Assessing the Linkages between Tree Species Composition and Stream Water Nitrate in a Reference Watershed in Central Appalachia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Species Composition and Stand N Uptake
2.3. 15N Labeling
2.4. Data Analysis
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | A |
|---|---|
| Acer rubrum | 0.149 |
| Acer saccharum | −0.097 |
| Fagus grandifolia | 0.301 |
| Liriodendron tulipifera | 0.338 |
| Magnolia acuminata | 0.276 |
| Nyssa sylvatica | −0.220 |
| Oxydendrum arboreum | 0.187 |
| Prunus serotina | 0.222 |
| Quercus alba | −0.041 |
| Quercus coccinea | 0.304 |
| Quercus prinus | 0.440 |
| Quercus rubra | 0.327 |
| Tilia americana | 0.000 |
| Other species | 0.168 |
References
- Driscoll, C.T.; Lawrence, G.B.; Bulger, A.J.; Butler, T.J.; Cronan, C.S.; Eagar, C.; Lambert, K.F.; Likens, G.E.; Stoddard, J.L.; Weathers, K.C. Acidic deposition in the northeastern United States: Sources and inputs, ecosystems effects, and management strategies. Bioscience 2001, 51, 180–198. [Google Scholar] [CrossRef]
- Galloway, J.; Dentener, F.; Capone, D.; Boyer, E.; Howarth, R.; Seitzinger, S.; Asner, G.; Cleveland, C.; Green, P.; Holland, E.; et al. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Aber, J.; McDowell, W.; Nadelhoffer, K.; Magill, A.; Bernston, G.; Kamakea, M.; McNulty, S.; Currie, W.; Rustad, L.; Fernandez, I. Nitrogen saturation in temperate forest ecosystems. Bioscience 1998, 48, 921–934. [Google Scholar] [CrossRef]
- Edwards, P.J.; Williard, K.W.J.; Wood, F.; Sharpe, W.E. Soil water and stream water chemical responses. In The Fernow Watershed Acidification Study; Adams, M., DeWalle, D., Hom, J., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 71–136. [Google Scholar]
- Adams, M.B.; Angradi, T.R.; Kochenderfer, J.N. Stream water and soil solution responses to 5 years of nitrogen and sulfur additions at the Fernow Experimental Forest, West Virginia. For. Ecol. Manag. 1997, 95, 79–91. [Google Scholar] [CrossRef]
- Boggs, J.L.; Mcnulty, S.G.; Gavazzi, M.J.; Myers, J.M. Tree growth, foliar chemistry, and nitrogen cycling across a nitrogen deposition gradient in southern Appalachian deciduous forests. Can. J. For. Res. 2005, 35, 1901–1913. [Google Scholar] [CrossRef]
- Likens, G.E.; Buso, D.C. Dilution and the elusive baseline. Environ. Sci. Technol. 2012, 46, 4382–4387. [Google Scholar] [CrossRef] [PubMed]
- Argerich, A.; Johnson, S.L.; Sebestyen, S.D.; Rhoades, C.C.; Greathouse, E.; Knoepp, J.D.; Adams, M.B.; Likens, G.E.; Campbell, J.L.; McDowell, W.H.; et al. Trends in stream nitrogen concentrations for forested reference catchments across the USA. Environ. Res. Lett. 2013, 8, 1–8. [Google Scholar] [CrossRef]
- Skjelkvåle, B.L.; Stoddard, J.L.; Jeffries, D.S.; Tørseth, K.; Høgåsen, T.; Bowman, J.; Mannio, J.; Monteith, D.T.; Mosello, R.; Rogora, M.; et al. Regional scale evidence for improvements in surface water chemistry 1990-2001. Environ. Pollut. 2005, 137, 165–176. [Google Scholar] [CrossRef]
- Fenn, M.E.; Poth, M.A.; Aber, J.D.; Baron, J.S.; Bormann, B.T.; Johnson, D.W.; Lemly, A.D.; Mcnulty, S.G.; Ryan, D.F.; Stottlemyer, R. Nitrogen excess in North American ecosystems: Predisposing factors, ecosystem responses, and management strategies. Ecol. Appl. 1998, 8, 706–733. [Google Scholar] [CrossRef]
- Nadelhoffer, K.; Downs, M.; Fry, B.; Magill, A.; Aber, J. Controls on N retention and exports in a forested watershed. Environ. Monit. Assess. 1999, 55, 187–210. [Google Scholar] [CrossRef]
- Goodale, C.L.; Fredriksen, G.; Weiss, M.S.; McCalley, C.K.; Sparks, J.P.; Thomas, S.A. Soil processes drive seasonal variation in retention of 15N tracers in a deciduous forest catchment. Ecology 2015, 96, 2653–2668. [Google Scholar] [CrossRef] [PubMed]
- Christ, M.; Peterjohn, W.; Cumming, J.; Adams, M. Nitrification potentials and landscape, soil and vegetation characteristics in two Central Appalachian watersheds differing in NO3− export. For. Ecol. Manag. 2002, 159, 145–158. [Google Scholar] [CrossRef]
- Lovett, G.M.; Weathers, K.C.; Arthur, M.A.; Schultz, J.C. Nitrogen cycling in a northern hardwood forest: Do species matter? Biogeochemistry 2004, 67, 289–308. [Google Scholar] [CrossRef]
- Peterjohn, W.T.; Harlacher, M.A.; Christ, M.J.; Adams, M.B. Testing associations between tree species and nitrate availability: Do consistent patterns exist across spatial scales? For. Ecol. Manag. 2015, 358, 335–343. [Google Scholar] [CrossRef]
- Lovett, G.M.; Weathers, K.C.; Arthur, M.A. Control of nitrogen loss from forested watersheds by soil carbon: Nitrogen ratio and tree species composition. Ecosystems 2002, 5, 712–718. [Google Scholar] [CrossRef]
- Campbell, J.L.; Hornbeck, J.W.; Mitchell, M.J.; Adams, M.B.; Castro, M.S.; Driscoll, C.T.; Kahl, J.S.; Kochenderfer, J.N.; Likens, G.E.; Lynch, J.A.; et al. Input-output budgets of inorganic nitrogen for 24 forest watersheds in the northeastern United States: A review. Water Air Soil Pollut. 2004, 151, 373–396. [Google Scholar] [CrossRef]
- Peterjohn, W.T.; Adams, M.B.; Gilliam, F.S. Symptoms of nitrogen saturation in two central Appalachian hardwood forest ecosystems. Biogeochemistry 1996, 35, 507–522. [Google Scholar] [CrossRef]
- Schuler, T.; Gillespie, A. Temporal patterns of woody species diversity in a central Appalachian forest from 1856 to 1997. J. Torrey Bot. Soc. 2000, 127, 149–161. [Google Scholar] [CrossRef]
- McFarlane, K.J.; Yanai, R.D. Measuring nitrogen and phosphorus uptake by intact roots of mature Acer saccharum Marsh., Pinus resinosa Ait., and Picea abies (L.) Karst. Plant Soil 2006, 279, 163–172. [Google Scholar] [CrossRef][Green Version]
- Templer, P.H.; Dawson, T.E. Nitrogen uptake by four tree species of the Catskill Mountains, New York: Implications for forest N dynamics. Plant Soil 2004, 262, 251–261. [Google Scholar] [CrossRef]
- Schulz, H.; Härtling, S.; Stange, C.F. Species-specific differences in nitrogen uptake and utilization by six European tree species. J. Plant Nutr. Soil Sci. 2011, 174, 28–37. [Google Scholar] [CrossRef]
- Jacob, A.; Leuschner, C. Complementarity in the use of nitrogen forms in a temperate broad-leaved mixed forest. Plant Ecol. Divers. 2014, 1–16. [Google Scholar] [CrossRef]
- Rennenberg, H.; Wildhagen, H.; Ehlting, B. Nitrogen nutrition of poplar trees. Plant Biol. 2010, 12, 275–291. [Google Scholar] [CrossRef] [PubMed]
- DesRochers, A.; van den Driessche, R.; Thomas, B.R. The interaction between nitrogen source, soil pH, and drought in the growth and physiology of three poplar clones. Can. J. Bot. 2007, 85, 1046–1057. [Google Scholar] [CrossRef]
- Buchmann, N.; Schultz, E.; Gebauer, G. 15N-ammonium and 15N-nitrate uptake of a 15-Year-Old Picea abies plantation. Oecologia 1995, 102, 361–370. [Google Scholar] [CrossRef]
- Gessler, A.; Schneider, S.; Von Sengbusch, D.; Weber, P.; Haneman, U.; Huber, C.; Rothe, A.; Kreutzer, K.; Rennenberg, H. Field and laboratory experiments on net uptake of nitrate and ammonium by the roots of spruce (Picea abies) and beech (Fagus sylvatica) trees. New Phytol. 1998, 138, 275–285. [Google Scholar] [CrossRef]
- Gessler, A.; Jung, K.; Gasche, R.; Papen, H.; Heidenfelder, A.; Börner, E.; Metzler, B.; Augustin, S.; Hildebrand, E.; Rennenberg, H. Climate and forest management influence nitrogen balance of European beech forests: Microbial N transformations and inorganic N net uptake capacity of mycorrhizal roots. Eur. J. For. Res. 2005, 124, 95–111. [Google Scholar] [CrossRef]
- Malagoli, M.; Canal, A.D.; Quaggiotti, S.; Pegoraro, P.; Bottacin, A. Differences in nitrate and ammonium uptake between Scots pine and European larch. Plant Soil 2000, 221, 1–3. [Google Scholar] [CrossRef]
- Gallet-Budynek, A.; Brzostek, E.; Rodgers, V.L.; Talbot, J.M.; Hyzy, S.; Finzi, A.C. Intact amino acid uptake by northern hardwood and conifer trees. Oecologia 2009, 160, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Lovett, G.; Mitchell, M. Sugar maple and nitrogen cycling in the forests of Eastern North America. Front. Ecol. Environ. 2004, 2, 81–88. [Google Scholar] [CrossRef]
- Rothstein, D.E.; Zak, D.R.; Pregitzer, K.S.; Url, S.; Zak, R. Nitrate deposition in northern hardwood forests and the nitrogen metabolism of Acer saccharum marsh. Oecologia 1996, 108, 338–344. [Google Scholar] [CrossRef] [PubMed]
- BassiriRad, H.; Prior, S.; Norby, R.; Rogers, H. A field method of determining NH4+ and NO3- uptake kinetics in intact roots: Effects of CO2 enrichment on trees and crop species. Plant Soil 1999, 217, 195–204. [Google Scholar] [CrossRef]
- Eddy, W.C.; Zak, D.R.; Holmes, W.E.; Pregitzer, K.S. Chronic Atmospheric NO3− Deposition Does Not Induce NO3− Use by Acer saccharum Marsh. Ecosystems 2008, 11, 469–477. [Google Scholar] [CrossRef]
- Crabtree, R.; Bazzaz, F. Seedling response of four birch species to simulated nitrogen deposition: Ammonium vs. nitrate. Ecol. Appl. 1993, 3, 315–321. [Google Scholar] [CrossRef]
- Kochenderfer, J.N. Fernow and the Appalachian Hardwood Region. In The Fernow Watershed Acidification Study; Adams, M., DeWalle, D., Hom, J., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 17–39. [Google Scholar]
- Reinhart, K.; Eschner, A.; Trimble, G. US Forest Service Research Paper NE-I. Effect on Streamflow of Four Forest Practices in the Mountains of West; Upper Darby: Virginia, PA, USA, 1963. [Google Scholar]
- Edwards, P.J.; Wood, F. USDA Forest Service Northern Forest Experimental Station General Technical Report NE-177. Field and Laboratory Quality Assurance/Quality Control Protocols and Accomplishments for the Fernow Experimental Forest Watershed Acidification Study; Forest Service: Radnor, PA, USA, 1993. [Google Scholar]
- Schuler, T.M.; Wood, F. Fernow Experimental Forest watershed 4 overstory tree data, 1959–2001; U.S. Department of Agriculture Forest Service, Northern Research Station: Parsons, WV, USA, 2015.
- Bremner, J.M.; Mulvaney, C.S. N—Total. In Methods of Soil Analysis. Part 2; Page, A.L., Ed.; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Brenneman, B.; Frederick, D.; Gardner, W.; Schoenhofen, L.; Marsh, P. Biomass of species and stands of West Virginia hardwoods. In Proceedings of the 2nd Central Hardwood Forest Conference, West Lafayette, IN, USA, 14–16 November 1978; pp. 159–178. [Google Scholar]
- McKane, R.B.; Johnson, L.C.; Shaver, G.R.; Nadelhoffer, K.J.; Rastetter, E.B.; Fry, B.; Giblin, A.E.; Kielland, K.; Kwiatkowski, B.L.; Laundre, J.A.; et al. Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 2002, 415, 68–71. [Google Scholar] [CrossRef]
- Thornton, B.; Osborne, S.M.; Paterson, E.; Cash, P. A proteomic and targeted metabolomic approach to investigate change in Lolium perenne roots when challenged with glycine. J. Exp. Bot. 2007, 58, 1581–1590. [Google Scholar] [CrossRef]
- Burnham, M.B.; Cumming, J.R.; Adams, M.B.; Peterjohn, W.T. Soluble soil aluminum alters the relative uptake of mineral nitrogen forms by six mature temperate broadleaf tree species: Possible implications for watershed nitrate retention. Oecologia 2017. [Google Scholar] [CrossRef]
- Fry, B. Stable Isotope Ecology, 1st ed.; Springer: New York, NY, USA, 2006; ISBN 0-387-33745-8. [Google Scholar]
- Kronzucker, H.J.; Siddiqi, M.Y.; Glass, A.D.M. Conifer root discrimination against soil nitrate and the ecology of forest succession. Nature 1997, 385, 59–61. [Google Scholar] [CrossRef]
- Chapman, N.; Miller, A.J.; Lindsey, K.; Whalley, W.R. Roots, water, and nutrient acquisition: Let’s get physical. Trends Plant Sci. 2012, 17, 701–710. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Adams, M.B. Effects of nitrogen on temporal and spatial patterns of nitrate in streams and soil solution of a central hardwood forest. ISRN Ecol. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Pastor, J.; Aber, J.D.; Mcclaugherty, C.A.; Melillo, J.M. Aboveground production and N and P cycling along a nitrogen mineralization gradient on Blackhawk Island, Wisconsin. Ecology 1984, 65, 256–268. [Google Scholar] [CrossRef]
- Zak, D.R.; Pregitzer, K.S. Spatial and temporal variability of nitrogen cycling in northern Lower Michigan. For. Sci. 1990, 36, 367–380. [Google Scholar]
- Ross, D.S.; Shanley, J.B.; Campbell, J.L.; Lawrence, G.B.; Bailey, S.W.; Likens, G.E.; Wemple, B.C.; Fredriksen, G.; Jamison, A.E. Spatial patterns of soil nitrification and nitrate export from forested headwaters in the northeastern United States. J. Geophys. Res. Biogeosci. 2012, 117, 1–14. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Zak, D.R.; Talhelm, A.F.; Burton, A.J.; Eikenberry, J.R. Nitrogen turnover in the leaf litter and fine roots of sugar maple. Ecology 2010, 91, 3456–3462. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, F.S.; Yurish, B.M.; Adams, M.B. Temporal and spatial variation of nitrogen transformations in nitrogen-saturated soils of a central Appalachian hardwood forest. Can. J. For. Res. 2001, 31, 1768–1785. [Google Scholar] [CrossRef]
- Mitchell, M.J. Nitrate dynamics of forested watersheds: Spatial and temporal patterns in North America, Europe and Japan. J. For. Res. 2011, 16, 333–340. [Google Scholar] [CrossRef]
- Peterjohn, W.T.; Foster, C.J.; Christ, M.J.; Adams, M.B. Patterns of nitrogen availability within a forested watershed exhibiting symptoms of nitrogen saturation. For. Ecol. Manag. 1999, 119, 247–257. [Google Scholar] [CrossRef]
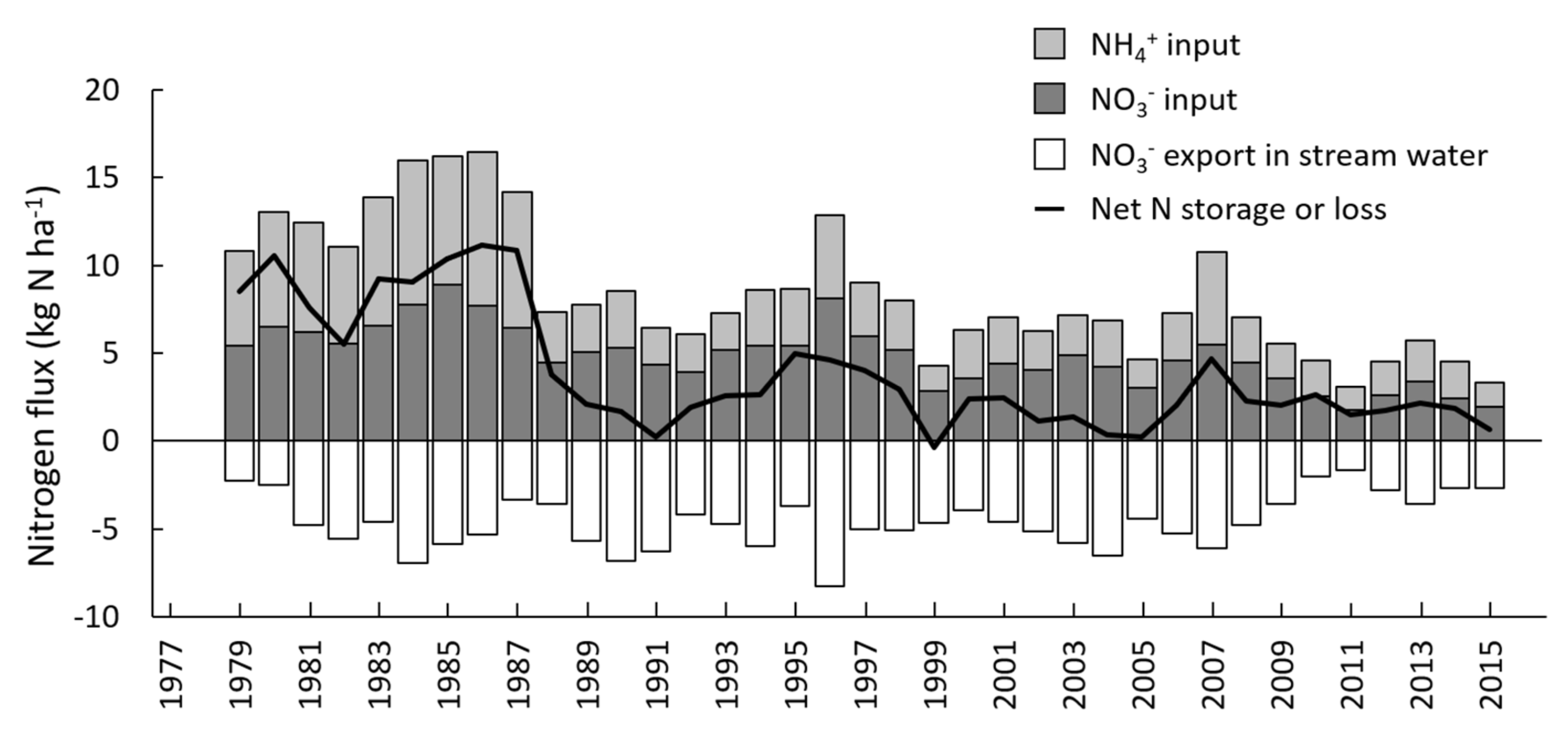
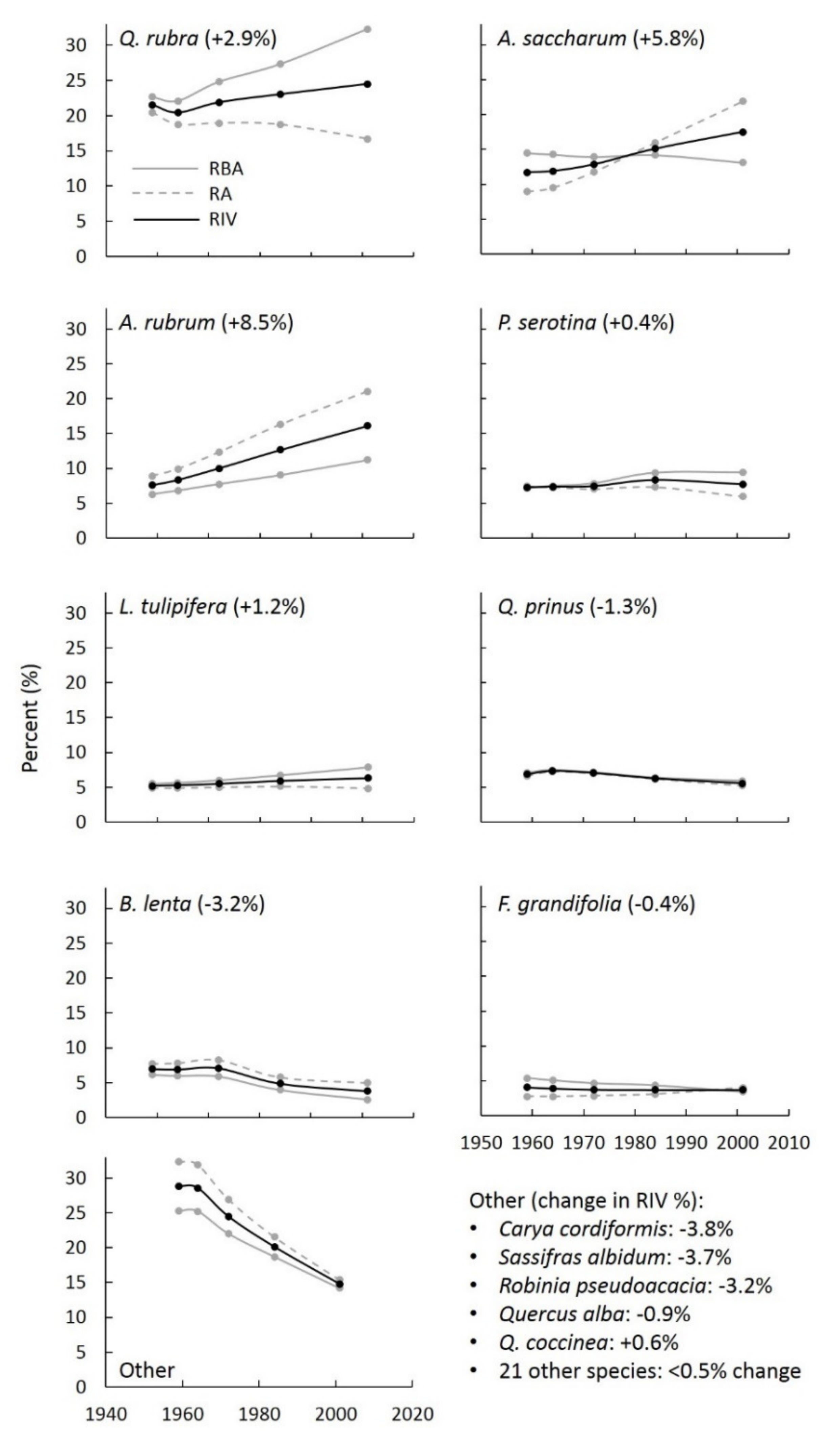
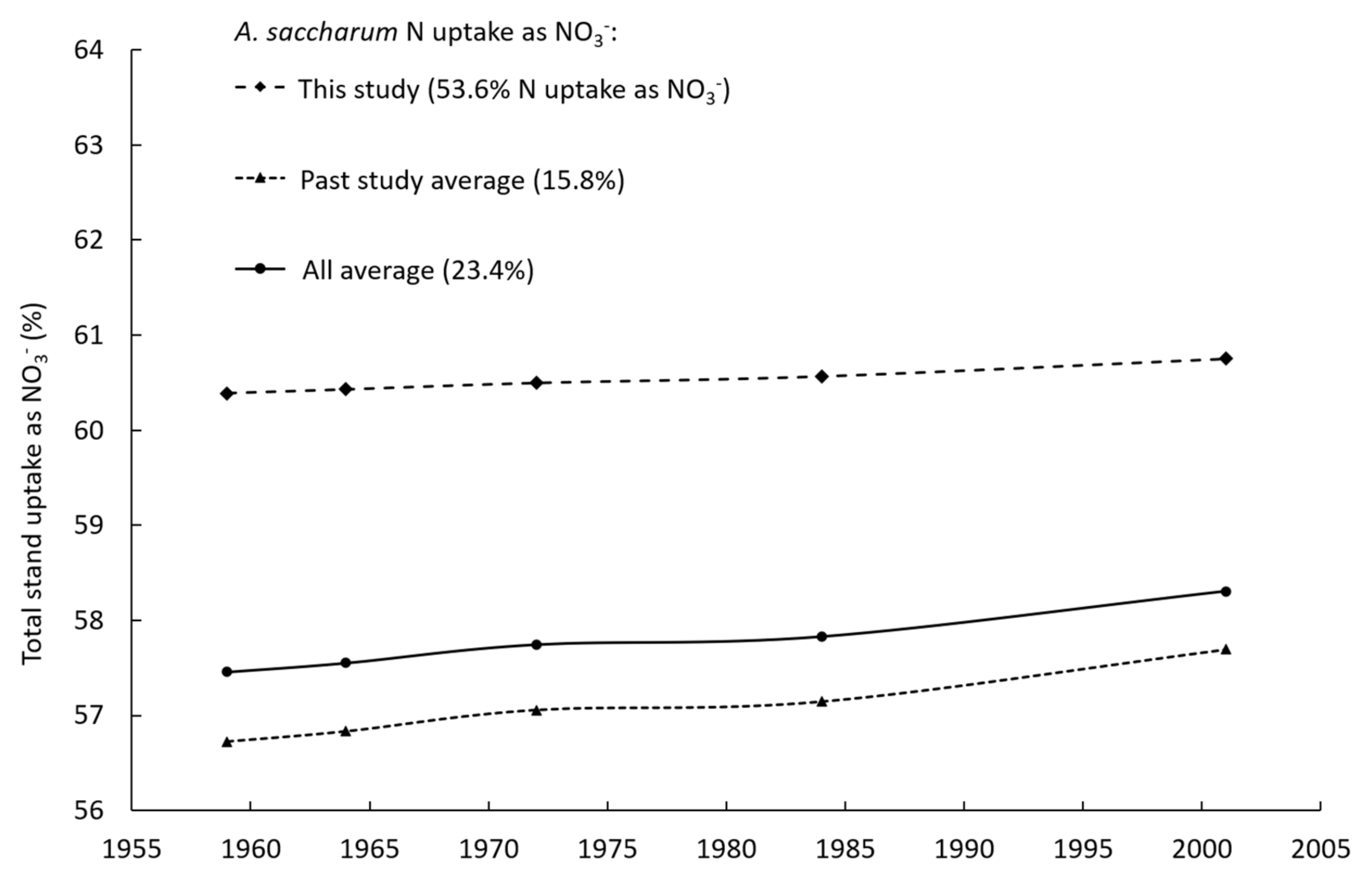
| Species | Percent of N Uptake as NO3− (±SE) |
|---|---|
| A. rubrum | 75.3 (±12.5) |
| A. saccharum | 53.6 (±16.0) |
| B. lenta | 54.7 (±11.5) |
| L. tulipifera | 52.7 (±13.0) |
| P. serotina | 61.6 (±11.3) |
| Q. rubra | 56.4 (±11.5) |
| Study | Method | A. saccharum N Uptake as NO3− (%) | Estimated Uptake Rate (μmol N g−1 h−1) |
|---|---|---|---|
| BassiriRad et al. (1999) | In situ N depletion, excavated intact roots, Vmax | 31 | 9 |
| Eddy et al. (2008) | Excised root 15N uptake, Vmax | 11.2 | 0.63 |
| Rothstein et al. (1996) | Excised root 15N uptake, Vmax | 3 | 1.0 |
| Templer and Dawson (2004) | 15N addition to seedlings, greenhouse, roots in native soil | 18 | 1.0 1 |
| This study | In situ 15N addition to mature trees, roots left in native soil | 53.6 | 11.6 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burnham, M.B.; Christ, M.J.; Adams, M.B.; Peterjohn, W.T. Assessing the Linkages between Tree Species Composition and Stream Water Nitrate in a Reference Watershed in Central Appalachia. Forests 2021, 12, 1116. https://doi.org/10.3390/f12081116
Burnham MB, Christ MJ, Adams MB, Peterjohn WT. Assessing the Linkages between Tree Species Composition and Stream Water Nitrate in a Reference Watershed in Central Appalachia. Forests. 2021; 12(8):1116. https://doi.org/10.3390/f12081116
Chicago/Turabian StyleBurnham, Mark B., Martin J. Christ, Mary Beth Adams, and William T. Peterjohn. 2021. "Assessing the Linkages between Tree Species Composition and Stream Water Nitrate in a Reference Watershed in Central Appalachia" Forests 12, no. 8: 1116. https://doi.org/10.3390/f12081116
APA StyleBurnham, M. B., Christ, M. J., Adams, M. B., & Peterjohn, W. T. (2021). Assessing the Linkages between Tree Species Composition and Stream Water Nitrate in a Reference Watershed in Central Appalachia. Forests, 12(8), 1116. https://doi.org/10.3390/f12081116






