Abstract
Wood-boring insects, such as Cerambyx welensii Küster, are involved in oak decline in Mediterranean areas. To advance our understanding of the olfactory perception of C. welensii, we recorded electroantennographic (EAG) responses from male and female antennae to 32 tree volatile organic compounds typical of emissions from its main Quercus L. hosts, and also analysed the dose-dependent response. Cerambyx welensii antennae responded to 24 chemicals. Eight odorants elicited the highest EAG responses (normalized values of over 98%): 1,8-cineole, limonene-type blend, β-pinene, pinene-type blend, sabinene, α-pinene, turpentine and (E)-2-hexenal. Cerambyx welensii exhibits a broad sensitivity to common tree volatiles. The high EAG responses to both limonene- and pinene-type blends suggest the detection of specific blends of the main foliar monoterpenes emitted by Q. suber L. and Q. ilex L. (limonene, α- and β-pinene, sabinene and myrcene), which could influence the intraspecific host choice by C. welensii, and in particular, females may be able to detect oak trees with a limonene-type chemotype. In addition, C. welensii showed high antennal activity to some odorants that characterize emissions from non-host tree species (1,8-cineole, β-pinene, α-pinene, turpentine, δ3-carene and camphene). The results obtained may be applicable to optimize monitoring and mass-trapping programmes in an integrated pest management context.
1. Introduction
Mediterranean Quercus suber L. and Quercus ilex L. open woodlands (called montados in Portugal and dehesas in Spain) have a high ecological, economic and cultural importance in Western Europe and Northern Africa. Tree decline in these Mediterranean Quercus stands is significantly influenced by wood-boring insects, such as Cerambyx welensii Küster (Coleoptera, Cerambycidae), as these beetles act as an aggravating factor [1,2,3]. Hence, the spread of wood-boring cerambycids such as C. welensii in open woodlands deserves special attention, particularly within the context of climate change [2,3]. Cerambyx welensii is a large cerambycid (up to 60 mm long) and its host trees are most notably Quercus species, but also Salix L., Populus L. and Ceratonia L. [4]. Adults have crepuscular and early nocturnal habits; the larvae bore into wood, mainly in weakened trees, causing tree branches and trunks to break [1]. In addition, these insects facilitate infection by both plant pathogens and wood-decaying fungi [5].
Locating a host plant is crucial for herbivorous insects to meet their nutritional requirements and find suitable oviposition sites [6,7]. The role of volatile organic compounds (VOCs) in host selection by cerambycid beetles has been investigated in more than 30 species and continues to be studied, with female search behaviour playing an important role in determining the host plant range [8,9]. Much of the research on host attractants of wood-boring insects has focused on bark beetles attacking conifers, largely due to their importance as forest pests [9]. However, little is known about the role of such compounds in host selection by wood-borers infesting Quercus species. Plant volatiles have been considered to be involved in the recognition of damaged and weakened hosts, with ethanol playing a role in that process [8]; in addition, visual cues have been proposed to play a role in host location by C. welensii [10]. Research into the chemical ecology of C. welensii may help improving the management of this pest and could, for instance, facilitate the improvement of lures [2,11]. Electroantennography (EAG) is a valuable screening tool for host plant volatiles [6,7] and has already been applied to cerambycid wood borers [8,9], including species colonizing Mediterranean oak trees, such as C. welensii and Prinobius myardi Mulsant [12,13,14].
The emission of isoprenoids (isoprene and monoterpenes) is widespread in the Quercus genus, with isoprene having been found the only isoprenoid emitted by a large amount of species (namely North American evergreen oaks and both North American and European deciduous oaks), while monoterpene emission has been found to be specific to evergreen oaks of the Mediterranean environment [15]. Severe environmental stress, which occurs in summer in Mediterranean areas, induces physiological and biochemical changes in trees that affect the monoterpene emission [16,17]. It has been proposed that constitutive terpene emissions could play a role in abiotic stress tolerance, with monoterpenes (limonene in particular) potentially mitigating the effects of oxidative stress [18,19]. Quercus ilex and Q. suber are strong emitters of foliar monoterpenes, especially limonene, α-pinene, β-pinene, sabinene and myrcene, with their relative emissions characterizing four genetically based leaf chemotypes: a limonene type, a pinene type, an intermediate limonene/pinene type (in Q. suber) and a myrcene type (mostly in Q. ilex) [20,21]. The colonization of Q. suber trees by C. welensii has been associated with trees exhibiting a limonene-type chemotype [22]. In addition, C. welensii have shown to be attracted to traps baited with fermentation odours [11,23]. To understand the olfactory sensitivity of C. welensii to plant volatiles, we analysed EAG responses of adult females and males to tree VOCs, namely, host-related odours.
2. Materials and Methods
2.1. Test Substances
The odorants tested were 32 synthetic VOCs, applied either alone or in mixtures that included the main compounds reported in Q. suber and/or Q. ilex foliar and bark emissions. The main group, 21 VOCs, were foliar monoterpenes ((+)-limonene, α-pinene, β-pinene, sabinene, β-myrcene, 1,8-cineole, (E)-β-ocimene, α-terpinene, γ-terpinene, α-phellandrene, ρ-cymene, α-terpineol, δ3-carene, linalool and camphene) [20,21,22] and green leaf volatiles ((E)-2-hexenal, (Z)-3-hexenol, (Z)-2-hexenol and (Z)-3-hexenyl acetate) [24,25], as well as acetic acid and ethanol [26,27]. Regarding compounds found in bark tissues, we tested gallic and ellagic acids, the triterpenes friedelin, betulin and lupeol, as well as erythrodiol and 3-methyl-butanol [28,29,30]. Turpentine (composition: 80% α-pinene, 16.2% β-pinene and 3.8% camphene) was used as a source of VOCs characteristic of pine trees and other conifers. Finally, three blends were created to simulate the main leaf chemotypes in Q. ilex and Q. suber, based on the percentages of limonene, α-pinene, β-pinene, sabinene and myrcene they contained [20]: a limonene type (56%, 19%, 12%, 7% and 6% of the aforementioned five monoterpenes, respectively), a pinene type (5%, 43%, 31%, 12% and 9%) and a myrcene type (5%, 11%, 8%, 3% and 73%). The purity of chemicals we tested was ≥94% (Sigma-Aldrich, Madrid, Spain).
2.2. Electroantennography
Cerambyx welensii adults were collected during the flight period, by hand picking, in a Q. suber dehesa (Huelva, SW Spain). Insects (4.6 ± 0.5 cm long, 2.7 ± 0.7 g weight; on average) were maintained in individual containers provided with a 4% sugar solution in semi-darkness (at 23–32 °C). EAG responses were recorded 1 to 8 days (mean of 4 days) after insect collection, as described in Sánchez-Osorio et al. [12,13,14]. Briefly, two capillary electrodes filled with KCl solution were placed in contact with antennae of intact insects, with one of the electrodes in contact with the distal end of the antenna and the other inserted through the membrane between scape and pedicel. Aliquots of hexane solutions (1:1 v/v 20-µL) were prepared with each odorant tested and applied to filter paper strips (Whatman No 1) (Sigma-Aldrich, Madrid, Spain). Previous studies demonstrated that 1:1 (v/v) concentrations did not saturate C. welensii antennae, while allowing stable recordings several hours in duration [12,13]; moreover, it enabled us to obtain reliable EAG recordings from the less antennally active VOCs. For stimulation, 10 mL puffs of air were blown through Pasteur pipettes into a constant stream of purified air flowing over the antenna (4.1 l min−1). EAG responses were recorded (in mV) using SynTech electrode holders, an IDAC-4 acquisition controller (SynTech, Hilversum, The Netherlands) and AutoSpike32 software (SynTech, Hilversum, The Netherlands).
Exposure to different odours can induce changes of sensitivity due to habituation (decrease in response to a stimulus after repeated presentations) and/or sensitization (increase in response to a stimulus of particular interest) [31]. To reduce such risk, EAG responses to the panel of odorants were recorded for series of 5–6 randomly puffed individual odour sources, with one replication per individual and per odour source. A 10 min rest period was allowed between series and a 1 min interval between stimulations within a series. The same thirteen insects (Nfemales = 7, Nmales = 6) were tested with all the compounds, except in the cases of δ3-carene and the three stimulant blends (pinene-type, limonene-type and myrcene-type) for which six different insects were used (Nfemales = 3, Nmales = 3).
To analyse the sensitivity threshold of the antenna of C. welensii females, we performed a dose-response study with three of the most EAG-active odorants—1,8-cineole, β-pinene and turpentine—besides the standard (E)-2-hexenal. These odorants were applied at five doses increasing by orders of magnitude from 10−4:1 (846–922 ηg depending on the odorant) to 1:1 v/v in hexane. For each odorant, dose-response was tested in five stimulation series with three replicates for each dose. There were 1 min intervals between series, as well as between replicates within series; and 10 min intervals between tests with different odorants. We calculated the mean response from the three replicates of each dose. The dose-response was tested for each of the four odorants using the same five C. welensii females (these individuals were different from those used in the previous experiment).
EAG responses to both hexane (20 μL, used as control) and (E)-2-hexenal (used as the standard stimulus) [12] were recorded at the beginning and end of each series. To compensate for solvent and/or mechanoreceptive artefacts, measured EAG responses were corrected by subtracting the mean response to the two hexane control injections (except when comparing responses to those of the hexane controls themselves). Corrected measures were then normalized to percentages relative to the mean EAG response to the two injections of the standard stimulus.
2.3. Statistical Analyses
Outliers detected using Grubbs’ tests revealed intraspecific variability in EAG response, which could be due to the different vitality of field-caught insects [12] and, hence, the hypotheses of normality and homoscedasticity were not met in either males or females. Robust statistical methods have been recommended in such cases [32]; in the present study, EAG responses were compared using functions implemented in the WRS2 package [32], based on 20% trimmed means (Mean0.2: mean obtained after removing the top and bottom 20% of values). Data were analysed using either heteroscedastic analysis of variance (ANOVA) for related samples (followed by modified Bonferroni corrections for multiple comparisons using Rom’s method) or a bootstrap version of Yuen’s test for unrelated samples (one-sided when the group sample sizes were unequal) [32]; when sample size did not enable to obtain Mean0.2 values, Mann-Whitney U-test was used. Taking into account differences we found between sexes, EAG data from males and females were treated separately for six odorants (1,8-cineole, (E)-β-ocimene, β-pinene, α-pinene, α-terpinene and the limonene-type blend), but pooled for the others. One-sided confidence intervals based on the Mean0.2 for related samples were used to assess whether normalized (uncorrected) EAG responses to individual compounds were larger than the response to the hexane controls.
All statistical analyses were performed using the R framework (version 3.6.0), with a cut-off of α = 0.05 for significance and α = 0.1 for marginal significance.
3. Results
EAG signals showed the typical waveform, consisting of a rapid depolarization followed by a slower recovery phase. Yuen’s bootstrap tests revealed significant differences between sexes only for two compounds, 1,8-cineole (t = 3.66, p = 0.011) and (E)-β-ocimene (t = 2.05, p = 0.038), while marginally significant differences between sexes were observed for another four odorants: β-pinene, α-pinene, α-terpinene and the limonene-type blend (EAG responses to this blend were compared by using a Mann-Whitney U-test) (1.64 ≤ t ≤ 2.16; 0.056 ≤ p ≤ 0.080, with the limonene-type blend and α-pinene at the lowest and highest ends of the range, respectively). (E)-2-hexenal elicited moderate responses (0.6 ± 0.07 mV, Mean0.2 ± Error0.2, n = 32), and responses to the hexane control were very weak (0.08 ± 0.03 mV, n = 32). There were significant differences in EAG responses to individual compounds (i.e., ANOVA for related samples on pooled sexes dataset −n = 25, with the same 13 insects tested−: F13 106= 42.53, p < 0.001) (Figure 1).
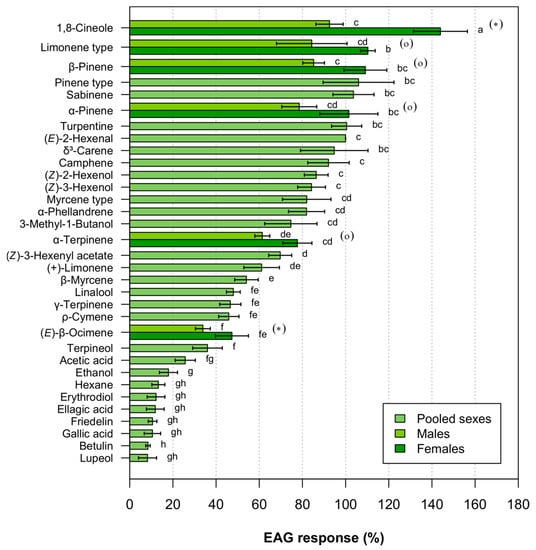
Figure 1.
Mean0.2 (± Error0.2) EAG responses of Cerambyx welensii Küster to tree volatiles. Responses are expressed as normalized percentages relative to the standard stimulus ((E)-2-hexenal), after subtracting the response to the hexane control. The response to the hexane control is included for comparison. Bars with the same letter were not significantly different (p > 0.05, heteroscedastic ANOVA; bootstrap version of Yuen’s test). Odorants that elicited EAG responses that differed between sexes are indicated with an asterisk or a circle (significant or marginally significant, respectively; bootstrap version of Yuen’s test or Mann-Whitney U-test).
Seven out of thirty-two compounds (all tested triterpenes and acids, as well as erythrodiol) elicited EAG responses that were not significantly different from hexane controls. All odorants eliciting significant responses had a more stimulating effect on females than males (normalized values being 24.9 ± 2.9% higher in females; mean ± SE), with the exception of sabinene and terpineol (103.6% and 103.8%; 34.4% and 39.2%, in females and males, respectively). Among the blends representing the three main leaf chemotypes in Q. suber and Q. ilex, EAG responses elicited by the pinene-type blend were similar to those evoked by α- and β-pinene individually. In contrast, the myrcene-type blend elicited higher EAG activity than the β-myrcene did alone (83.2% and 53.1%, respectively), and female antennae responded to the limonene-type blend more intensely than they did to limonene alone (110.5% and 61.1%, respectively) (Figure 1).
We were able to group odorants that were significantly active into three groups based on levels of EAG intensity (absolute Mean0.2 responses indicated within parentheses; normalised Mean0.2 responses presented in Figure 1): a first group of 10 compounds producing medium-intensity signals (40–75%) (compact letter display showing “d” and/or “f”, Figure 1), those including two of the main foliar volatiles of Q. ilex and Q. suber: (+)-limonene and β-myrcene (0.37 mV and 0.32 mV, respectively), as well as the branched alcohol 3-methyl-1-butanol (0.45 mV); a second group that contained six odorants eliciting responses of over 80%, which included the myrcene-type blend (0.49 mV); lastly, a notable group of eight odorants eliciting the highest normalised EAG responses (> 98%): 1,8-cineole (0.86 mV and 0.56 mV, females and males, respectively), the limonene-type blend (0.66 mV and 0.51 mV, females and males, respectively), β-pinene (0.65 mV and 0.54 mV, females and males, respectively), the pinene-type blend (0.64 mV), sabinene (0.62 mV), α-pinene (0.61 mV and 0.47 mV, females and males, respectively), turpentine (0.60 mV) and (E)-2-hexenal. The three strongest antennal responses were found in females after stimulation with 1,8-cineole, the limonene-type blend and β–pinene.
The response to 1,8-cineole, β-pinene, turpentine and (E)-2-hexenal was significantly dose-dependent (Figure 2), with a significant interaction between dose and odorant (ANOVA: p < 0.001, p = 0.137 and p < 0.001; respectively, for dose, odorant and dose × odorant). No or negligible responses were elicited at 10−4:1 and low responses were elicited at 10−3:1. EAG responses elicited by 1,8-cineole were higher than those elicited by β-pinene, turpentine and (E)-2-hexenal at 10−2:1 (p < 0.04) and 1:1 (p < 0.02). Dose–response profiles did not suggest that any saturation threshold had been reached.
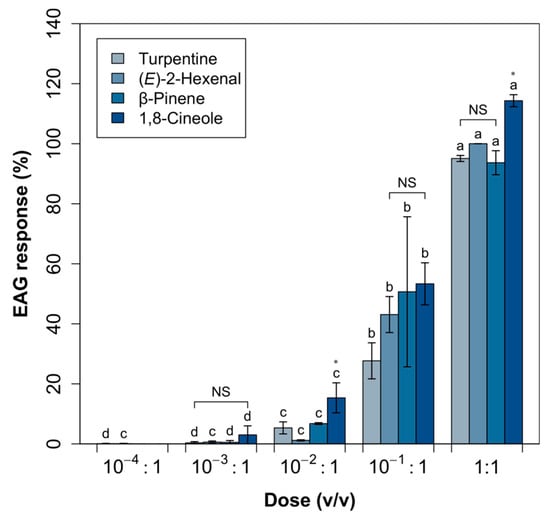
Figure 2.
Dose-response EAG profiles (Mean0.2 ± Error0.2) of Cerambyx welensii females. Responses are expressed as normalized percentages relative to the standard stimulus (E)-2-hexenal. For each odorant, letters on the columns denote differences between doses. Differences between odorants at each dose are indicated by an asterisk (NS indicates no significant differences) (Heteroscedastic ANOVA for related samples, n = 5).
4. Discussion
Cerambycid species have shown to be highly selective of host trees regardless of forest type, especially saproxylophagous insects, as they have to deal with a feeding resource that changes constantly in quality and contains defensive compounds [33,34]; in addition, wood borers make adaptive decisions regarding the selection of suitable host plants both before and after landing on potential hosts, as reported in Anoplophora malasiaca (Thomson) [35]. Monoterpenes have been considered to play a role in host selection by wood-boring cerambycids infesting deciduous tree species [8,9]. Foliar emissions by the main C. welensii hosts (Q. suber and Q. ilex) were strongly dominated by limonene, α-pinene, β-pinene, sabinene and myrcene, with these five compounds accounting for more than 87% of the overall monoterpene emission by each tree in the Q. suber dehesa where we collected the insects studied [22]. Using olfactory receptor neurons for common VOCs allowed insects to evaluate a greater range of potential hosts [6,7]. Our results demonstrated that C. welensii had an olfactory sensitivity to a wide range of common tree VOCs, with the three pinene-related compounds (α-pinene, β-pinene and sabinene) being among the eight odorants that were most antennally active of all those tested. Limonene and myrcene elicited moderate EAG responses (normalised values < 62%). Differences in foliar monoterpene emissions were reported between Q. suber trees highly infested by C. welensii and non-infested neighbouring trees, the former having high emissions of limonene and the latter of pinene-type compounds [22]. In C. welensii, both limonene- and pinene-type blends were more antennally active than the myrcene-type blend (this chemotype being rare in Q. suber); further, female antennae showed a high sensitivity to the limonene-type blend, as well as to both β-pinene and α-pinene.
Antennally active odorants may mediate behavioural responses other than attraction and may even be repellent, some conifer-related monoterpenes having been shown to discourage species attacking deciduous hosts [9]. Moreover, the same volatile odorants can function as both host and non-host cues, depending upon the context (the complete blend of compounds emitted by each plant species) in which they are perceived [36]. Olea europaea L., Pinus pinea L. and Pinus halepensis Miller are some of the non-host tree species that are most frequently found in the same stands as Q. suber and Q. ilex. All these three species are low monoterpene emitters, their foliar emissions being just 3.2–7.7% (O. europaea and P. halepensis, respectively) of those reported for Q. suber [37]. The terpene pool in these Pinus species mostly contains α-pinene, β-pinene, camphene, δ3-carene, myrcene, 1,8-cineole and linalool [16,17], while O. europaea foliar emissions have been reported to comprise only (E)- and (Z)-ocimene [37]. It has been suggested that the recognition of non-host-related compounds is a relevant cue in host selection by Cerambycidae [9], with such a process having been tentatively proposed for P. myardi, a wood-boring cerambycid sympatric to C. welensii [14]. Cerambyx welensii showed high antennal activity in response to VOCs characterizing non-host emissions, such as 1,8-cineole, the three pinene-related compounds, the turpentine blend, camphene and δ3-carene; in addition, C. welensii females exhibited a greater antennal sensitivity to (E)-β-ocimene than males.
Cerambyx welensii has been found to mainly colonize weak, damaged or old trees, as have been reported for other Cerambycidae species [1,9]. Traps baited with synthetic blends mimicking emissions from fermenting plant material, such as those from bark exudates frequently found in Q. ilex and Q. suber, proved to be attractant to C. welensii; while lures exclusively based on monoterpenes (limonene, α-pinene and β-pinene, both individually and combined) were less effective in attracting this species [23]. It also showed an antennal response to two compounds included in the synthetic blends, namely, 3-methyl-butanol and ethanol (normalized EAG responses: 74.2% and 18.3%, respectively). Another two antennally active compounds, (E)-2-hexenal and (E)-β-ocimene, have been associated with emissions from wounded leaves of Quercus species [38].
EAG studies are useful for understanding the sensitivity of insect antennae to volatile compounds, even though EAG responses provide no insight into behavioural responses. A potential problem in establishing the relative effectiveness of stimuli in EAG screenings is the sometimes wide difference in volatility among test compounds [39]. In our study, the poor responses to ellagic and gallic acids, as an example, could have been due to their low volatility. The ranking of EAG responses in Dioryctria abietivorella (Grote) to (E)-2-hexenal and (E)-3-hexenyl acetate changed relative to those elicited by some terpenes ((-)-α-pinene, (-)-limonene and (-)-E-caryophyllene) when the stimuli solutions were corrected to present equimolar airborne concentrations; however, the correction of concentrations had no effect on the ranking of responses to these monoterpenes and sesquiterpene relative to each other [39]. EAG amplitudes have been proposed to depend mainly on the sensitivity of the olfactory system [40], and references therein, even though differences in volatility of test odorants may result in a different amount of molecules reaching the EAG preparation. Our results allow us to formulate a tentative three-factor hypothesis regarding reliance on common tree volatiles in host selection by C. welensii in Quercus open woodlands, which should be further explored. As a first factor, the recognition of individual or specific blends of strong EAG-active ubiquitous volatiles ―β-pinene, α-pinene, 1,8-cineole, camphene and δ3-carene―may help distinguish suitable from unsuitable hosts on a long-range scale. As a second factor, specific ratios of the main host-related monoterpenes (α- and β-pinene, sabinene, limonene and myrcene) may help C. welensii to discriminate between host leaf chemotypes, females possibly playing a key role in detecting the limonene-type chemotype. As a third factor, C. welensii may use specific ratios of odorants eliciting low-to-moderate EAG responses to identify blends of volatiles characterizing wounded trees (e.g., ethanol, 3-methyl-butanol, (E)-β-ocimene, or some GLVs), which could also favour conspecific encounters.
Semiochemicals, such as host plant volatiles, that mediate insect behaviours have potential applications in integrated pest management for monitoring and mass-trapping programmes [8,9]. Our results are promising for such applications in C. welensii, especially in the context of declining oak stands, as is the case for Q. ilex and Q. suber open woodlands. Nonetheless, future research should focus on improving electrophysiological testing (especially larger dose-response analyses) for tree volatiles, as well as conducting behavioural bioassays (field trials with baited traps and olfactometric studies) to thoroughly investigate the activity induced by EAG-active odorants.
Author Contributions
Conceptualization, I.S.-O., R.T., L.D. and G.L.-P.; methodology, I.S.-O. and R.T.; formal analysis, R.T., L.D. and G.L.-P.; investigation, I.S.-O., R.T., L.D., G.L.-P. and M.d.M.G.; writing—original draft preparation, I.S.-O.; writing—review and editing, I.S.-O., R.T., L.D. and G.L.-P.; supervision, R.T.; project administration, I.S.-O.; funding acquisition, I.S.-O. and G.L.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded primarily by the Andalusian Regional Council of Environment and Spatial Planning and conducted through a collaborative agreement between the University of Huelva and University of Córdoba (Ref.15-2003-UHu).
Data Availability Statement
The data presented in this study are openly available in Open Science Framework repository at https://doi.org/10.17605/OSF.IO/6NU8H (accessed on 19 July 2021), reference number 6nu8h.
Acknowledgments
We thank Peter Gordon and Julia G. Fenn for commenting on an earlier version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- López-Pantoja, G.; Domínguez, L.; Sánchez-Osorio, I. Mark-recapture estimates of the survival and recapture rates of Cerambyx welensii Küster (Coleoptera, Cerambycidae) in a cork oak dehesa in Huelva (Spain). Cent. Eur. J. Biol. 2008, 3, 431–441. [Google Scholar] [CrossRef]
- Sallé, A.; Bouget, C. Victims or perpetrators: Contribution and response of insects to forest diebacks and declines. Ann. For. Sci. 2020, 77, 104. [Google Scholar] [CrossRef]
- Duque-Lazo, J.; Navarro-Cerrillo, R.M. What to save, the host or the pest? The spatial distribution of xylophage insects within the Mediterranean oak woodlands of Southwestern Spain. For. Ecol. Manag. 2017, 392, 90–104. [Google Scholar] [CrossRef]
- Vives, E. Coleoptera, Cerambycidae. In Fauna Ibérica; Ramos, M.A., Ed.; Museo Nacional de Ciencias Naturales, CSIC: Madrid, Spain, 2000; Volume 12. [Google Scholar]
- Martin, J.; Cabezas, J.; Buyolo, T.; Patón, D. The relationship between Cerambyx spp. damage and subsequent Biscogniauxia mediterranum infection on Quercus suber forests. For. Ecol. Manag. 2005, 216, 166–174. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Smart, L.E.; Aradottir, G.I.; Bruce, T.J.A. Role of semiochemicals in Integrated Pest Management. In Integrated Pest Management Current Concepts and Ecological Perspective; Abrol, D., Ed.; Elsevier: London, UK, 2013; pp. 93–109. [Google Scholar]
- Allison, J.; Borden, J.; Seybold, J. A review of the chemical ecology of the Cerambycidae (Coleoptera). Chemoecology 2004, 14, 123–150. [Google Scholar] [CrossRef]
- Millar, J.G.; Hanks, L.M. Chemical ecology of cerambycids. In Cerambycidae of the World: Biology and Pest Management; Wang, Q., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 161–208. [Google Scholar]
- Torres-Vila, L.M.; Mendiola-Díaz, F.J.; Sánchez-González, Á. Dispersal differences of a pest and a protected Cerambyx species (Coleoptera: Cerambycidae) in oak open woodlands: A mark–recapture comparative study. Ecol. Entomol. 2017, 42, 18–32. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Sánchez-González, Á.; Merino-Martínez, J.; Ponce-Escudero, F.; Conejo-Rodríguez, Y.; Martín-Vertedor, D.; Ferrero-García, J.J. Mark-recapture of Cerambyx welensii in dehesa woodlands: Dispersal behaviour, population density, and mass trapping efficiency with low trap densities. Entomol. Exper. Appl. 2013, 149, 273–281. [Google Scholar] [CrossRef]
- Sánchez-Osorio, I.; Tapias, R.; Domínguez, L.; López-Pantoja, G. Intraspecific variability of olfactory responses in Cerambyx welensii Küster (Coleoptera, Cerambycidae). Influence of anatomical, physiological and experimental factors. Invest. Agrar. Sist. Y Recur. For. 2009, 18, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Osorio, I.; Tapias, R.; Domínguez, L.; López, G. Caracterización de la respuesta electroantenográfica de Cerambyx welensii Küster y Prinobius germari Dejean (Coleoptera, Cerambycidae). Investig. Agraria. Sist. Y Recur. For. 2007, 16, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Osorio, I.; Domínguez, L.; López-Pantoja, G.; Tapias, R. Antennal response of Prinobius myardi to synthetic tree volatiles. Silva Fenn. 2015, 49, 1305. [Google Scholar] [CrossRef] [Green Version]
- Loreto, F. Distribution of isoprenoid emitters in the Quercus genus around the world: Chemo-taxonomical implications and evolutionary considerations based on the ecological function of the trait. Perspect. Plant Ecol. Evol. Syst. 2002, 5, 185–192. [Google Scholar] [CrossRef]
- Niinemets, U.; Reichstein, M.; Staudt, M.; Seufert, G.; Tenhunen, J.D. Stomatal constraints may affect emission of oxygenated monoterpenoids from the foliage of Pinus pinea. Plant Physiol. 2002, 130, 1371–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanch, J.S.; Peñuelas, J.; Sardans, J.; Llusia, J. Drought, warming and soil fertilization effects on leaf volatile terpene concentrations in Pinus halepensis and Quercus ilex. Acta Physiol. Plant. 2009, 31, 207–218. [Google Scholar] [CrossRef]
- Delfine, S.; Csiky, O.; Seufert, G.; Loreto, F. Fumigation with exogenous monoterpenes of a non-isoprenoid-emitting oak (Quercus suber): Monoterpene acquisition, translocation, and effect on the photosynthetic properties at high temperatures. New Phytol. 2000, 146, 27–36. [Google Scholar] [CrossRef]
- Llusià, J.; Peñuelas, J.; Asensio, D.; Munné-Bosch, S. Airborne limonene confers limited thermotolerance to Quercus ilex. Physiol. Plant. 2005, 123, 40–48. [Google Scholar] [CrossRef]
- Staudt, M.; Mir, C.; Joffre, R.; Rambal, S.; Bonin, A.; Landais, D.; Lumaret, R. Isoprenoid emissions of Quercus spp. (Q. suber and Q. ilex) in mixed stands contrasting in interspecific genetic introgression. New Phytol. 2004, 163, 573–584. [Google Scholar] [CrossRef]
- Loreto, F.; Pollastri, S.; Fineschi, S.; Velikova, V. Volatile isoprenoids and their importance for protection against environmental constraints in the Mediterranean area. Environ. Exp. Bot. 2014, 103, 99–106. [Google Scholar] [CrossRef]
- Sánchez-Osorio, I.; López-Pantoja, G.; Tapias, R.; Pareja-Sánchez, E.; Domínguez, L. Monoterpene emission of Quercus suber L. highly infested by Cerambyx welensii Küster. Ann. For. Sci. 2019, 76, 98. [Google Scholar] [CrossRef]
- Sánchez-Osorio, I.; Paramio, A.M.; Lencina, J.L.; Gallego, D.; Domínguez, L. Field attraction of Cerambyx welensii to fermentation odors and host monoterpenes. J. Pest. Sci. 2016, 89, 59–68. [Google Scholar] [CrossRef]
- Fürstenau, B.; Rosell, G.; Guerrero, A.; Quero, C. Electrophysiological and behavioral responses of the black-banded oak borer, Coroebus florentinus, to conspecific and host-plant volatiles. J. Chem. Ecol. 2012, 38, 378–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paris, C.I.; Llusia, J.; Peñuelas, J. Indirect effects of tending ants on holm oak volatiles and acorn quality. Plant Signal Behav. 2011, 6, 547–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriel, R.; Schäfer, L.; Gerlach, C.; Rausch, T.; Kesselmeier, J. Factors controlling the emissions of volatile organic acids from leaves of Quercus ilex L. (Holm oak). Atmos. Environ. 1999, 33, 1347–1355. [Google Scholar] [CrossRef]
- Holzinger, R.; Sandoval-Soto, L.; Rottenberge, S.; Crutzen, P.J.; Kesselmeier, J. Emissions of volatile organic compounds from Quercus ilex L. measured by Proton Transfer Reaction Mass Spectrometry under different environmental conditions. J. Geophys. Res. 2000, 105, 20573–20579. [Google Scholar] [CrossRef]
- Monaco, P.; Previtera, L. Isoprenoids from the leaves of Quercus suber. J. Nat. Prod. 1984, 47, 673–676. [Google Scholar] [CrossRef]
- Rocha, S.; Delgadillo, I.; Correia, A. GC-MS study of volatiles of normal and microbiologically attacked cork from Quercus suber L. J. Agric. Food. Chem. 1996, 44, 865–871. [Google Scholar] [CrossRef]
- Busta, L.; Serra, O.; Kim, O.T.; Molinas, M.; Peré-Fossoul, I.; Figueras, M.; Jetter, R. Oxidosqualene cyclases involved in the biosynthesis of triterpenoids in Quercus suber cork. Sci. Rep. 2020, 10, 8011. [Google Scholar] [CrossRef]
- Quero, C.; Vidal, B.; Guerrero, Á. EAG responses increase of Spodoptera littoralis antennae after a single pheromone pulse. Nat. Prod. Commun. 2014, 9, 1099–1101. [Google Scholar] [CrossRef] [Green Version]
- Mair, P.; Wilcox, R.R. Robust Statistical Methods in R Using the WRS2 Package. Behav. Res. Methods 2020, 52, 464–488. [Google Scholar] [CrossRef]
- Wende, B.; Gossner, M.M.; Grass, I.; Arnstadt, T.; Hofrichter, M.; Floren, A.; Linsenmair, K.E.; Weisser, W.W.; Steffan-Dewenter, I. Trophic level, successional age and trait matching determine specialization of deadwood-based interaction networks of saproxylic beetles. Proc. R. Soc. B 2017, 284, 1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos-Robles, M.; Vargas-Cardoso, O.R.; Corona-López, A.M.; Flores-Palacios, A.; Toledo-Hernández, V.H. Spatio-temporal variation of Cerambycidae-host tree interaction networks. PLoS ONE 2020, 15, 228880. [Google Scholar] [CrossRef] [Green Version]
- Yasui, H. Chemical communication in mate location and recognition in the white-spotted longicorn beetle, Anoplophora malasiaca (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 2009, 44, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Webster, B.; Bruce, T.; Pickett, J.; Hardie, J. Volatiles functioning as host cues in a blend become nonhost cues when presented alone to the black bean aphid. Anim. Behav. 2010, 79, 451–457. [Google Scholar] [CrossRef]
- Bracho-Nunez, A.; Knothe, N.M.; Welter, S.; Staudt, M.; Costa, M.R.; Liberato, M.A.R.; Piedade, M.T.F.; Kesselmeier, J. Leaf level emissions of volatile organic compounds (VOC) from some Amazonian and Mediterranean plants. Biogeosciences 2013, 10, 5855–5873. [Google Scholar] [CrossRef] [Green Version]
- Pearse, I.S.; Gee, W.S.; Beck, J.J. Headspace volatiles from 52 oak species advertise induction, species identity, and evolution, but not defense. J. Chem. Ecol. 2013, 39, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Brockerhoff, E.G.; Grant, G.G. Correction for differences in volatility among olfactory stimuli and effect on EAG responses of Dioryctria abietivorella to plant volatiles. J. Chem. Ecol. 1999, 25, 1353–1366. [Google Scholar] [CrossRef]
- Germinara, G.S.; Ganassi, S.; Pistillo, M.O.; Di Domenico, C.; De Cristofaro, A.; Di Palma, A.M. Antennal olfactory responses of adult meadow spittlebug, Philaenus spumarius, to volatile organic compounds (VOCs). PLoS ONE 2017, 12, 190454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).