Complete Chloroplast Genome Sequence of Fortunella venosa (Champ. ex Benth.) C.C.Huang (Rutaceae): Comparative Analysis, Phylogenetic Relationships, and Robust Support for Its Status as an Independent Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Acquisition and Chloroplast Genome Sequencing
2.2. Assembly of the Genome
2.3. Annotation of the Genome
2.4. Repeat Sequence and Codon Usage
2.5. Comparative Genome Analysis and Sequence Divergence
2.6. Adaptive Evolution and Substitution Rate
2.7. Phylogeny
3. Results
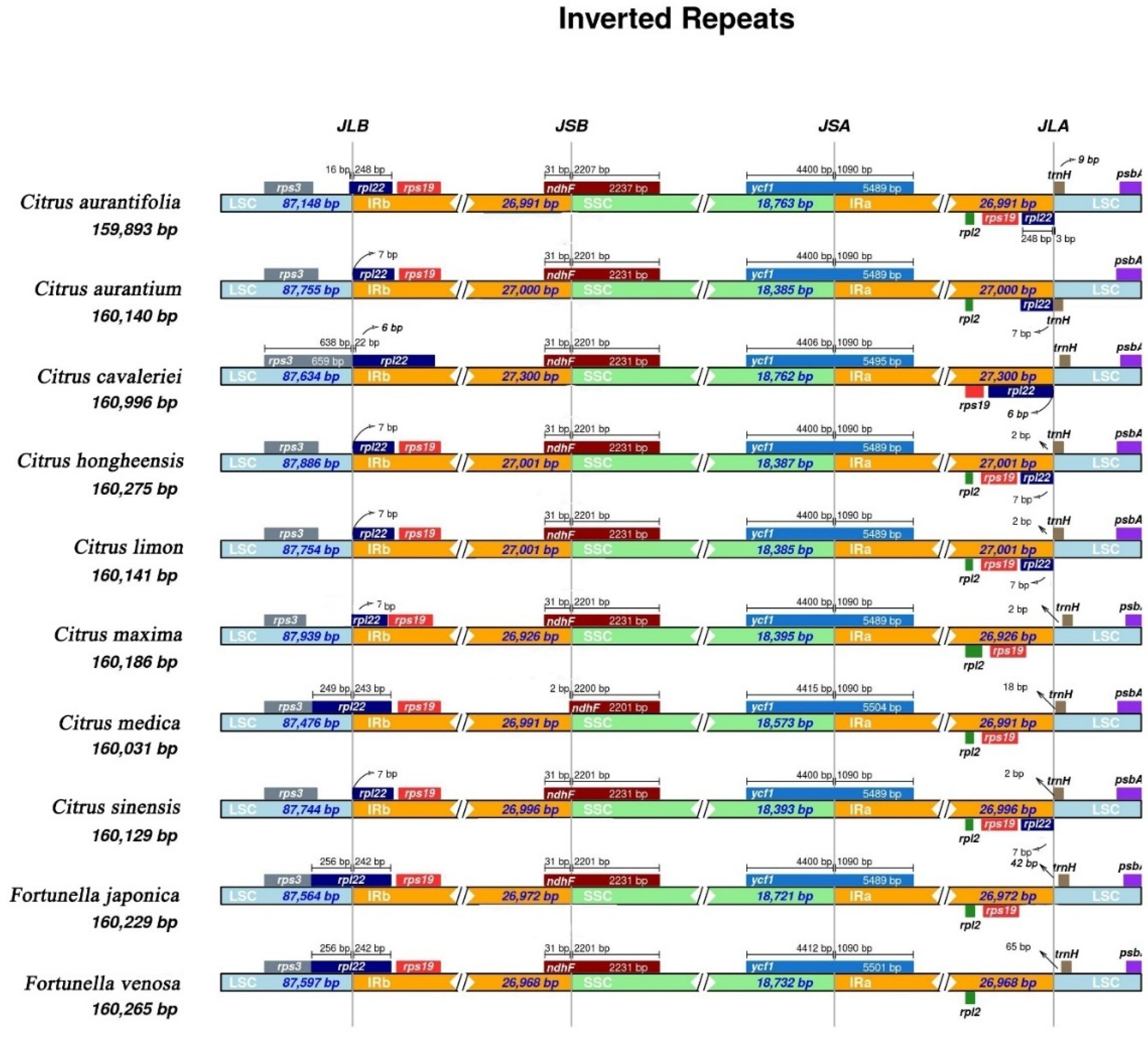
3.1. Analysis of Chloroplast Genome Structure
3.2. Repeat Sequence Analysis
3.3. SSR Analysis
3.4. Codon Usage Analysis
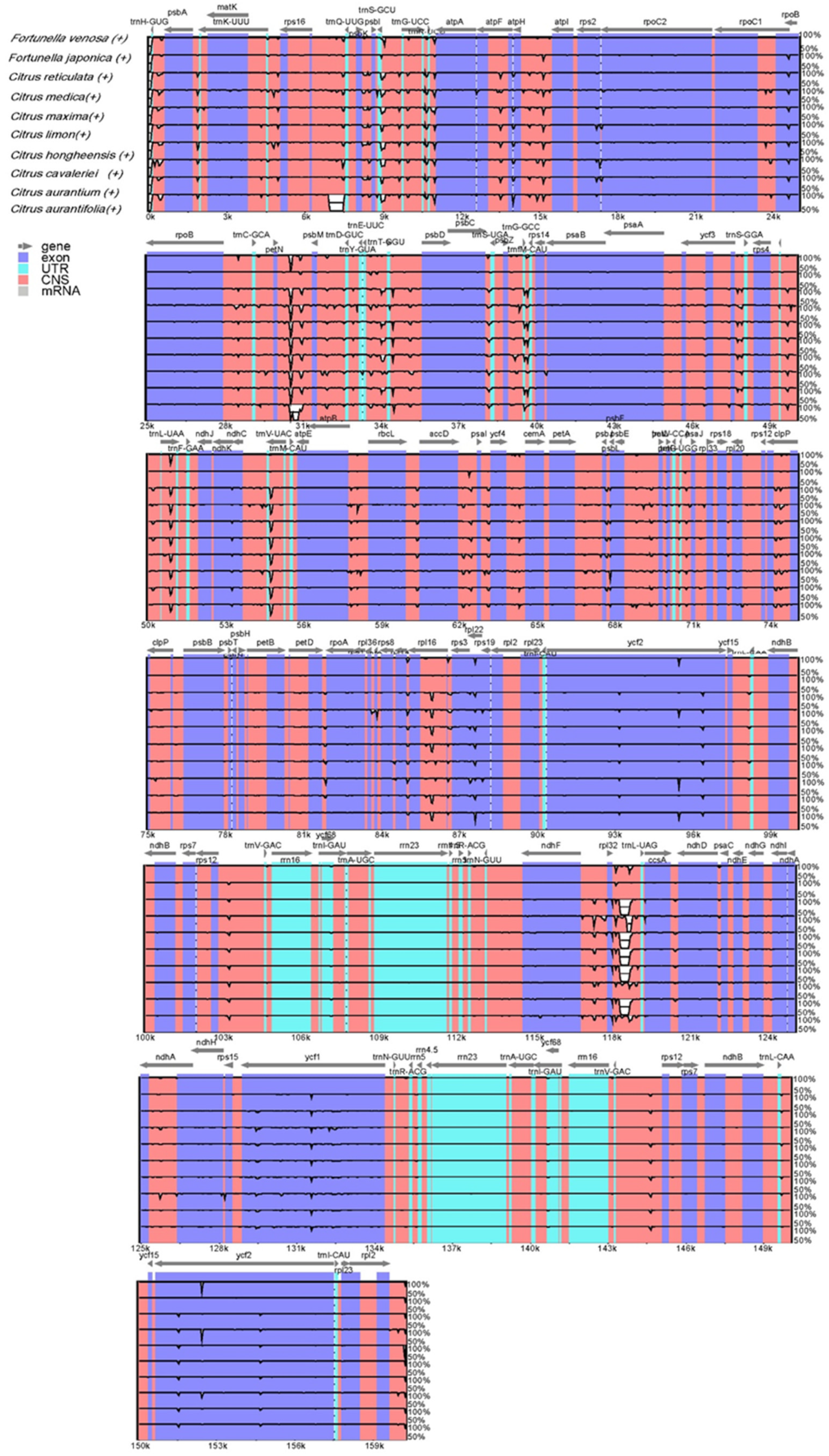
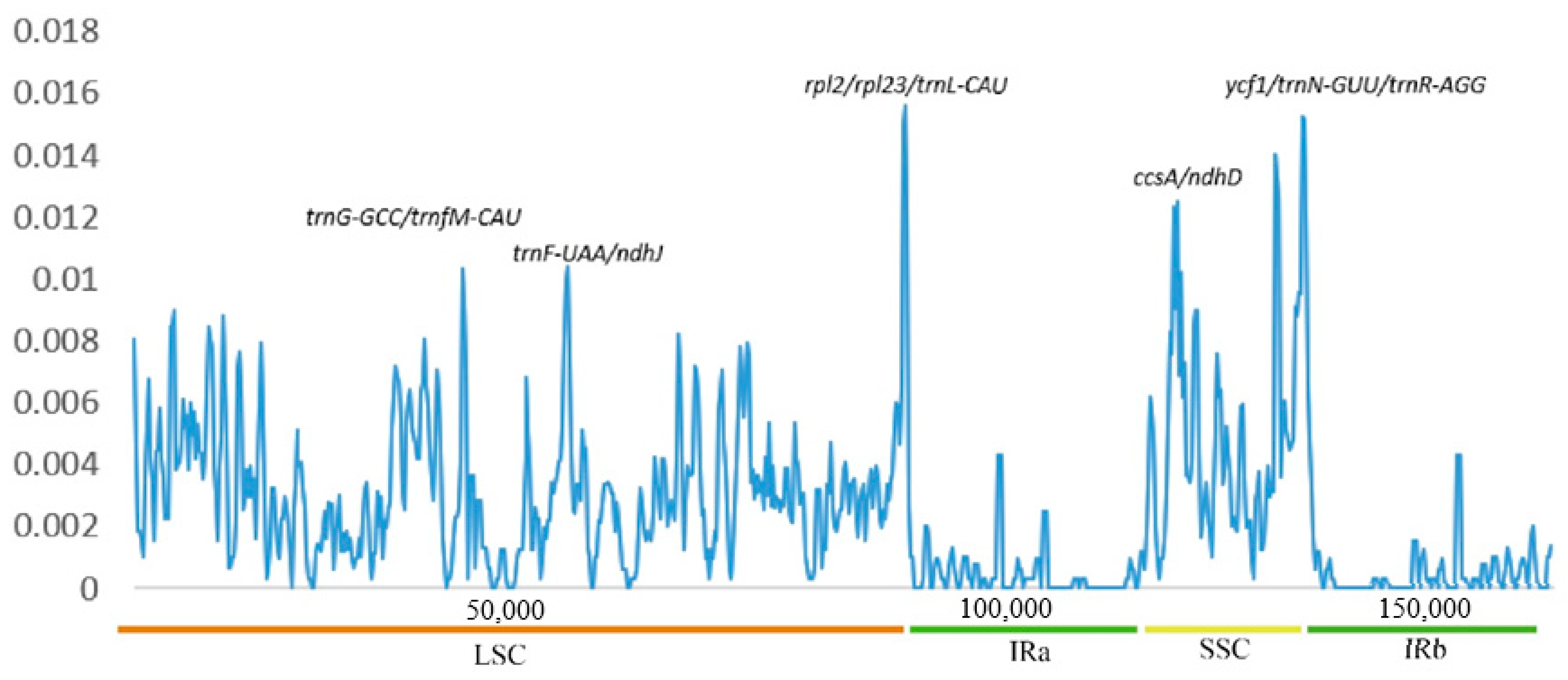
3.5. Comparative Genome and Sequence Divergence Analysis
3.6. Adaptive Evolution Analysis
3.7. Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brocks, J.J.; Logan, G.A.; Buick, R.; Summons, R.E. Archean molecular fossils and the early rise of eukaryotes. Science 1999, 285, 1033–1036. [Google Scholar] [CrossRef] [Green Version]
- Kostianovsky, M. Evolutionary origin of eukaryotic cells. Ultrastruct. Pathol. 2000, 24, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Embley, T.M.; Martin, W. Eukaryotic evolution, changes and challenges. Nature 2006, 440, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.C.; Clarke, J.L. Process in chloroplast genome analysis. Prog. Biochem. Biophys. 2008, 35, 21–28. [Google Scholar]
- Daniell, H.; Lin, C.S.; Yu, M.; Chang, W.J. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 2016, 17, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravi, V.; Khurana, J.P.; Tyagi, A.K.; Khurana, P. An update on chloroplast genomes. Plant Syst. Evol. 2008, 271, 101–122. [Google Scholar] [CrossRef]
- Wang, L.; Dong, W.P.; Zhou, S.L. Structural mutations and reorganizations in chloroplast genomes of flowering plants. Acta Bot. Boreali-Occident. Sin. 2012, 32, 1282–1288. [Google Scholar]
- Zhang, T.; Fang, Y.; Wang, X.; Deng, X.; Zhang, X.; Hu, S.; Yu, J. The complete chloroplast and mitochondrial genome sequences of Boea hygrometrica: Insights into the evolution of plant organellar genomes. PLoS ONE 2012, 7, e30531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinozaki, K.; Ohme, M.; Tanaka, M.; Wakasugi, T.; Hayashida, N.; Matsubayashi, T.; Zaita, N.; Chunwongse, J.; Obokata, J.; Yamaguchi-Shinozaki, K.; et al. The complete nucleotide sequence of the tobacco chloroplast genome: Its gene organization and expression. EMBO J. 1986, 5, 2043–2049. [Google Scholar] [CrossRef]
- Liu, L.X.; Li, R.; Worth, J.R.P.; Li, X.; Li, P.; Cameron, K.M.; Fu, C.X. The complete chloroplast genome of Chinese bayberry (Morella rubra, Myricaceae): Implications for understanding the evolution of Fagales. Front. Plant Sci. 2017, 8, 968. [Google Scholar] [CrossRef]
- McCauley, D.E.; Stevens, J.E.; Peroni, P.A.; Raveill, J.A. The spatial distribution of chloroplast DNA and allozyme polymorphisms within a population of Silene alba (Caryophyllaceae). Am. J. Bot. 1996, 83, 727–731. [Google Scholar] [CrossRef]
- Small, R.L.; Cronn, R.C.; Wendel, J.F. Use of nuclear genes for phylogeny reconstruction in plants. Aust. Syst. Bot. 2004, 17, 145–170. [Google Scholar] [CrossRef]
- Korpelainen, H. The evolutionary processes of mitochondrial and chloroplast genomes differ from those of nuclear genomes. Naturwissenschaften 2004, 91, 505–518. [Google Scholar] [CrossRef]
- Shaw, J.; Lickey, E.B.; Beck, J.T.; Farmer, S.B.; Liu, W.; Miller, J.; Siripun, K.C.; Winder, C.T.; Schilling, E.E.; Small, R.L. The tortoise and the hare II: Relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. Am. J. Bot. 2005, 92, 142–166. [Google Scholar] [CrossRef] [Green Version]
- Hollingsworth, P.M.; Graham, S.W.; Little, D.P. Choosing and using a plant DNA barcode. PLoS ONE 2011, 6, e19254. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.P.; Xu, C.; Li, C.H.; Sun, J.H.; Zuo, Y.J.; Shi, S.; Cheng, T.; Guo, J.J.; Zhou, S.L. ycf1, the most promising plastid DNA barcode of land plants. Sci. Rep. 2015, 5, 8348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.-J.; Liu, J.-S.; Luo, L.; Wei, X.P.; Zhang, J.; Qi, Y.-D.-; Zhang, B.-G.; Liu, H.-T.; Xiao, P.-G. Complete chloroplast genome sequences of Schisandra chinensis: Genome structure, comparative analysis, and phylogenetic relationship of basal angiosperms. Sci. China Life Sci. 2017, 11, 1286–1290. [Google Scholar] [CrossRef]
- Wang, S.; Yang, C.P.; Zhao, X.Y.; Chen, S.; Qu, G.Z. Complete chloroplast genome sequence of Betula platyphylla: Gene organization, RNA editing, and comparative and phylogenetic analyses. BMC Genom. 2018, 19, 950. [Google Scholar] [CrossRef] [Green Version]
- Rono, P.C.; Dong, X.; Yang, J.X.; Mutie, F.M.; Oulo, M.A.; Malombe, I.; Kirika, P.M.; Hu, G.W.; Wang, Q.F. Initial Complete Chloroplast Genomes of Alchemilla (Rosaceae): Comparative Analysis and Phylogenetic Relationships. Front. Genet. 2020, 11, 1390. [Google Scholar] [CrossRef]
- Zhou, H.; Gao, X.; Woeste, K.; Zhao, P.; Zhang, S. Comparative Analysis of the Complete Chloroplast Genomes of Four Chestnut Species (Castanea). Forests 2021, 12, 861. [Google Scholar] [CrossRef]
- Wanga, V.O.; Dong, X.; Oulo, M.A.; Mkala, E.M.; Wang, Q.F. Complete chloroplast genomes of Acanthochlamys bracteata (china) and Xerophyta (Africa) (Velloziaceae): Comparative genomics and phylogenomic placement. Front. Plant Sci. 2021, 12, 691833. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-J. Flora Reipublicae Popularis Sinicae; Science Press: Beijing, China, 1997; Volume 43, Part 2; pp. 169–175. [Google Scholar]
- Liu, R.-L. Fortunella venosa (Champ. ex Benth.) Huang. South China For. Sci. 2014, 42, 2. [Google Scholar]
- Huang, X.-Z.; Lei, Y.; Chen, X.-M.; Wei, X.-X.; Lu, X.-K.; Ye, X.; Zheng, H.-Z. Investigation and Analysis of Wild Fortunella hindsii and Fortunella venosa Resources in Fujian. J. Plant Genet. Resour. 2010, 11, 509–513. [Google Scholar]
- Wang, S.; Xie, Y. China Species Red List; Higher Education Press: Beijing, China, 2004; Volume 1, p. 346. [Google Scholar]
- Yasuda, K.; Yahata, M.; Kunitake, H. Phylogeny and Classification of Kumquats (Fortunella spp.) Inferred from CMA Karyotype Composition. Hortic. J. 2016, 85, 115–121. [Google Scholar] [CrossRef]
- Tanaka, T. Revisio Aurantiacearum—I. Bull. Société Bot. Fr. 1928, 75, 708–715. [Google Scholar] [CrossRef]
- Zhang, D.; Hartley, T.G.; Mabberley, D.J. Flora of China; Wu, C.-Y., Raven, P.H., Hong, D.-Y., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MI, USA, 2008; Volume 11, pp. 92–93. [Google Scholar]
- Araújo, E.F.D.; Queiroz, L.P.D.; Machado, M.A. What is Citrus? Taxonomic implications from a study of cp-DNA evolution in the tribe Citreae (Rutaceae subfamily Aurantioideae). Org. Divers. Evol. 2003, 3, 55–62. [Google Scholar]
- Khalvashi, N.; Memarne, G. Morphological peculiarities of the genus Fortunella swingle and perspectives of its application. Mod. Phytomorphology 2014, 6, 221–224. [Google Scholar]
- Xu, S.-R.; Zhang, Y.-Y.; Liu, F.; Tian, N.; Pan, D.-M.; Bei, X.-J.; Cheng, C.-Z. Characterization of the complete chloroplast genome of the Hongkong kumquat (Fortunella hindsii Swingle). Mitochondrial. Dna. Part B 2019, 4, 2612–2613. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.-X.; Tang, Y.; Deng, C.-L. Progress on study of Citrus palynology. South China Fruits 2017, 46, 148–153. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2013. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 7 April 2021).
- Jin, J.-J.; Yu, W.-B.; Yang, J.-B.; Song, Y.; dePamphilis, C.W.; Yi, T.-S.; Li, D.-Z. GetOrganelle: A fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020, 21, 241. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ning, M.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wick, R.R.; Schultz, M.B.; Zobel, J.; Holt, K.E. Bandage: Interactive visualization of de novo genome assemblies: Figure 1. Bioinformatics 2015, 31, 3350–3352. [Google Scholar] [CrossRef] [Green Version]
- Qu, X.-J.; Moore, M.J.; Li, D.-Z.; Yi, T.-S. PGA: A software package for rapid, accurate, and flexible batch annotation of Plastomes. Plant Methods 2019, 15, 50. [Google Scholar] [CrossRef] [Green Version]
- Lohse, M.; Drechsel, O.; Bock, R. OrganellarGenomeDRAW(OGDRAW): A tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr. Genet. 2007, 52, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.; Drechsel, O.; Kahlau, S.; Bock, R. OrganellarGenomeDRAW—A suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 2013, 41, W575–W581. [Google Scholar] [CrossRef]
- Kurtz, S.; Choudhuri, J.V.; Ohlebusch, E.; Schleiermacher, C.; Stoye, J.; Giegerich, R. REPuter: The manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001, 29, 4633–4642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benson, G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef] [Green Version]
- Thiel, T.; Michalek, W.; Varshney, R.; Graner, A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). Theor. Appl. Genet. 2003, 106, 411–422. [Google Scholar] [CrossRef]
- Beier, S.; Thiel, T.; Munch, T.; Scholz, U.; Mascher, M. MISA-web: A web server for microsatellite prediction. Bioinformatics 2017, 33, 2583–2585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stothard, P. The Sequence Manipulation Suite: JavaScript programs for analyzing and formatting protein and DNA sequences. Biotechniques 2000, 28, 1102–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipskj, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Mayor, C.; Brudno, M.; Schwartz, J.R.; Poliakov, A.; Rubin, E.M.; Frazer, K.A.; Pachter, L.S.; Dubchak, I. VISTA: Visualizing global DNA sequence alignments of arbitrary length. Bioinformatics 2000, 16, 1046–1047. [Google Scholar] [CrossRef] [Green Version]
- Frazer, K.A.; Pachter, L.; Poliakov, A.; Rubin, E.M.; Dubchak, I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004, 32, W273–W279. [Google Scholar] [CrossRef]
- Amiryousefi, A.; Hyvönen, J.; Poczai, P. IRscope: An online program to visualize the junction sites of chloroplast genomes. Bioinformatics 2018, 34, 3030–3031. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Gao, F.-L.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-H. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol 2007, 24, 1586–1591. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.-L.; Chen, C.-J.; Arab, D.A.; Du, Z.-G.; He, Y.-H.; Ho, S.Y.W. EasyCodeML: A visual tool for analysis of selection using CodeML. Ecol. Evol. 2019, 9, 3891–3898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambaut, A. FigTree v1.4.4. Institute of Evolutionary Biology; University of Edinburgh: Edinburgh, UK, 2018. [Google Scholar]
- Buschiazzo, E.; Ritland, C.; Bohlmann, J.; Ritland, K. Slow but not low: Genomic comparisons reveal slower evolutionary rate and higher dN/dS in conifers compared to angiosperms. BMC Evol. Biol. 2012, 12, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, H.-J.; Hogenhout, S.A.; Al-Sadi, A.M.; Kuo, C.-H. Complete Chloroplast Genome Sequence of Omani Lime (Citrus aurantiifolia) and Comparative Analysis within the Rosids. PLoS ONE 2014, 9, e113049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bausher, M.G.; Singh, N.D.; Lee, S.B.; Jansen, R.K.; Daniell, H. The complete chloroplast genome sequence of Citrus sinensis (L.) Osbeck var ‘Ridge Pineapple’: Organization and phylogenetic relationships to other angiosperms. BMC Plant Biol. 2006, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Ni, Z.-X.; Ye, Y.-J.; Bai, T.-D.; Xu, M.; Xu, L.-A. Complete Chloroplast Genome of Pinus massoniana (Pinaceae): Gene Rearrangements, Loss of ndh Genes, and Short Inverted Repeats Contraction, Expansion. Molecules 2017, 22, 1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Qamar, M.T.; Feng, J.-W.; Ding, Y.-D.; Wang, S.; Wu, G.-Z.; Ke, L.-J.; Xu, Q.; Chen, L.-L. Comparative analysis of miniature inverted–repeat transposable elements (MITEs) and long terminal repeat (LTR) retrotransposons in six Citrus species. BMC Plant Biol. 2019, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Kanamoto, H.; Yoshimura, H.; Tomizawa, K.-I.; Kitajima, S.; Ujihara, T.; Matsuda, Y.; Sugimura, Y. Sequence variation in the rbcL-accD region in the chloroplast genome of Moraceae. Plant Biotechnol. 2005, 22, 231. [Google Scholar]
- Zheng, X.-M.; Wang, J.-R.; Feng, L.; Liu, S.; Pang, H.-B.; Qi, L.; Li, J.; Sun, Y.; Qiao, W.-H.; Zhang, L.-F.; et al. Inferring the evolutionary mechanism of the chloroplast genome size by comparing whole-chloroplast genome sequences in seed plants. Sci. Rep. 2017, 7, 1555. [Google Scholar]
- Cavalier-Smith, T. Chloroplast evolution: Secondary symbiogenesis and multiple losses. Curr. Biol. 2002, 12, R62–R64. [Google Scholar] [CrossRef] [Green Version]
- Yi, X.; Gao, L.; Wang, B.; Su, Y.J.; Wang, T. The complete chloroplast genome sequence of Cephalotaxus oliveri (Cephalotaxaceae): Evolutionary comparison of Cephalotaxus chloroplast DNAs and insights into the loss of inverted repeat copies in gymnosperms. Genome Biol. Evol. 2013, 5, 688–698. [Google Scholar] [CrossRef] [Green Version]
- Levinson, G.; Gutman, G.A. Slipped-strand mispairing: A major mechanism for DNA sequence evolution. Mol. Biol. Evol. 1987, 4, 203–221. [Google Scholar]
- Cheng, Y.-J.; Guo, W.-W.; Deng, X.-X. cpSSR: A New Tool to Analyze Chloroplast Genome of Citrus Somatic Hybrids. J. Integr. Plant Biol. 2003, 45, 906–909. [Google Scholar]
- Wang, H.-K.; Lou, X.-M.; Zhang, Z. Application in germplasm resource research using chloroplast simple sequence repeat. MPB 2006, S1, 92–98. [Google Scholar]
- Zhou, S.; Dong, W.; Chen, X.; Zhang, X.; Wen, J.; Schneider, H. How many species of bracken (Pteridium) are there? Assessing the Chinese brackens using molecular evidence. Taxon 2014, 63, 509–521. [Google Scholar] [CrossRef]
- Qi, W.-C.; Lin, F.; Liu, Y.-H.; Huang, B.-Q.; Cheng, J.-H.; Zhang, W.; Zhao, H. High-throughput development of simple sequence repeat markers for genetic diversity research in Crambe abyssinica. BMC Plant Biol. 2016, 16, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Komivi, D.; Wang, L.; Zhang, Y.; Wei, X.; Liao, B.; Zhang, X. PMDBase: A database for studying microsatellite DNA and marker development in plants. Nucleic Acids Res. 2017, 45, D1046–D1053. [Google Scholar] [CrossRef] [Green Version]
- Chai, S.-F.; Chen, Z.-G.; Hong, C.-X. Construction and Optimization of ISSR Reaction System of Medicinal Rutaceae Plants. J. Anhui Agric. Sci. 2008, 36, 14433–14435. [Google Scholar]
- Powell, W.; Morgante, M.; McDevitt, R.; Vendramin, G.G.; Rafalski, J.A. Polymorphic simple sequence repeats regions in chloroplast genomes: Applications to the population genetics of Pines. Proc. Natl. Acad. Sci. USA 1995, 92, 7759–7763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro, J.A.; Sternberg, R. von. Why repetitive DNA is essential to genome function. Biol. Rev. Camb. Philos. Soc. 2005, 80, 227–250. [Google Scholar] [CrossRef] [Green Version]
- Axel, C.; Romain, K.; Julien, M. The 3D folding of metazoan genomes correlates with the association of similar repetitive elements. Nucleic Acids Res. 2016, 44, 245–255. [Google Scholar]
- Kuang, D.-Y.; Wu, H.; Wang, Y.-L.; Gao, L.-M.; Zhang, S.-Z.; Lu, L. Complete chloroplast genome sequence of Magnolia kwangsiensis (Magnoliaceae): Implication for DNA barcoding and population genetics. Genome 2011, 54, 663–673. [Google Scholar] [CrossRef] [Green Version]
- Asaf, S.; Khan, A.L.; Khan, M.A.; Waqas, M.; Kang, S.-M.; Yun, B.-W.; Lee, I.J. Chloroplast genomes of Arabidopsis halleri ssp. gemmifera and Arabidopsis lyrata ssp. petraea: Structures and comparative analysis. Sci. Rep. 2017, 7, 7556. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Tan, W.; Zhang, H.; Gao, H.; Wang, W.; Tian, X. Complete Chloroplast Genomes of Ampelopsis humulifolia and Ampelopsis japonica: Molecular Structure, Comparative Analysis, and Phylogenetic Analysis. Plants 2019, 8, 410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Yu, Y.; Liu, Y.-M.; Xie, D.-F.; He, X.-J.; Zhou, S.-D. Comparative Chloroplast Genomics of Fritillaria (Liliaceae), Inferences for Phylogenetic Relationships between Fritillaria and Lilium and Plastome Evolution. Plants 2020, 9, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, B.R. The role of context-dependent mutations in generating compositional and codon usage bias in grass chloroplast DNA. J. Mol. Evol. 2003, 56, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xing, H.; Yuan, Y.; Wang, X.; Muhammad, S.; Tao, J.; Wei, F.; Zhang, G.; Song, X.; Sun, X. Genome- wide analysis of codon usage bias in four sequenced cotton species. PLoS ONE 2018, 13, e0194372. [Google Scholar] [CrossRef] [Green Version]
- Sharp, P.M.; Li, W.-H. The codon adaptation index-a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987, 15, 1281–1295. [Google Scholar] [CrossRef] [Green Version]
- Jose, C.C.; Roberto, A.; Victoria, I.; Javier, T.; Manuel, T.; Joaquin, D. A Phylogenetic Analysis of 34 Chloroplast Genomes Elucidates the Relationships between Wild and Domestic Species within the Genus Citrus. Mol. Biol. Evol. 2015, 32, 2015–2035. [Google Scholar]
- Wang, W.-C.; Chen, S.-Y.; Zhang, X.-Z. Whole-Genome Comparison Reveals Divergent IR Borders and Mutation Hotspots in Chloroplast Genomes of Herbaceous Bamboos (Bambusoideae: Olyreae). Molecules 2018, 23, 1537. [Google Scholar] [CrossRef] [Green Version]
- Li, D.-M.; Ye, Y.-J.; Xu, Y.-C.; Liu, J.-M.; Zhu, G.-F. Complete chloroplast genomes of Zingiber montanum and Zingiber zerumbet: Genome structure, comparative and phylogenetic analyses. PLoS ONE 2020, 15, e0236590. [Google Scholar] [CrossRef]
- Wu, J.-W.; Liu, F.; Tian, N.; Liu, J.-P.; Shi, X.-B.; Bei, X.-J.; Cheng, C.-Z. Characterization of the complete chloroplast genome of Fortunella Crassifolia Swingle and phylogenetic relationships. Mitochondrial DNA Part B 2019, 4, 3538–3539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
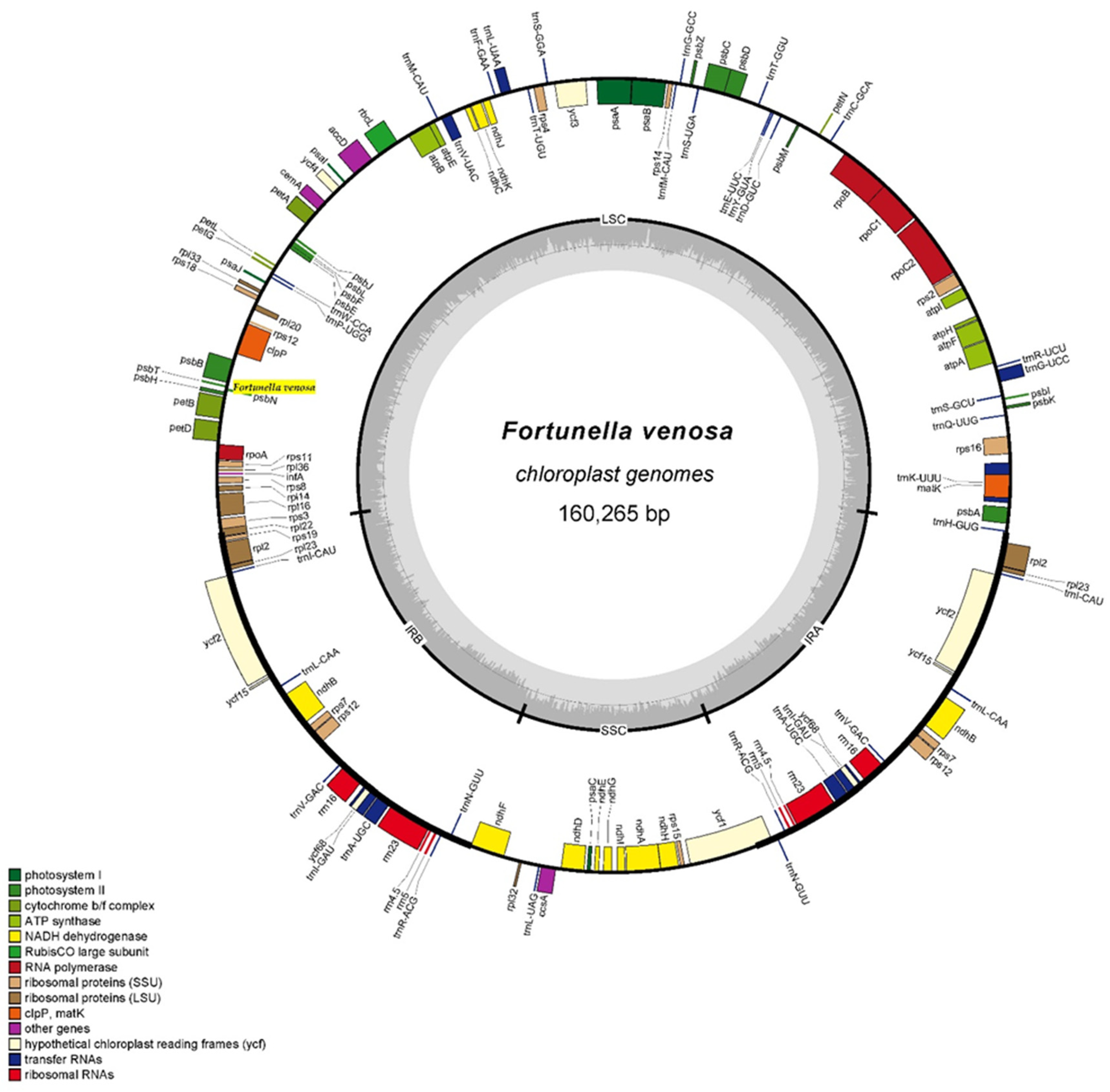
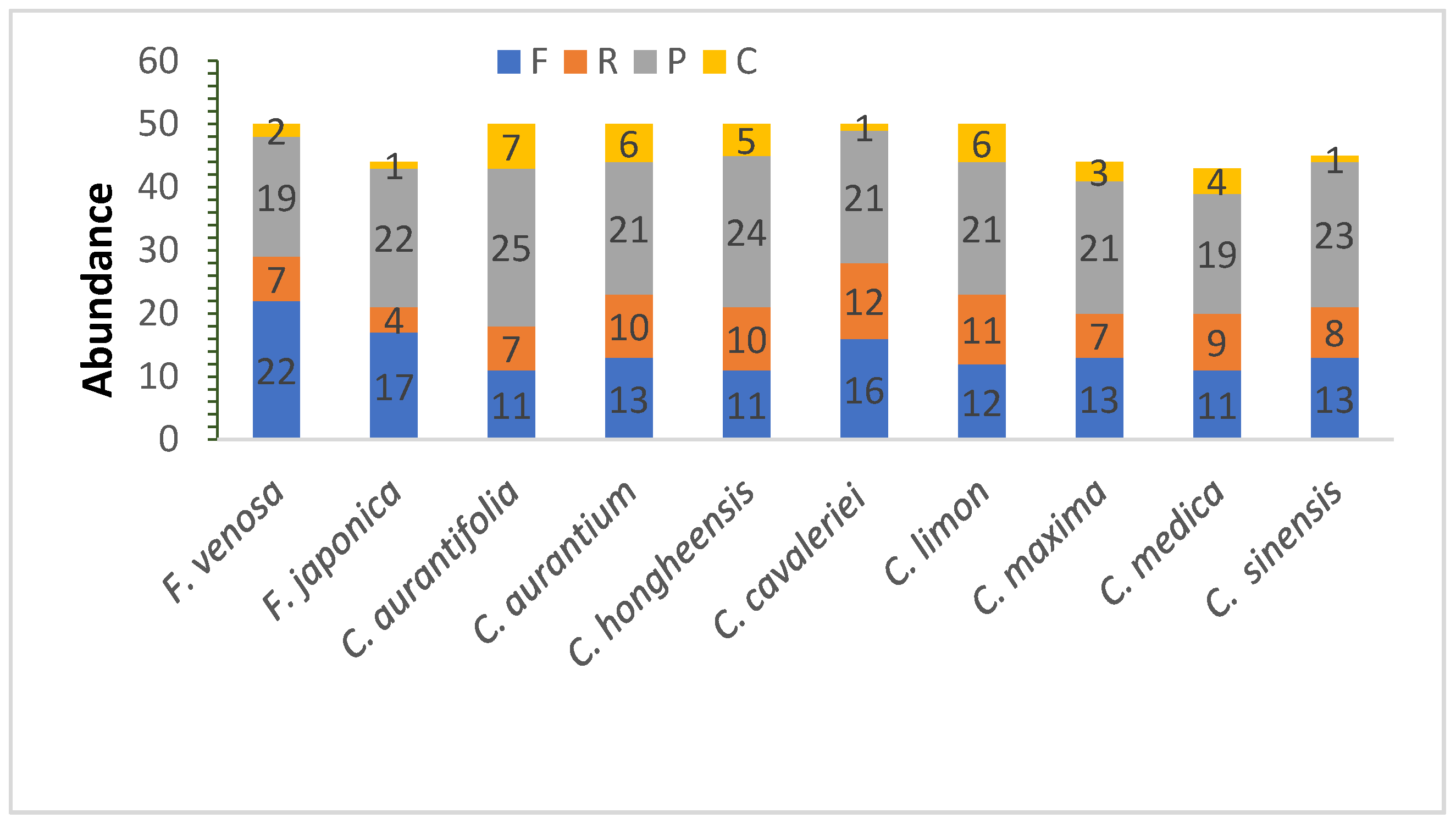

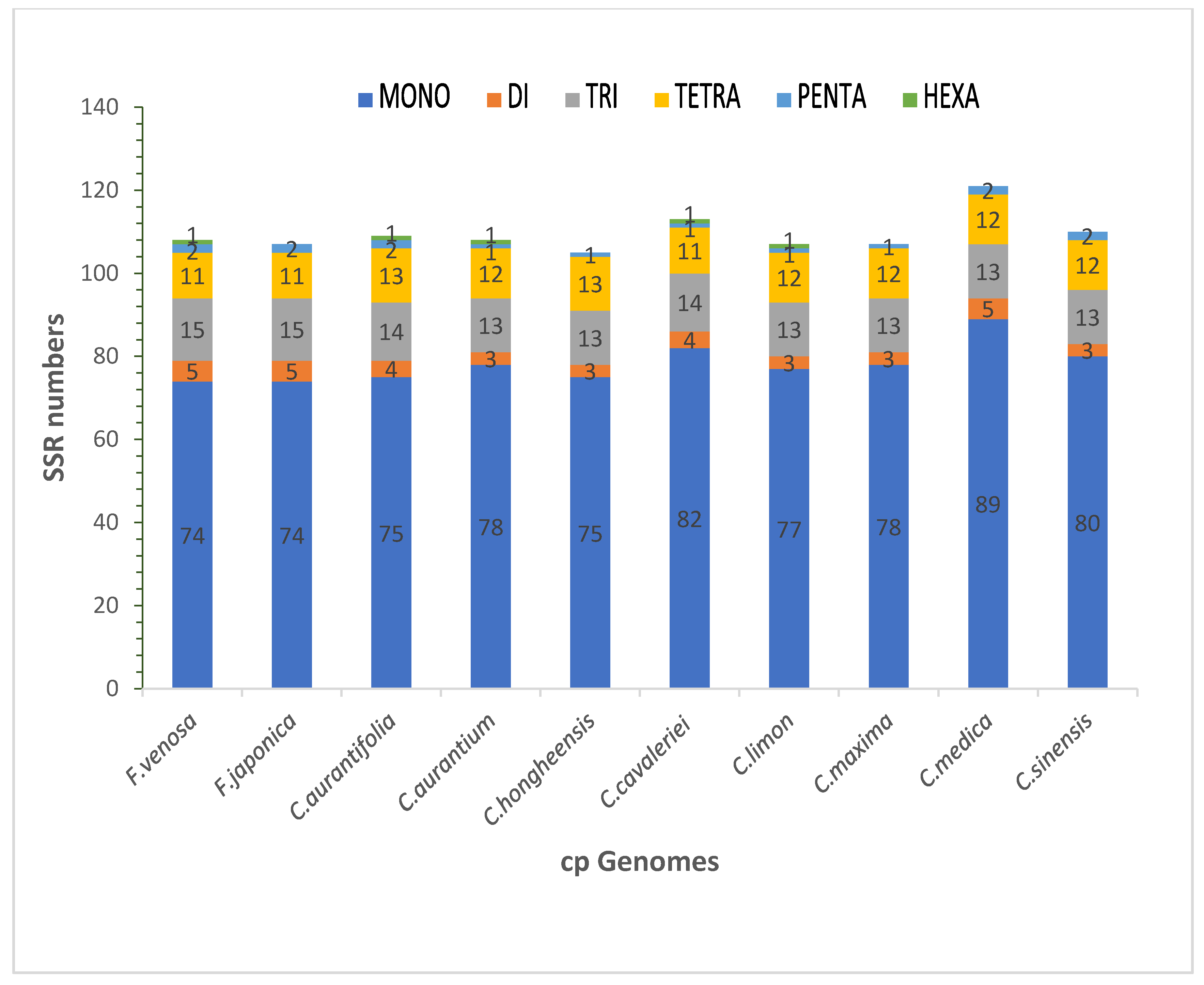
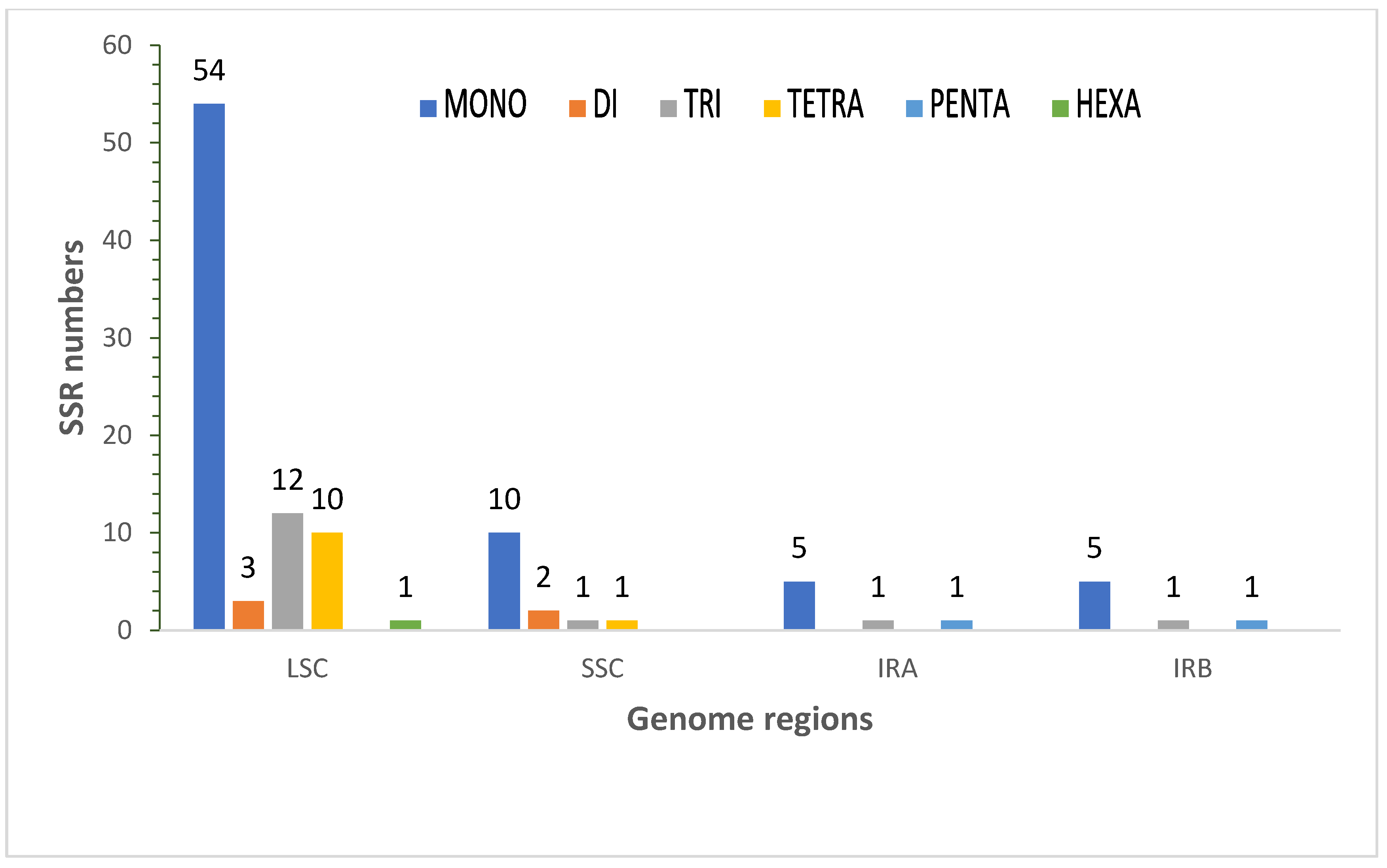



| Species/Taxa | Fortunella venosa | Fortunella japonica | Citrus aurantifolia | Citrus aurantium | Citrus hongheensis | Citrus cavaleriei | Citrus limon | Citrus maxima | Citrus medica | Citrus sinensis | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Accession number | MZ457935 | MN495932 | KJ865401 | MT702983 | MT880607 | MT880606 | MT880608 | MN782007 | MT106673 | DQ864733 | |
| Total Number of Genes | 134 | 135 | 138 | 132 | 135 | 135 | 135 | 125 | 134 | 134 | |
| Genome | Total GC content (%) | 38.4 | 38.4 | 38.4 | 38.5 | 38.5 | 38.5 | 38.5 | 38.5 | 38.4 | 38.5 |
| Total Length(bp) | 160,265 | 160,229 | 159,893 | 160,140 | 160,275 | 160,996 | 160,141 | 160,186 | 160,031 | 160,129 | |
| CDS | number | 89 | 90 | 93 | 87 | 89 | 89 | 89 | 88 | 89 | 89 |
| Length(bp) | 79,983 | 80,568 | 81,363 | 80,097 | 79,509 | 80,097 | 79,509 | 79,971 | 80,370 | 79,971 | |
| GC (%) | 38.9 | 38.8 | 39 | 38.8 | 38.9 | 38.8 | 38.9 | 38.8 | 39 | 38.8 | |
| tRNA | number | 37 | 37 | 37 | 37 | 37 | 37 | 37 | 37 | 37 | 37 |
| Length(bp) | 2790 | 2792 | 2802 | 2793 | 2792 | 2792 | 2792 | 2800 | 2802 | 2802 | |
| GC (%) | 53.3 | 53.3 | 53.3 | 53.3 | 53.4 | 53.3 | 53.4 | 53.3 | 53.2 | 53.3 | |
| rRNA | number | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Length(bp) | 9044 | 9048 | 9048 | 9044 | 9048 | 9050 | 9048 | 9046 | 9048 | 9048 | |
| GC (%) | 55.7 | 55.7 | 55.7 | 55.7 | 55.7 | 55.7 | 55.7 | 55.7 | 55.7 | 55.7 | |
| Category | Group of Genes | Name of Genes |
|---|---|---|
| Self-replication | Ribosomal protein (LSU) | * rpl2, rpl14, * rpl16, rpl20, rpl22, rpl23, rpl33, rpl32, rpl36 |
| Ribosomal proteins (SSU) | rps2, rps3, rps4, rps7, rps8, rps11, * rps12, rps14, rps15, * rps16, rps18, rps19 | |
| DNA-dependent RNA polymerase | rpoA, rpoB, * rpoC1, rpoC2 | |
| rRNA genes | rrn4.5, rrn5, rrn16, rrn23 | |
| tRNA genes | * trnA-UGC, trnC-GCA, trnD-GUC, trnE-UUC, trnF-GAA, trnfM-CAU, | |
| trnG-GCC, * trnG-UCC, trnH-GUG, trnI-CAU, * trnI-GAU, trnK-UUU, | ||
| trnL-CAA, * trnL-UAA, trnL-UAG, trnM-CAU, trnN-GUU, trnP-UGG, | ||
| trnQ-UUG, trnR-ACG, trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, | ||
| trnT-UGU, trnV-GAC, * trnV-UAC, trnW-CCA, trnY-GUA | ||
| Photosynthesis | Photosystem I | psaA, psaB, psaC, psaI, psaJ |
| Photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, | |
| psbN, psbT, psbZ | ||
| NADPH dehydrogenase | * ndhA, * ndhB, ndhC, ndhD, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK | |
| ATP synthase | atpA, atpB, atpE, * atpF, atpH, atpI | |
| Cytochrome c-type synthesis | petA, * petB, * petD, petG, petL, petN | |
| Rubisco | rbcL | |
| Other genes | Maturase | matK |
| Cytochrome c-type synthesis | ccsA | |
| Carbon metabolism | cemA | |
| Fatty acid synthesis | accD | |
| Transfer initiation factor | infA | |
| Proteolysis | ** clpP | |
| unknown | Conserved open reading frames | ycf1, ycf2, ** ycf3, ycf4, ycf68, ycf15 |
| Codon | Count | RSCU | Codon | Count | RSCU | Codon | Count | RSCU | Codon | Count | RSCU |
|---|---|---|---|---|---|---|---|---|---|---|---|
| UUU(F) | 975 | 1.27 | UCU(S) | 551 | 1.6 | UAU(Y) | 774 | 1.59 | UGU(C) | 233 | 1.48 |
| UUC(F) | 556 | 0.73 | UCC(S) | 343 | 1 | UAC(Y) | 197 | 0.41 | UGC(C) | 82 | 0.52 |
| UUA(L) | 832 | 1.76 | UCA(S) | 384 | 1.12 | UAA (*) | 51 | 1.72 | UGA (*) | 15 | 0.51 |
| UUG(L) | 586 | 1.24 | UCG(S) | 243 | 0.71 | UAG (*) | 23 | 0.78 | UGG(W) | 455 | 1 |
| CUU(L) | 590 | 1.24 | CCU(P) | 407 | 1.45 | CAU(H) | 461 | 1.43 | CGU(R) | 321 | 1.15 |
| CUC(L) | 222 | 0.47 | CCC(P) | 243 | 0.87 | CAC(H) | 183 | 0.57 | CGC(R) | 131 | 0.47 |
| CUA(L) | 395 | 0.83 | CCA(P) | 321 | 1.14 | CAA(Q) | 706 | 1.53 | CGA(R) | 386 | 1.38 |
| CUG(L) | 219 | 0.46 | CCG(P) | 152 | 0.54 | CAG(Q) | 215 | 0.47 | CGG(R) | 153 | 0.55 |
| AUU(I) | 1071 | 1.47 | ACU(T) | 528 | 1.56 | AAU(N) | 961 | 1.51 | AGU(S) | 398 | 1.16 |
| AUC(I) | 461 | 0.63 | ACC(T) | 264 | 0.78 | AAC(N) | 313 | 0.49 | AGC(S) | 147 | 0.43 |
| AUA(I) | 651 | 0.89 | ACA(T) | 391 | 1.16 | AAA(K) | 1041 | 1.47 | AGA(R) | 493 | 1.77 |
| AUG(M) | 632 | 1 | ACG(T) | 168 | 0.5 | AAG(K) | 371 | 0.53 | AGG(R) | 189 | 0.68 |
| GUU(V) | 527 | 1.45 | GCU(A) | 626 | 1.71 | GAU(D) | 848 | 1.58 | GGU(G) | 552 | 1.2 |
| GUC(V) | 181 | 0.5 | GCC(A) | 248 | 0.68 | GAC(D) | 226 | 0.42 | GGC(G) | 198 | 0.43 |
| GUA(V) | 536 | 1.48 | GCA(A) | 390 | 1.07 | GAA(E) | 1016 | 1.47 | GGA(G) | 698 | 1.52 |
| GUG(V) | 209 | 0.58 | GCG(A) | 200 | 0.55 | GAG(E) | 366 | 0.53 | GGG(G) | 394 | 0.86 |
| Gene Name | M8 | |
|---|---|---|
| Selected Sites | Pr (w > 1) | |
| ndhB | 4084R | 0.990 ** |
| ndhI | 6657M | 1.000 ** |
| 6658S | 1.000 ** | |
| psbZ | 11729L | 0.990 ** |
| rpoC2 | 16680Y | 0.999 ** |
| 16682C | 0.999 ** | |
| 16683I | 0.999 ** | |
| 16703T | 0.998 ** | |
| 16705R | 0.991 ** | |
| 16706A | 0.998 ** | |
| 16714G | 0.998 ** | |
| 16725Y | 0.997 ** | |
| rps18 | 17492N | 0.999 ** |
| rps19 | 17529A | 0.990 ** |
| rps2 | 17766Y | 1.000 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Kuang, R.-P.; Wang, X.-H.; Liang, X.-L.; Wanga, V.O.; Liu, K.-M.; Cai, X.-Z.; Hu, G.-W. Complete Chloroplast Genome Sequence of Fortunella venosa (Champ. ex Benth.) C.C.Huang (Rutaceae): Comparative Analysis, Phylogenetic Relationships, and Robust Support for Its Status as an Independent Species. Forests 2021, 12, 996. https://doi.org/10.3390/f12080996
Wang T, Kuang R-P, Wang X-H, Liang X-L, Wanga VO, Liu K-M, Cai X-Z, Hu G-W. Complete Chloroplast Genome Sequence of Fortunella venosa (Champ. ex Benth.) C.C.Huang (Rutaceae): Comparative Analysis, Phylogenetic Relationships, and Robust Support for Its Status as an Independent Species. Forests. 2021; 12(8):996. https://doi.org/10.3390/f12080996
Chicago/Turabian StyleWang, Ting, Ren-Ping Kuang, Xiao-Hui Wang, Xiao-Li Liang, Vincent Okelo Wanga, Ke-Ming Liu, Xiu-Zhen Cai, and Guang-Wan Hu. 2021. "Complete Chloroplast Genome Sequence of Fortunella venosa (Champ. ex Benth.) C.C.Huang (Rutaceae): Comparative Analysis, Phylogenetic Relationships, and Robust Support for Its Status as an Independent Species" Forests 12, no. 8: 996. https://doi.org/10.3390/f12080996
APA StyleWang, T., Kuang, R.-P., Wang, X.-H., Liang, X.-L., Wanga, V. O., Liu, K.-M., Cai, X.-Z., & Hu, G.-W. (2021). Complete Chloroplast Genome Sequence of Fortunella venosa (Champ. ex Benth.) C.C.Huang (Rutaceae): Comparative Analysis, Phylogenetic Relationships, and Robust Support for Its Status as an Independent Species. Forests, 12(8), 996. https://doi.org/10.3390/f12080996







