Nutrient Release through Litterfall in Short Rotation Poplar Crops in Mediterranean Marginal Land
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location and Weather Conditions
2.2. Soil Analysis
2.3. Agronomical Practices
2.4. Biomass Sampling
2.5. Decay Assay
2.6. Analysis
2.7. Calculations and Data Analysis
3. Results and Discussion
3.1. Biomass Production and Composition
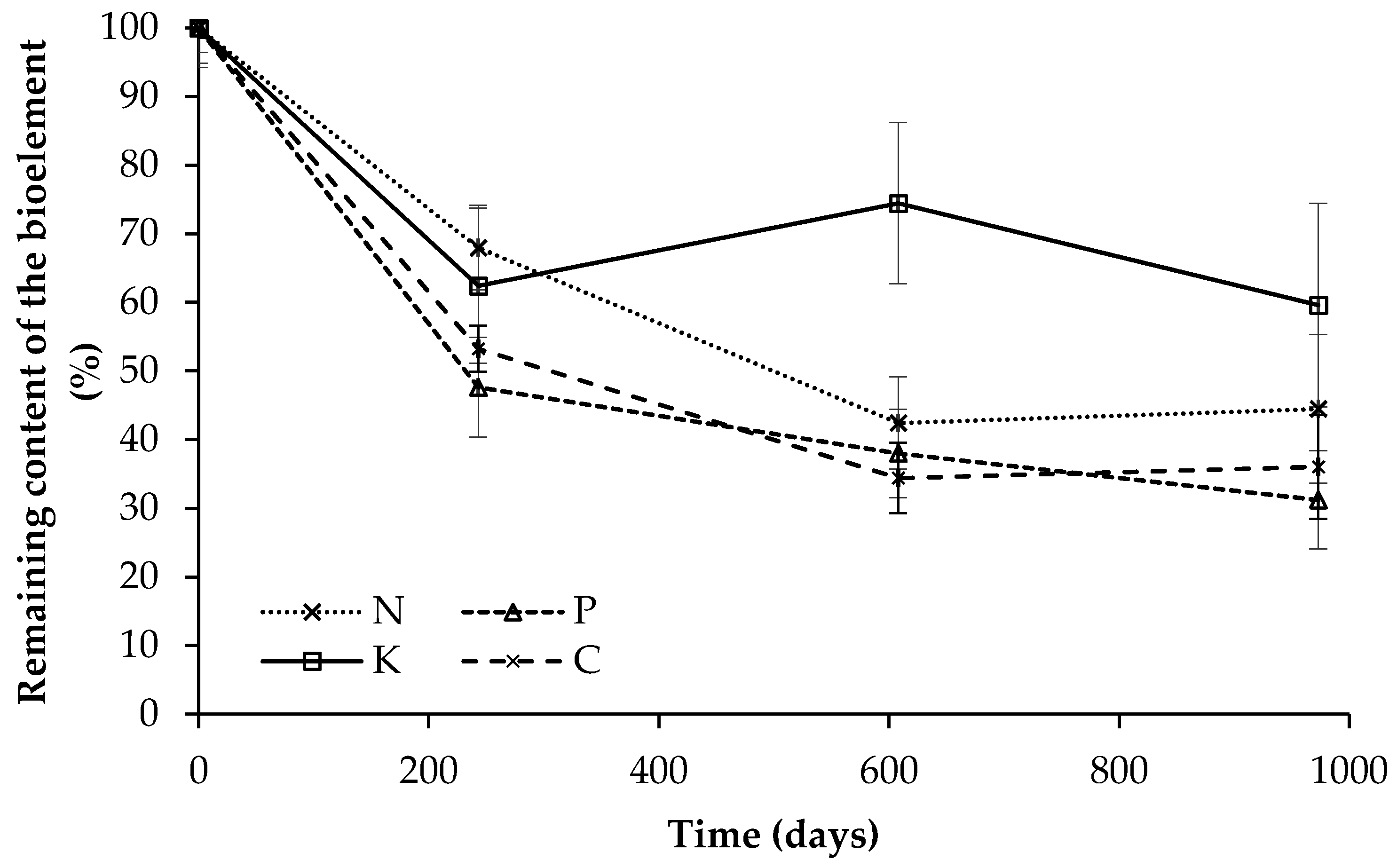
3.2. Leaf Litter Decomposition
3.3. Nutrient Dynamics Associated with Leaf Litter Decomposition
3.4. Carbon Dynamics
3.5. Soil
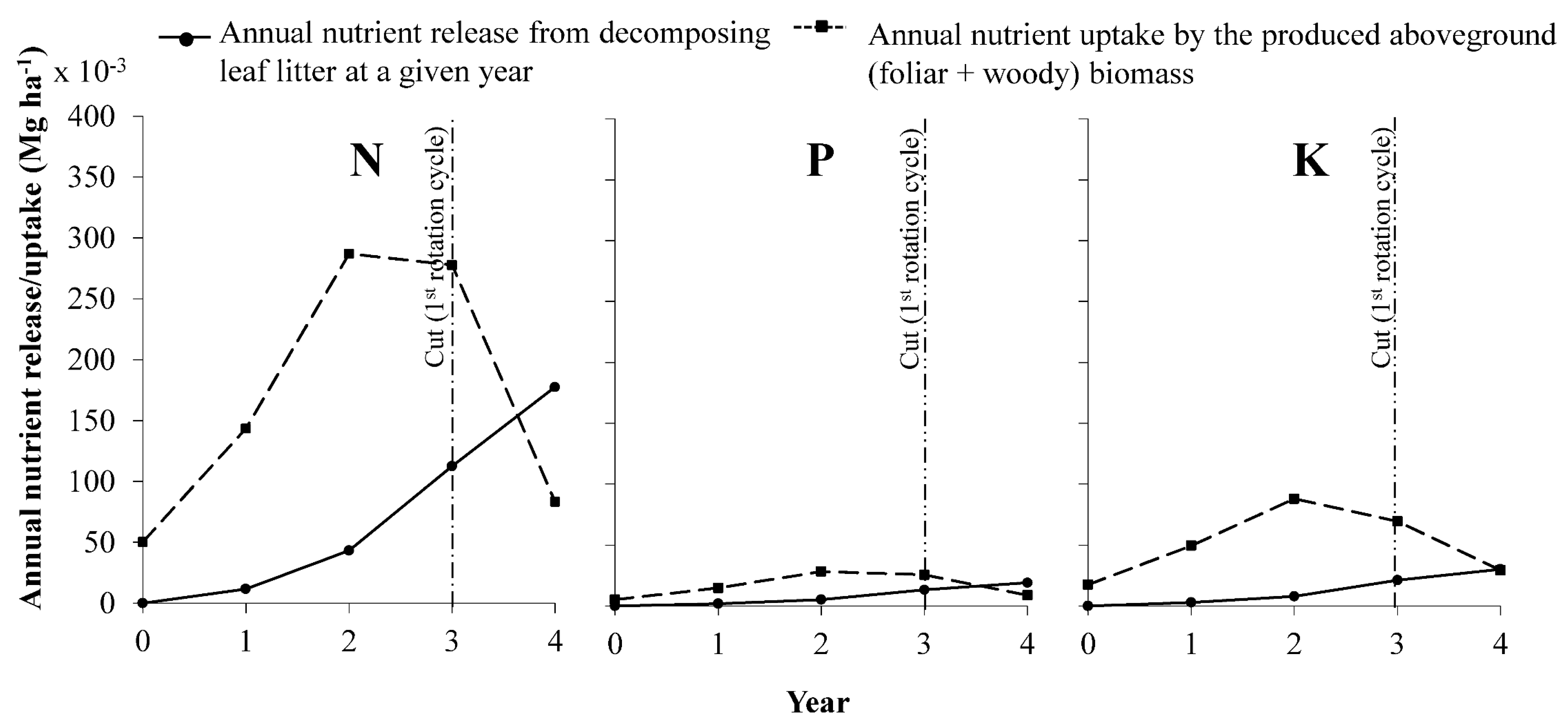
3.6. Nutrient Balance between Litter Decomposition and Crop Uptake
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- European Union. Directive 2018/2001/EC of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources. Off. J. Eur. Unión 2018, L 328, 82–209. [Google Scholar]
- Sierra, M.; Martínez, F.J.; Verde, R.; Martín, F.J.; Macías, F. Soil-C sequestration and soil-C fractions, comparison between poplar plantations and corn crops in south-eastern Spain. Soil Till. Res. 2013, 130, 1–6. [Google Scholar] [CrossRef]
- Galatsidas, S.; Gounaris, N.; Vlachaki, D.; Dimitriadis, E.; Kiourtosis, F.; Keramitzis, D. Revealing Bioenergy Potentials: Mapping Marginal Lands in Europe—The Seemla Approach. 26th European Biomass Conference and Exhibition, Copenhagen, Denmark, 14–17 May 2018; Persson, M., Scarlat, N., Grassi, A., Helm, P., Eds.; ETA-Florence: Florenz, Italy, 2018; pp. 31–37. [Google Scholar]
- Ciria, C.S.; Sanz, M.; Carrasco, J.; Ciria, P. Identification of arable marginal lands under rainfed conditions for bioenergy purposes in Spain. Sustainability 2019, 111, 1833. [Google Scholar] [CrossRef] [Green Version]
- Mehmood, M.A.; Ibrahim, M.; Rashid, U.; Nawaz, M.; Ali, S.; Hussain, A.; Gull, M. Biomass production for bioenergy using marginal lands. Sustain. Prod. Consum. 2017, 9, 3–21. [Google Scholar] [CrossRef]
- Fernández, M.J.; Barro, R.; Pérez, J.; Losada, J.; Ciria, P. Influence of the agricultural management practices on the yield and quality of poplar biomass (a 9-year study). Biomass Bioenerg. 2016, 93, 87–96. [Google Scholar] [CrossRef]
- Bassam, N.E. Handbook of Bioenergy Crops: A Complete Reference to Species. Dev. Appl. 2010. [Google Scholar] [CrossRef]
- Fernández, M.J.; Barro, R.; Pérez, J.; Ciria, P. Production and composition of biomass from short rotation coppice in marginal land: A 9-year study. Biomass Bioenerg. 2020, 134, 105478. [Google Scholar] [CrossRef]
- FAO (The Food and Agriculture Organization). Poplars and Other Fast-Growing Trees—Renewable Resources for Future Green Economies. Synthesis of Country Progress Reports. In Proceedings of the 25th Session of the International Poplar Commission, Berlin, Germany, 13–16 September 2016; International Poplar Commission Working Paper IPC/15 . Forestry Policy and Resources Division FAO: Rome, Italy, 2016. [Google Scholar]
- Pérez-Corona, M.E.; Pérez, M.C.; Bermúdez de Castro, F. Decomposition of alder, ash, and poplar litter in a Mediterranean riverine area. Commun. Soil Sci. Plant Anal. 2006, 37, 1111–1125. [Google Scholar] [CrossRef]
- Rodríguez, C.R.; Durán, V.H.; Muriel, J.L.; Martín, F.J.; Franco, D. Litter decomposition and nitrogen release in a sloping Mediterranean subtropical agroecosystem on the coast of Granada (SE, Spain): Effects of floristic and topographic alteration on the slope. Agric. Ecosyst. Environ. 2009, 134, 79–88. [Google Scholar] [CrossRef]
- Aerts, R.; De Caluwe, H. Nutritional and plant-mediated controls on leaf litter decomposition of Carex species. Ecology 1997, 78, 244–260. [Google Scholar] [CrossRef]
- Berg, B.; Staaf, H. Release of nutrients from decomposing white birch leaves and Scots pine needle litter. Pedobiologia 1987, 30, 55–63. [Google Scholar]
- Berg, B. Litter decomposition and organic matter turnover in northern forest soils. Forest Ecol. Manag. 2000, 133, 13–22. [Google Scholar] [CrossRef]
- Bessaad, A.; Korboulewsky, N. How much does leaf leaching matter during the pre-drying period in a whole-tree harvesting system? Forest Ecol. Manag. 2020, 447, 11. [Google Scholar] [CrossRef]
- Zeng, D.-H.; Mao, R.; Chang, S.X.; Li, L.-J.; Yang, D. Carbon mineralization of tree leaf litter and crop residues from poplar-based agroforestry systems in Northeast China: A laboratory study. Appl. Soil Ecol. 2010, 44, 133–137. [Google Scholar] [CrossRef]
- Ngao, J.; Bernhard-Reversat, F.; Loumeto, J.J. Changes in eucalypt litter quality during the first three months of field decomposition in a Congolese plantation. Appl. Soil Ecol. 2009, 42, 191–199. [Google Scholar] [CrossRef]
- Ghezehei, S.B.; Ewald, A.L.; Hazel, D.W.; Zalesny, R.S., Jr.; Nichols, E.G. Productivity and Profitability of Poplars on Fertile and Marginal Sandy Soils under Different Density and Fertilization Treatments. Forests 2021, 12, 869. [Google Scholar] [CrossRef]
- Adams, M.B.; Angradi, T.R. Decomposition and nutrient dynamics of hardwood leaf litter in the Fernow Whole-Watershed Acidification Experiment. Forest Ecol. Manag. 1996, 83, 61–69. [Google Scholar] [CrossRef]
- AEMET (Agencia Estatal de Meteorología) and IM (Instituto de Meteorología de Portugal). Iberian Climate Atlas Air temperature and precipitation 1971–2000; AEMET, IM, Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Spain, 2011. [Google Scholar] [CrossRef]
- Guía Resumida del Clima en España (1981–2010). Available online: http://www.aemet.es/documentos/es/conocermas/recursos_en_linea/publicaciones_y_estudios/publicaciones/guia_resumida_2010/GuiaResumidaDelClima.zip (accessed on 11 May 2021).
- Ciria, M.P. El Chopo (Populus spp) Como Cultivo Energético; Hoja Divulgadora nº 2131; Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Spain, 2009; p. 32. [Google Scholar]
- Lodhiyal, L.S.; Lodhiyal, N. Variation in biomass and net primary productivity in short rotation high density central Himalayan poplar plantations. Forest Ecol. Manag. 1997, 98, 167–179. [Google Scholar] [CrossRef]
- Lodhiyal, L.S.; Singh, R.P.; Singh, S.P. Productivity and nutrient cycling in poplar stands in central Himalaya, India. Can. J. Forest. Res. 1994, 24, 1199–1209. [Google Scholar] [CrossRef]
- Sastre, C.M.; Barro, R.; González-Arechavala, Y.; Santos-Montes, A.; Ciria, P. Life Cycle Assessment and soil nitrogen balance of different N fertilizers for top dressing rye as energy crop for electricity generation. Agron J. 2021, 11, 844. [Google Scholar] [CrossRef]
- Van Wesemael, B. Litter decomposition and nutrient distribution in humus profiles in some Mediterranean forests in southern Tuscany. Forest Ecol. Manag. 1993, 57, 99–114. [Google Scholar] [CrossRef]
- Guo, L.B.; Sims, R.E.H. Litter decomposition and nutrient release via litter decomposition in New Zealand eucalypt short rotation forests. Agr. Ecosyst. Environ. 1999, 75, 133–140. [Google Scholar] [CrossRef]
- Harmon, M.E.; Silver, W.L.; Fasth, B.; Chen, H.; Burke, I.C.; Parton, W.J.; Hart, S.C.; Currie, W.S. Long-term patterns of mass loss during the decomposition of leaf and fine root litter: An intersite comparison. Glob. Chang. Biol. 2009, 15, 1320–1338. [Google Scholar] [CrossRef] [Green Version]
- Stochlová, P.; Novotná, K.; Costa, M.; Rodrigues, A. Biomass production of poplar short rotation coppice over five and six rotations and its aptitude as a fuel. Biomass Bioenerg. 2019, 122, 183–192. [Google Scholar] [CrossRef]
- Abelho, M.; Graça, M.A.S. Litter in a first-order stream of a temperate deciduous forest (Margaraça Forest, central Portugal). Hydrobiologia 1998, 386, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Meiresonne, L.; De Schrijver, A.; De Vos, B. Nutrient cycling in a poplar plantation (Populus trichocarpa x Populus deltoides’Beaupré´) on former agricultural land in northern Belgium. Can J. Forest Res. 2007, 37, 141–155. [Google Scholar] [CrossRef]
- Abelho, M. Effects of leaf litter species on macroinvertebrate colonization during decomposition in a Portuguese stream. Int. Rev. Hydrobiol. 2008, 93, 358–371. [Google Scholar] [CrossRef]
- Bohara, M.; Yadav, R.K.P.; Dong, W.; Cao, J.; Hu, C. Nutrient and Isotopic Dynamics of Litter Decomposition from Different Land Uses in Naturally Restoring Taihang Mountain, North China. Sustainability 2019, 11, 1752. [Google Scholar] [CrossRef] [Green Version]
- Lavelle, P.; Blanchart, E.; Martin, A.; Martin, S.; Spain, A.; Toutain, F.; Barois, I.; Schaefer, R. A hierarchical model for decomposition in terrestrial ecosystems. Applications to soils of the humid tropics. Biotropica 1993, 25, 130–150. [Google Scholar] [CrossRef]
- Aranda, Y.; Serrano, J.M.; Bermúdez de Castro, F. Degradación de la hojarasca de Populus nigra L. Revue D’écologie et de Biologie du Sol 1990, 27, 395–406. [Google Scholar]
- Jonczak, J.; Dziadowiec, H.; Kacprowicz, K.; Czarnecki, A. An assessment of the influence of poplar clones Hybrid 275 and Robusta on soil cover based on the characteristics of their plant litter fall. Pol. J. Soil Sci. 2010, 43, 9–19. [Google Scholar]
- Kim, C.; Lim, J.H.; Shin, J.H. Nutrient dynamics in litterfall and decomposing leaf litter at the Kwangneung deciduous broad-leaved natural forest. Korean J. Agric. For. Meteorol. 2003, 5, 87–93. [Google Scholar]
- Sartori, F.; Lal, R.; Ebinger, M.H.; Eaton, J.A. Changes in soil C and nutrient pools along a chronosequence of poplar plantations in the Columbia Plateau, Oregon, USA. Agric. Ecosyst. Environ. 2007, 122, 325–339. [Google Scholar] [CrossRef] [Green Version]
- Rytter, R.M. The potential of willow and poplar plantations as C sinks in Sweden. Biomass Bioener. 2012, 36, 86–95. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Cui, Y.; Pei, Z. Litter decomposition in a subtropical plantation in Qianyanzhou, China. J. Res. 2011, 16, 8–15. [Google Scholar] [CrossRef]
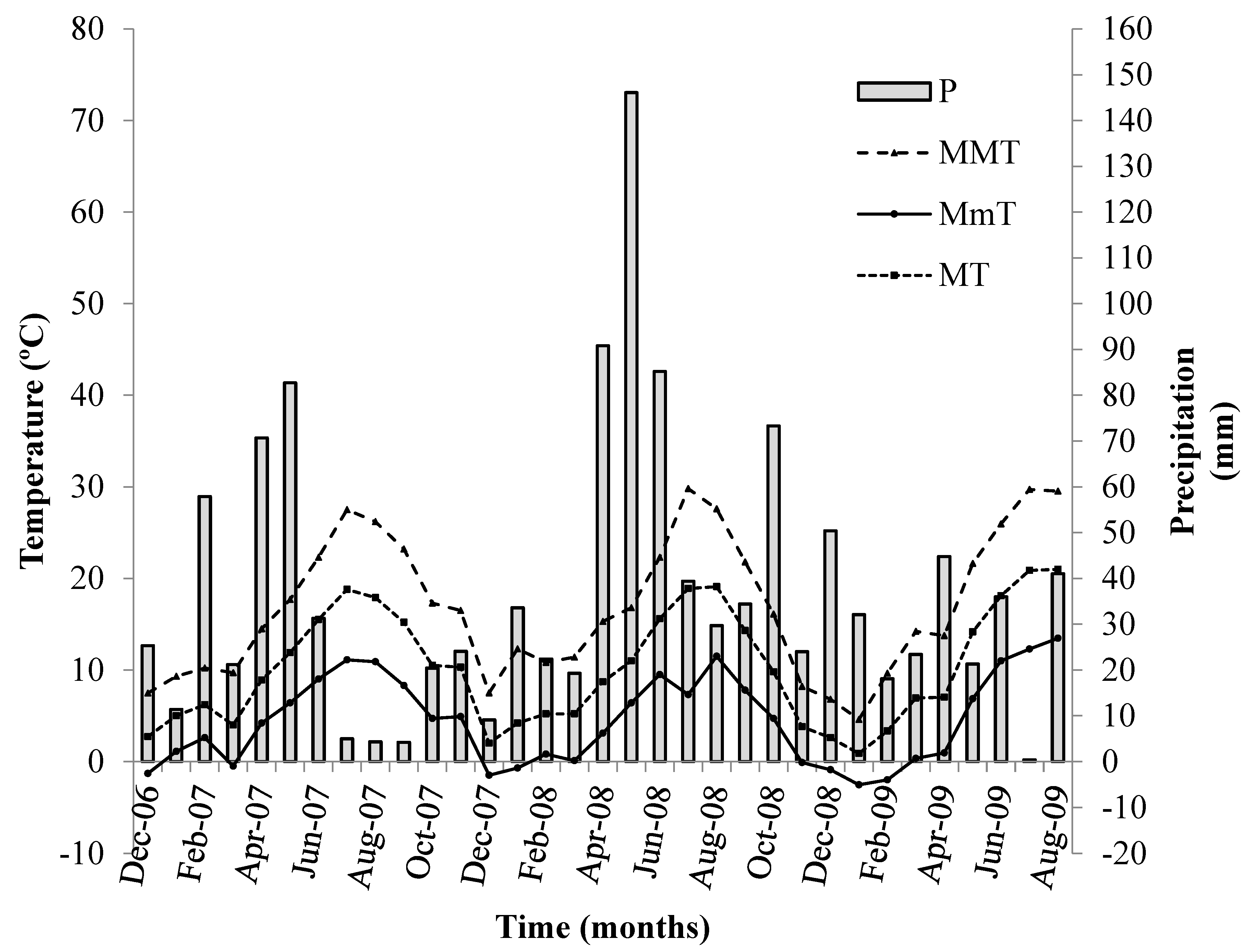

| Year | P (mm) | MT (°C) | RH (%) | |
|---|---|---|---|---|
| 2006 | 0 | 554 | 11.6 | 69.0 |
| 2007 | 1 | 342 | 10.0 | 68.0 |
| 2008 | 2 | 649 | 9.9 | 70.7 |
| 2009 | 3 | 390 | 10.8 | 64.8 |
| 2010 | 4 | 599 | 9.6 | 68,0 |
| Mean | 507 | 10.4 | 68.1 | |
| Sampling | Initial | Final | Initial | Final | Initial | Final | |
|---|---|---|---|---|---|---|---|
| Depth (cm) | 0 to 20 | 20 to 40 | 40 to 60 | ||||
| pH | 6.8 ± 0.2 a | 6.3 ± 0.2 b | 6.5 ± 0.2 a | 6.1 ± 0.2 b | 6.4 ± 0.5 a | 6.1 ± 0.5 b | |
| Nt | (mg kg−1) | 320 ± 110 a | 675 ± 210 b | 200 ± 100 a | 610 ± 90 b | 180 ± 90 a | 650 ± 110 b |
| Pav | (mg kg−1) | 14 ± 2 a | 43 ± 2 b | 12 ± 4 a | 33 ± 6 b | 36 ± 5 a | 24 ± 3 b |
| Kav | (mg kg−1) | 61 ± 12 a | 92 ± 18 b | 66 ± 15 a | 65 ± 15 a | 65 ± 19 a | 61 ± 17 a |
| Organic C | (%) | 0.5 ± 0.2 a | 0.6 ± 0.1 a | 0.2 ± 0.1 a | 0.6 ± 0.2 b | 0.06 ± 0.01 a | 0.49± 0.05 b |
| Organic matter | (%) | 0.8 ± 0.3 a | 1.0 ± 0.2 a | 0.4 ± 0.2 a | 1.0 ± 0.3 b | 0.11± 0.09 a | 0.84 ± 0.08 b |
| C/N ratio | 15 ± 3 a | 9 ± 3 b | 12 ± 4 a | 10 ± 3 a | 4 ± 1 a | 8 ± 2 b | |
| Year | Annual DM Production | C | N | P | K | |
|---|---|---|---|---|---|---|
| (Mg ha−1 year−1) | (g/kg, db) | |||||
| Woody biomass | 0 (cut) | 2.6 ± 1.3 | 489 | 5.1 | 0.77 | 3.6 |
| 1 | 7.5 ± 2.5 | 493 | 4.8 | 0.76 | 3.1 | |
| 2 | 10.9 ± 5.8 | 492 | 4.1 | 0.63 | 2.8 | |
| 3 (cut) | 4.3 ± 2.5 | 490 | 3.1 | 0.90 | 3.3 | |
| 4 | 4.6 ± 1.0 | 490 | 4.9 | 0.76 | 3.6 | |
| Mean | 6.0 ± 3.2 | 491 | 4.4 | 0.77 | 3.3 | |
| Foliar biomass | 0 (cut) | 1.6 ± 0.8 | 445 | 22.6 | 1.94 | 4.7 |
| 1 | 4.7 ± 1.6 | 443 | 22.4 | 1.99 | 4.6 | |
| 2 | 10.3 ± 4.6 | 447 | 23.2 | 1.85 | 4.7 | |
| 3 (cut) | 11.4 ± 6.7 | 446 | 22.8 | 1.95 | 4.7 | |
| 4 | 2.6 ± 0.6 | 447 | 22.5 | 1.96 | 4.8 | |
| Mean | 6.1 ± 2.7 | 446 | 22.7 | 1.94 | 4.7 | |
| Time | Leaf Litter Mass in Bags | C | N | P | K |
|---|---|---|---|---|---|
| (days) | (dry g) | (mg g−1, db) | |||
| 0 | 7.2 ± 0.0 a | 447 ± 14 a | 23 ± 2 a | 1.9 ± 0.2 a | 4.7 ± 0.5 a |
| 243 | 5.3 ± 1.0 b | 325 ± 37 b | 21 ± 2 ab | 1.2 ± 0.2 b | 3.9 ± 0.8 b |
| 608 | 4.1 ± 0.9 c | 271 ± 61 c | 17 ± 3 c | 1.3 ± 0.2 b | 5.6 ± 0.7 c |
| 973 | 3.8 ± 1.5 c | 304 ± 27 bc | 18 ± 3 bc | 1.1 ± 0.1 b | 4.6 ± 0.5 ab |
| Diff (%) | −47 | −32 | −18 | −42 | −2 |
| Parameters | Units | |
|---|---|---|
| Woody biomass production (2006–2009) | Mg ha−1 | 25.3 |
| Foliar biomass production (2006–2009) | Mg ha−1 | 28.0 |
| Underground biomass production (2006–2009) | Mg ha−1 | 5.6 |
| Accumulated C in woody biomass (2006–2009) | Mg ha−1 | 12.4 |
| C sequestered by underground biomass (2006–2009) | Mg ha−1 | 3.1 |
| C content in the upper layer of the soil (2006–2009) | Mg ha−1 | 2.8 |
| Annual C foliar production | Mg ha−1 | 3.2 |
| Annual C output by litter decomposition | Mg ha−1 | 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, P.; Barro, R.; Pérez, J.; Fernández, M.J.; Moyano, A.; Ciria, P. Nutrient Release through Litterfall in Short Rotation Poplar Crops in Mediterranean Marginal Land. Forests 2021, 12, 1185. https://doi.org/10.3390/f12091185
Pérez P, Barro R, Pérez J, Fernández MJ, Moyano A, Ciria P. Nutrient Release through Litterfall in Short Rotation Poplar Crops in Mediterranean Marginal Land. Forests. 2021; 12(9):1185. https://doi.org/10.3390/f12091185
Chicago/Turabian StylePérez, Paloma, Ruth Barro, Javier Pérez, Miguel J. Fernández, Amelia Moyano, and Pilar Ciria. 2021. "Nutrient Release through Litterfall in Short Rotation Poplar Crops in Mediterranean Marginal Land" Forests 12, no. 9: 1185. https://doi.org/10.3390/f12091185
APA StylePérez, P., Barro, R., Pérez, J., Fernández, M. J., Moyano, A., & Ciria, P. (2021). Nutrient Release through Litterfall in Short Rotation Poplar Crops in Mediterranean Marginal Land. Forests, 12(9), 1185. https://doi.org/10.3390/f12091185






