specific example. For. Ecol. Manag. 2010, 259, 831–838. [Google Scholar] [CrossRef]
- Ippolitov, I.I.; Kabanov, M.V.; Loginov, S.V. Spatiotemporal scales of warming observed in Siberia. Dokl. Earth Sci. 2007, 413, 248–251. [Google Scholar] [CrossRef]
- Ippolitov, I.I.; Loginov, S.V.; Kharyutkina, E.V.; Moraru, E.I. The changes of climate of Asian part of Russia in 1975–2012. Geogr. Nat. Resour. 2014, 4, 13–21. [Google Scholar]
- Kleshchenko, L.K. About relationship between average-season air temperature and fluctuations of large-scale atmosphere circulation in Russia in the second half of XX century. In Proceedings of RIHMI-WDC. Iss. 176; Sherstukov, B.G., Ed.; RIHMI-WDC: Obninsk, Russia, 2012; pp. 194–213. [Google Scholar]
- Salugashvili, R.S. The climatic regions of Eurasia in the area of influence of Northern Atlantic. In Proceedings of RIHMI-WDC. Iss. 176; Sherstukov, B.G., Ed.; RIHMI-WDC: Obninsk, Russia, 2012; pp. 53–72. [Google Scholar]
- Kholoptsev, V.; Semenov, V.; Kononova, N.K. Long arctic invasion and El-Nino—Southern Oscillation. Izv. Ross. Akad. Nauk. Seriya Geogr. 2018, 4, 22–32. [Google Scholar] [CrossRef]
- Mokhov, I.I.; Smirnov, D.A. Study of the mutual influence of the El Niño-Southern Oscillation processes and the North Atlantic and Arctic Oscillations. Izv. Atmos. Ocean. Phys. 2006, 42, 598–614. [Google Scholar] [CrossRef]
- Rust, H.W.; Richling, A.; Bissolli, P.; Ulbrich, U. Linking teleconnection patterns to European temperature—A multiple linear regression model. Meteorol. Z. 2015, 24, 411–423. [Google Scholar] [CrossRef]
- Westgarth-Smith, A.R.; Roy, D.B.; Scholze, M.; Tucker, A.; Sumpter, J.P. The role of the North Atlantic Oscillation in controlling U.K. butterfly population size and phenology. Ecol. Entomol. 2012, 37, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Nojarov, P. The increase in September precipitation in the Mediterranean region as a result of changes in atmospheric circulation. Meteorol. Atmos. Phys. 2017, 129, 145–156. [Google Scholar] [CrossRef]
- D’Agostino, R.; Lionello, P. Evidence of global warming impact on the evolution of the Hadley Circulation in ECMWF centennial reanalyses. Clim. Dyn. 2017, 48, 3047–3060. [Google Scholar] [CrossRef]
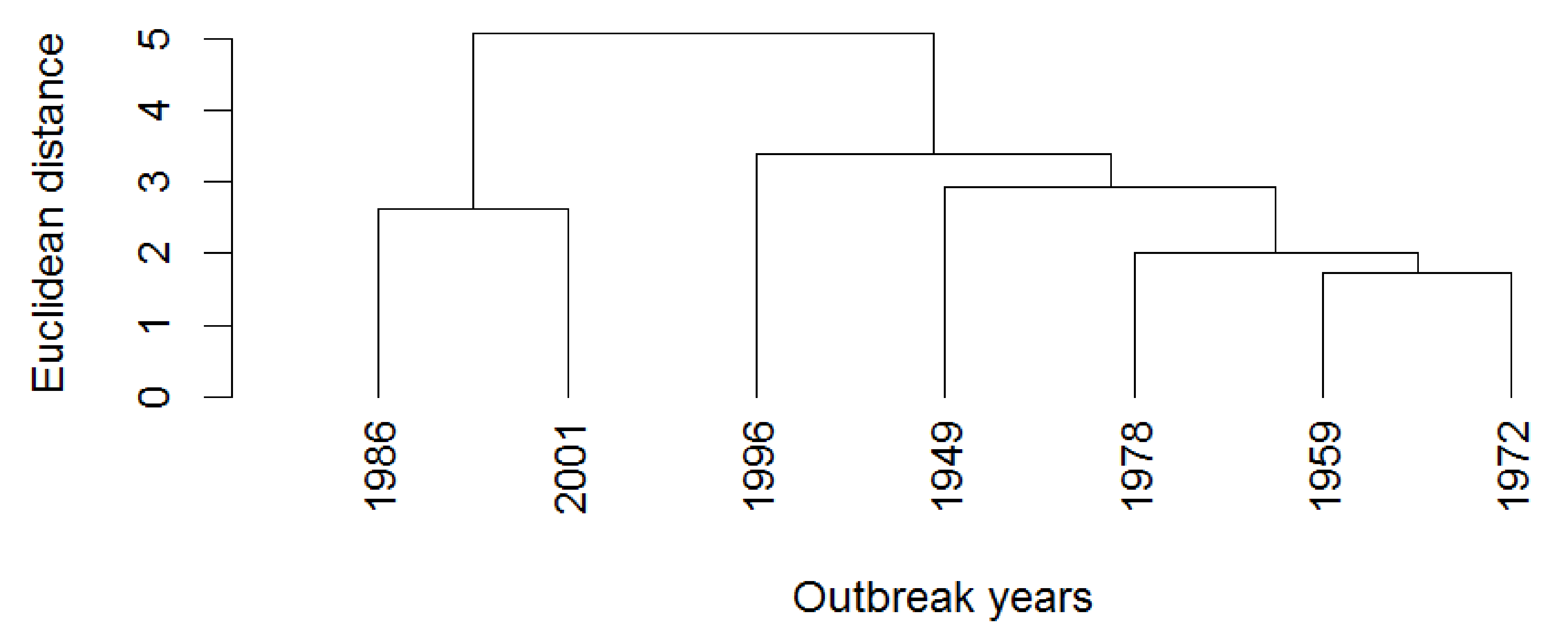
| Model | Coefficients | AIC | CVadj | Acc | Sens | Spec | R2MF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | HTCJJA t − 4 | HTCS t − 4 | TempAM t − 4 | TempSO t − 4 | HTCJJA t − 2 | PrecJJA t − 2 | |||||||
| R | −744.75 | −63.38 | 63.51 | 143.57 | −81.33 | 380.71 | −7.32 | 14 | 0.058 | 1.000 | 1.000 | 1.000 | 0.957 |
| B | −21.33 | −2.40 | 2.60 | 3.04 | −1.54 | 0.96 | 26 | 0.091 | 0.974 | 0.857 | 0.986 | 0.659 | |
| Years | HTCJJAt − 4 | TempAMt − 4 | TempSOt − 4 | HTCJJAt − 2 |
|---|---|---|---|---|
| 1949, 1959, 1972, 1978, 1996 | 2.09 | 8.66 | 5.70 | 5.22 |
| 1986, 2001 | 2.54 | 11.35 | 8.55 | 2.89 |
| Years with no further outbreaks | 3.80 | 7.44 | 6.95 | 3.52 |
| Oscillation | Weather Characteristics | |||
|---|---|---|---|---|
| TempAM | HTCJJA | TempSO | HTCS | |
| AO | 4.14, 5.85–6.06, 6.50 | |||
| NAO | 4.00, 5.85–6.50 | 6.28–6.50 | 4.00–4.29, 7.46–8.00 | |
| PDO | - | - | ||
| PNAI | 4.44–4.76, 8.88–9.51 | 4.00, 6.06–6.27 | ||
| SOI | 7.73–8.00, 8.88–9.19 | |||
| SCAND | 4.00–4.14, 5.66–6.50 | 4.00–4.14, 8.00 | 4.14–4.29, 7.46–8.88 | |
| EA/WR | 8.57–8.88 | 6.06–6.50 | 6.06 | |
| POL | 8.88 | |||
| WP | 6.06 | 4.00, 5.86–6.06, 6.73–6.96 | 6.06, 7.46 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demidko, D.A.; Sultson, S.M.; Mikhaylov, P.V.; Verkhovets, S.V. Influence of Weather Conditions and Climate Oscillations on the Pine Looper Bupalus piniaria (L.) Outbreaks in the Forest-Steppe of the West Siberian Plain. Forests 2022, 13, 15. https://doi.org/10.3390/f13010015
Demidko DA, Sultson SM, Mikhaylov PV, Verkhovets SV. Influence of Weather Conditions and Climate Oscillations on the Pine Looper Bupalus piniaria (L.) Outbreaks in the Forest-Steppe of the West Siberian Plain. Forests. 2022; 13(1):15. https://doi.org/10.3390/f13010015
Chicago/Turabian StyleDemidko, Denis A., Svetlana M. Sultson, Pavel V. Mikhaylov, and Sergey V. Verkhovets. 2022. "Influence of Weather Conditions and Climate Oscillations on the Pine Looper Bupalus piniaria (L.) Outbreaks in the Forest-Steppe of the West Siberian Plain" Forests 13, no. 1: 15. https://doi.org/10.3390/f13010015
APA StyleDemidko, D. A., Sultson, S. M., Mikhaylov, P. V., & Verkhovets, S. V. (2022). Influence of Weather Conditions and Climate Oscillations on the Pine Looper Bupalus piniaria (L.) Outbreaks in the Forest-Steppe of the West Siberian Plain. Forests, 13(1), 15. https://doi.org/10.3390/f13010015






