Marked Differences in Butterfly Assemblage Composition between Forest Types in Central Amazonia, Brazil
Abstract
1. Introduction
2. Materials and Methods
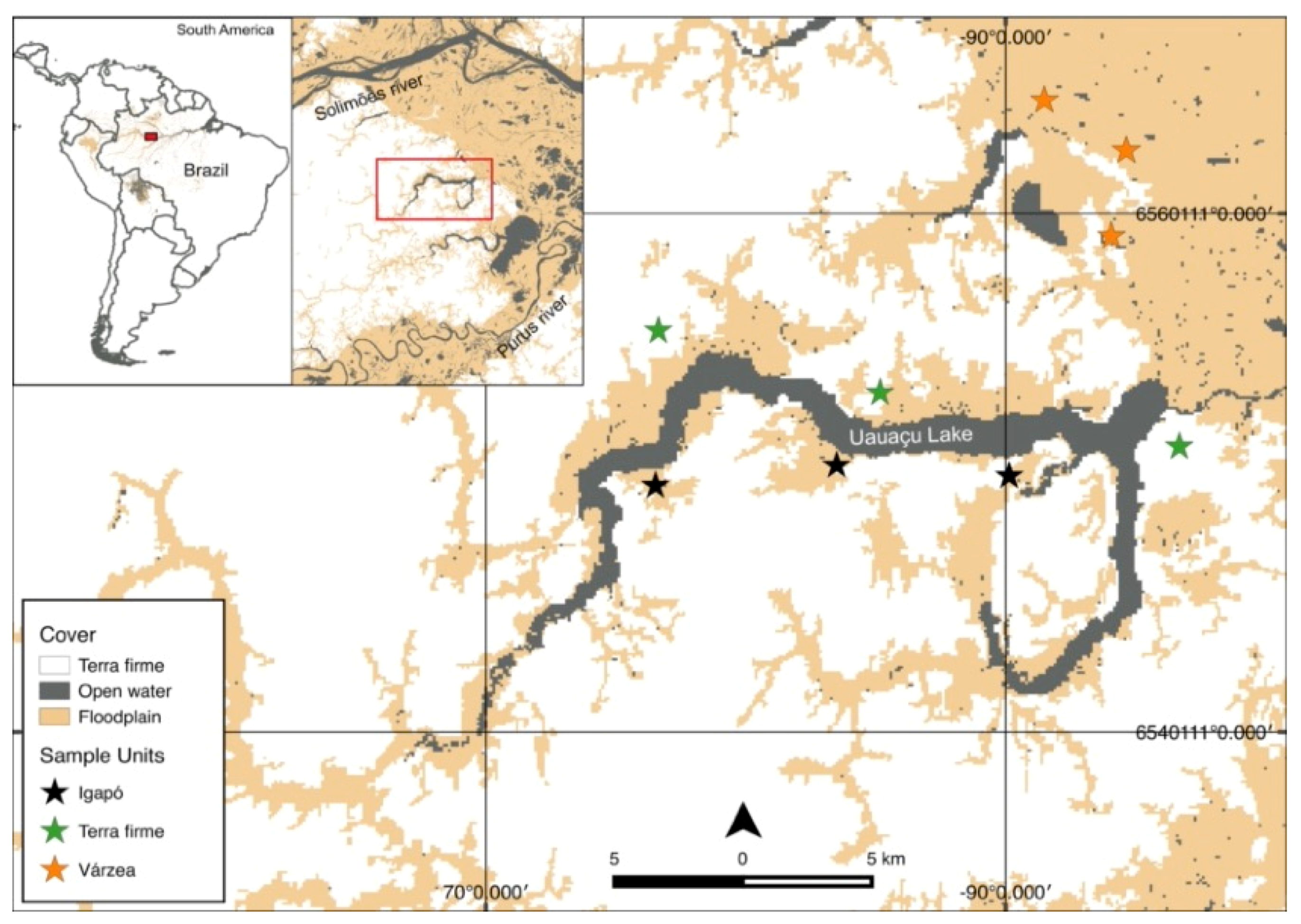
2.1. Study Area
2.2. Sampling Design
2.3. Statistical Analysis
3. Results
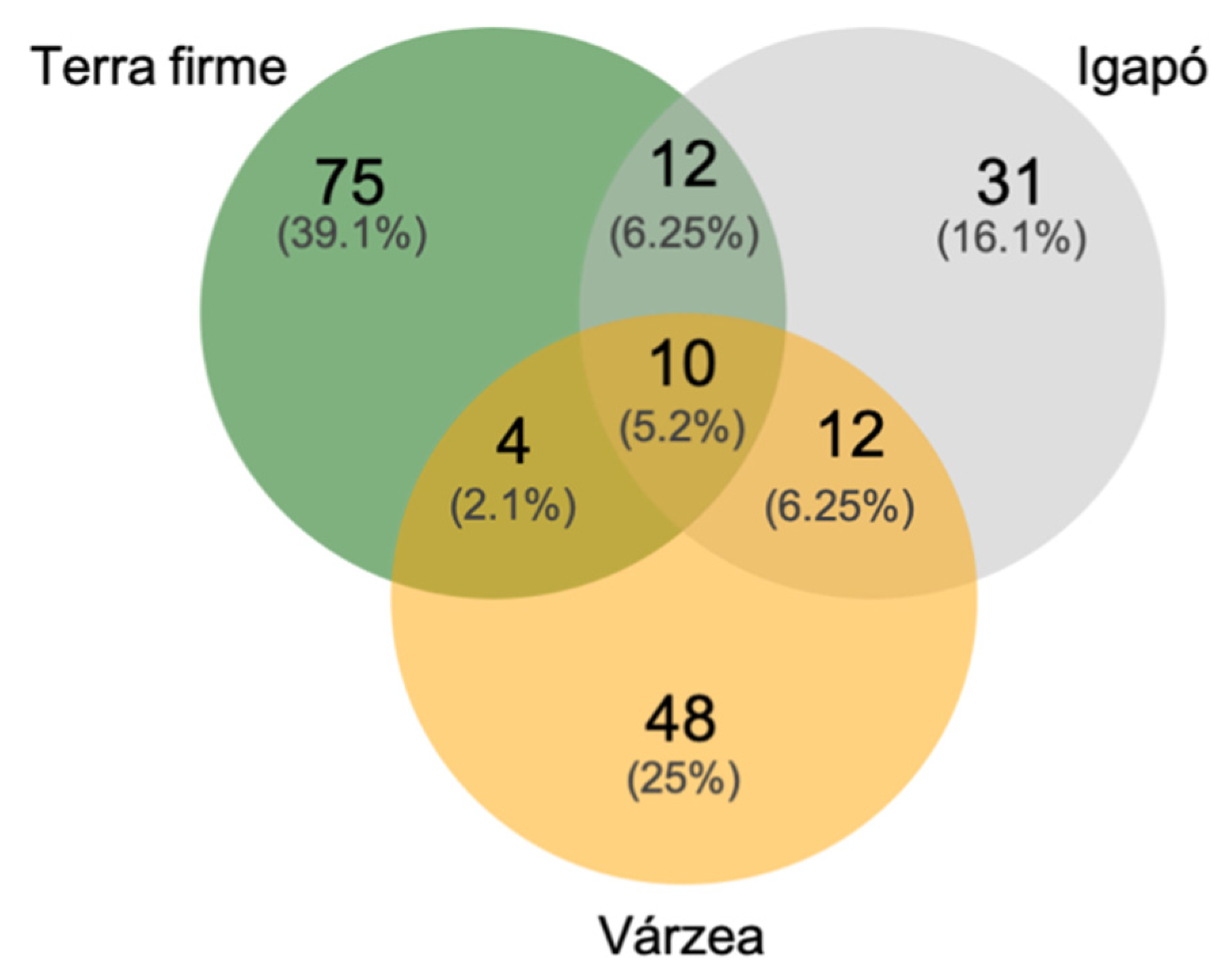
3.1. The Uauaçu Lake Butterfly Community
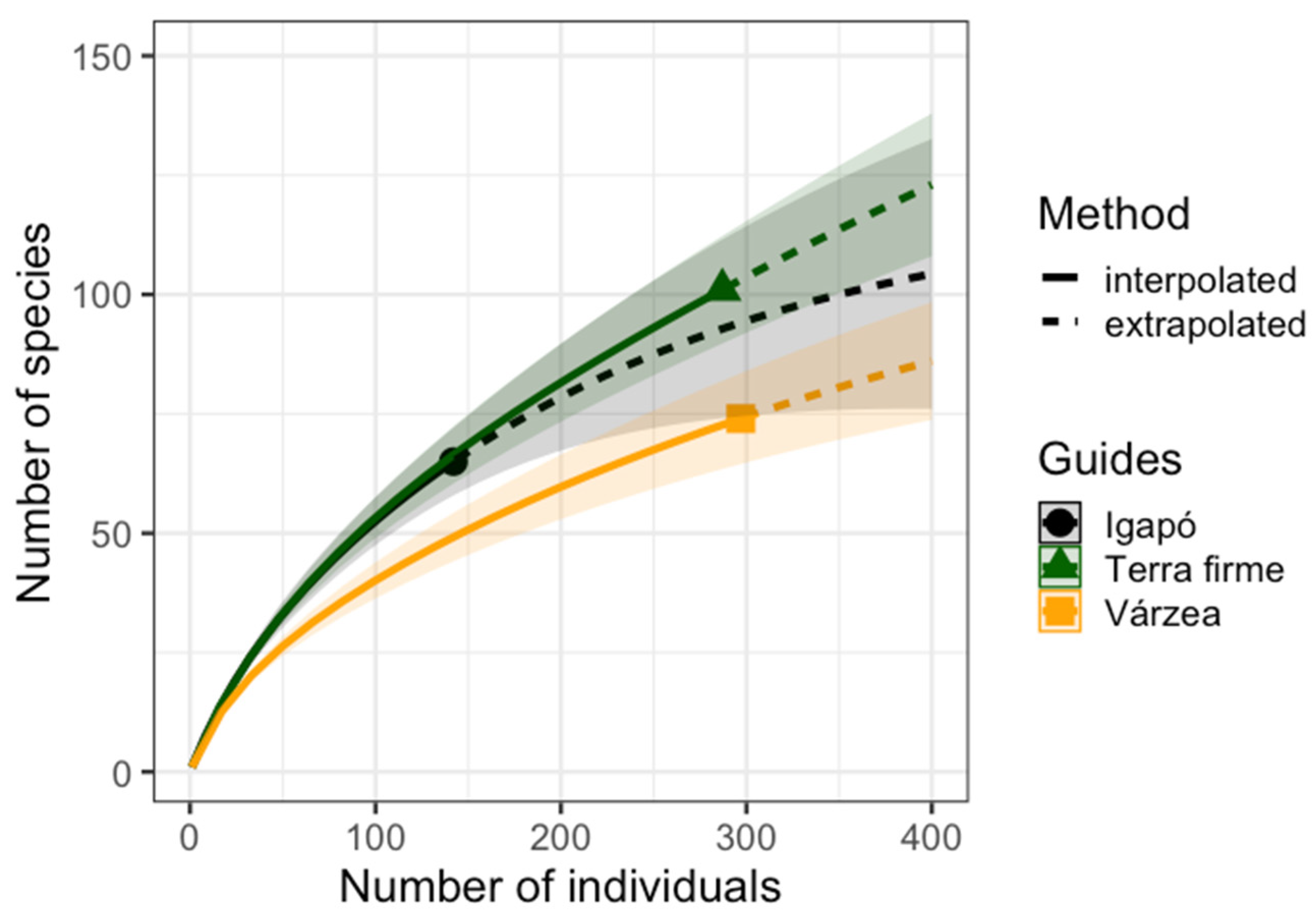
3.2. Butterfly Richness and Abundance
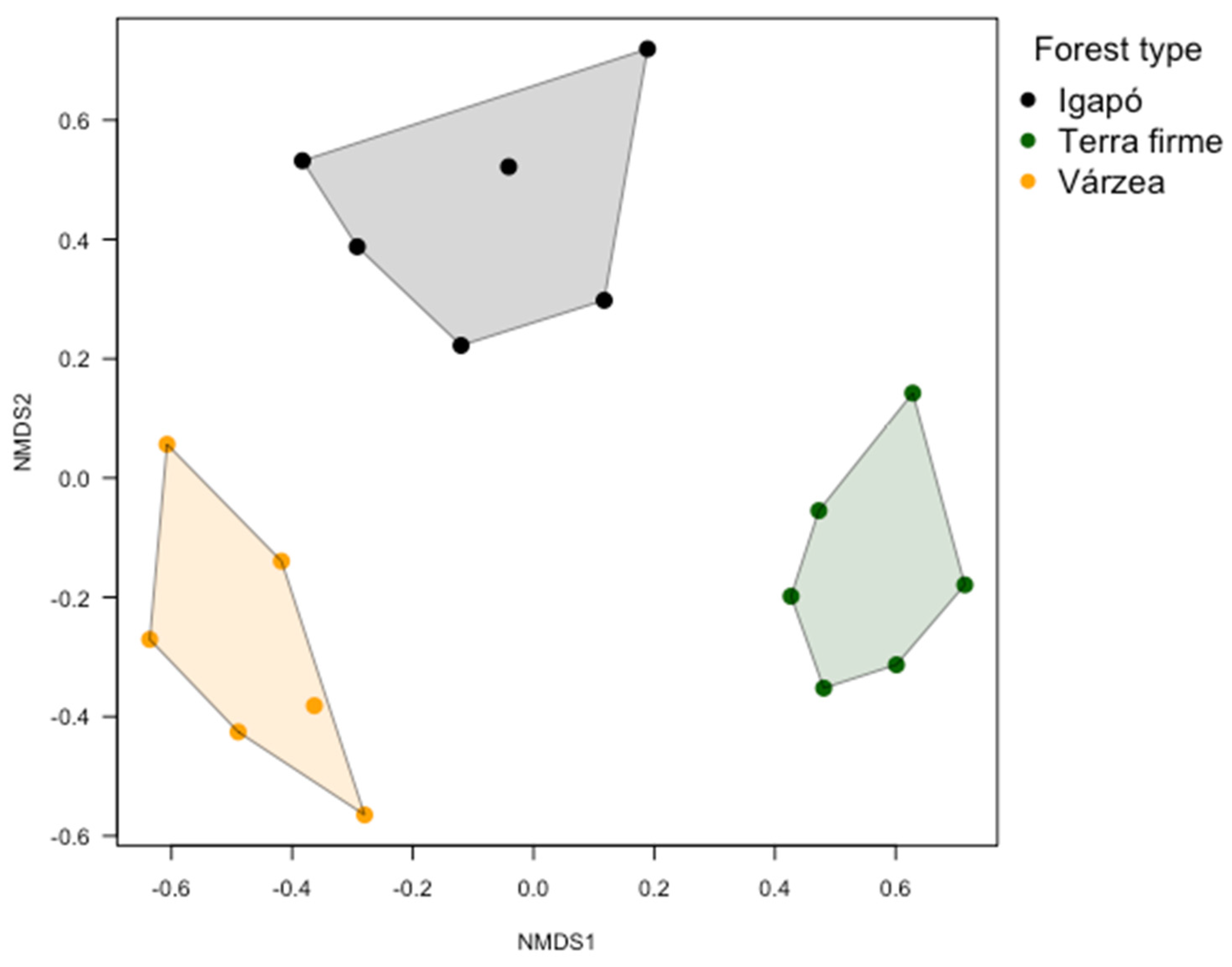
3.3. Butterfly Assemblage Composition
4. Discussion
4.1. Species Richness and Abundance
4.2. Butterfly Assemblage Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-Resolution Global Maps of 21st-Century Forest Cover Change. Science 2013, 342, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.N.; Pimm, S.L.; Joppa, L.N. Global patterns of terrestrial vertebrate diversity and conservation. Proc. Natl. Acad. Sci. USA 2013, 110, E2603–E2610. [Google Scholar] [CrossRef]
- Antonelli, A.; Zizka, A.; Carvalho, F.A.; Scharn, R.; Bacon, C.D.; Silvestro, D.; Condamine, F.L. Amazonia is the primary source of Neotropical biodiversity. Proc. Natl. Acad. Sci. USA 2018, 115, 6034–6039. [Google Scholar] [CrossRef] [PubMed]
- Ter Steege, H.; Pitman, N.; Sabatier, D.; Castellanos, H.; Van Der Hout, P.; Daly, D.C.; Silveira, M.; Phillips, O.; Vasquez, R.; van Andel, T.; et al. A spatial model of tree alpha-diversity and tree density for the Amazon. Biodivers. Conserv. 2003, 12, 2255–2277. [Google Scholar] [CrossRef]
- Quesada, C.A.; Lloyd, J.; Schwarz, M.; Baker, T.R.; Phillips, O.L.; Patiño, S.; Czimczik, C.; Hodnett, M.G.; Herrera, R.; Arneth, A.; et al. Regional and large-scale patterns in Amazon forest structure and function are mediated by variations in soil physical and chemical properties. Biogeosci. Discuss. 2009, 6, 3993–4057. [Google Scholar]
- Tuomisto, H.; Van Doninck, J.; Ruokolainen, K.; Moulatlet, G.M.; Figueiredo, F.O.G.; Sirén, A.; Cárdenas, G.; Lehtonen, S.; Zuquim, G. Discovering floristic and geoecological gradients across Amazonia. J. Biogeogr. 2019, 46, 1734–1748. [Google Scholar] [CrossRef]
- Dambros, C.; Zuquim, G.; Moulatlet, G.M.; Costa, F.R.C.; Tuomisto, H.; Ribas, C.C.; Azevedo, R.; Baccaro, F.; Bobrowiec, P.E.D.; Dias, M.S.; et al. The role of environmental filtering, geographic distance and dispersal barriers in shaping the turnover of plant and animal species in Amazonia. Biodivers. Conserv. 2020, 29, 3609–3634. [Google Scholar] [CrossRef]
- Melack, J.M.; Hess, L.L. Remote Sensing of the Distribution and Extent of Wetlands in the Amazon Basin. In Amazonian Floodplain Forests: Ecophysiology, Biodiversity and Sustainable Management, Ecological Studies; Springer: Dordrecht, The Netherlands, 2010; Volume 210, pp. 43–59. [Google Scholar]
- Hess, L.L.; Melack, J.M.; Affonso, A.G.; Barbosa, C.; Gastil-Buhl, M.; Novo, E.M.L.M. Wetlands of the Lowland Amazon Basin: Extent, Vegetative Cover, and Dual-season Inundated Area as Mapped with JERS-1 Synthetic Aperture Radar. Wetlands 2015, 35, 745–756. [Google Scholar] [CrossRef]
- Wittmann, F.; Schöngart, J.; Junk, W.J. Phytogeography, Species Diversity, Community Structure and Dynamics of Central Amazonian Floodplain Forests. In Amazonian Floodplain Forests: Ecophysiology, Biodiversity and Sustainable Management, Ecological Studies; Springer: Dordrecht, The Netherlands, 2010; Volume 210, pp. 61–102. [Google Scholar]
- Prance, G.T. Notes on the Vegetation of Amazonia III. The Terminology of Amazonian Forest Types Subject to Inundation. Brittonia 1979, 31, 26–38. [Google Scholar] [CrossRef]
- Junk, W.J.; Wittmann, F.; Schöngart, J.; Piedade, M.T.F. A classification of the major habitats of Amazonian black-water river floodplains and a comparison with their white-water counterparts. Wetl. Ecol. Manag. 2015, 23, 677–693. [Google Scholar] [CrossRef]
- Junk, W.J.; Bayley, P.B.; Sparks, R.E. The Flood Pulse Concept in River-Flooding Systems. Can. Spec. Publ. Fish. Aquat. Sci. 1989, 78, 110–127. [Google Scholar]
- Junk, W.J.; Piedade, M.T.F.; Schöngart, J.; Cohn-Haft, M.; Adeney, J.M.; Wittmann, F. A classification of major naturally-occurring amazonian lowland wetlands. Wetlands 2011, 31, 623–640. [Google Scholar] [CrossRef]
- Haugaasen, T.; Peres, C.A. Floristic, edaphic and structural characteristics of flooded and unflooded forests in the lower Rio Purús region of central Amazonia, Brazil. Acta Amaz. 2006, 36, 25–36. [Google Scholar] [CrossRef]
- Pringle, E.G.; Santos TF dos Gonçalves, M.S.; Hawes, J.E.; Peres, C.A.; Baccaro, F.B. Arboreal ant abundance tracks primary productivity in an Amazonian whitewater river system. Ecosphere 2019, 10, 10. [Google Scholar] [CrossRef]
- Haugaasen, T.; Peres, C.A. Primate assemblage structure in Amazonian flooded and unflooded forests. Am. J. Primatol. 2005, 67, 243–258. [Google Scholar] [CrossRef]
- Pereira, M.J.R.; Marques, J.T.; Santana, J.; Santos, C.D.; Valsecchi, J.; De Queiroz, H.L.; Beja, P.; Palmeirim, J. Structuring of Amazonian bat assemblages: The roles of flooding patterns and floodwater nutrient load. J. Anim. Ecol. 2009, 78, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Bobrowiec, P.E.D.; dos Santos Rosa, L.; Gazarini, J.; Haugaasen, T. Phyllostomid Bat Assemblage Structure in Amazonian Flooded and Unflooded Forests. Biotropica 2014, 46, 312–321. [Google Scholar] [CrossRef]
- Laranjeiras, T.O.; Naka, L.N.; Cohn-Haft, M. Using river color to predict Amazonian floodplain forest avifaunas in the world’s largest blackwater river basin. Biotropica 2019, 51, 330–341. [Google Scholar] [CrossRef]
- Costa, H.C.M.; Peres, C.A.; Abrahams, M.I. Seasonal dynamics of terrestrial vertebrate abundance between Amazonian flooded and unflooded forests. PeerJ 2018, 6, e5058. [Google Scholar] [CrossRef]
- Wittmann, F.; Schongart, J.; Montero, J.C.; Motzer, T.; Junk, W.J.; Piedade, M.T.F.; Queiroz, H.L.; Worbes, M. Tree species composition and diversity gradients in white-water forests across the Amazon Basin. J. Biogeogr. 2006, 33, 1334–1347. [Google Scholar] [CrossRef]
- Wittmann, F.; Junk, W.J.; Piedade, M.T.F. The várzea forests in Amazonia: Flooding and the highly dynamic geomorphology interact with natural forest succession. For. Ecol. Manag. 2004, 196, 199–212. [Google Scholar] [CrossRef]
- Worbes, M.; Klinge, H.; Revilla, J.D.; Martius, C. On the dynamics, floristic subdivision and geographical distribution of várzea forests in Central Amazonia. J. Veg. Sci. 1992, 3, 553–564. [Google Scholar] [CrossRef]
- Assis, R.L.; Wittmann, F.; Piedade, M.T.F.; Haugaasen, T. Effects of hydroperiod and substrate properties on tree alpha diversity and composition in Amazonian floodplain forests. Plant Ecol. 2015, 216, 41–54. [Google Scholar] [CrossRef]
- Takeuchi, M. The structure of the Amazonian vegetation VI. Igapó. J. Fac. Sci. Tokyo III 1962, 8, 297–304. [Google Scholar]
- Keel, S.H.; Prance, G.T. Studies of the vegetation of a black-water Igapó. Acta Amaz. 1979, 9, 645–655. [Google Scholar] [CrossRef]
- Kubitzki, K.; Ziburski, A. Seed Dispersal in Flood Plain Forests of Amazonia. Biotropica 1994, 26, 30–43. [Google Scholar] [CrossRef]
- Haugaasen, T.; Peres, C.A. Tree phenology in adjacent Amazonian flooded and unflooded forests. Biotropica 2005, 37, 620–630. [Google Scholar] [CrossRef]
- Vasconcelos, H.L.; Macedo, A.C.C.; Vilhena, J.M.S. Influence of topography on the distribution of ground-dwelling ants in an Amazonian forest. Stud. Neotrop. Fauna Environ. 2003, 38, 115–124. [Google Scholar] [CrossRef]
- Rojas-Ahumada, D.P.; Landeiro, V.L.; Menin, M. Role of environmental and spatial processes in structuring anuran communities across a tropical rain forest. Austral Ecol. 2021, 37, 865–873. [Google Scholar] [CrossRef]
- Capaverde, U.D.; do Amaral Pereira, L.G.; da Cunha Tavares, V.; Magnusson, W.E.; Baccaro, F.B.; Bobrowiec, P.E.D. Subtle changes in elevation shift bat-assemblage structure in Central Amazonia. Biotropica 2018, 50, 674–683. [Google Scholar] [CrossRef]
- Kinap, N.M.; Nagy, M.; Paulo, R.; Bobrowiec, E.D.; Gordo, M.; Spironello, W.R. Influence of topography gradient and seasonality on primate habitat use in Central Amazonia. Mamm. Biol. 2021, 101, 251–259. [Google Scholar] [CrossRef]
- Schulze, C.H.; Waltert, M.; Kessler, P.J.A.; Pitopang, R.; Shahabuddin, S.; Veddeler, D.; Mühlenberg, M.; Gradstein, S.R.; Leuschner, C.; Steffan-Dewenter, I.; et al. Biodiversity indicator groups of tropical land-use systems: Comparing plants, birds, and insects. Ecol. Appl. 2004, 14, 1321–1333. [Google Scholar] [CrossRef]
- Bonebrake, T.C.; Ponisio, L.C.; Boggs, C.L.; Ehrlich, P.R. More than just indicators: A review of tropical butterfly ecology and conservation. Biol. Conserv. 2010, 143, 1831–1841. [Google Scholar] [CrossRef]
- De Vries, P.J. Hostplant records and Natural History Notes on Costa Rican Butterflies (Papilionidae, Pieridae e Nymphalidae). J. Res. Lepid. 1986, 24, 290–333. [Google Scholar]
- Ackery, P.R. Hostplants and classification: A review of nymphalid butterflies. Biol. J. Linn. Soc. 1988, 33, 95–203. [Google Scholar] [CrossRef]
- Brown, K.S.; Freitas, A.V.L. Atlantic forest butterflies: Indicators for landscape conservation. Biotropica 2000, 32, 934–956. [Google Scholar] [CrossRef]
- Freitas, A.V.L.; Francini, R.B.; Brown, K.S. Insetos como Indicadores Ambientais. In Métodos de Estudos em Biologia da Conservação e Manejo da Vida Silvestre; Cullen, L., Jr., Rudran, R., Valladares-Pádua, C., Eds.; Fundação Boticário e editora da UFPR: Curitiba, Brasil, 2003; pp. 19–41. [Google Scholar]
- Koh, L.P.; Sodhi, N.S.; Brook, B.W. Ecological correlates of extinction proneness in tropical butterflies. Conserv. Biol. 2004, 18, 1571–1578. [Google Scholar] [CrossRef]
- Ferrer-Paris, J.R.; Sánchez-Mercado, A.; Viloria, Á.L.; Donaldson, J. Congruence and Diversity of Butterfly-Host Plant Associations at Higher Taxonomic Levels. PLoS ONE 2013, 8, e63570. [Google Scholar] [CrossRef]
- DeVries, P.J.; Alexander, L.G.; Chacon, I.A.; Fordyce, J.A. Similarity and difference among rainforest fruit-feeding butterfly communities in Central and South America. J. Anim. Ecol. 2012, 81, 472–482. [Google Scholar] [CrossRef]
- Brito, M.M.; Ribeiro, D.B.; Raniero, M.; Hasui, É.; Ramos, F.N.; Arab, A. Functional composition and phenology of fruit-feeding butterflies in a fragmented landscape: Variation of seasonality between habitat specialists. J. Insect Conserv. 2014, 18, 547–560. [Google Scholar] [CrossRef]
- Graça, M.B.; Pequeno, P.A.C.L.; Franklin, E.; Morais, J.W. Coevolution between flight morphology, vertical stratification and sexual dimorphism: What can we learn from tropical butterflies? J. Evol. Biol. 2017, 30, 1862–1871. [Google Scholar] [CrossRef]
- Santos, E.C.; Mielke, O.H.H.; Casagrande, M.M. Butterfly inventories in Brazil: The state of the art and the priority-areas model for research aiming at conservation. Nat. Conserv. 2008, 6, 178–200. [Google Scholar]
- Casagrande, M.M.; Mielke, O.H.H.; Carneiro, E.; Rafael, J.A.; Hutchings, R.W. Hesperioidea e Papilionoidea (Lepidoptera) coligidos em expedição aos Rios Nhamundá e Abacaxis, Amazonas, Brasil: Novos subsídios para o conhecimento da biodiversidade da Amazônia Brasileira. Rev. Bras. Entomol. 2012, 56, 23–28. [Google Scholar] [CrossRef][Green Version]
- Rydon, A. Notes on the use of butterfly traps in East Africa. J. Lepid. Soc. 1964, 18, 51–58. [Google Scholar]
- Ribeiro, D.B.; Williams, M.R.; Specht, A.; Freitas, A.V.L. Vertical and temporal variability in the probability of detection of fruit-feeding butterflies and moths (Lepidoptera) in tropical forest. Austral Entomol. 2016, 55, 112–120. [Google Scholar] [CrossRef]
- Pollard, E. A method for assessing changes in the abundance of butterflies. Biol. Conserv. 1977, 12, 115–134. [Google Scholar] [CrossRef]
- Van Swaay, C.; Regan, E.; Ling, M.; Bozhinovska, E.; Fernandez, M.; Huertas, B.; Chooi-Khim, P.; Kőrösi, Á.; Marini-Filho, O.J.; Meerman, J.; et al. Guidelines for Standardised Global Butterfly Monitoring. Group on Earth Observations Biodiversity Observation Network, Leipzig, Germany; GEO BON Technical Series; GEO BON: Leipzig, Germany, 2015; pp. 1–32. [Google Scholar]
- D’Abrera, B. Part I. Papilionidae and Pieridae. In Butterflies of the Neotropical Region; Hill House: Victoria, Australia, 1981; pp. 2–169. [Google Scholar]
- D’Abrera, B. Part III. Brassolidae, Acraeidae and Nymphalidae. In Butterflies of the Neotropical Region; Hill House: Victoria, Australia, 1987; pp. 386–521. [Google Scholar]
- D’Abrera, B. Part IV. Nymphalidae. In Butterflies of the Neotropical Region; Hill House: Victoria, Australia, 1987; pp. 528–678. [Google Scholar]
- D’Abrera, B. Part V. Nymphalidae and Satyridae. In Butterflies of the Neotropical Region; Hill House: Victoria, Australia, 1988; pp. 679–873. [Google Scholar]
- Jiggins, C.D. The Ecology and Evolution of Heliconius Butterflies; Oxford University Press: Oxford, UK, 2017; pp. 1–321. [Google Scholar]
- Constantino, L. Revisión de la tribu Haeterini Herrich-Schäffer, 1864 en Colombia. SHILAP Rev. Lepidopterol. 1995, 23, 49–76. [Google Scholar]
- Constantino, L.M. Analisis Morfologicos, Moleculares y Biogeograficos en la Validación de Nuevas Especies y Resolución de Problemas Taxonómicos en Lepidoptera; 43° Congreso Sociedad Colombiana de Entomología–SOCOLEN: Manizales, Colombia, 2016; pp. 209–228. [Google Scholar]
- Penz, C.M.; Mohammadi, N. Diversidade de padrão das asas em Brassolini (Nymphalidae, Satyrinae). Biota Neotrop. 2013, 13, 154–180. [Google Scholar] [CrossRef]
- Penz, C.M.; Alexander, L.G.; DeVries, P.J. Revised species definitions and nomenclature of the rose colored Cithaerias butterflies (Lepidoptera, Nymphalidae, Satyrinae). Zootaxa 2014, 3873, 541–559. [Google Scholar] [CrossRef]
- Zacca, T.; Casagrande, M.; Huertas, B.; Barbosa, E.; Magaldi, L.; Espeland, M.; Mielke, O.; Freitas, A.; Nakahara, S.; Willmott, K. Systematics of the butterfly genus Cissia Doubleday, 1848 (Lepidoptera: Nymphalidae: Satyrinae) using an integrative approach. Arthropod Syst. Phylogeny 2018, 76, 349–376. [Google Scholar]
- Zacca, T.; Casagrande, M.M.; Mielke, O.H.H.; Huertas, B.; Barbosa, E.P.; Freitas, A.V.L.; Lamas, G.; Espeland, M.; Brévignon, C.; Nakahara, S. Systematics of the Neotropical butterfly genus Paryphthimoides (Lepidoptera: Nymphalidae: Satyrinae), with descriptions of seven new taxa. Insect Syst. Evol. 2020, 1964, 1–55. [Google Scholar] [CrossRef]
- Zacca, T.; Siewert, R.R.; Casagrande, M.M.; Mielke, O.H.H.; Paluch, M. Taxonomic revision of the “Pierella lamia species group” (Lepidoptera: Nymphalidae: Satyrinae) with descriptions of four new species from Brazil. Zootaxa 2016, 4078, 366–386. [Google Scholar] [CrossRef]
- Nakahara, S.; Lamas, G.; Tyler, S.; Marín, M.A.; Huertas, B.; Willmott, K.R.; Mielke, O.H.H.; Espeland, M. A revision of the new genus Amiga Nakahara, Willmott & Espeland, gen. N., described for Papilio arnaca fabricius, 1776 (Lepidoptera, Nymphalidae, Satyrinae). ZooKeys 2018, 2019, 85–152. [Google Scholar]
- Colwell, R.K.; Chao, A.; Gotelli, N.J.; Lin, S.Y.; Mao, C.X.; Chazdon, R.L.; Longino, J.T. Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J. Plant Ecol. 2021, 5, 3–21. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R package version 2.5-6. 2019. Available online: https://CRAN.R-project.org/package=vegan.
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species the need for flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Cáceres, M.; De Legendre, P.; Moretti, M. Improving indicator species analysis by combining groups of sites. Oikos 2010, 119, 1674–1684. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Beja, P.; Santos, C.D.; Santana, J.; Pereira, M.J.; Marques, J.T.; Queiroz, H.L.; Palmeirim, J.M. Seasonal patterns of spatial variation in understory bird assemblages across a mosaic of flooded and unflooded Amazonian forests. Biodivers. Conserv. 2010, 19, 129–152. [Google Scholar] [CrossRef]
- Morais, H.C.; Sujii, E.R.; Almeida-Neto, M.; De-Carvalho, P.S.; Hay, J.D.; Diniz, I.R. Host plant specialization and species turnover of caterpillars among hosts in the Brazilian cerrado. Biotropica 2011, 43, 467–472. [Google Scholar] [CrossRef]
- Haugaasen, T.; Peres, C.A. Vertebrate responses to fruit production in Amazonian flooded and unflooded forests. Biodivers. Conserv. 2007, 16, 4165–4190. [Google Scholar] [CrossRef]
- Hawes, J.E.; Peres, C.A. Patterns of plant phenology in Amazonian seasonally flooded and unflooded forests. Biotropica 2016, 48, 465–475. [Google Scholar] [CrossRef]
- Haugaasen, T.; Peres, C.A. Population abundance and biomass of large-bodied birds in Amazonian flooded and unflooded forests. Bird Conserv. Int. 2008, 18, 87–101. [Google Scholar] [CrossRef]
- Junk, W.J.; Piedade, M.T.F.; Wittmann, F.; Schöngart, J. Várzeas Amazônicas: Desafios para um Manejo Sustentável; Editora do INPA: Manaus, Brazil, 2020; pp. 1–310. [Google Scholar]
- Kirschner, J.A.; Hoorn, C. The onset of grasses in the Amazon drainage basin, evidence from the fossil record. Front. Biogeogr. 2020, 12, e44827. [Google Scholar] [CrossRef]
- Ferreira, L.V. Effects of the duration of flooding on species richness and floristic composition in three hectares in the Jaú National Park in floodplain forests in central Amazonia. Biodivers. Conserv. 1997, 6, 1353–1363. [Google Scholar] [CrossRef]
- Beccaloni, G.W.; Viloria, Á.L.; Hall, S.K.; Robinson, G.S. Catalogue of the hostplants of the Neotropical butterflies/Catálogo de las plantas huésped de las mariposas neotropicales. Monogr. Terc. Milen. 2008, 8, 539. [Google Scholar]
- Muyshondt, A. Notes on the life cycle and natural history of butterflies of El Salvador. VII. Archaeoprepona demophon centralis (Nymphalidae). J. Lepid. Soc. 1979, 30, 23–32. [Google Scholar]
- Brown, K.J.; Hutchings, R.W. Disturbance, fragmentation, and the dynamics of diversity in Amazonian forest butterflies. In Tropical Forest Remnants: Ecology, Management, and Conservation of Fragmented Communities; Laurance, F., Bierregaard, R.O., Jr., Eds.; The University of Chicago Press: Chicago, IL, USA, 1997; pp. 91–110. [Google Scholar]
- Graça, M.B.; Pequeno, P.A.C.L.; Franklin, E.; Souza, J.L.P.; Morais, J.W. Taxonomic, functional, and phylogenetic perspectives on butterfly spatial assembly in northern Amazonia. Ecol. Entomol. 2017, 42, 816–826. [Google Scholar] [CrossRef]
- Brown, K.J. The biology of Heliconius and related genera. Ann. Rev. Entomol. 1981, 26, 427–456. [Google Scholar] [CrossRef]
- Aurivillius, P.O.C. Wissenschaftliche Ergebnisse der schwedischen entomologischen Reisen des Herrn Dr. A. Roman in Amazonas 1914–1915 und 1923–1924. 13. Rhopalocera. Entomol. Tidskrift. 1929, 50, 153–168. [Google Scholar]
- Bates, H.W. Contributions to an insect fauna of the Amazon Valley, Lepidoptera: Heliconidae. Trans. Linn. Soc. Lond. 1862, 23, 495–566. [Google Scholar] [CrossRef]
- Bates, H.W. The Naturalist on the River Amazons; University of California Press: Berkeley, CA, USA, 1864; p. 465. [Google Scholar]
- Ribeiro, D.B.; Freitas, A.V.L.L. The effect of reduced-impact logging on fruit-feeding butterflies in Central Amazon, Brazil. J. Insect Conserv. 2012, 16, 733–744. [Google Scholar] [CrossRef]
- Graça, M.B.; Morais, J.W.; Franklin, E.; Pequeno, P.A.C.L.; Souza, J.L.P.; Bueno, A.S. Combining Taxonomic and Functional Approaches to Unravel the Spatial Distribution of an Amazonian Butterfly Community. Environ. Entomol. 2016, 45, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Spaniol, R.L.; da Silva Duarte, L.; de Souza Mendonça, M.; Iserhard, C.A. Combining functional traits and phylogeny to disentangling Amazonian butterfly assemblages on anthropogenic gradients. Ecosphere 2019, 10, e02837. [Google Scholar] [CrossRef]
- Spaniol, R.L.; de Souza Mendonça, M.; Hartz, S.M.; Iserhard, C.A.; Stevens, M. Discolouring the Amazon Rainforest: How deforestation is affecting butterfly coloration. Biodivers. Conserv. 2020, 29, 2821–2838. [Google Scholar] [CrossRef]
- Ramos, F.A. Nymphalid butterfly communities in an amazonian forest fragment. J. Res. Lepid. 2000, 35, 29–41. [Google Scholar]
- DeVries, P.; Walla, T. Species diversity and community structure in neotropical fruit-feeding butterflies. Biol. J. Linn. Soc. 2001, 74, 1–15. [Google Scholar] [CrossRef]
- Barlow, J.; Overal, W.L.; Araujo, I.S.; Gardner, T.A.; Peres, C.A. The value of primary, secondary and plantation forests for fruit-feeding butterflies in the Brazilian Amazon. J. Appl. Ecol. 2007, 44, 1001–1012. [Google Scholar] [CrossRef]
- da Costa Araujo, E.; Martins, L.P.; Duarte, M.; Azevedo, G.G. Temporal distribution of fruit-feeding butterflies (Lepidoptera, Nymphalidae) in the eastern extreme of the Amazon region. Acta Amaz. 2020, 50, 12–23. [Google Scholar] [CrossRef]
- Emmel, T.C.; Austin, G.T. The Tropical Rain Forest Butterfly Fauna of Rondonia, Brazil: Species Diversity and Conservation. Trop. Lepidotera 1990, 1, 1–12. [Google Scholar]
| Indicator Species | Forest Type | Records | Stat | p |
|---|---|---|---|---|
| Nubila nortia (Hewitson, 1862) | Terra firme | 20 (6) | 100% | 0.001 |
| Bia actorion (Linnaeus, 1763) | Terra firme | 19 (5) | 91.3% | 0.004 |
| Pierella lena brasiliensis (C. Felder & R. Felder, 1862) | Terra firme | 21 (5) | 91.3% | 0.005 |
| Cithaerias aurora (C. Felder & R. Felder, 1862) | Terra firme | 9 (4) | 81.6% | 0.009 |
| Nymphidium baeotia (Hewitson, 1853) | Terra firme | 7 (7) | 83.3% | 0.010 |
| Haetera piera (Linnaeus, 1758) | Terra firme | 5 (4) | 81.6% | 0.014 |
| Pierella chalybaea (Godman, 1905) | Terra firme | 18 (4) | 81.6% | 0.018 |
| Scriptor sphenophorus (Lamas & Nakahara, 2020) | Terra firme | 6 (4) | 81.6% | 0.018 |
| Taygetis laches (Fabricius, 1793) | Terra firme | 4 (4) | 81.6% | 0.019 |
| Pseudodebis marpessa (Hewitson, 1862) | Várzea | 22 (6) | 100% | 0.001 |
| Taygetis mermeria (Cramer, 1776) | Várzea | 42 (5) | 91.3% | 0.004 |
| Magneuptychia ocnus (A. Butler, 1867) | Várzea | 15 (4) | 81.6% | 0.018 |
| Taygetis rufomarginata (Staudinger, 1888) | Várzea | 9 (4) | 81.6% | 0.016 |
| Pseudodebis valentina (Cramer, 1779) | Várzea | 22 (7) | 80.2% | 0.020 |
| Archaeoprepona demophon demophon (Linnaeus, 1758) | Igapó | 6 (5) | 91.3% | 0.002 |
| Heliconius antiochus (Linnaeus, 1767) | Igapó | 12 (6) | 83.3% | 0.014 |
| Hermeuptychia undulata1 (A. Butler, 1867) | Igapó | 6 (4) | 81.6% | 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, I.F.; Baccaro, F.B.; Werneck, F.P.; Zacca, T.; Haugaasen, T. Marked Differences in Butterfly Assemblage Composition between Forest Types in Central Amazonia, Brazil. Forests 2021, 12, 942. https://doi.org/10.3390/f12070942
Oliveira IF, Baccaro FB, Werneck FP, Zacca T, Haugaasen T. Marked Differences in Butterfly Assemblage Composition between Forest Types in Central Amazonia, Brazil. Forests. 2021; 12(7):942. https://doi.org/10.3390/f12070942
Chicago/Turabian StyleOliveira, Isabela Freitas, Fabricio Beggiato Baccaro, Fernanda P. Werneck, Thamara Zacca, and Torbjørn Haugaasen. 2021. "Marked Differences in Butterfly Assemblage Composition between Forest Types in Central Amazonia, Brazil" Forests 12, no. 7: 942. https://doi.org/10.3390/f12070942
APA StyleOliveira, I. F., Baccaro, F. B., Werneck, F. P., Zacca, T., & Haugaasen, T. (2021). Marked Differences in Butterfly Assemblage Composition between Forest Types in Central Amazonia, Brazil. Forests, 12(7), 942. https://doi.org/10.3390/f12070942






