High Diversity of Mites (Acari: Oribatida, Mesostigmata) Supports the High Conservation Value of a Broadleaf Forest in Eastern Norway
Abstract
1. Introduction
2. Material and Methods
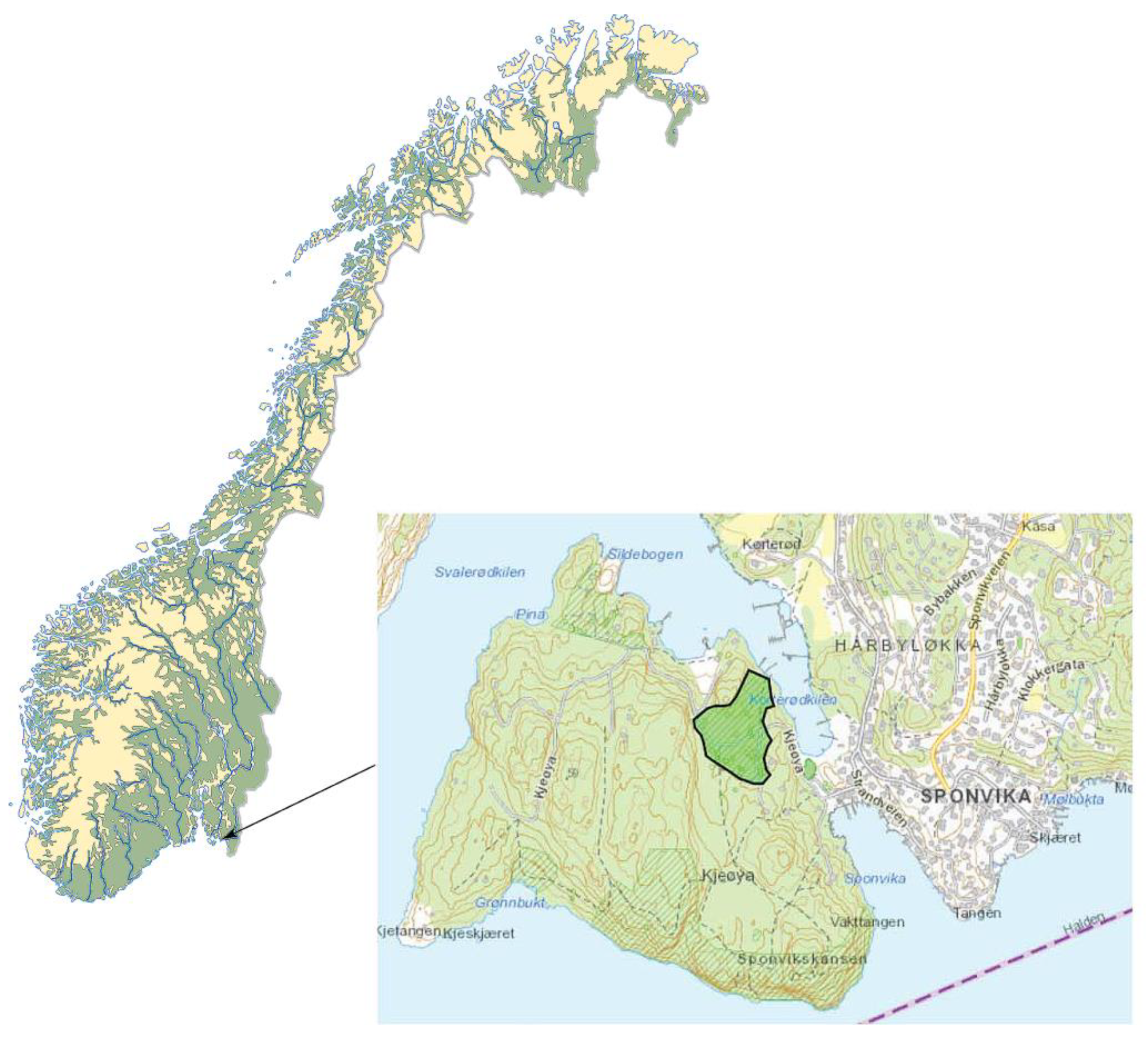
2.1. Study Site
2.2. Sampling and Identification
2.3. Statistical Analyses
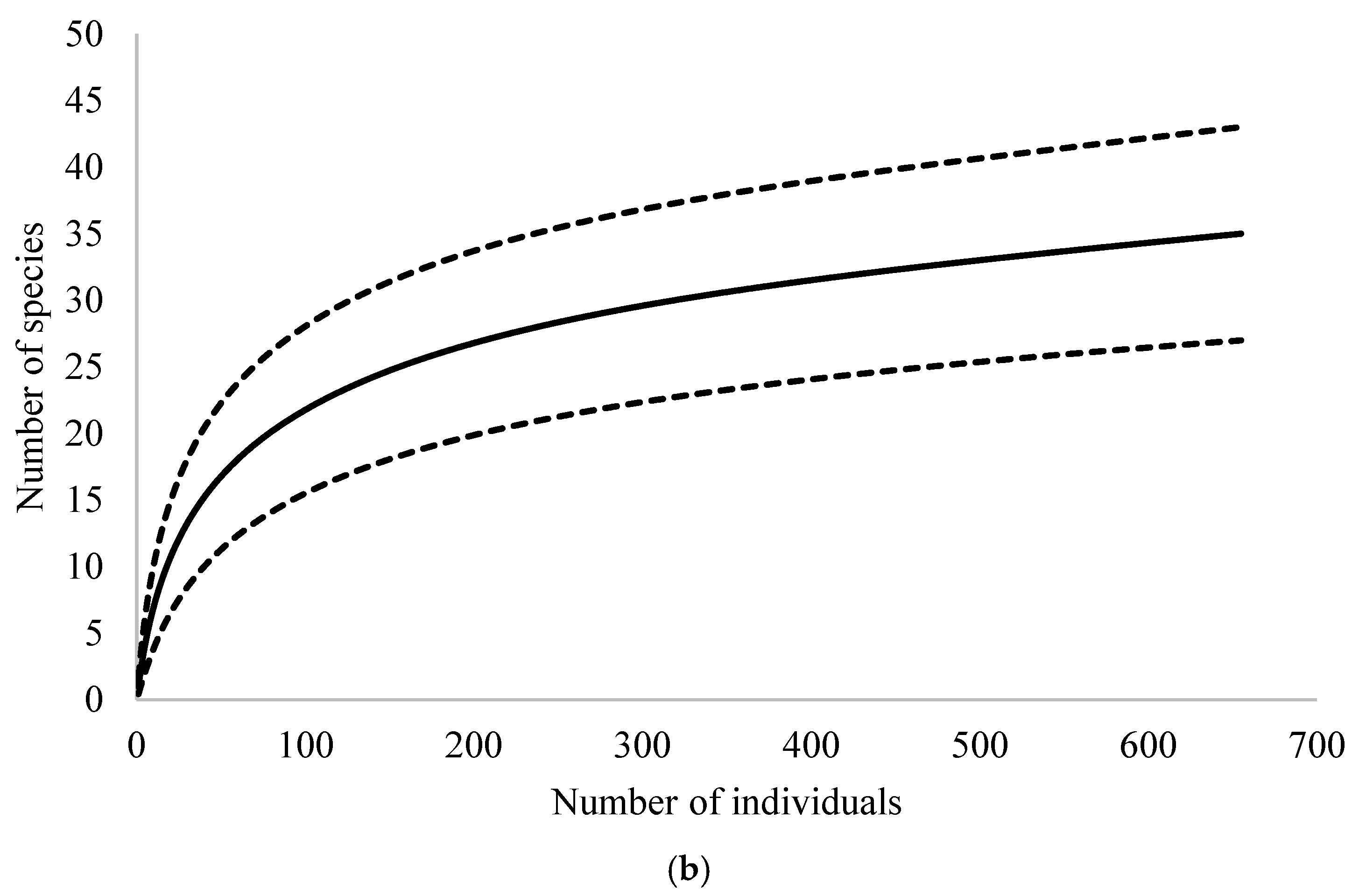
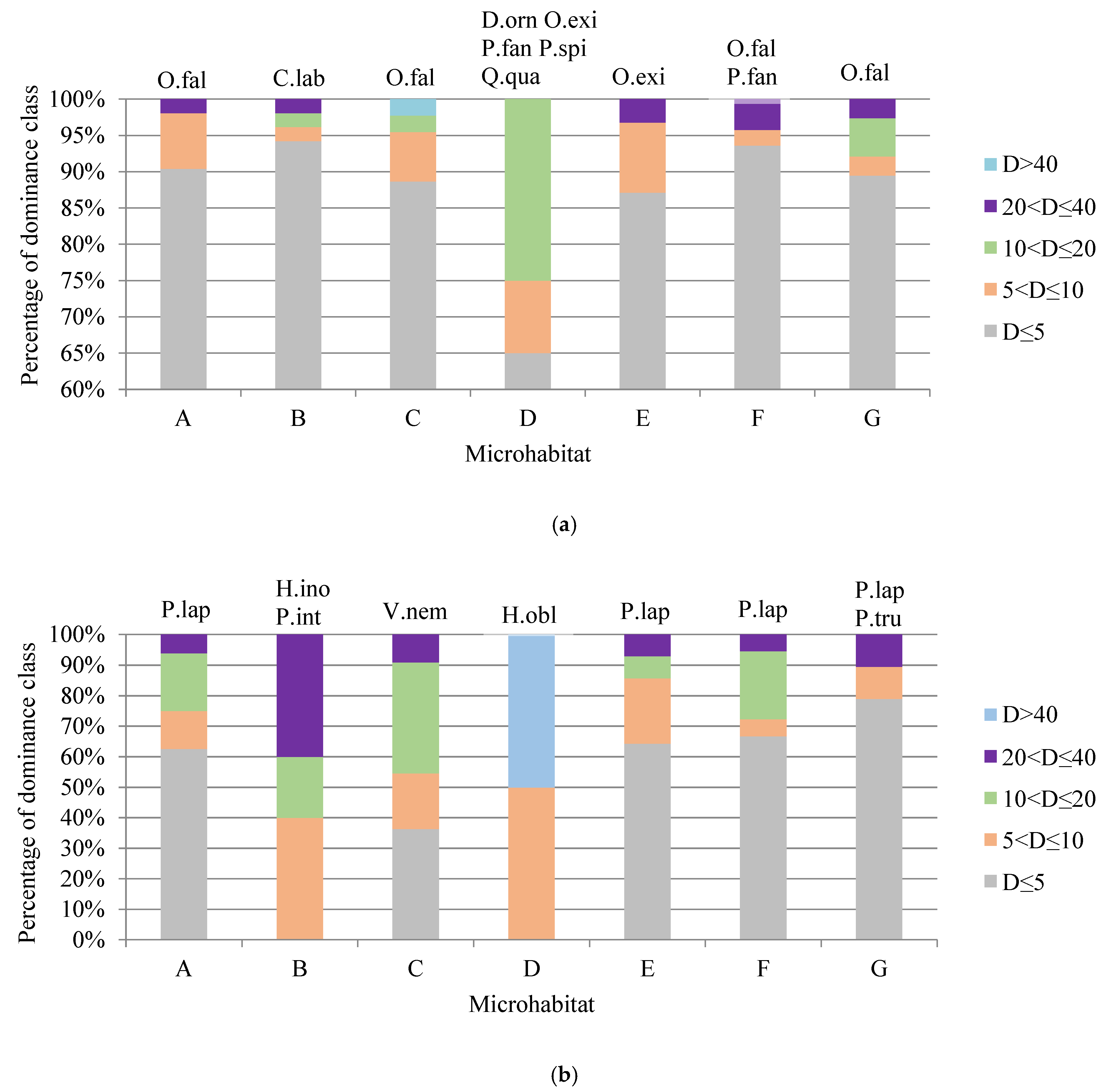
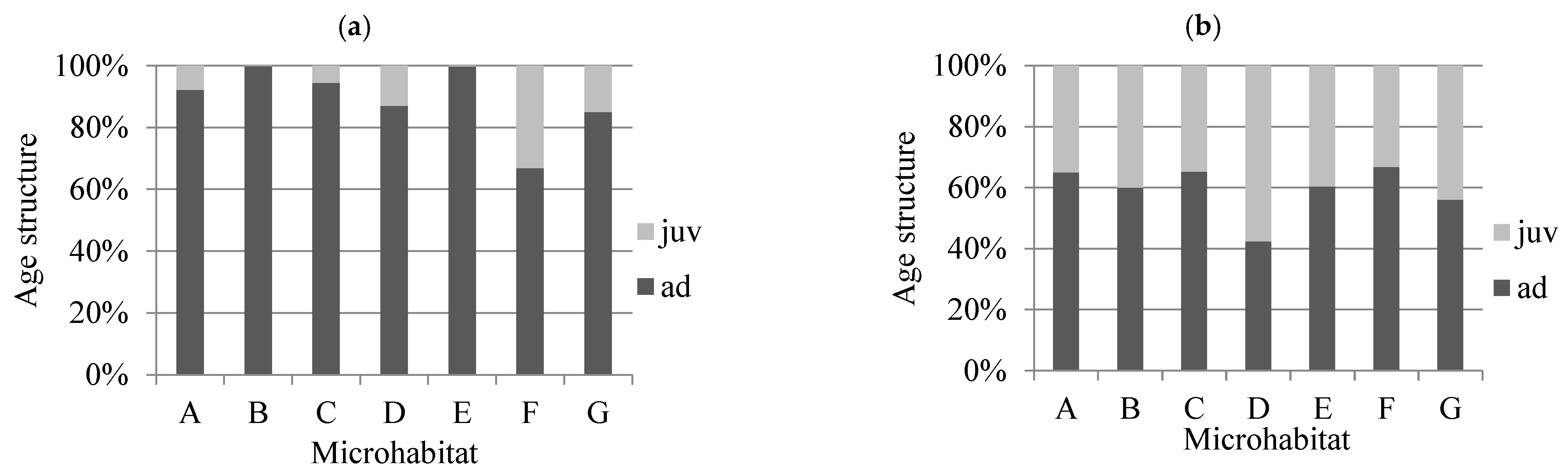
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aerts, R.; Honnay, O. Forest restoration, biodiversity and ecosystem functioning. BMC Ecol. 2011, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Paquette, A.; Messier, C. The effect of biodiversity on tree productivity: From temperate to boreal forests. Glob. Ecol. Biogeogr. 2011, 20, 170–180. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Barbaro, L.; Castagneyrol, B.; Forrester, D.I.; Gardiner, B.; Gonzalez, J.R.; Lyver, P.O.B.; Meurisse, N.; Oxbrough, A.; Taki, H.; et al. Forest biodiversity, ecosystem functioning and the provision of ecosystem services. Biodivers. Conserv. 2017, 26, 3005–3035. [Google Scholar] [CrossRef]
- Norwegian Ministry of the Environment 2011: Norway’s Environmental Targets. Available online: https://www.regjeringen.no (accessed on 8 June 2021).
- Gundersen, V.; Rolstad, J. Truete arter i skog. En gjennomgang av rødlistearter i forhold til norsk skogbruk. Nor. For. Res. Inst. Oppdragsrapp. 1998, 6, 1–90. [Google Scholar]
- Henriksen, S.; Hilmo, O. (Eds.) Norsk Rødliste for Arter 2015; Artsdatabanken: Trondheim, Norway, 2015; pp. 1–193. [Google Scholar]
- Håpnes, A. Background Note: Natural Forest Heritage in Norway. WWF Norway. 2003. Available online: http://wwf.panda.org/wwf_news/?6748/Background-paper-Natural-forest-heritage-in-Norway (accessed on 8 June 2021).
- De Deyn, G.B.; Van der Putten, W.H. Linking aboveground and belowground diversity. Trends Ecol. Evol. 2005, 20, 625–633. [Google Scholar] [CrossRef]
- Orgiazzi, A.; Singh, B.; Wall, D.; Barrios, E.; Kandeler, E.; Moreira, F.; De Deyn, G.; Chotte, J.; Six, J.; Hedlund, K.; et al. Global Soil Biodiversity Atlas; Publications Office of the European Union: Luxembourg, 2015; pp. 1–176. [Google Scholar]
- Walter, D.E.; Behan-Pelletier, V. Mites in forest canopies: Filling the size distribution shortfall? Annu. Rev. Entomol. 1999, 44, 1–19. [Google Scholar] [CrossRef]
- Skubała, P. Microhabitats and oribatid fauna: Comparison of 2 sampling approaches. Biol. Lett. 2016, 53, 31–47. [Google Scholar] [CrossRef]
- Manu, M.; Băncilă, R.I.; Onete, M. Importance of moss habitats for mesostigmatid mites (Acari: Mesostigmata) in Romania. Turk. J. Zool. 2018, 42, 673–683. [Google Scholar] [CrossRef]
- Seniczak, S.; Graczyk, R.; Seniczak, A.; Faleńczyk-Koziróg, K.; Kaczmarek, S.; Marquardt, T. Microhabitat preferences of Oribatida and Mesostigmata (Acari) inhabiting lowland beech forest in Poland and the trophic interactions between these mites. Eur. J. Soil Biol. 2018, 87, 25–32. [Google Scholar] [CrossRef]
- Seniczak, A.; Bolger, T.; Roth, S.; Seniczak, S.; Djursvoll, P.; Jordal, B.H. Diverse mite communities (Acari: Oribatida, Mesostigmata) from a broadleaf forest in western Norway. Ann. Zool. Fenn. 2019, 56, 121–136. [Google Scholar] [CrossRef]
- Pollierer, M.M.; Langel, R.; Körner, C.; Maraun, M.; Scheu, S. The underestimated importance of belowground carbon input for forest soil food webs. Ecol. Lett. 2007, 10, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, G.; Otte, V.; Langel, R.; Scheu, S.; Maraun, M. The trophic structure of bark-living oribatid mite communities analysed with stable isotopes (15N; 13C) indicates strong niche differentiation. Exp. Appl. Acarol. 2007, 41, 1–10. [Google Scholar] [CrossRef]
- Heidemann, K.; Scheu, S.; Ruess, L.; Maraun, M. Molecular detection of nematode predation and scavenging in oribatid mites: Laboratory and field experiments. Soil. Biol. Biochem. 2011, 43, 2229–2236. [Google Scholar] [CrossRef]
- Norton, R.A.; Behan-Pelletier, V.M. Suborder Oribatida. In A Manual of Acarology; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 430–564. [Google Scholar]
- Walter, D.E.; Proctor, H.C. Mites: Ecology, Evolution and Behaviour; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Bolger, T.; Arroyo, J.; Kenny, J.; Caplice, M. Hierarchical analysis of mite community structures in Irish forests—A study of the relative contribution of location, forest type and microhabitat. Appl. Soil. Ecol. 2014, 83, 39–43. [Google Scholar] [CrossRef]
- Klarner, B.; Maraun, M.; Scheu, S. Trophic diversity and niche partitioning in a species rich predator guild—Natural variations in stable isotope ratios (C-13/C-12, N-15/N-14) of mesostigmatid mites (Acari, Mesostigmata) from Central European beech forests. Soil. Biol. Biochem. 2013, 57, 327–333. [Google Scholar] [CrossRef]
- Moser, J.C.; Bogenschütz, H. A key to the mites associated with flying Ips typographus in South Germany. Zeit. Angew. Entomol. 1984, 97, 437–450. [Google Scholar] [CrossRef]
- Moser, J.C.; Eidmann, H.H.; Regnander, J.R. The mites associated with Ips typographus in Sweden. Ann. Entomol. Fenn. 1989, 55, 23–27. [Google Scholar]
- Kaczmarek, S.; Michalski, J. Roztocze (Acari, Gamasida) w żerowiskach kornika drukarza (Ips typographus L.) w Polsce. PTPN Wydział Kom. Nauk. Rol. Leśnych 1994, 78, 75–82. [Google Scholar]
- Kaczmarek, S.; Michalski, J. Roztocze Acari, Gamasida występujące w żerowiskach korników Coleoptera, Scolytidae z rodzaju Ips na terenie wybranych parków narodowych. Parki Nar. Rezerw. Przyr. 1995, 13, 35–42. [Google Scholar]
- Bluhm, C.; Scheu, S.; Maraun, M. Oribatid mite communities on the bark of dead wood vary with log type, surrounding forest and regional factors. Appl. Soil Ecol. 2015, 89, 102–112. [Google Scholar] [CrossRef]
- Hofstetter, R.W.; Dinkins-Bookwalter, J.; Davis, T.S.; Klepzig, K.D. Symbiotic associations of bark beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Elsevier: Amsterdam, The Netherland, 2015; pp. 209–245. [Google Scholar]
- Chaires-Grijalva, M.P.; Estrada-Venegas, E.G.; Quiroz-Ibáñez, I.F.; Equihua-Martínez, A.; Moser, J.C.; Blomquist, S.R. Acarine biodiversity associated with bark beetles in Mexico. Acarol. Stud. 2019, 1, 152–160. [Google Scholar]
- Seniczak, S.; Seniczak, A.; Gwiazdowicz, D.J.; Coulson, S.J. Community structure of oribatid and gamasid mites (Acari) in moss-grass tundra in Svalbard (Spitsbergen, Norway). Arctic Antarct. Alpine Res. 2014, 46, 591–599. [Google Scholar] [CrossRef]
- Huhta, V.; Siira-Pietikäinen, A.; Penttinen, R. Importance of dead wood for soil mite (Acarina) communities in boreal old-growth forests. Soil Org. 2012, 84, 499–512. [Google Scholar]
- Nordén, B.; Jordal, J.B.; Bratli, H. Bacidia incompta, Pyrenula nitidella and Schismatomma de-colorans, three lichen species on old deciduous trees new to Norway. Grap. Scr. 2013, 25, 44–47. [Google Scholar]
- Hauge, E.; Meidell, B.; Solhøy, T. Edelløvskog på Vestlandet. Evertebrater. I–III Miljøverndepartement Rapport; Zoologisk Museum, Universitetet i Bergen: Bergen, Norway, 1975; pp. 1–277. [Google Scholar]
- Skarpaas, O.; Diserud, O.; Sverdrup-Thygeson, A.; Ødegaard, F. Predicting hotspots for red-listed species: Multivariate regression models for oak-associated beetles. Insect Conserv. Divers. 2011, 4, 53–59. [Google Scholar] [CrossRef]
- Sverdrup-Thygeson, A.; Bratli, H.; Brandrud, T.E.; Endrestøl, A.; Evju, M.; Hanssen, O.; Skarpaas, O.; Stabbetorp, O.E.; Ødegaard, F. Hule Eiker-et Hotspot-Habitat; Sluttrapport under ARKO-Prosjektets Periode II. NINA Rapp. 2011, 710, 1–47. [Google Scholar]
- Bolger, T.; Devlin, M.; Seniczak, A. First records of ten species of Mesostigmata (Acari, Mesostigmata) added to the published Norwegian species list. Nor. J. Entomol. 2018, 65, 94–100. [Google Scholar]
- Seniczak, A.; Niedbała, W.; Iturrondobeitia, J.C.; Seniczak, S.; Roth, S.; Jordal, B.H. Type of broadleaf forest matters most for ptyctimous mite communities (Acari, Oribatida) in Norway. Biodivers. Conserv. 2021, 1–25. [Google Scholar] [CrossRef]
- Meteorological Institute. Available online: https://www.met.no (accessed on 8 June 2021).
- Moen, A.; Lillethun, A. National Atlas of Norway: Vegetation; Norwegian Mapping Authorities: Hønefoss, Norway, 1999. [Google Scholar]
- Wergeland Krog, O.M.; Laugsand, A. Naturtypekartlegging i Halden Kommune 2009–2010. Available online: http://www.wkn.no/Publikasjoner/WKN_Rapport_2010_2.pdf (accessed on 2 June 2021).
- Miljødirektoratet. Available online: https://faktaark.naturbase.no/?id=BN00107976 (accessed on 6 August 2021).
- Ghiljarov, M.S.; Krivolutskij, D.A. Opredelitel Obitajuščich v Počve Kleščej. Sarcoptiformes; Nauka: Moskva, Russia, 1975; pp. 1–492. [Google Scholar]
- Pérez-Íñigo, C. Acari: Oribatei, Poronota. In Fauna Ibérica; Ramos, M.A., Ed.; Museo Nacional de Ciencias Naturales CSIC: Madrid, Spain, 1993; Volume 3, pp. 1–320. [Google Scholar]
- Pérez-Íñigo, C. Acari: Oribatei, Gymnonota I. In Fauna Ibérica; Ramos, M.A., Ed.; Museo Nacional de Ciencias Naturales CSIC: Madrid, Spain, 1997; Volume 9, pp. 1–373. [Google Scholar]
- Weigmann, G. Hornmilben (Oribatida). Die Tierwelt Deutschlands; Goecke and Evers: Keltern, Germany, 2006; Volume 76, pp. 1–520. [Google Scholar]
- Shaldybina, E.S. Biology of Melanozetes mollicomus (Koch) (Oribatei, Ceratozetidae). Zool. Zhurnal. 1967, 46, 1659–1667. [Google Scholar]
- Seniczak, S. The morphology of juvenile stages of moss mites of the family Pelopidae Ewing (Acarida: Oribatida), II. Ann. Zool. 1988, 41, 383–393. [Google Scholar]
- Seniczak, S.; Solhøy, T. The morphology of juvenile stages of moss mites of the family Chamobatide Thor (Acarida: Oribatida), I. Ann. Zool. 1988, 41, 491–502. [Google Scholar]
- Seniczak, S.; Żelazna, E. The Morphology of Juvenile Stages of Moss Mites of the Family Nothridae (Acari, Oribatida). II. Zool. Anz. 1992, 229, 149–162. [Google Scholar]
- Ermilov, S.G.; Łochyńska, M. Morphology of juvenile stages of Epidamaeus kamaensis (Sellnick, 1925) and Porobelba spinosa (Sellnick, 1920) (Acari: Oribatida: Damaeidae). Ann. Zool. 2009, 59, 527–544. [Google Scholar] [CrossRef]
- Ermilov, S.G.; Łochyńska, M. Morphology of juvenile stages, duration of the development of Nanhermannia cf. coronata Berlese, 1913 (Acari, Oribatida, Nanhermaniidae). Acarologia 2007, 47, 61–68. [Google Scholar]
- Seniczak, S.; Seniczak, A. Morphology of juvenile stages of Parachipteria bella (Sellnick, 1928) and P. willmanni Hammen, 1952 (Acari: Oribatida: Achipteriidae). Ann. Zool. 2007, 57, 533–540. [Google Scholar]
- Seniczak, S.; Seniczak, A. Differentiation of external morphology of Damaeidae (Acari: Oribatida) in light of the ontogeny of three species. Zootaxa 2011, 2775, 1–36. [Google Scholar] [CrossRef]
- Seniczak, A.; Seniczak, S. Comparison of morphology and ontogeny of Chamobates subglobulus (Oudemans, 1900) and Euzetes globulus (Nicolet, 1855) (Acari: Oribatida). Int. J. Acarol. 2014, 40, 274–295. [Google Scholar] [CrossRef]
- Seniczak, A.; Seniczak, S. Morphological ontogeny of Caleremaeus monilipes (Michael, 1882) (Acaria: Oribatida: Caleremaeidae) with comments on Caleremaeus Berlese. Syst. Appl. Acarol. 2019, 24, 1995–2009. [Google Scholar] [CrossRef]
- Seniczak, S.; Norton, R.A.; Seniczak, A. Morphology of Eniochthonius minutissimus (Berlese, 1904) and Hypochthonius rufulus C.L. Koch, 1835 (Acari: Oribatida: Hypochthonioidea). Ann. Zool. 2009, 59, 373–386. [Google Scholar] [CrossRef]
- Seniczak, A.; Seniczak, S.; Sgardelis, S.; Graczyk, R. Morphological ontogeny, distribution and ecology of Edwardzetes edwardsii and Sphaerozetes orbicularis (Acari, Oribatida, Ceratozetidae). Syst. Appl. Acarol. 2016, 21, 713–744. [Google Scholar]
- Seniczak, A.; Seniczak, S.; Graczyk, R.; Bukowski, G. Morphological ontogeny, ecology and some biological parameters of Achipteria magna (Acari: Oribatida: Achipteriidae). Syst. Appl. Acarol. 2017, 22, 980–992. [Google Scholar] [CrossRef]
- Pfingstl, T.; Krisper, G. Juvenile stages of the arboricolous mite Cymbaeremaeus cymba (Nicolet, 1855) (Acari: Oribatida: Cymbaeremaeidae). Int. J. Acarol. 2011, 37, 175–189. [Google Scholar] [CrossRef]
- Pfingstl, T.; Krisper, G. No difference in the juveniles of two Tectocepheus species (Acari: Oribatida, Tectocepheidae). Acarologia 2011, 51, 199–218. [Google Scholar] [CrossRef][Green Version]
- Ermilov, S.G.; Kolesnikov, V.B. Morphology of juvenile instars of Furcoribula furcillata and Zygoribatula exilis (Acari, Oribatida). Acarina 2012, 20, 48–59. [Google Scholar]
- Schatz, H. Catalogue of oribatid mites (Acari: Oribatida) from Vorarlberg (Austria). Zootaxa 2020, 4783, 1–106. [Google Scholar] [CrossRef]
- Subías, L.S. Listado sistemático, sinonímico y biogeográfico de los Ácaros Oribátidos (Acariformes, Oribatida) del mundo (1758−2002). Graellsia 2004, 60, 3–305. [Google Scholar] [CrossRef]
- Niedbała, W.; Liu, D. Catlogue of ptyctimous mites (Acari, Oribatida) of the world. Zootaxa 2018, 4393, 1–238. [Google Scholar] [CrossRef]
- Farrier, M.H. A Revision of the Veigaiidae (Acarina); North Carolina Agricultural Experiment Station: Salisbury, NC, USA, 1957; Volume 124, pp. 1–103. [Google Scholar]
- Bhattacharyya, S.K. A revision of the British mites of the genus Pergamasus Berlese s lat. (Acari: Mesostigmata). Bull. Br. Mus. nat. Hist. Zool. 1963, 11, 133–242. [Google Scholar]
- Hirschmann, W.; Zirngiebl-Nicol, I. Gangsystematik der Parasitiformes. Teil 43. Zwei neuen Dinychus-Arten. Acarologie 1969, 12, 39–40. [Google Scholar]
- Micherdziński, W. Die Familie Parasitidae Oudemans 1901 (Acarina, Mesostigmata); PWN: Warszawa, Poland, 1969; pp. 1–690. [Google Scholar]
- Halašková, V. Zerconidae of Czechoslovakia (Acari: Mesostigmata). Acta Univ. Carol. Biol. 1970, 3, 175–352. [Google Scholar]
- Błaszak, C. Zerconidae (Acari, Mesostigmata) Polski. Monogr. Fauny Pol. 1974, 3, 1–315. [Google Scholar]
- Ghiljarov, M.S.; Bregetova, N.G. Opredelitel Obitajuščich v Počve Kleščej. Mesostigmata; Nauka: Moskva, Russia, 1977; pp. 1–718. [Google Scholar]
- Hyatt, K.H. Mites of the subfamily Parasitinae (Mesostigmata: Parasitidae) in the British Isles. Bull. Brit. Mus. Nat. Hist. Zool. 1980, 38, 237–378. [Google Scholar]
- Hirschmann, W.; Wiśniewski, J. Weltweite Revision der Gattungen Dendrolaelaps Halbert 1915 und Longoseius Chant 1961 (Parasitiformes). Beschreibung der Untergattungen und Arten, Bestimmungstabellen, Chaetotaxie, Porotaxie. Acarologie 1982, 29, 1–190. [Google Scholar]
- Hyatt, K.H.; Emberson, R.M. A review of the Macrochelidae (Acari: Mesostigmata) of the British Isles. Bull. Br. Mus. Nat. Hist. Zool. 1988, 54, 63–125. [Google Scholar]
- Denmark, H.A.; Muma, M.H. A Revision of the Genus Amblyseius Berlese, 1914 (Acari: Phytoseiidae). In Occasional Papers of the Florida State Collection of Arthropods; Florida Department of Agriculture and Consumer Services, Division of Plant Industry: Gainesville, FL, USA, 1989; Volume 4, pp. 1–149. [Google Scholar]
- Karg, W. Acari (Acarina), Milben, Unterordnung Parasitiformes (Anactinochaeta), Uropodina Kramer, Schildkrötenmilben; Gustav Fischer Verlag: Jena, Germany, 1989; pp. 1–203. [Google Scholar]
- Karg, W. Raubmilben: Acari (Acarina), Milben Parasitiformes (Allactinochaeta) Cohors Gamasina Leach; Gustav Fischer Verlag: Jena, Germany, 1993; 523p. [Google Scholar]
- Hirschmann, W.; Wiśniewski, J.; Kaczmarek, S. Weltweite Revision der Ganggattung Sejus C.L. Koch 1836 (Trichopygidiina). Gangsystematik der Parasitiformes. Acarologie 1991, 38, 136–214. [Google Scholar]
- Mašán, P. Macrochelid Mites of Slovakia (Acari: Mesostigmata: Macrochelidae); Institute of Zoology, Slovak Academy of Sciences: Bratislava, Slovakia, 2003; pp. 1–149. [Google Scholar]
- Mašán, P. Roztoče kohorty Uropodina (Acarina, Mesostigmata) Slovenska. Annot. Zool. Bot. 2001, 223, 1–320. [Google Scholar]
- Mašán, P.; Fenďa, P. Zerconid Mites of Slovakia (Acari, Mesostigmata, Zerconidae); Institute of Zoology, Slovak Academy of Sciences: Bratislava, Slovakia, 2004; pp. 1–238. [Google Scholar]
- Kalúz, S.; Fenďa, P. Mites (Acari: Mesostigmata) of the Family Ascidae of Slovakia; Institute of Zoology, Slovak Academy of Sciences: Bratislava, Slovakia, 2005; pp. 1–166. [Google Scholar]
- Gwiazdowicz, D.J. Ascid Mites (Acari, Mesostigmata) from Selected Forest Ecosystems and Microhabitats in Poland; Wydawnictwo Akademii Rolniczej im. Augusta Cieszkowskiego: Poznań, Poland, 2007; pp. 1–248. [Google Scholar]
- Witaliński, W. Key to the world species of Holoparasitus Oudemans, 1936 (Acari: Parasitiformes: Parasitidae). Zootaxa 2017, 4277, 301–351. [Google Scholar] [CrossRef] [PubMed]
- Khaustov, V.A. Review of Amblyseius Berlese (Acari: Phytoseiidae) in Western Siberia, Russia. Acarologia 2020, 60, 769–805. [Google Scholar] [CrossRef]
- Beck, L.; Horak, F.; Woas, S. Zur Taxonomie der Gattung Phthiracarus Perty, 1841 (Acari, Oribatida) in Südwestdeutschland. Carolinea 2014, 72, 109–132. [Google Scholar]
- Schatz, H. Hornmilben (Acari, Oribatida) vom Fohramoos (Vorarlberg, Österreich). Ina. Forsch. 2015, 18, 1–17. [Google Scholar]
- Weigmann, G.; Horak, F.; Franke, K.; Christian, A. Verbreitung und Ökologie der Hornmilben (Oribatida) in Deutschland; Senckenberg, Museum Für Naturkunde, Peckiana: Görlitz, Germany, 2015; Volume 10, pp. 1–171. [Google Scholar]
- Hirschmann, W.; Wiśniewski, J. Die Uropodiden der Erde. Acari Parasitiformes. Supercohors Atrichopygidiina, Hirschmann 1975. Acarologie 1993, 40, 1–466. [Google Scholar]
- Salmane, I.; Brumelis, G. Species list and habitat preference of Mesostigmata mites (Acari, Parasitiformes) in Latvia. Acarologia 2010, 50, 373–394. [Google Scholar] [CrossRef]
- Huhta, V. Catalogue of the Mesostigmata mites in Finland. Memo. Soc. Fauna Flora Fenn. 2016, 92, 129–148. [Google Scholar]
- Bolger, T.; Arroyo, J.; Piotrowska, K. A catalogue of the species of Mesostigmata (Arachnida, Acari, Parasitiformes) recorded from Ireland including information on their geographical distribution and habitats. Zootaxa 2018, 4519, 1–220. [Google Scholar] [CrossRef] [PubMed]
- Mehl, R. Checklist of Norwegian ticks and mites (Acari). Fauna Norv. B 1979, 26, 31–45. [Google Scholar]
- Seniczak, A.; Seniczak, S.; Iturrondobeitia, J.C.; Solhøy, T.; Flatberg, K.I. Diverse Sphagnum mosses support rich moss mite communities (Acari, Oribatida) in mires of western Norway. Wetlands 2020, 40, 1339–1351. [Google Scholar] [CrossRef]
- Lundqvist, L. Bibliografi och checklist över Sveriges oribatider (Acari: Oribatei). Entomol. Tidskr. 1987, 108, 3–12. [Google Scholar]
- Niemi, R.; Karppinen, E.; Uusitalo, M. Catalogue of the Oribatida (Acari) of Finland. Acta Zool. Fenn. 1997, 207, 1–39. [Google Scholar]
- Koponen, S.; Rinne, V.; Clayhills, T. Arthropods on oak branches in SW Finland, collected by a new trap type. Entomol. Fenn. 1997, 8, 177–183. [Google Scholar] [CrossRef]
- Huhta, V.; Sulkava, P.; Viberg, K. Interactions between enchytraeid (Cognettia sphagnetorum), microarthropod and nematode populations in forest soil at different moistures. Appl. Soil Ecol. 1998, 9, 53–58. [Google Scholar] [CrossRef]
- Huhta, V.; Räty, M.; Ahlroth, P.; Hänninen, S.M.; Mattila, J.; Penttinen, R.; Rintala, T. Soil fauna of deciduous forests in central Finland. Memo. Soc. Fauna Flora Fenn. 2005, 81, 52–70. [Google Scholar]
- Huhta, V.; Siira-Pietikäinen, A.; Penttinen, R.; Räty, M. Soil fauna of Finland: Acarina, Collembola and Enchytraeidae. Memo. Soc. Fauna Flora Fenn. 2010, 86, 59–82. [Google Scholar]
- Huhta, V.; Penttinen, R.; Pitkänen, E. Cultural factors in the distribution of soil mites in Finland. Memo. Soc. Fauna Flora Fenn. 2012, 88, 52–70. [Google Scholar]
- Fröberg, L.; Solhøy, T.; Baur, A.; Baur, B. Lichen specificity of Oribatid mites (Acari; Oribatida) on limestone walls in the Great Alvar of Gland, Sweden. Entomol. Tidskr. 2003, 124, 177–182. [Google Scholar]
- Huhta, V.; Niemi, R. Communities of soil mites (Acarina) in planted birch stands as compared with natural forests in central Finland. Can. J. For. Res. 2003, 33, 171–180. [Google Scholar] [CrossRef]
- Penttinen, R.; Siira-Pietikäinen, A.; Huhta, V. Oribatid mites in eleven different habitats in Finland. In Integrative Acarology Montpellier: Proceedings of the 6th European Congress of the EURAAC 2008; European Association of Acarologists: Montpellier, France, 2008; pp. 237–244. [Google Scholar]
- Siira-Pietikäinen, A.; Penttinen, R.; Huhta, V. Oribatid mites (Acari: Oribatida) in boreal forest floor and decaying wood. Pedobiologia 2008, 52, 111–118. [Google Scholar] [CrossRef]
- Penttinen, R.; Huhta, V. Ptyctima (Acari, Oribatida) in various habitats in Finland. In Trends in Acarology; Sabelis, M.W., Bruin, J., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 167–170. [Google Scholar]
- Elo, R.A.; Penttinen, R.; Sorvari, J. A comparative study of oribatid mite communities in red wood ant Formica polyctena nests and surrounding soil in a Finnish oak forest. Insect Conserv. Divers. 2016, 9, 210–223. [Google Scholar] [CrossRef]
- Elo, R.A.; Penttinen, R.; Sorvari, J. Distribution of oribatid mites is moisture-related within red wood ant Formica polyctena nest mounds. Appl. Soil Ecol. 2018, 124, 203–210. [Google Scholar] [CrossRef]
- Gwiazdowicz, D.J.; Gulvik, M.E. Checklist of Norwegian mesostigmatid mites (Acari, Mesostigmata). Nor. J. Entomol. 2005, 52, 117–125. [Google Scholar]
- Gwiazdowicz, D.J.; Gulvik, M.E. The first records of five mite species (Acari, Mesostigmata) in Norway. Nor. J. Entomol. 2007, 54, 125–127. [Google Scholar]
- Gwiazdowicz, D.J.; Solhøy, T.; Kaasa, K. Five mesostigmatid mites (Acari, Mesostigmata) new to the Norwegian fauna. Nor. J. Entomol. 2013, 60, 8–10. [Google Scholar]
- Swedish Taxonomy Initiative. Available online: https://artfakta.se (accessed on 8 June 2021).
- Odum, E.P. Podstawy Ekologii; PWRiL: Warszawa, Poland, 1982; pp. 1–661. [Google Scholar]
- Niedbała, W.; Błoszyk, J.; Gutowski, J.M.; Konwerski, S.; Napierała, A. A characteristic of a community of ptyctimous mites (Acari: Oribatida) in the Białowieża Primeval Forest, Central Europe. In Mites (Acari) of the Białowieża Primeval Forest; Błoszyk, J., Napierała, A., Eds.; Wydawnictwo Kontekst: Poznan, Poland, 2020; pp. 61–87. [Google Scholar]
- Bray, J.R.; Curtis, J.T. An ordination of upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Kovach Computing Services. MVSP: A Multivariate Statistical Package for Windows, ver. 3.0. Pentraeth; Kovach Computing Services: Wales, UK, 2010. [Google Scholar]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Version 9. User’s Guide and Application. 2013. Available online: http://purl.oclc.org/estimates (accessed on 20 June 2021).
- Lekander, B.; Bejer-Petersen, B.; Kangas, E.; Bakke, A. The distribution of bark beetles in the Nordic countries. Entomol. Fenn. 1977, 32, 1–37. [Google Scholar]
- Väisänen, R.; Heliövaara, K. Hot-spots of insect diversity in Northern Europe. Ann. Zool. Fenn. 1994, 31, 71–81. [Google Scholar]
- Olsen, S.L.; Hedger, R.D.; Hendrichsen, D.; Nowell, M.; Dillinger, B.; Syverhuset, A.O.; Evju, M. Hotspots for Truede Arter i Norge: Karplanter, Insekter og Edderkoppdyr, Sopp, Lav og Moser; NINA Temahefte Norsk Institutt for Naturforskning: Trondheim, Norway, 2020; Volume 75. [Google Scholar]
- Moe, B. Studies of the alpine flora along an east-west gradient in central Western Norway. Nord. J. Bot. 1995, 15, 77–89. [Google Scholar] [CrossRef]
- Kyrkjeeide, M.O.; Stenøien, H.K.; Flatberg, K.I.; Hassel, K. Glacial refugia and post-glacial colonization patterns in European bryophytes. Lindbergia 2014, 37, 47–59. [Google Scholar] [CrossRef]
- Corral-Hernández, E.; Balanzategui, I.; Iturrondobeitia, J.C. Ecosystemic, climatic and temporal differences in oribatid communities (Acari: Oribatida) from forest soils. Exp. Appl. Acarol. 2016, 69, 389–401. [Google Scholar] [CrossRef]
- Erdmann, G.; Scheu, S.; Maraun, M. Regional factors rather than forest type drive the community structure of soil living oribatid mites (Acari, Oribatida). Exp. Appl. Acarol. 2012, 57, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Wunderle, I. Die Oribatiden-Gemeinschaften (Acari) der verschiedenen Habitate eines Buchenwaldes. Carolinea 1992, 50, 79–144. [Google Scholar]
- Arroyo, J.; Kenny, J.; Bolger, T. Variation between mite communities in Irish forest types—Importance of bark and moss cover in canopy. Pedobiologia 2013, 56, 241–250. [Google Scholar] [CrossRef]
- Skubała, P. Comparison of adult oribatid mites (Acari, Oribatida) from three mountain forests in Poland: I. Abundance, biomass and species richness. In Ecology and Evolution of Acari; Bruin, J., Van der Geest, L.P.S., Sabelis, M.W., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 547–555. [Google Scholar]
- Hansen, R.A. Effects of habitat complexity and composition on a diverse litter microarthropod assemblage. Ecology 2000, 81, 1120–1132. [Google Scholar] [CrossRef]
- Eissfeller, V.; Langenbruch, C.; Jacob, A.; Maraun, M.; Scheu, S. Tree identity surpasses tree diversity in affecting the community structure of oribatid mites (Oribatida) of deciduous temperate forests. Soil Biol. Biochem. 2013, 63, 154–162. [Google Scholar] [CrossRef]
- Murvanidze, M.; Mumladze, L.; Arabuli, T.; Barjadze, S.; Salakaia, M. Oribatida diversity in different microhabitats of Mtirala National Park. J. Acarol. Soc. Jpn. 2016, 25, 35–49. [Google Scholar] [CrossRef]
- Skubała, P. Oribatid fauna in Norway spruce stumps. Are there saproxylophilic oribatid species? In Integrative Acarology: Proceedings of the 6th European Congress; IEEE: New York, NY, USA, 2008; pp. 250–260. [Google Scholar]
- Hirschmann, W.; Wiśniewski, J. Lebensräume der Dendrolaelaps- und Longoseius-Arten. Acarologie 1983, 30, 21–33. [Google Scholar]
- Skubała, P.; Duras, M. Do decaying logs represent habitat islands? Oribatid mite communities in dead wood. Ann. Zool. 2008, 58, 453–466. [Google Scholar] [CrossRef]
- Skubała, P.; Marzec, A. Importance of different types of beech dead wood for soil microarthropod fauna. Pol. J. Ecol. 2013, 61, 545–560. [Google Scholar]
- Wunderle, I. Vertical distribution and life stages of oribatid communities on beech trees. In The Acari; Springer: Dordrecht, The Netherland, 1991; pp. 437–440. [Google Scholar] [CrossRef]
- Beaulieu, F.; Dowling, A.P.G.; Klompen, H.; De Moraes, G.J.; Walter, D.E. Superorder Parasitiformes Reuter, 1909. Zootaxa 2011, 3148, 123–128. [Google Scholar] [CrossRef]
- Maraun, M.; Schatz, H.; Scheu, S. Awesome or ordinary? Global diversity patters of oribatid mites. Ecography 2007, 30, 209–216. [Google Scholar] [CrossRef]
- Mendenhall, C.D.; Karp, D.S.; Meyer, C.F.J.; Hadly, E.A.; Daily, G.C. Predicting biodiversity change and averting collapse in agricultural landscapes. Nature 2014, 509, 213–217. [Google Scholar] [CrossRef]



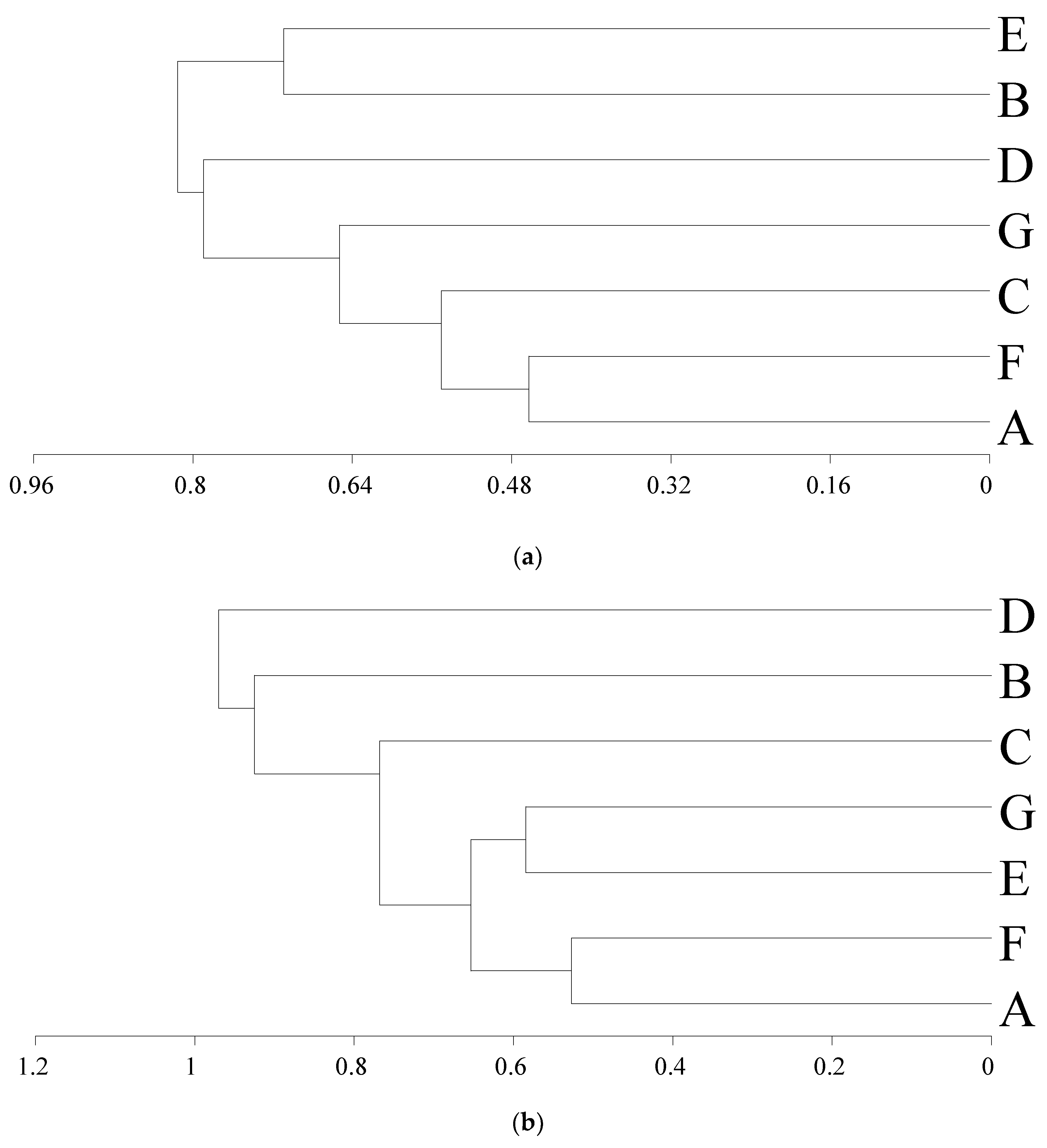
| Family | Family/Species | Habitat Preferences | No | F | Microhabitat |
|---|---|---|---|---|---|
| Oribatida | |||||
| Brachychthoniidae | Liochthonius brevis (Michael, 1888) | eu (si) el ge | 67 | 22 | A C |
| Neobrachychthonius magnus Moritz, 1976 | si el ge | 1 | 6 | A | |
| Sellnickochthonius immaculatus (Forsslund, 1942) | si ar el ge | 14 | 28 | A C | |
| S. suecicus (Forsslund, 1942) | si ge | 2 | 6 | C | |
| S. zellawaiensis (Sellnick, 1928) | si ge | 9 | 22 | A F | |
| Hypochthoniidae | Hypochthonius rufulus C.L. Koch, 1835 † | eu mh el ge | 16 | 17 | C F |
| Oribotritiidae | Oribotritia berlesei (Michael, 1898) | ? mu ge | 1 | 6 | B |
| Euphthiracaridae | Acrotritia duplicata (Grandjean, 1953) † | si ar ge | 10 | 17 | C |
| Euphthiracarus cribrarius (Berlese, 1904) | si ar el ge | 58 | 28 | A E F G | |
| E. monodactylus (Willmann, 1919) | si ar el ge | 32 | 17 | A F G | |
| Phthiracaridae | Phthiracarusboresetosus Jacot, 1930 | si ge | 4 | 22 | A F G |
| P. bryobius Jacot, 1930 † | si ar el ge mu | 45 | 72 | A B C D E F G | |
| P. clavatus Parry, 1979 † | si ge | 48 | 22 | A B E G | |
| P. crinitus (C.L. Koch, 1841) † | si ar el ge mu xy | 43 | 39 | A C F G | |
| P. globosus (C.L. Koch, 1841) | eu (si) ar el ge | 21 | 39 | A B C E F G | |
| P. laevigatus (C.L. Koch, 1844) † | si ar ge mu xy | 33 | 50 | A B C E F G | |
| P. longulus (C.L. Koch, 1841) † | si el ge | 221 | 83 | A B C D E F G | |
| P. nitens (Nicolet, 1855) | si el ge | 2 | 11 | B F | |
| Steganacaridae | Atropacarus striculus (C.L. Koch, 1835) | si pr ty hy el ge | 29 | 11 | A C |
| Steganacarus applicatus (Sellnick, 1920) | si ar el ge | 104 | 28 | A B | |
| S. carinatus (C.L. Koch, 1841) | si ge | 16 | 28 | A B | |
| Nanhermanniidae | Nanhermannia coronata Berlese, 1913 † | eu (ty si) mh ar ge | 104 | 6 | C |
| Nothridae | Nothrus silvestris Nicolet, 1855 | eu ar ge | 28 | 11 | C G |
| Damaeidae | Damaeus clavipes (Hermann, 1804) | eu (si) hy ar ge mu | 7 | 33 | A B F |
| D. gracilipes (Kulczynski, 1902) | si mh ar ge | 1 | 6 | A | |
| D. onustus C.L. Koch, 1844 † | si pr ar el ge | 4 | 17 | A | |
| Porobelba spinosa (Sellnick, 1920) † | si li ar ge mu xe | 169 | 22 | A C D | |
| Caleremaeidae | Caleremaeus monilipes (Michael, 1882) † | si ar ep ge | 145 | 39 | A C D F G |
| Eremaeidae | Eueremaeus oblongus (C.L. Koch, 1835) | si ar el ep ge mu xe | 3 | 6 | E |
| E. silvestris (Forsslund, 1956) | si ge | 1 | 6 | A | |
| Eueremaeus sp. 2 | ? | 3 | 11 | A B | |
| Eueremaeus sp. 3 | ? | 2 | 6 | B | |
| E. valkanovi (Kunst, 1957)†,‡ | si ar ge mu xe | 20 | 28 | A B C F | |
| Astegistidae | Cultroribula bicultrata (Berlese, 1905) | si ar el ge | 8 | 6 | G |
| Liacaridae | Adoristes ovatus (C.L. Koch, 1839) † | eu ar ep ge | 55 | 39 | A B E F |
| Liacarus coracinus (C.L. Koch, 1841) † | eu (si pr) ar el ge | 82 | 50 | B D E F G | |
| Xenillus tegeocranus (Hermann, 1804) † | si ar el ge | 5 | 22 | B C E F | |
| Carabodidae | Carabodes areolatus Berlese, 1916 † | si mh ar el ge mu | 64 | 61 | A B C D F G |
| C. coriaceus C.L. Koch, 1835 | si ar el ep ge | 1 | 6 | E | |
| C. femoralis (Nicolet, 1855) † | si ty ar ge | 60 | 39 | A B C F G | |
| C. labyrinthicus (Michael, 1879) † | eu (si) ar el ep ge li mu | 457 | 67 | A B D E F G | |
| C. marginatus (Michael, 1884) † | si mh ar ge mu | 26 | 33 | A B F | |
| C. ornatus Štorkán, 1925 | si ty er ep ge | 6 | 11 | B | |
| C. reticulatus Berlese, 1913 † | si mu ge | 26 | 28 | B E F | |
| C. subarcticus Trägårdh, 1902 | si ar ge | 2 | 11 | B D | |
| C. willmanni Bernini, 1975 | si ty ar ge | 198 | 17 | A B | |
| Odontocepheus elongatus (Michael, 1879) | si ar ge mu xe | 4 | 11 | B F | |
| Autognetidae | Autogneta longilamellata (Michael, 1885) | si ar ge | 2 | 6 | E |
| A. parva Forsslund, 1947 | si ar ge | 4 | 6 | B | |
| Conchogneta dalecarlica (Forsslund, 1947) | si ep ge | 18 | 6 | C | |
| C. traegardhi (Forsslund, 1947) † | si ge | 27 | 22 | C F G | |
| Oppiidae | Dissorhina ornata (Oudemans, 1900) † | eu si ar el ge | 499 | 61 | A B C D E G |
| Moritzoppia unicarinata (Paoli, 1908) | si ty ar el ge | 37 | 28 | B E F G | |
| Oppiella falcata (Paoli, 1908) | si mh ar ep ge | 2938 | 78 | A B C E F G | |
| O. nova (Oudemans, 1902) † | eu ar el ep ge li | 386 | 50 | A B C E F G | |
| Ramusella furcata (Willmann, 1928) | pr ty ge | 5 | 6 | G | |
| Rhinoppia subpectinata (Oudemans, 1900) | eu (si) ar ge | 112 | 39 | A C D | |
| Subiasella quadrimaculata (Evans, 1952) | si ge | 40 | 11 | B D | |
| Quadroppiidae | Coronoquadroppia monstruosa (Hammer, 1979) | si er ge | 232 | 61 | A B C D F |
| Q. quadricarinata (Michael, 1885) † | eu ar el ep ge | 296 | 83 | A B C E F G D | |
| Suctobelbidae | Suctobelba regia Moritz, 1970 † | si ar el li ge | 79 | 67 | A B D E F G |
| Suctobelbella falcata (Forsslund, 1941) | si ty ar el ge | 13 | 17 | B C G | |
| S. palustris (Forsslund, 1953) | pr ty aq hy ge li | 1 | 6 | G | |
| S. similis (Forsslund, 1941) | si ty ar ge | 2 | 6 | G | |
| S. subcornigera (Forsslund, 1941) † | eu (si) ar el ge | 58 | 56 | A B C F G | |
| S. subtrigona (Oudemans, 1916) † | eu (si) ar ge | 8 | 22 | A C | |
| Tectocepheidae | Tectocepheus velatus (Michael, 1880) † | eu ar el ep ge | 227 | 50 | A B C E F G |
| Cymbaeremaeidae | Cymbaeremaeus cymba (Nicolet, 1855) | xe ar el ge li mu | 2 | 11 | D E |
| Licneremaeidae | Licneremaeus licnophorus (Michael, 1882) | si ar el ge | 1 | 6 | C |
| Phenopelopidae | Eupelops torulosus (C.L. Koch, 1839) * | si ty ar el ge | 3 | 6 | A |
| Achipteriidae | Achipteria coleoptrata (Linnaeus, 1758) | eu ar el ge | 4 | 6 | B |
| A. magna (Sellnick, 1928) | si ar | 107 | 33 | B D F G | |
| A. nitens (Nicolet, 1855) | si ar ge | 1 | 6 | G | |
| Parachipteria fanzagoi Jacot, 1929 | ty si el ge | 1741 | 67 | A B C D E F G | |
| Oribatellidae | Oribatella quadricornuta Michael, 1880 | pr si xe ar el ge | 7 | 17 | B D F |
| Ophidiotrichus tectus (Michael, 1884) † | si ar ge mu xe | 19 | 22 | A C F G | |
| Oribatulidae | Oribatula exilis (Nicolet, 1855) † | eu ar bo el ep mu | 765 | 67 | A B C D E F |
| O. tibialis (Nicolet, 1855) | eu ar el ep ge | 3 | 11 | C | |
| Phauloppia lucorum (C.L. Koch, 1841) | ar el ep li ge xe | 2 | 6 | B | |
| Phauloppia rauschenensis (Sellnick, 1908) | si ar ge el | 1 | 6 | E | |
| Parakalummidae | Neoribates aurantiacus (Oudemans, 1914) | si pr mh ar el ge | 1 | 6 | B |
| Scheloribatidae | Scheloribates ascendens Weigmann et Wunderle, 1990 | eu ar ge el | 2 | 6 | B |
| S. initialis (Berlese, 1908) † | eu ar el ep ge | 53 | 39 | A B E F G | |
| S. laevigatus (C.L. Koch, 1835) † | eu ar el ep ge | 30 | 17 | E F | |
| Ceratozetidae | Ceratozetella sellnicki (Rajski, 1958) | pr ge | 13 | 6 | C |
| Ceratozetes gracilis (Michael, 1884) | eu ar ge | 28 | 11 | A C | |
| Melanozetes mollicomus (C.L. Koch, 1839) † | si ty ar el ep ge mu | 121 | 33 | B E F G | |
| Sphaerozetes orbicularis (C.L. Koch, 1835) † | si ar el ep ge mu xe | 53 | 50 | A B C F G | |
| Chamobatidae | Chamobates borealis Trägårdh, 1902 † | eu si ar el ep ge | 161 | 61 | A B C E F G |
| C. cuspidatus (Michael, 1884) † | eu si ar el ep ge | 252 | 61 | A B C E F G | |
| C. rastratus (Hull, 1914) | si ar ge el | 2 | 11 | C E | |
| Euzetidae | Euzetes globulus (Nicolet, 1855) | eu ar el ge | 7 | 28 | A C G |
| Punctoribatidae | Minunthozetes pseudofusiger (Schweizer, 1922) | si ar el ep ge li mu xe | 216 | 56 | A B C D F G |
| M. semirufus (C.L. Koch, 1841) | eu el ep ge | 4 | 11 | A B | |
| Galumnidae | Pergalumna nervosa (Berlese, 1914) | eu mu ar el ge | 3 | 11 | F |
| Mesostigmata | |||||
| Microgyniidae | Microgynium rectangulatum Trägårdh, 1942 | xy, mer, si | 2 | 11 | F G |
| Sejidae | Sejus togatus C.L. Koch, 1836 | xy, mer, si | 7 | 17 | E F G |
| Epicriidae | Epicrius mollis (Kramer, 1876) | mu, si | 3 | 11 | C |
| Zerconidae | Parazercon radiatus (Berlese, 1914) | eu, mu, pr, si | 15 | 17 | A C G |
| Prozercon kochi Sellnick, 1943 | eu, mh, mu, xy, si, pr | 19 | 33 | A C E F G | |
| Zercon berlesei Sellnick, 1958 * | eu, pr, si, xe | 4 | 11 | A F | |
| Z. triangularis C.L. Koch, 1836 | eu, mu, pr, si | 56 | 28 | A C F | |
| Z. zelawaiensis Sellnick, 1944 | mu, si, ty | 38 | 39 | A C D F | |
| Macrochelidae | Geholaspis longispinosus (Kramer, 1876) † | mu, li, pr, xe | 6 | 28 | A B F G |
| G. mandibularis Berlese, 1904 | mu, li, si, xy | 1 | 6 | A | |
| Parasitidae | Holoparasitus inornatus (Berlese, 1906) † | mu, si | 54 | 61 | A B C E F G |
| Paragamasus integer (Bhattacharyya, 1963) † | mu, si | 10 | 28 | B F G | |
| P. lapponicus (Trägårdh, 1910) † | mu, si | 136 | 56 | A C E F G | |
| P. truncus Schweizer, 1961 | mu, si, pr | 105 | 44 | A C F G | |
| Pergamasus crassipes (Linnaeus, 1758) | mu, xy, pr, si | 11 | 33 | A E F | |
| Vulgarogamasus kraepelini (Berlese, 1905) | mu, xy, si, pr | 6 | 17 | A E G | |
| Veigaiidae | Veigaia cerva (Kramer, 1876) †,* | mu, si | 1 | 6 | E |
| V. kochi (Trägårdh, 1901) | mu, si | 1 | 6 | A | |
| V. nemorensis (C.L. Koch, 1839) † | mu, pr, si | 29 | 39 | A G E F C | |
| V. transisalae (Oudemans, 1902) † | mu, hy, ty, si | 5 | 17 | E G | |
| Digamasellidae | Dendrolaelaps cornutulus Hirschmann, 1960 | xy, mer, si | 36 | 22 | C F G |
| D. insignis Hirschmann, 1960 | xy, si | 9 | 6 | G | |
| D. multidentatus (Leitner, 1949) | mer, pr | 1 | 6 | E | |
| D. rectus Karg, 1962 | pr | 24 | 17 | F G | |
| D. spinosus Hirschmann, 1960 | mer, si | 4 | 6 | G | |
| D. tenuipilus Hirschmann, 1960 | mer, si | 1 | 6 | E | |
| Ascidae | Zerconopsis michaeli Evans et Hyatt, 1960 | mer, si | 3 | 6 | E |
| Z. remiger (Kramer, 1876) | mu, mh, pr, si | 4 | 6 | E | |
| Laelapidae | Hypoaspis oblonga (Halbert, 1915) | mu, si | 30 | 6 | D |
| Phytoseiidae | Amblyseius silvaticus (Chant, 1959) | ar, si | 1 | 6 | B |
| Trachytidae | Trachytes aegrota (C.L. Koch, 1841) | eu, mu, xe, mh, pr, si | 10 | 17 | A B G |
| Urodinychidae | Dinychus perforatus Kramer, 1882 | eu, xy, pr, si | 1 | 6 | G |
| D. woelkei Hirschmann et Zirngiebl-Nicol, 1969 * | xy, si | 1 | 6 | F | |
| Trematuridae | Trichouropoda ovalis (C.L. Koch, 1839) | eu, xe, pr, si | 17 | 28 | C E F G |
| Very Frequent (F > 75%) | Frequent (30−75%) | Infrequent (15−30%) | Very Rare (F ≤ 15%) | |
|---|---|---|---|---|
| Numerous (20% < D) | O. falcata | |||
| Abundant (10 < D ≤ 20%) | P. truncus | |||
| Sparse (1 < D ≤ 10%) | M. pseudofusiger C. monstruosa | D. rectus | ||
| Few (D ≤ 1%) | E. valkanovi | C. sellnicki C. rastratus C. dalecarlica C. bicultrata N. magnus R. furcata S. ascendens S. suecicus S. quadrimaculata D. insignis D. multidentatus D. spinosus D. tenuipilus D. woelkei Z. berlesei Z. michaeli Z. remiger |
| Locality/Forest Type | Number and Volume of Samples | Number of Samplings | Microhabitat Sampled | Abundance on Ground (indiv. /m2) | Diversity Measures (indiv./spp./H′) | Diversity Measures (indiv./spp.) per 500 cm3 | Reference |
|---|---|---|---|---|---|---|---|
| Eastern Norway/ rich broadleaf forest | 18 × 500 cm3 | 1 | Moss, lichens, decaying wood | O: 44,000 M: 3600 | O: 10,843/95/2.97 M: 655/35/2.74 | O: 602/5.3 M: 36/1.9 | Present study |
| Western Norway/ low-herb broadleaf forest | 14 × 500 cm3 | 1 | Moss, decaying wood | O: 32,700 M: 5400 | O: 6350/67/2.54 M: 559/22/1.52 | O: 453/4.8 M: 39/1.6 | [15] |
| Northern Poland/beech forest (nature reserve) | 42 × 500 cm3 | 1 | Soil litter, moss, decaying wood, tree bark | O: 82,300 M: 7000 | O: 71,124/79/2.20 M: 3309/66/1.70 | O: 1693/1.9 M: 79/1.6 | [14] |
| Northern Spain/ 18 forests, 5 types, different regions | 54 × 2000 cm3 | 3 (3 years.) | Soil | Na | O: 50,307/260/- | O: 233/1.2 | [123] |
| Ireland/5 oak forests, different regions | 45 (different volume) | 1 | Moss and tree bark from canopy, moss from ground, soil | Na | O + M: 5906/59/- | Na | [126] |
| Ireland/5 ash forests, different regions | 45 (different volume) | 1 | Moss and tree bark from canopy, moss from ground, soil | Na | O + M: 2863/32/- | Na | [126] |
| Germany/3 beech forests in different regions | 24 × 157 cm3 | 1 | Soil | O: 30,000 *,† | O: -/15–20/- *,‡ | O: -/2.0–2.6 *,‡ | [124] |
| Southern Poland/beech forest (nature reserve) | 1080 × 135 cm3 | 36 (3 years.) | Soil | O: 20,000 * | O: -/77/- * | O: -/0.3 * | [127] |
| Southern Germany/beech forest | Over 100 (different volume) | 8 (2 years.) | Soil litter, moss, decaying wood, tree bark | O: 61,500 *,† | O: -/119/- | Na | [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seniczak, A.; Seniczak, S.; Starý, J.; Kaczmarek, S.; Jordal, B.H.; Kowalski, J.; Roth, S.; Djursvoll, P.; Bolger, T. High Diversity of Mites (Acari: Oribatida, Mesostigmata) Supports the High Conservation Value of a Broadleaf Forest in Eastern Norway. Forests 2021, 12, 1098. https://doi.org/10.3390/f12081098
Seniczak A, Seniczak S, Starý J, Kaczmarek S, Jordal BH, Kowalski J, Roth S, Djursvoll P, Bolger T. High Diversity of Mites (Acari: Oribatida, Mesostigmata) Supports the High Conservation Value of a Broadleaf Forest in Eastern Norway. Forests. 2021; 12(8):1098. https://doi.org/10.3390/f12081098
Chicago/Turabian StyleSeniczak, Anna, Stanisław Seniczak, Josef Starý, Sławomir Kaczmarek, Bjarte H. Jordal, Jarosław Kowalski, Steffen Roth, Per Djursvoll, and Thomas Bolger. 2021. "High Diversity of Mites (Acari: Oribatida, Mesostigmata) Supports the High Conservation Value of a Broadleaf Forest in Eastern Norway" Forests 12, no. 8: 1098. https://doi.org/10.3390/f12081098
APA StyleSeniczak, A., Seniczak, S., Starý, J., Kaczmarek, S., Jordal, B. H., Kowalski, J., Roth, S., Djursvoll, P., & Bolger, T. (2021). High Diversity of Mites (Acari: Oribatida, Mesostigmata) Supports the High Conservation Value of a Broadleaf Forest in Eastern Norway. Forests, 12(8), 1098. https://doi.org/10.3390/f12081098





