Soil Microbial Functional Diversity under the Single-Season Influence of Traditional Forest Management in a Sessile Oak Forest of Central Europe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Field and Laboratory Work
2.4. Data Analysis
3. Results
3.1. Differences between 2017 and 2018
3.2. Specifications of the Treatments
3.3. Diversity Indices and Multiple Relations
4. Discussion
4.1. Soil under Influence of Traditional Management
4.2. Factors Influencing Soil Response Measure
4.2.1. Soil Spatial Diversity
4.2.2. Temporal Aspect of Changes in Soil Environment
4.2.3. Microclimate Limitations
4.3. Substrate Consumption Level, Diversity Indices and Metabolic Profile
4.4. Multivariate Soil Responses and Disturbance Regime
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jabiol, B.; Jévy, G.; Bonneau, M.; Brêthes, A. Comprendre les Sols Pour Mieux Gérer les Forêts, 1st ed.; AgroParisTech ENGREF: Nancy Cedex, France, 2009; 624p. [Google Scholar]
- Dzwonko, Z.; Gawroński, S. Effect of litter removal on species richness and acidification of a mixed oak-pine woodland. Biol. Conserv. 2002, 106, 389–398. [Google Scholar] [CrossRef]
- Gimmi, U.; Bürgi, M.; Stuber, M. Reconstructing anthropogenic disturbance regimes in forest ecosystems: A case study from the Swiss Rhone valley. Ecosystems 2008, 11, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Koerner, W.; Dupouey, J.L.; Dambrine, E.; Benoit, M. Influence of Past Land Use on the Vegetation and Soils of Present Day Forest in the Vosges Mountains, France. J. Ecol. 1997, 85, 351–358. [Google Scholar] [CrossRef]
- Dupouey, J.L.; Dambrine, E.; Laffite, J.D.; Moares, C. Irreversible Impact of past Land Use on Forest Soils and Biodiversity. Ecology 2002, 83, 2978–2984. [Google Scholar] [CrossRef]
- Aggestam, F.; Konczal, A.; Sotirov, M.; Wallin, I.; Paillet, Y.; Spinelli, R.; Lindner, M.; Derks, J.; Hanewinkel, M.; Winkel, G. Can nature conservation and wood production be reconciled in managed forests? A review of driving factors for integrated forest management in Europe. J. Environ. Manag. 2020, 268, 110670. [Google Scholar] [CrossRef]
- Douda, J.; Boublík, K.; Doudová, J.; Kyncl, M. Traditional forest management practices stop forest succession and bring back rare plant species. J. Appl Ecol. 2017, 54, 761–771. [Google Scholar] [CrossRef]
- Kadavý, J.; Adamec, Z.; Uherková, B.; Kneifl, M.; Knott, R.; Kučera, A.; Friedl, M.; Dařenová, E.; Skládánka, J.; Drápela, K. Growth response of sessile oak and European hornbeam to traditional coppice-with-standards management. Forests 2019, 10, 515. [Google Scholar] [CrossRef] [Green Version]
- Vild, O.; Kalwij, J.M.; Hédl, R. Effects of simulated historical tree litter raking on the understorey vegetation in a central European forest. Appl. Veg. Sci. 2015, 18, 569–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forest Act No. 289/1995 Coll., On Forests, Under Article 20. Available online: https://www.zakonyprolidi.cz/cs/1995-289 (accessed on 17 September 2020).
- Debussche, M.; Debussche, G.; Lepart, J. Changes in the vegetation of Quercus pubescens woodland after cessation of coppicing and grazing. J. Veg. Sci. 2001, 12, 81–92. [Google Scholar] [CrossRef]
- Bürgi, M.; Gimmi, U. Three objectives of historical ecology: The case of litter collecting in Central European forests. Landsc. Ecol. 2007, 22, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Mayer, M.; Prescott, C.E.; Abaker, W.E.A.; Augusto, L.; Cécillon, L.; Ferreira, G.W.D.; James, J.; Jandl, R.; Katzensteiner, K.; Laclau, J.-P.; et al. Tamm Review: Influence of forest management activities on soil organic carbon stocks: A knowledge synthesis. For. Ecol. Manag. 2020, 466, 118127. [Google Scholar] [CrossRef]
- Hédl, R.; Kopecký, M.; Komárek, J. Half a century of succession in a temperate oakwood: From species-rich community to mesic forest. Divers. Distrib. 2010, 16, 267–276. [Google Scholar] [CrossRef]
- Gimmi, U.; Poulter, B.; Wolf, A.; Portner, H.; Weber, P.; Bürgi, M. Soil carbon pools in Swiss forests show legacy effects from historic forest litter raking. Landsc. Ecol. 2013, 28, 835–846. [Google Scholar] [CrossRef] [Green Version]
- Hofmeister, J.; Oulehle, F.; Krám., P.; Hruška, J. Loss of nutrients due to litter raking compared to the effect of acidic deposition in two spruce stands, Czech Republic. Biogeochemistry 2008, 88, 139–151. [Google Scholar] [CrossRef]
- Schulz, K.; Voigt, K.; Beusch, C.; Almeida-Cortez, J.S.; IngoKowarik, I.; Walz, A.; Cierjacks, A. Grazing deteriorates the soil carbon stocks of Caatinga forest ecosystems in Brazil. For. Ecol. Manag. 2016, 367, 62–70. [Google Scholar] [CrossRef]
- Orefice, J.; Smith, R.G.; Carroll, J.; Asbjornsen, H.; Kelting, D. Soil and understory plant dynamics during conversion of forest to silvopasture, open pasture, and woodlot. Agrofor. Syst. 2017, 91, 729–739. [Google Scholar] [CrossRef]
- Stewart, A.; Coble, A.; Contosta, A.R.; Orefice, J.N.; Smith, R.G.; Asbjornsen, H. Forest conversion to silvopasture and open pasture: Effects on soil hydraulic properties. Agrofor. Syst. 2020, 94, 869–879. [Google Scholar] [CrossRef]
- Kadavý, J.; Servus, M.; Knott, R.; Hurt, V.; Flora, M. Nízký a Střední les Jako Plnohodnotná Alternativa Hospodaření Malých a Středních Vlastníků Lesa, 1st ed.; Lesnická Práce, s.r.o.: Kostelec nad Černými lesy, Czech Republic, 2011; 294p. [Google Scholar]
- Zhu, W.Z.; Xiang, J.S.; Wang, S.G.; Li, M.H. Resprouting ability and mobile carbohydrate reserves in an oak shrubland decline with increasing elevation on the eastern edge of the Qinghai-Tibet Plateau. For. Ecol. Manag. 2012, 278, 118–126. [Google Scholar] [CrossRef]
- Müllerová, J.; Szabó, P.; Hédl, R. The rise and fall of traditional forest management in southern Moravia: A history of the past 700 years. For. Ecol. Manag. 2014, 331, 104–115. [Google Scholar] [CrossRef] [Green Version]
- Pelíšek, J. Stanovištní poměry pařezin v oblasti ČSR. Sborník Ceskoslov. Akad Zemědělských Věd 1957, 3, 85–108. [Google Scholar]
- Kupec, P.; Kučera, A.; Kadavý, J.; Kneifl, M. Contribution to a discussion on the influence of coppicing on soil environment. J. For. Sci. 2015, 61, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Chodak, M.; Klimek, B.; Azarbad, H.; Jaźwa, M. Functional diversity of soil microbial communities under Scots pine, Norway spruce, silver birch and mixed boreal forests. Pedobiologia 2015, 58, 81–88. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Villarreal Ruiz, L.; Vasco-Palacios, A.M.; Quang Thu, P.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [Green Version]
- Ladygina, N.; Hedlund, K. Plant species influence microbial diversity and carbon allocation in the rhizosphere. Soil Biol. Biochem. 2010, 42, 162–168. [Google Scholar] [CrossRef]
- Zak, J.C.; Willig, M.R.; Moorhead, D.L.; Wildman, H.G. Functional diversity of microbial communities: A quantitative approach. Soil Biol. Biochem. 1994, 26, 1101–1108. [Google Scholar] [CrossRef]
- Aerts, R.; Honnay, O. Forest restoration, biodiversity and ecosystem functioning. BMC Ecol. 2011, 11, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadotte, M.W.; Carscadden, K.; Mirotchnick, N. Beyond species: Functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 2011, 48, 1079–1087. [Google Scholar] [CrossRef]
- Thom, D.; Taylor, A.R.; Seidl, R.; Thuiller, W.; Wang, J.; Robideau, M.; Keeton, W.S. Forest structure, not climate, is the primary driver of functional diversity in northeastern North America. Sci. Total Environ. 2021, 762, 143070. [Google Scholar] [CrossRef] [PubMed]
- Duveneck, M.J.; Scheller, R.M.; White, M.A. Effects of alternative forest management on biomass and species diversity in the face of climate change in the northern Great Lakes region (USA). Can. J. For. Res. 2014, 44, 700–710. [Google Scholar] [CrossRef]
- Escalas, A.; Hale, L.; Voordeckers, J.W.; Yang, Y.; Firestone, M.K.; Alvarez-Cohen, L.; Zhou, J. Microbial functional diversity: From concepts to applications. Ecol. Evol. 2019, 9, 12000–12016. [Google Scholar] [CrossRef] [Green Version]
- Klimek, B.; Chodak, M.; Jaźwa, M.; Solak, A.; Tarasek, A.; Niklińska, M. The relationship between soil bacteria substrate utilisation patterns and the vegetation structure in temperate forests. Eur. J. For. Res. 2016, 135, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Tomao, A.; Bonet, J.A.; de-Miguel, C.S. How does forest management affect fungal diversity and community composition? Current knowledge and future perspectives for the conservation of forest fungi. For. Ecol. Manag. 2020, 457, 117678. [Google Scholar] [CrossRef]
- Viewegh, J.; Kusbach, A.; Mikeska, M. Czech forest ecosystem classification. J. For. Sci. 2003, 49, 85–93. [Google Scholar] [CrossRef] [Green Version]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 1st ed.; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2014; p. 203. [Google Scholar]
- Jabiol, B.; Brêthes, A.; Toutain, F.; Ponge, J.-F.; Brun, J.J. L´Humus Sous Toutes Ses Formes, 1st ed.; École Nationale du Génie Rural des Eaux et Forêts: Nancy, France, 1995; 63p. [Google Scholar]
- Van Calster, H.; Baeten, L.; Verheyen, K.; De Keersmaeker, L.; Dekeyser, S.; Rogister, J.E.; Hermy, M. Diverging effects of overstorey conversion scenarios on the understorey vegetation in a former coppice-with-standards forest. For. Ecol. Manag. 2008, 256, 519–528. [Google Scholar] [CrossRef]
- Adams, F.; Evans, C.E. A rapid method for measuring lime requirement of yellow podzolic soils. Soil Sci Soc. Am. J. 1990, 26, 355–357. [Google Scholar] [CrossRef]
- Sokolov, A.V. Determination of exchangeable Al in soil. Chem. Soc. Zemled. 1939, 7, 30–35. [Google Scholar]
- Mehlich, A. New extractant for soil test evaluation of phosphorus, potassium, magnesium, calcium, sodium, manganese and zinc 1. Commun. Soil Sci. Plant. Anal. 1978, 9, 477–492. [Google Scholar] [CrossRef]
- Zbíral, J.; Malý, S.; Váňa, M. Soil Analysis, Soil Survey Laboratory Methods Manual; Central Institute for Supervising and Testing in Agriculture: Brno, Czech Republic, 2011; Volume 3, p. 290. [Google Scholar]
- Kirk, P.L. Kjeldahl Method for Total Nitrogen. Anal. Chem. 1950, 22, 354–358. [Google Scholar] [CrossRef]
- Konova, M.; Bělčikova, N. Uskorennyje metody opredelenija sostava gumusa mineralnych počv. Počvovedenie 1961, 10, 75–87. [Google Scholar]
- White, R.E. Principles and Practice of Soil Science: The Soil as a Natural Resource, 4th ed.; Wiley-Blackwell: Oxford, UK, 2006; 376p. [Google Scholar]
- Rejšek, K.; Formánek, P.; Pavelka, M. Estimation of protease activity in soils at low temperatures by casein amendment and with substitution of buffer by demineralized water. Amino Acids 2008, 35, 411–417. [Google Scholar] [CrossRef]
- Kučera, A.; Holík, L.; Marosz, K.; Martiník, A.; Vavříček, D. Changes in forms of available nitrogen and respiration in soil of beech forest as a reaction to a deforestation resulting from wind storm. Acta Univ. Agric. Silvic. Mendel. Brun. 2013, 61, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Rejšek, K. Acid phosphomonoesterase activity of ectomycorrhizal roots in Norway spruce pure stands exposed to pollution. Soil Biol. Biochem. 1991, 23, 667–671. [Google Scholar] [CrossRef]
- Islam, K.R.; Weil, R.R. Microwave irradiation of soil for routine measurement of microbial biomass carbon. Biol. Fertil. Soils 1998, 27, 408–416. [Google Scholar] [CrossRef]
- Xu, W.; Ge, Z. Application and Optimization of Biolog EcoPlates in Functional Diversity Studies of Soil Microbial Communities. MATEC Web Conf. 2015, 22, 1–6. [Google Scholar] [CrossRef] [Green Version]
- StatSoft Inc. STATISTICA (Data Analysis Software System); Version 12; StatSoft: Tulsa, OK, USA, 2013; Available online: http://www.statsoft.com (accessed on 17 September 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: http://www.R-project.org/ (accessed on 17 September 2020).
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1996, 13, 131–144. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communications, 1st ed.; University of Illinois Press: Urbana, IL, USA, 1963; 131p. [Google Scholar]
- Oksanen, J.; Blanchet, F.H.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. ‘Vegan’ Community Ecology Package; Version 2.5–6; 2019. Available online: https://cran.r-project.org; https://github.com/vegandevs/vegan (accessed on 18 September 2020).
- Wickham, H.; Lionel, H.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. Create Elegant Data Visualisations Using the Grammar of Graphics. Package ggplot2 Version 3.3.0 for R Software for Statistical Computing. 2020. Available online: https://ggplot2.tidyverse.org/ (accessed on 21 September 2020).
- Rogers, P. Disturbance Ecology and Forest Management: A review of the Literature; General Technical Report INT-GTR-336; U.S. Department of Agriculture, Forest Service, Intermountain Research Station: Ogden, UT, USA, 1996; 16p. [Google Scholar]
- Van Calster, H.; Baeten, L.; De Schrijver, A.; Keersmaeker, L.D.; Rogister, J.E.; Verheyen, K.; Hermy, M. Management driven changes (1967–2005) in soil acidity and the understorey plant community following conversion of a coppice-with-standards forest. For. Ecol. Manag. 2007, 241, 258–271. [Google Scholar] [CrossRef]
- Chen, F.; Zheng, H.; Kai, Z.; Ouyang, Z.; Lan, J.; Li, H.; Shi, Q. Changes in soil microbial community structure and metabolic activity following conversion from native Pinus massoniana plantations to exotic Eucalyptus plantations. For. Ecol. Manag. 2013, 291, 65–72. [Google Scholar] [CrossRef]
- Mohr, D. Soil Degradation in simple Oak Coppice Forests of the Ahr-Eifel. Implications for Forest Management Derived from Soil Ecological Studies. Ph.D. Thesis, Universität zu Köln, Cologne, Germany, 2004. [Google Scholar]
- Sasaki, N.; Putz, F.E. Critical need for new definitions of “forest” and “forest degradation” in global climate change agreements. Conserv. Lett. 2009, 2, 226–232. [Google Scholar] [CrossRef]
- Papaioannou, A.G. Assessment of the Empirical Management Method of Coppice Chestnut (Castanea sativa Mill.) Forests Practiced by the Monks and its Effect on the Availability of Forest Soil Resources in Mount Athos, Greece. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Salomón, R.; Rodríguez-Calcerrada, J.; Zafra, E.; Morales-Molino, C.; Rodríguez-García, A.; González-Doncel, I.; Oleksyn, J.; Zytkowiak, R.; López, R.; CarlosMiranda, J.; et al. Unearthing the roots of degradation of Quercus pyrenaica coppices: A root-to-shoot imbalance caused by historical management? For. Ecol. Manag. 2016, 363, 200–211. [Google Scholar] [CrossRef]
- Vacca, A.; Aru, F.; Ollesch, G. Short-term Impact of Coppice Management on Soil in a Quercus ilex l. Stand of Sardinia. Land Degrad. Dev. 2017, 28, 553–565. [Google Scholar] [CrossRef] [Green Version]
- Zlatník, A. Výmladkové lesy s hlediska proměn lesů pod vlivem člověka a úloha ekologie pří přeměnách a převodech výmladkových lesů (in Czech). Sborník Ceskoslov. Akad Zemědělských Věd 1957, 3, 109–123. [Google Scholar]
- Hölscher, D.; Schade, E.; Leuschner, C. Effects of coppicing in temperate deciduous forests on ecosystem nutrient pools and soil fertility. Basic Appl Ecol. 2001, 2, 155–164. [Google Scholar] [CrossRef]
- Pignataro, A.; Moscatelli, M.C.; Mocali, S.; Benedetti, A.; Grego, S. Effect of oak forest coppicing on soil biochemical properties in mediterranean environment. Ann. Univ. Crainova 2011, 41, 162–172. [Google Scholar]
- Wilke, B.; Bogenrieder, A.; Wilmanns, O. Differenzierte Streuverteilung im Walde, ihre Ursachen und Folgen. Phytocoenologia 1993, 23, 129–155. [Google Scholar] [CrossRef]
- Darenova, E.; Čater, M. Effect of spatial scale and harvest on heterogeneity of forest floor CO2 efflux in a sessile oak forest. Catena 2020, 188, 104455. [Google Scholar] [CrossRef]
- Petersen, P.M. Importance of site conditions and time since abandonment for coppice vegetation on Langeland, Denmark. Nord. J. Bot. 2003, 22, 463–481. [Google Scholar] [CrossRef]
- Logli, F.; Joffre, R. Individual variability as related to stand structure and soil condition in a Mediterranean oak coppice. For. Ecol. Manag. 2001, 142, 53–63. [Google Scholar] [CrossRef]
- Kaiser, C.H.; Fuchslueger, L.; Koranda, M.; Gorfer, M.; Stange, C.F.; Kitzler, B.; Rasche, F.; Strauss, J.; Sessitsch, A.; Zechmeister-Boltenstern, S.; et al. Plants control the seasonal dynamics of microbial N cycling in a beech forest soil by belowground C allocation. Ecology 2011, 92, 1036–1051. [Google Scholar] [CrossRef]
- Hédl, R.; Rejšek, K. Soil changes after forty years of succession in an abandoned coppice in the Czech Republic. Acta Agron. Hung. 2007, 55, 453–474. [Google Scholar] [CrossRef]
- Kučera, A.; Šikl, J.; Oulehle, F.; Šamonil, P.; Marosz, K.; Hleb, R.; Houška, J.; Hruška, J. Comparison of modern and traditional methods of soilsorption complex measurement: The basis of long-term studies and modelling. Ekol. Bratisl. 2014, 33, 48–59. [Google Scholar] [CrossRef]
- Cullotta, S.; Bagarello, V.; Baiamonte, G.; Gugliuzza, G.; Iovino, M.; La Mela Veca, D.S.; Maetzke, F.; Palmeri, V.; Sferlazza, S. Comparing Different Methods to Determine Soil Physical Quality in a Mediterranean Forest and Pasture Land. Soil Sci. Soc. Am. J. 2016, 80, 1038–1056. [Google Scholar] [CrossRef]
- Boyle, J.R.; Powers, R.F. Forest Soils and Ecosystem Sustainability, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2001; 474p. [Google Scholar]
- Hallett, L.M.; Stein, C.; Suding, K.N. Functional diversity increases ecological stability in a grazed grassland. Oecologia 2017, 183, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Chazarenc, F.; Brisson, J.; Merlin, G. Seasonal and spatial changes of microorganism communities in constructed wetlands: A community level physiological profiling analysis. Int. J. Chem. Eng. 2010, 2010, 490240. [Google Scholar] [CrossRef] [Green Version]
- Howes, A.S. Environmental Factors, Not Plants, Contribute to Functional Diversity of Soil Bacteria in the Dunes of Lake Michigan. Master’s Thesis, University of Louisville, Louisville, KY, USA, May 2017. [Google Scholar]
- Rosíková, J.; Darenova, E.; Kučera, A.; Volarik, D.; Vránová, V. Effect of different dolomitic limestone dosages on soil respiration in a mid-altitudinal Norway spruce stand. iForest 2019, 12, 357–365. [Google Scholar] [CrossRef]
- Preston-Mafham, J. Analysis of microbial community functional diversity using sole-carbon-source utilisation profiles—A critique. FEMS Microbiol. Ecol. 2002, 42, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Yokokawa, T.; Ke, P.-J.; Hsieh, I.-F.; Hsieh, C.-H.; Kume, T.; Yoneya, K.; Matsui, K. Statistical recipe for quantifying microbial functional diversity from EcoPlate metabolic profiling. Ecol. Res. 2018, 33, 249–260. [Google Scholar] [CrossRef]
- Biolog Inc. Microbial Community Analysis: EcoPlateTM; Biolog Inc.: Hayward, CA, USA, 2007; p. 2. [Google Scholar]
- Pignataro, A.B.; Moscatelli, M.C.; Mocali, S.; Grego, S.; Benedetti, A. Assessment of soil microbial functional diversity in a coppiced forest system. Appl. Soil Ecol. 2012, 62, 115–123. [Google Scholar] [CrossRef]
- Pignataro, A. Assessment of microbial functional and genetic diversity of forest soils in Central Italy. Ph.D. Thesis, Università Degli Studi Della Tuscia, Viterbo, Italy, 27 April 2012. [Google Scholar]
- Gömöryová, E.; Fleischer, P.; Gömöry, D. Soil microbial community responses to windthrow disturbance in Tatra National Park (Slovakia) during the period 2006–2013 / Odozva pôdneho mikrobiálneho spoločenstva na veternú kalamitu v Tatranskom národnom parku (Slovensko) v období rokov 2006–2013. Cent. Eur. For. J. 2014, 60, 137–142. [Google Scholar] [CrossRef]
- Vuong, T.M.D.; Zeng, J.Y.; Man, X.L. Soil fungal and bacterial communities in southern boreal forests of the Greater Khingan Mountains and their relationship with soil properties. Sci. Rep. 2010, 10, 22025. [Google Scholar] [CrossRef] [PubMed]
- Błońska, E.; Lasota, J.; Gruba, P. Effect of temperate forest tree species on soil dehydrogenase and urease activities in relation to other properties of soil derived from loess and glaciofluvial sand. Ecol. Res. 2016, 31, 655–664. [Google Scholar] [CrossRef] [Green Version]
- Adamczyk, B.; Adamczyk, S.; Kukkola, M.; Tamminen, P.; Smolander, A. Logging residue harvest may decrease enzymatic activity of boreal forest soils. Soil Biol. Biochem. 2015, 82, 74–80. [Google Scholar] [CrossRef]
- Klose, S.; Tabatabai, M.A. Response of phosphomono-esterases in soils to chloroform fumigation. J. Plant. Nutr. Soil Sci. 2002, 165, 429–434. [Google Scholar] [CrossRef]
- Tan, X.; Chang, S.X.; Kabzems, R. Soil compaction and forest floor removal reduced microbial biomass and enzyme activities in a boreal aspen forest soil. Biol. Fertil. Soils 2008, 44, 471–479. [Google Scholar] [CrossRef]
- Šarapatka, B.; Kršková, M. Interactions between phos-phatase activity and soil characteristics at some locations in the Czech Republic. Plant. Prod. 1997, 43, 415–419. [Google Scholar]
- Attiwill, P.M. The disturbance of forest ecosystems: The ecological basis for conservative management. For. Ecol. Manag. 1993, 63, 247–300. [Google Scholar] [CrossRef]
- Marshall, V.G. Impacts of forest harvesting on biological processes in northern forest soils. For. Ecol. Manag. 2000, 133, 43–60. [Google Scholar] [CrossRef]
- Zhang, N.; Wan, S.; Li, L.; Bi, J.; Zhao, M.; Ma, K. Impacts of urea N addition on soil microbial community in a semi-arid temperate steppe in northern China. Plant. Soil 2008, 311, 19–28. [Google Scholar] [CrossRef]


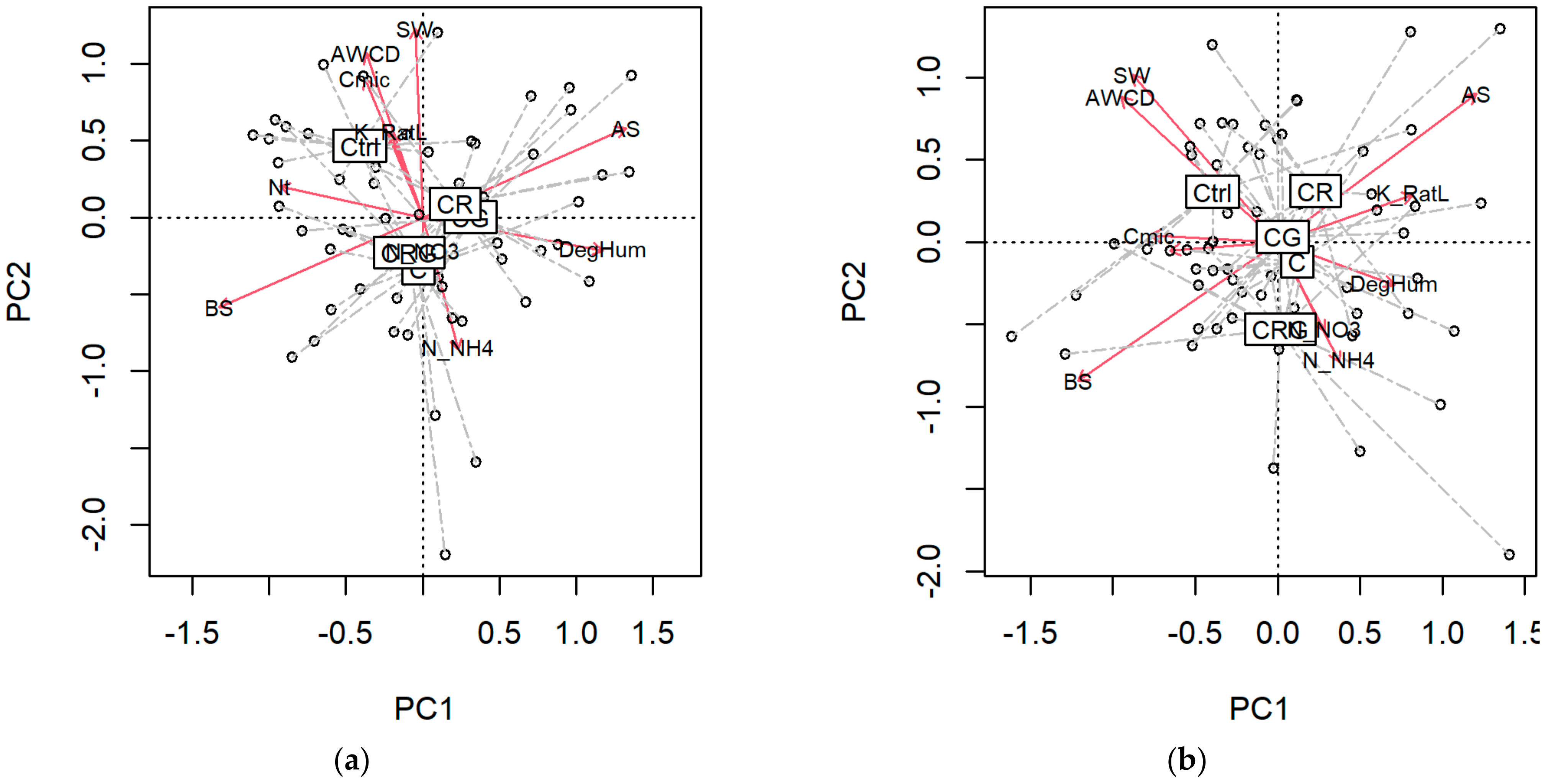


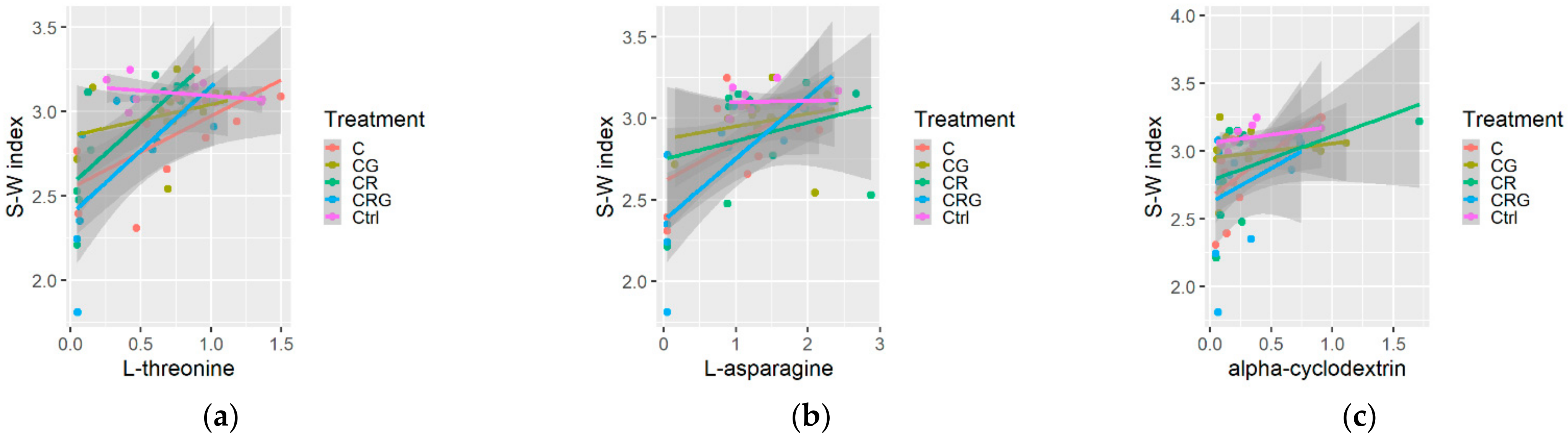
| C | CR | CG | CRG | Ctrl | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Mean | Δ | t-test | Mean | Δ | t-test | Mean | Δ | t-test | Mean | Δ | t-test | Mean | Δ | t-test | |||||
| 2017 | p | 2017 | p | 2017 | p | 2017 | p | 2017 | p | |||||||||||
| pH/H2O | 5.58 | + | 0.29 | * | 5.43 | − | 0.09 | n-s | 5.61 | + | 0.33 | · | 5.28 | + | 0.57 | ** | 5.42 | + | 0.58 | ** |
| pH/KCl | 4.83 | + | 0.28 | · | 4.65 | − | 0.17 | n-s | 4.93 | + | 0.38 | n-s | 4.53 | + | 0.65 | * | 4.73 | + | 0.6 | * |
| Corg | 4.56 | + | 0.65 | n-s | 3.77 | + | 1.025 | * | 3.78 | + | 0.32 | n-s | 3.7 | + | 1.07 | * | 4.51 | + | 0.7 | · |
| Nt | 0.31 | + | 0.076 | ** | 0.28 | + | 0.089 | ** | 0.29 | + | 0.062 | ** | 0.27 | + | 0.098 | *** | 0.32 | + | 0.1 | ** |
| C/N | 15 | − | 1.23 | n-s | 13.83 | - | 0.808 | n-s | 13.75 | − | 1.61 | * | 13.92 | − | 0.89 | n-s | 14.75 | − | 2.33 | * |
| C-THS | 1.15 | + | 0.09 | n-s | 0.94 | + | 0.19 | · | 0.97 | + | 0.072 | n-s | 0.87 | + | 0.22 | ** | 1.11 | + | 0.018 | n-s |
| C-HA | 0.51 | + | 0.055 | n-s | 0.36 | + | 0.16 | * | 0.39 | + | 0.068 | · | 0.33 | + | 0.14 | ** | 0.46 | + | 0.024 | n-s |
| C-FA | 0.61 | + | 0.039 | · | 0.56 | + | 0.023 | n-s | 0.57 | + | 0.028 | n-s | 0.5 | + | 0.103 | ** | 0.61 | − | 0.007 | n-s |
| C-HA/FA | 0.85 | + | 0.03 | n-s | 0.65 | + | 0.245 | * | 0.68 | + | 0.078 | n-s | 0.67 | + | 0.11 | n-s | 0.75 | + | 0.095 | n-s |
| DegHum | 25.47 | − | 1.38 | n-s | 25.48 | − | 1.44 | n-s | 25.74 | − | 0.49 | n-s | 24.21 | − | 1.01 | n-s | 24.75 | − | 2.9 | ** |
| P | 45.33 | + | 1 | n-s | 46.33 | + | 19.17 | * | 38.75 | + | 14.42 | ** | 47.33 | + | 25.42 | * | 57.25 | + | 14.17 | * |
| Mg | 191.17 | + | 72.42 | ** | 181.92 | + | 1.33 | n-s | 238.5 | + | 51.17 | ** | 171.17 | + | 93.92 | ** | 240.92 | + | 70.25 | n-s |
| Ca | 2066.4 | + | 1172.25 | ** | 1614.5 | + | 593.6 | * | 1966.2 | + | 758.8 | * | 1513.67 | + | 1142.5 | ** | 1876.25 | + | 963.58 | * |
| K | 152.25 | − | 21.5 | n-s | 128.75 | + | 2.42 | n-s | 125.17 | + | 5.67 | n-s | 123.67 | + | 59.08 | n-s | 181.42 | − | 23.75 | * |
| H+ | 65.25 | − | 17.25 | ** | 69.17 | − | 4.58 | n-s | 60.92 | − | 22.42 | * | 70.08 | − | 26.25 | ** | 72.33 | − | 26 | ** |
| Al3+ | 7.67 | − | 5.67 | · | 10.75 | + | 0.08 | n-s | 4.75 | − | 8.33 | * | 8.75 | − | 7.5 | * | 5.33 | − | 4.17 | · |
| CEC | 195.17 | + | 40.75 | ** | 178.25 | + | 25.25 | * | 186.25 | + | 11.25 | n-s | 171.17 | + | 32 | · | 195.5 | + | 22.67 | n-s |
| AS | 34.24 | − | 12.16 | ** | 40.37 | + | 3.11 | n-s | 32.86 | − | 11.54 | * | 41.48 | − | 18.22 | ** | 38.86 | − | 15.86 | ** |
| Bc | 123 | + | 63.58 | ** | 98.83 | + | 29.75 | · | 121 | + | 42.17 | * | 92.75 | + | 66.25 | ** | 118.08 | + | 53.25 | * |
| BS | 61.88 | + | 16.03 | ** | 53.22 | + | 5.49 | n-s | 64.78 | + | 18.02 | ** | 53.69 | + | 22.93 | ** | 58.21 | + | 18.89 | ** |
| K-rat.law | 0.37 | − | 0.02 | * | 0.302 | − | 0.006 | n-s | 0.358 | − | 0.01 | n-s | 0.344 | − | 0.007 | n-s | 0.463 | − | 0.023 | ** |
| Ca/K | 13.63 | + | 13.18 | ** | 12 | + | 5.065 | · | 16.23 | + | 5.29 | * | 10.43 | + | 4.78 | * | 10.43 | + | 7.845 | ** |
| Ca/Mg | 127.38 | + | 31.25 | · | 100.72 | + | 27.22 | · | 113.15 | + | 21.7 | n-s | 105.88 | + | 16.19 | n-s | 100.26 | + | 11.07 | n-s |
| Mg/K | 1.3 | + | 0.74 | *** | 1.46 | − | 0.03 | n-s | 1.94 | + | 0.29 | · | 1.44 | + | 0.3 | · | 1.36 | + | 0.64 | · |
| N-NH4+ | 9.09 | + | 9.17 | ** | 9.03 | + | 2.014 | n-s | 9.64 | + | 15.23 | · | 9.4 | + | 14.86 | · | 10.43 | + | 0.41 | n-s |
| N-NO3- | 0.45 | + | 1.058 | ** | 0.78 | + | 1.58 | *** | 0.27 | + | 1.3 | ** | 0.39 | + | 3.39 | * | 0.68 | + | 0.95 | * |
| Phosphatase | 429.9 | − | 184.81 | *** | 357.62 | − | 83.17 | * | 408.56 | − | 78.58 | * | 375.47 | − | 69.72 | · | 457.67 | − | 63.19 | * |
| Urease | 75.98 | − | 20.58 | * | 70.1 | + | 10.75 | n-s | 70.75 | − | 12.84 | · | 66.83 | + | 9.06 | n-s | 90.07 | − | 1.01 | n-s |
| Protease | 128.04 | − | 37.02 | n-s | 106.47 | + | 33.64 | n-s | 130.83 | + | 16.22 | n-s | 97.49 | + | 84.12 | * | 130 | + | 50.29 | · |
| Cmic | 3707.7 | − | 1020 | n-s | 3264 | + | 265.9 | n-s | 3280 | - | 200.1 | n-s | 2844.5 | + | 1145.3 | n-s | 4160.6 | + | 41.17 | n-s |
| Index | C | CR | CG | CRG | Ctrl | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Mean | Median | Mean | Median | Mean | Median | Mean | Median | ||
| pH/H2O–delta 3 | ab | 0.300 | b | 0.150 | ab | 0.350 | a | 0.450 | a | 0.600 | 0.03611 |
| pH/KCl–delta 3 | ab | 0.300 | b | 0.150 | ab | 0.400 | a | 0.600 | ab | 0.500 | 0.04771 |
| N-NO3−–delta 3 | ab | 1.011 | ab | 1.675 | ab | 1.241 | a | 1.796 | b | 0.860 | 0.04461 |
| Phosphatase–delta 3 | b | −174.18 | ab | −106.06 | ab | −89.37 | a | −95.89 | a | −72.48 | 0.01691 |
| Cmic–delta 3 | b | −1207.99 | ab | 177.48 | ab | −131.402 | a | 789.72 | ab | −6.19 | 0.04871 |
| AWCD–2018 | 0.487 b | 0.482 | 0.544 ab | 0.532 | 0.605 ab | 0.600 | 0.464 b | 0.477 | 0.715 a | 0.725 | 0.01841 |
| S-W–2018 | 2.859 | 2.934 ab | 2.910 | 3.067 ab | 2.986 | 3.013 ab | 2.756 | 2.886 b | 3.102 | 3.083 a | 0.03302 |
| 2017 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil Property | Block (Treatment) (n = 12/Block) | C | CG | CR | CRG | Ctrl | ||||||||||
| Plot (n = 4/Plot) | 1B | 4B | 6B | 2B | 3B | 5B | 1A | 4A | 6A | 2A | 3A | 5A | K1 | K2 | K3 | |
| pH/H2O | sd (block) | 0.68 | 0.80 | 0.55 | 0.61 | 0.56 | ||||||||||
| sd (plot) | 0.34 | 0.40 | 0.26 | 0.15 | 0.44 | 0.64 | 0.10 | 0.36 | 0.17 | 0.15 | 0.59 | 0.28 | 0.23 | 0.64 | 0.70 | |
| pH/KCl | sd (block) | 0.85 | 0.98 | 0.70 | 0.72 | 0.68 | ||||||||||
| sd (plot) | 0.41 | 0.54 | 0.36 | 0.17 | 0.59 | 0.73 | 0.12 | 0.48 | 0.21 | 0.12 | 0.75 | 0.37 | 0.36 | 0.77 | 0.83 | |
| N-NO3- | sd (block) | 0.43 | 0.55 | 0.11 | 0.27 | 0.44 | ||||||||||
| sd (plot) | 0.03 | 0.25 | 0.62 | 0.65 | 0.18 | 0.67 | 0.16 | 0.13 | 0.14 | 0.19 | 0.10 | 0.12 | 0.17 | 0.43 | 0.62 | |
| Phosph. | sd (block) | 47 | 74 | 72 | 76 | 113 | ||||||||||
| sd (plot) | 33 | 41 | 52 | 44 | 29 | 25 | 12 | 61 | 103 | 46 | 93 | 44 | 76 | 84 | 140 | |
| Cmic | sd (block) | 609 | 859 | 627 | 1031 | 1759 | ||||||||||
| sd (plot) | 628 | 524 | 735 | 957 | 1015 | 486 | 747 | 573 | 574 | 490 | 1348 | 158 | 534 | 953 | 1776 | |
| 2018 | ||||||||||||||||
| pH/H2O | sd (block) | 0.55 | 0.60 | 0.64 | 0.43 | 0.46 | ||||||||||
| sd (plot) | 0.06 | 0.38 | 0.45 | 0.45 | 0.67 | 0.72 | 0.32 | 0.25 | 0.24 | 0.60 | 0.43 | 0.10 | 0.26 | 0.72 | 0.42 | |
| pH/KCl | sd (block) | 0.63 | 0.72 | 0.68 | 0.42 | 0.50 | ||||||||||
| sd (plot) | 0.17 | 0.46 | 0.59 | 0.52 | 0.74 | 0.91 | 0.36 | 0.39 | 0.19 | 0.63 | 0.42 | 0.17 | 0.30 | 0.79 | 0.44 | |
| N-NO3- | sd (block) | 0.95 | 0.14 | 0.67 | 4.33 | 1.08 | ||||||||||
| sd (plot) | 0.19 | 0.78 | 1.60 | 0.41 | 0.60 | 1.15 | 0.35 | 0.51 | 0.63 | 2.27 | 6.93 | 0.29 | 0.33 | 1.69 | 0.29 | |
| Phosph. | sd (block) | 40 | 64 | 81 | 77 | 89 | ||||||||||
| sd (plot) | 36 | 43 | 34 | 38 | 81 | 36 | 105 | 20 | 29 | 64 | 82 | 26 | 36 | 62 | 82 | |
| Cmic | sd (block) | 854 | 1094 | 1215 | 1727 | 964 | ||||||||||
| sd (plot) | 938 | 774 | 697 | 667 | 1547 | 719 | 1105 | 442 | 901 | 1341 | 1175 | 492 | 768 | 1237 | 621 | |
| AWCD | sd (block) | 0.18 | 0.17 | 0.21 | 0.24 | 0.15 | ||||||||||
| sd (plot) | 0.19 | 0.26 | 0.10 | 0.13 | 0.12 | 0.13 | 0.31 | 0.14 | 0.14 | 0.33 | 0.15 | 0.15 | 0.11 | 0.15 | 0.15 | |
| S-W | sd (block) | 0.28 | 0.19 | 0.33 | 0.41 | 0.07 | ||||||||||
| sd (plot) | 0.24 | 0.40 | 0.21 | 0.13 | 0.22 | 0.67 | 0.47 | 0.17 | 0.34 | 0.40 | 0.48 | 0.08 | 0.01 | 0.04 | 0.05 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kučera, A.; Holík, L.; Rosíková, J.; Volařík, D.; Kneifl, M.; Vichta, T.; Knott, R.; Friedl, M.; Uherková, B.; Kadavý, J. Soil Microbial Functional Diversity under the Single-Season Influence of Traditional Forest Management in a Sessile Oak Forest of Central Europe. Forests 2021, 12, 1187. https://doi.org/10.3390/f12091187
Kučera A, Holík L, Rosíková J, Volařík D, Kneifl M, Vichta T, Knott R, Friedl M, Uherková B, Kadavý J. Soil Microbial Functional Diversity under the Single-Season Influence of Traditional Forest Management in a Sessile Oak Forest of Central Europe. Forests. 2021; 12(9):1187. https://doi.org/10.3390/f12091187
Chicago/Turabian StyleKučera, Aleš, Ladislav Holík, Jana Rosíková, Daniel Volařík, Michal Kneifl, Tomáš Vichta, Robert Knott, Michal Friedl, Barbora Uherková, and Jan Kadavý. 2021. "Soil Microbial Functional Diversity under the Single-Season Influence of Traditional Forest Management in a Sessile Oak Forest of Central Europe" Forests 12, no. 9: 1187. https://doi.org/10.3390/f12091187
APA StyleKučera, A., Holík, L., Rosíková, J., Volařík, D., Kneifl, M., Vichta, T., Knott, R., Friedl, M., Uherková, B., & Kadavý, J. (2021). Soil Microbial Functional Diversity under the Single-Season Influence of Traditional Forest Management in a Sessile Oak Forest of Central Europe. Forests, 12(9), 1187. https://doi.org/10.3390/f12091187






