Structure and Composition of Terra Firme and Seasonally Flooded Várzea Forests in the Western Brazilian Amazon
Abstract
1. Introduction
2. Materials and Methods
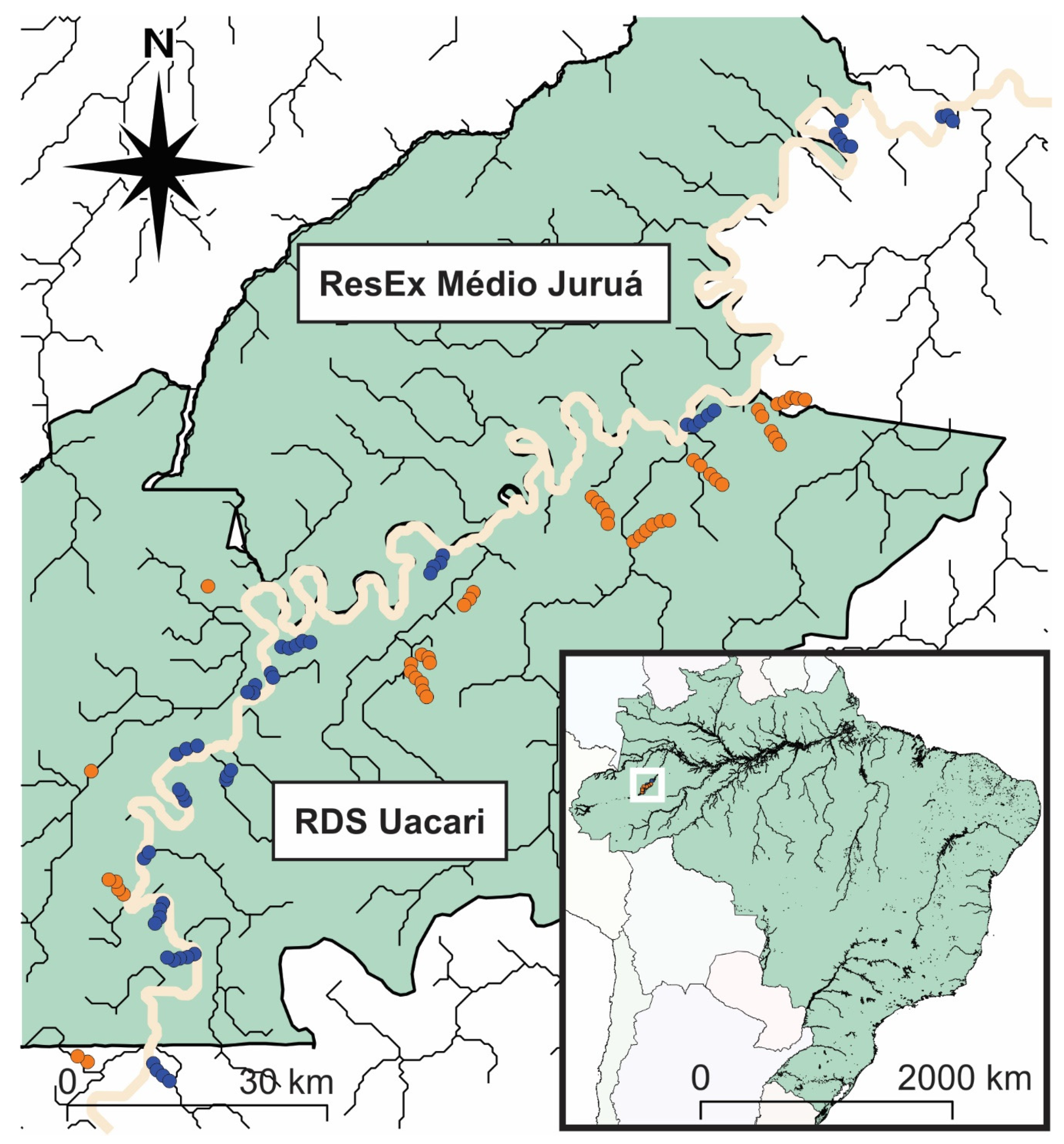
2.1. Study Area
2.2. Floristic Inventories and Measurements
2.3. Data Analyses
3. Results
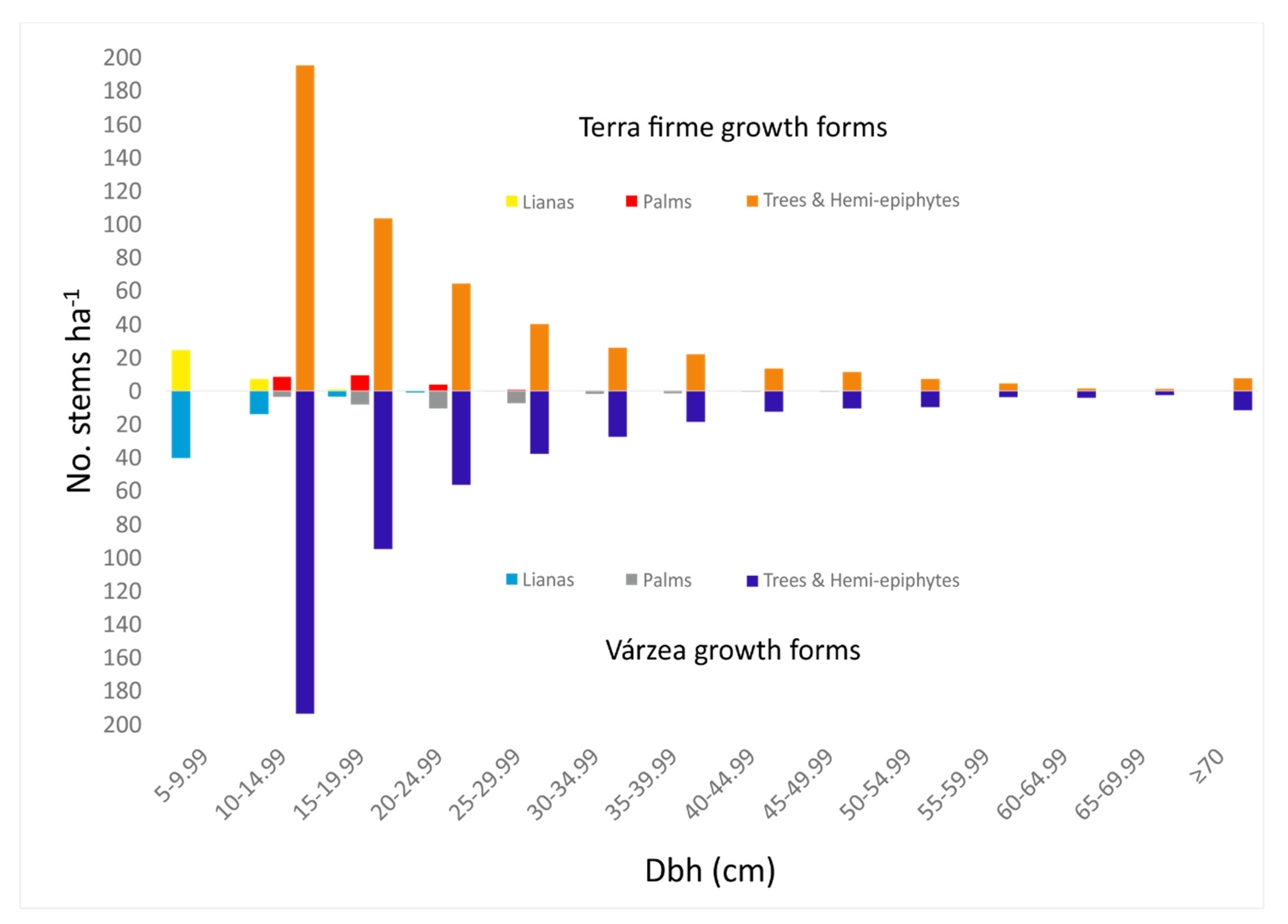
3.1. Forest Structure
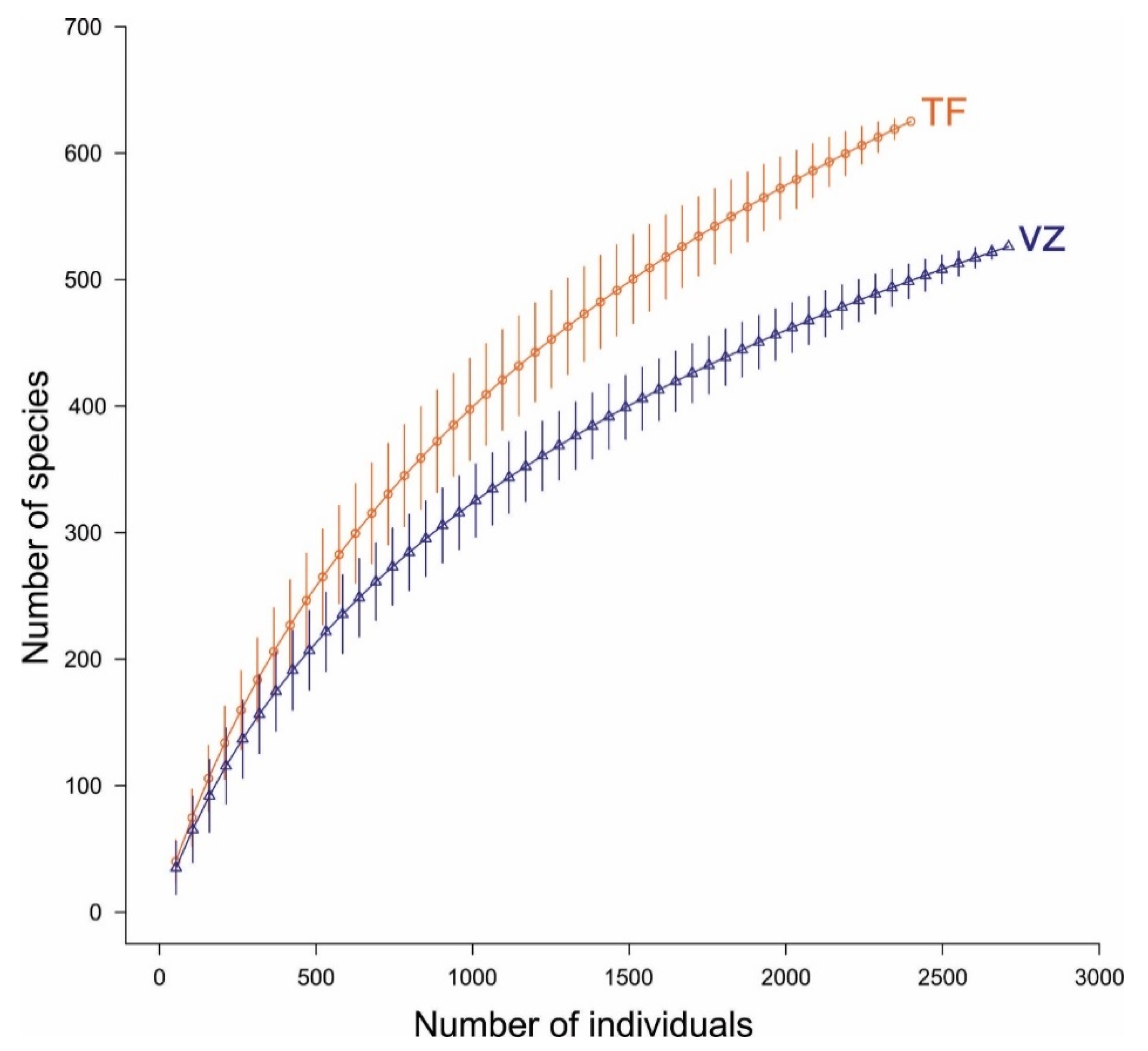
3.2. Floristic Diversity
3.3. Family Importance Value
3.4. Species Importance Value Index
3.5. Community Composition
4. Discussion
4.1. Forest Structure
4.2. Floristic Composition and Diversity
4.3. Important Families and Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Prance, G.T. A comparison of the efficacy of higher taxa and species numbers in the assessment of biodiversity in the neotropics. Philos. Trans. R. Soc. B Biol. Sci. 1994, 345, 89–99. [Google Scholar] [CrossRef]
- Gentry, A.H. Changes in plant community diversity and floristic composition on environmental and geographical gradients. Ann. Mo. Bot. Gard. 1988, 75, 1–34. [Google Scholar] [CrossRef]
- Ter Steege, H.; De Oliveira, S.M.; Pitman, N.; Sabatier, D.; Antonelli, A.; Andino, J.E.G.; Aymard, G.A.; Salomão, R.P. Towards a dynamic list of Amazonian tree species. Sci. Rep. 2019, 9, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, D.; Särkinen, T.; Alexander, S.; Amorim, A.M.; Bittrich, V.; Celis, M.; Daly, D.C.; Fiaschi, P.; Funk, V.A.; Giacomin, L.L.; et al. Amazon plant diversity revealed by a taxonomically verified species list. Proc. Natl. Acad. Sci. USA 2017, 114, 10695–10700. [Google Scholar] [CrossRef] [PubMed]
- Feeley, K.J. Are we filling the data void? An assessment of the amount and extent of plant collection records and census data available for tropical South America. PLoS ONE 2015, 10, e0125629. [Google Scholar] [CrossRef] [PubMed]
- Luize, B.G.; Magalhães, J.L.L.; Queiroz, H.; Lopes, M.A.; Venticinque, E.M.; de Moraes Novo, E.M.L.; Silva, T.S.F. The tree species pool of Amazonian wetland forests: Which species can assemble in periodically waterlogged habitats? PLoS ONE 2018, 13, e0198130. [Google Scholar] [CrossRef]
- Hopkins, M.J.G. Are we close to knowing the plant diversity of the Amazon? An. Acad. Bras. Ciênc. 2018, 91, e20190396. [Google Scholar] [CrossRef]
- Hopkins, M.J.G. Modelling the known and unknown plant biodiversity of the Amazon basin. J. Biogeogr. 2007, 34, 1400–1411. [Google Scholar] [CrossRef]
- Wallace, A.R. A Narrative of Travels on the Amazon and Rio Negro; Ward, Lock: London, UK, 1853; p. 541. [Google Scholar]
- Kalamandeen, M.; Gloor, E.; Mitchard, E.T.A.; Quincey, D.; Ziv, G.; Spracklen, D.; Spracklen, B.; Adami, M.; Aragão, L.; Galbraith, D. Pervasive rise of small-scale deforestation in Amazonia. Sci. Rep. 2018, 8, 1600. [Google Scholar] [CrossRef]
- Aragão, L.E.O.C.; Malhi, Y.; Barbier, N.; Lima, A.; Shimabukuro, Y.; Anderson, L.; Saatchi, S. Interactions between rainfall, deforestation and fires during recent years in the Brazilian Amazonia. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1779–1785. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Kirchhoff, F.T.; Oliveira, B.C.; Ribeiro, J.G.; Sales, M.H. Long-term annual surface water change in the Brazilian Amazon biome: Potential links with deforestation, infrastructure development and climate change. Water 2019, 11, 566. [Google Scholar] [CrossRef]
- de Area Leão Pereira, E.J.; Silveira Ferreira, P.J.; de Santana Ribeiro, L.C.; Sabadini Carvalho, T.; de Barros Pereira, H.B. Policy in Brazil (2016–2019) threaten conservation of the Amazon rainforest. Environ. Sci. Policy 2019, 100, 8–12. [Google Scholar] [CrossRef]
- Esquivel-Muelbert, A.; Baker, T.R.; Dexter, K.G.; Lewis, S.L.; Brienen, R.J.W.; Feldpausch, T.R.; Lloyd, J.; Monteagudo-Mendoza, A.; Arroyo, L.; Álvarez-Dávila, E.; et al. Compositional response of Amazon forests to climate change. Glob. Chang. Biol. 2019, 25, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.H.; Coe, M.T.; Guyot, J.L.; Batistella, M.; Artaxo, P.; Nobre, C.; Bustamante, M.; Luizao, F.J. Effects of climatic variability and deforestation on surface water regimes. In Sea Ice; American Geophysical Union (AGU): Washington, DC, USA, 2009; pp. 543–553. [Google Scholar]
- Hilker, T.; Lyapustin, A.I.; Tucker, C.J.; Hall, F.G.; Myneni, R.B.; Wang, Y.; Bi, J.; De Moura, Y.M.; Sellers, P.J. Vegetation dynamics and rainfall sensitivity of the Amazon. Proc. Natl. Acad. Sci. USA 2014, 111, 16041–16046. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, F.; Marques, M.C.M.; Júnior, G.D.; Budke, J.C.; Piedade, M.T.F.; Wittmann, A.D.O.; Montero, J.C.; De Assis, R.L.; Targhetta, N.; Parolin, P.; et al. The Brazilian freshwater wetscape: Changes in tree community diversity and composition on climatic and geographic gradients. PLoS ONE 2017, 12, e0175003. [Google Scholar] [CrossRef]
- Aleixo, I.; Norris, D.; Hemerik, L.; Barbosa, A.; Prata, E.; Costa, F.; Poorter, L. Amazonian rainforest tree mortality driven by climate and functional traits. Nat. Clim. Chang. 2019, 9, 384–388. [Google Scholar] [CrossRef]
- Aragão, L.E.O.C.; Poulter, B.; Barlow, J.B.; Anderson, L.O.; Malhi, Y.; Saatchi, S.; Phillips, O.L.; Gloor, E. Environmental change and the carbon balance of Amazonian forests. Biol. Rev. 2014, 89, 913–931. [Google Scholar] [CrossRef]
- Sombroek, W. Amazon Soils, Wageningen; Netherlands, Centre for Agricultural Publications and Documentation: Wageningen, The Netherlands, 1965. [Google Scholar]
- Sombroek, W. Amazon landforms and soils in relation to biological diversity. Acta Amaz. 2000, 30, 81. [Google Scholar] [CrossRef]
- Pires, J.M.; Prance, G.T. The vegetation types of the Brazilian Amazon. In Key Environments: AMAZONIA; Prance, G.T., Lovejoy, T.E., Eds.; Pergamon Press: Oxford, UK, 1985; pp. 109–145. [Google Scholar]
- Parolin, P.; Ferreira, L.V.; Albernaz, A.L.K.; Almeida, S.S. Tree species distribution in Várzea forests of Brazilian Amazonia. Folia Geobot. Phytotaxon. 2004, 39, 371–383. [Google Scholar] [CrossRef]
- Junk, W.J.; Piedade, M.T.F. An introduction to South American wetland forests: Distribution, definitions and general characterization. In Forest-Water Interactions; Springer Science and Business Media LLC: Berlin, Germany, 2010; pp. 3–25. [Google Scholar]
- Räsänen, M.E.; Salo, J.S.; Kalliola, R.J. Fluvial perturbance in the western Amazon basin: Regulation by long-term Sub-Andean tectonics. Science 1987, 238, 1398–1401. [Google Scholar] [CrossRef]
- Irion, G. Soil infertility in the Amazonian rain forest. Naturwissenschaften 1978, 65, 515–519. [Google Scholar] [CrossRef]
- Furch, K.; Klinge, H. Chemical relationship between vegetation, soil and water in contrasting inundation areas of Amazonia. In Mineral Nutrients in Tropical Forest and Savanna Ecosystems; Proctor, J., Ed.; Blackwell Scientific Publications: Oxford, UK, 1989; pp. 189–204. ISBN 0-632-02559-X. [Google Scholar]
- Furch, K.; Junk, W.J. Physiochemical conditions in the floodplains. In The Central Amazon Floodplain: Ecology of a Pulsing System; Junk, W.J., Ed.; Springer: Berlin, Germany, 1997; pp. 69–108. ISBN 978-3-642-08214-6. [Google Scholar]
- Wittmann, F.; Schongart, J.; Montero, J.C.; Motzer, T.; Junk, W.J.; Piedade, M.T.F.; Queiroz, H.L.; Worbes, M. Tree species composition and diversity gradients in white-water forests across the Amazon basin. J. Biogeogr. 2006, 33, 1334–1347. [Google Scholar] [CrossRef]
- Normand, S.; Vormisto, J.; Svenning, J.; Grández, C.; Balslev, H. Geographical and environmental controls of palm beta diversity in paleo-riverine terrace forests in Amazonian Peru. Plant Ecol. 2006, 186, 161–176. [Google Scholar] [CrossRef]
- Hawes, J.E.; Peres, C.A. Patterns of plant phenology in Amazonian seasonally flooded and unflooded forests. Biotropica 2016, 48, 465–475. [Google Scholar] [CrossRef]
- GADM Database. 2015. Available online: www.gadm.org (accessed on 4 May 2020).
- HAGLÖF SWEDEN AB Vertex IV and Transponder T3 Manual January 2007, v.1.0; Haglöf Sweden AB: Långsele, Sweden, 2007; pp. 1–27.
- Mori, S.A.; Boom, B.M.; De Carvalino, A.M.; Dos Santos, T.S. Ecological importance of Myrtaceae in an eastern Brazilian wet forest. Biotropica 1983, 15, 68. [Google Scholar] [CrossRef]
- Worbes, M.; Klinge, H.; Revilla, J.D.; Martius, C. On the dynamics, floristic subdivision and geographical distribution of várzea forests in central Amazonia. J. Veg. Sci. 1992, 3, 553–564. [Google Scholar] [CrossRef]
- Kindt, R.; Coe, R. Tree Diversity Analysis. A Manual and Software for Common Statistical Methods for Ecological and Biodiversity Studies; World Agroforestry Centre (ICRAF): Nairobi, Kenya, 2005; ISBN 92-9059-179-X. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; R Package, 2019; Available online: https://CRAN.R-project.org/package=vegan (accessed on 4 May 2020).
- Anderson, M.J. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 2005, 62, 245–253. [Google Scholar] [CrossRef]
- Anderson, M.J.; Ellingsen, K.E.; McArdle, B.H. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 2006, 9, 683–693. [Google Scholar] [CrossRef]
- Stier, A.; Geange, S.W.; Hanson, K.M.; Bolker, B.M. Predator density and timing of arrival affect reef fish community assembly. Ecology 2013, 94, 1057–1068. [Google Scholar] [CrossRef]
- O’Neill, M.E.; Mathews, K.L. A weighted least squares approach to Levene’s test of homogeneity of variance. Aust. N. Z. J. Stat. 2000, 42, 81–100. [Google Scholar] [CrossRef]
- R Environment R Core Team. R: A Language and Environment for Statistical Computing; R Environment R Core Team, 2018; Available online: https://www.r-project.org/ (accessed on 4 May 2020).
- Wittmann, A.D.O.; Schöngart, J.; Junk, W.J. Phytogeography, species diversity, community structure and dynamics of central Amazonian floodplain forests. In Forest-Water Interactions; Springer Science and Business Media LLC: Berlin, Germany, 2010; pp. 61–102. [Google Scholar]
- Montgomery, R.A.; Chazdon, R.L. Forest structure, canopy architecture, and light transmittance in tropical wet forests. Ecology 2001, 82, 2707–2718. [Google Scholar] [CrossRef]
- Myster, R.W. The physical structure of forests in the Amazon basin: A review. Bot. Rev. 2016, 82, 407–427. [Google Scholar] [CrossRef]
- Laurance, W.F.; Pérez-Salicrup, D.; Delamônica, P.; Fearnside, P.M.; D’Angelo, S.; Jerozolinski, A.; Pohl, L.; Lovejoy, T.E. Rain forest fragmentation and the structure of Amazonian liana communities. Ecology 2001, 82, 105–116. [Google Scholar] [CrossRef]
- Campbell, M.J.; Magrach, A.; Laurance, S. Liana diversity and the future of tropical forests. In Sustainable Development and Biodiversity; Springer Science and Business Media LLC: Berlin, Germany, 2015; Volume 5, pp. 255–274. [Google Scholar]
- Gaglioti, A.L.; Aguiar, D.P.P. Cecropia in Flora do Brasil 2020 em construção. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB24951 (accessed on 4 September 2020).
- Parolin, P.; Oliveira, A.C.; Piedade, M.T.F.; Wittmann, F.; Junk, W.J. Pioneer trees in Amazonian floodplains: Three key species form monospecific stands in different habitats. Folia Geobot. Phytotaxon. 2002, 37, 225–238. [Google Scholar] [CrossRef]
- Parolin, P. Life history and environment of Cecropia latiloba in Amazonian floodplains. Rev. Biol. Trop. 2002, 50, 531–545. [Google Scholar]
- De Castilho, C.V.; Magnusson, W.E.; De Araújo, R.N.O.; Luizão, R.C.; Luizão, F.J.; Lima, A.P.; Higuchi, N. Variation in aboveground tree live biomass in a central Amazonian Forest: Effects of soil and topography. For. Ecol. Manag. 2006, 234, 85–96. [Google Scholar] [CrossRef]
- Emilio, T.; Quesada, C.A.; Costa, F.R.C.; Magnusson, W.E.; Schietti, J.; Feldpausch, T.R.; Brienen, R.J.W.; Baker, T.R.; Chave, J.; Álvarez, E.; et al. Soil physical conditions limit palm and tree basal area in Amazonian forests. Plant Ecol. Divers. 2013, 7, 215–229. [Google Scholar] [CrossRef]
- Nebel, G.; Kvist, L.P.; Vanclay, J.K.; Christensen, H.; Freitas, L.; Ruíz, J. Structure and floristic composition of flood plain forests in the Peruvian Amazon. For. Ecol. Manag. 2001, 150, 27–57. [Google Scholar] [CrossRef]
- Parolin, P. Radial gradients in wood specific gravity in trees of central Amazonian floodplains. IAWA J. 2002, 23, 449–457. [Google Scholar] [CrossRef]
- Muller-Landau, H.C. Interspecific and inter-site variation in wood specific gravity of tropical trees. Biotropica 2004, 36, 20. [Google Scholar] [CrossRef]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef]
- Bredin, Y.K.; Peres, C.A.; Haugaasen, T. Forest type affects the capacity of Amazonian tree species to store carbon as woody biomass. For. Ecol. Manag. 2020, 473, 118297. [Google Scholar] [CrossRef]
- Schietti, J.; Martins, D.; Emilio, T.; Souza, P.F.; Levis, C.; Baccaro, F.B.; Pinto, J.L.P.d.V.; Moulatlet, G.M.; Stark, S.C.; Sarmento, K.; et al. Forest structure along a 600 km transect of natural disturbances and seasonality gradients in central-southern Amazonia. J. Ecol. 2016, 104, 1335–1346. [Google Scholar] [CrossRef]
- de Jesus Veiga Carim, M.; Wittmann, F.K.; Piedade, M.T.F.; a Silva Guimarães, J.R.; de Cássia Leôncio Tostes, L. Composition, diversity, and structure of tidal “Várzea” and “Igapó” floodplain forests in eastern Amazonia, Brazil. Braz. J. Bot. 2016, 40, 115–124. [Google Scholar] [CrossRef]
- Parolin, P.; De Simone, O.; Haase, K.; Waldhoff, D.; Rottenberger, S.; Kuhn, U.; Kesselmeier, J.; Kleiss, B.; Schmidt, W.; Pledade, M.T.F.; et al. Central Amazonian floodplain forests: Tree adaptations in a pulsing system. Bot. Rev. 2004, 70, 357–380. [Google Scholar] [CrossRef]
- Junk, W.J.; Piedade, M.T.F.; Schöngart, J.; Wittmann, F.; Parolin, P. (Eds.) Part II Ecological and ecophysiological aspects of Amazonian floodplain forests. In Amazonian Floodplain Forests Ecophysiology, Biodiversity and Sustainable Management; Springer: Berlin, Germany, 2010; pp. 105–313. ISBN 978-90-481-8724-9. [Google Scholar]
- Parolin, P. Submerged in darkness: Adaptations to prolonged submergence by woody species of the Amazonian floodplains. Ann. Bot. 2008, 103, 359–376. [Google Scholar] [CrossRef]
- Parolin, P. Morphological and physiological adjustments to waterlogging and drought in seedlings of Amazonian floodplain trees. Oecologia 2001, 128, 326–335. [Google Scholar] [CrossRef]
- Wittmann, F.; Anhuf, D.; Funk, W.J. Tree species distribution and community structure of central Amazonian várzea forests by remote-sensing techniques. J. Trop. Ecol. 2002, 18, 805–820. [Google Scholar] [CrossRef]
- Hess, L. Dual-season mapping of wetland inundation and vegetation for the central Amazon basin. Remote Sens. Environ. 2003, 87, 404–428. [Google Scholar] [CrossRef]
- Ter Steege, H. Contribution of current and historical processes to patterns of tree diversity and composition in the Amazon. In Amazonia: Landscape and Species Evolution. A Look into the Past; Hoorn, C., Wesselingh, F.P., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; ISBN 9781444306408. [Google Scholar]
- Quesada, C.A.; Lloyd, J.; Schwarz, M.; Patiño, S.; Baker, T.R.; Czimczik, C.; Fyllas, N.M.; Martinelli, L.; Nardoto, G.B.; Schmerler, J.; et al. Variations in chemical and physical properties of Amazon forest soils in relation to their genesis. Biogeosciences 2010, 7, 1515–1541. [Google Scholar] [CrossRef]
- Zuquim, G.; Costa, F.R.C.; Tuomisto, H.; Moulatlet, G.M.; Figueiredo, F.O.G. The importance of soils in predicting the future of plant habitat suitability in a tropical forest. Plant Soil 2019, 450, 151–170. [Google Scholar] [CrossRef]
- Ter Steege, H.; Pitman, N.C.A.; Phillips, O.L.; Chave, J.; Sabatier, D.; Duque, A.; Molino, J.-F.; Prévost, M.-F.; Spichiger, R.; Castellanos, H.; et al. Continental-scale patterns of canopy tree composition and function across Amazonia. Nat. Cell Biol. 2006, 443, 444–447. [Google Scholar] [CrossRef]
- Quesada, C.A.; Phillips, O.L.; Schwarz, M.; Czimczik, C.I.; Baker, T.R.; Patiño, S.; Fyllas, N.M.; Hodnett, M.G.; Herrera, R.; Almeida, S.; et al. Basin-wide variations in Amazon forest structure and function are mediated by both soils and climate. Biogeosciences 2012, 9, 2203–2246. [Google Scholar] [CrossRef]
- Ruokolainen, K.; Moulatlet, G.M.; Zuquim, G.; Hoorn, C.; Tuomisto, H. Geologically recent rearrangements in central Amazonian river network and their importance for the riverine barrier hypothesis. Front. Biogeogr. 2019, 11, e45046. [Google Scholar] [CrossRef]
- Wittmann, F.; Junk, W.J.; Piedade, M.T. The várzea forests in Amazonia: Flooding and the highly dynamic geomorphology interact with natural forest succession. For. Ecol. Manag. 2004, 196, 199–212. [Google Scholar] [CrossRef]
- Haugaasen, T.; Peres, C.A. Floristic, edaphic and structural characteristics of flooded and unflooded forests in the lower Rio Purús region of central Amazonia, Brazil. Acta Amaz. 2006, 36, 25–35. [Google Scholar] [CrossRef]
- Prance, G.T.; Rodrigues, W.A.; Da Silva, M.F. Inventário florestal de um hectare de mata de terra firme km 30 da Estrada Manaus-Itacoatiara. Acta Amaz. 1976, 6, 9–35. [Google Scholar] [CrossRef]
- Lima Filho, D.A.; Matos, F.D.A.; Amaral, L.; Revilla, J.D.; Coelho, L.S.; Ramos, J.F.; Santos, J.L. Inventário florístico de floresta ombrófila densa de terra firme, na região do Rio Urucu-Amazonas, Brasil. Acta Amaz. 2001, 31, 565. [Google Scholar] [CrossRef]
- Balslev, H.; Luteyn, J.L.; Øllgaard, B.; Holm-Nielsen, L.B. Composition and structure of adjacent unflooded and floodplain forest in Amazonian Ecuador. Opera Bot. 1987, 92, 37–57. [Google Scholar]
- Boom, B.M. A forest inventory in Amazonian Bolivia. Biotropica 1986, 18, 287. [Google Scholar] [CrossRef]
- Faber-Langendoen, N.; Gentry, A.H. The structure and diversity of rain forests at Bajo Calima, Choco region, western Colombia. Biotropica 1991, 23, 2–11. [Google Scholar] [CrossRef]
- Campbell, D.G.; Daly, D.C.; Prance, G.T.; Maciel, U.N. Quantitative ecological inventory of terra firme and varzea tropical forest on the Rio Xingu, Brazilian Amazon. Brittonia 1986, 38, 369–393. [Google Scholar] [CrossRef]
- Almeida, S.S.; Lisboa, P.L.B.; Silva, A.S.L. Diversidade florística de uma comunidade arbórea na estação cientifíca ‘Ferreira Penna’, em Caxiuanã (Pará). Bol. Mus. Para. Emílio Goeldi Sér. Bot. 1993, 9, 93–128. [Google Scholar]
- De Assis, R.; Wittmann, A.D.O.; Bredin, Y.K.; Schöngart, J.; Quesada, C.A.N.; Piedade, M.T.F.; Haugaasen, T. Above-ground woody biomass distribution in Amazonian floodplain forests: Effects of hydroperiod and substrate properties. For. Ecol. Manag. 2019, 432, 365–375. [Google Scholar] [CrossRef]
- ter Steege, H.; Prado, P.I.; Lima, R.A.F.d.; Pos, E.; de Souza Coelho, L.; de Andrade Lima Filho, D.; Salomão, R.P.; Amaral, I.L.; de Almeida Matos, F.D.; Castilho, C.V.; et al. Biased-corrected richness estimates for the Amazonian tree flora. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Peacock, J.; Cerón, C.E.; Aragão, L.E.O.C.; Réjou-Méchain, M.; Levis, C.; van der Hout, P.; van der Meer, P.J.; de Oliveira, E.A.; Huamantupa-Chuquimaco, I.; Laurance, S.G.W.; et al. Hyperdominance in Amazonian forest carbon cycling. Nat. Commun. 2015, 6, 6857. [Google Scholar] [CrossRef]
- Albernaz, A.K.M.; Ayres, J.M. Selective logging along the middle Solimoes River. Adv. Econ. Bot. 1999, 13, 135–151. [Google Scholar]
- Marinho, T.A.D.S.; Piedade, M.T.F.; Wittmann, A.D.O. Distribution and population structure of four central Amazonian high-várzea timber species. Wetl. Ecol. Manag. 2010, 18, 665–677. [Google Scholar] [CrossRef]
- Mesquita de Azevedo, L.A.; da Silva Cruz, F.A.; Dias, A.; Batista, G.; Jeanne, G.d.S.; de Amaral Carvalho, J.; Kasecker, T.; Santiago, H.C.; Lederman, M.R.; Bendezú Estupiñán, G.M.; et al. Plano de gestão da reserva de desenvolvimento sustentável de Uacari. Management Plan, 222; Governo do estado; Secretária de estado do meio ambiente e desenvolvimento sustentável; Centro estadual de unidades de conservação: Amazonas, Carauari, Brazil, 2010.
- Menores, A.; Grande, R.; Reserva, N. Myristicaceae. In Flora da Reserva Ducke: Guia de identificação das plantas vasculares de uma floresta de terra-firme na Amazônia central; Ribeiro, J.E.L.d.S., Hopkins, M.J.C., Virentini, A., Suthers, C.A., Costa, M.A.d.S., de Brito, J.M., de Souza, M.A.D., Martins, H.P., Lohmann, L.G., Assuncão, P.A.C.L., et al., Eds.; INPA: Manaus, Brazil, 1999; pp. 136–145. [Google Scholar]
- Mundo, V. Lecythidaceae. In Flora da Reserva Ducke: Guia de identificação das plantas vasculares de uma floresta de terra-firme na Amazônia central; Ribeiro, J.E.L.d.S., Hopkins, M.J.C., Virentini, A., Suthers, C.A., Costa, M.A.d.S., de Brito, J.M., de Souza, M.A.D., Martins, H.P., Lohmann, L.G., Assuncão, P.A.C.L., et al., Eds.; INPA: Manaus, Brazil, 1999; pp. 274–287. ISBN 13 9788521100119. [Google Scholar]
- Puig, H.; Fabre, A. Survival and growth of Iryanthera hostmannii seedlings and juveniles in the tropical rainforest of French Guyana. J. Trop. Ecol. 1997, 13, 139–143. [Google Scholar] [CrossRef]
- Marimon, B.S.; Felfili, J.M.; Haridasan, M. Studies in monodominant forests in eastern Mato Grosso, Brazil: I. A forest of Brosimum rubescens Taub. Edinb. J. Bot. 2001, 58, 123–137. [Google Scholar] [CrossRef]
- Wittmann, F.; Schöngart, J.; De Brito, J.M.; de Oliveira Wittmann, A.; Fernandez Piedade, M.T.; Parolin, P.; Junk, W.J.W.J.; Guillaumet, J.-L.J.L. Manual of Trees from Central Amazonian Várzea Floodplains; Cohn-Haft, M., Kossmann Ferraz, I.D., Eds.; Editora INPA: Manaus, Brazil, 2010; ISBN 978-85-211-0067-6. [Google Scholar]

| TF | VZ | Total | ||
|---|---|---|---|---|
| Plots | 46 | 51 | 97 | |
| Ha. | 4.60 | 5.10 | 9.70 | |
| Stems | Trees (%) | 2288 (89.80) | 2443 (83.24) | 4731 (86.28) |
| Hemi-ep. (%) | 5 (0.20) | 22 (0.75) | 27 (0.49) | |
| Palms (%) | 104 (4.08) | 170 (5.79) | 274 (5.00) | |
| Lianas (%) | 151 (5.93) | 300 (10.22) | 451 (8.23) | |
| Total (%) | 2548 (100.00) | 2935 (100.00) | 5483 (100.00) | |
| Hollow (%) | 34 (1.33) | 63 (2.15) | 97 (1.77) | |
| Plot mean ± sd | 55.39 ± 11.07 | 57.55 ± 12.29 | 56.53 ± 11.72 | |
| Inds. | Trees (%) | 2282 (89.77) | 2408 (83.12) | 4690 (86.23) |
| Hemi-ep. (%) | 5 (0.20) | 20 (0.69) | 25 (0.46) | |
| Palms (%) | 104 (4.09) | 170 (5.87) | 274 (5.04) | |
| Lianas (%) | 151 (5.94) | 299 (10.32) | 450 (8.27) | |
| Total (%) | 2542 (100.00) | 2897 (100.00) | 5439 (100) | |
| Multi-stemmed (%) | 4 (0.16) | 30 (1.04) | 34 (0.63) | |
| Mean dbh ± sd, cm | Trees | 21.85 ± 13.40 | 22.71 ± 16.09 | 22.29 ± 14.85 |
| Hemi-ep. | 27.28 ± 9.36 | 44.05 ± 42.81 | 40.94 ± 39.22 | |
| Palms *** | 16.65 ± 4.60 | 22.93 ± 6.84 | 20.54 ± 6.80 | |
| Lianas | 8.48 ± 2.79 | 9.14 ± 4.04 | 8.92 ± 3.68 | |
| Total | 20.85 ± 13.17 | 21.50 ± 15.93 | 21.40 ± 14.71 | |
| BA, m2 | Tree | 118.03 | 148.59 | 266.62 |
| Hemi-ep. | 0.32 | 6.38 | 6.70 | |
| Palm | 2.44 | 7.64 | 10.08 | |
| Liana | 0.94 | 2.35 | 3.29 | |
| Total | 121.73 | 164.96 | 286.69 | |
| Plot mean ± sd * | 2.65 ± 0.71 | 3.23 ± 1.18 | 2.96 ± 1.03 | |
| Mean height ± sd, m | Tree *** | 20.16 ± 7.40 | 16.20 ± 7.71 | 18.12 ± 7.81 |
| Hemi-ep. | 27.67 ± 8.74 | 24.30 ± 7.95 | 24.89 ± 7.92 | |
| Palm | 17.88 ± 5.94 | 16.90 ± 6.12 | 17.27 ± 6.06 | |
| Overall *** | 20.07 ± 7.36 | 16.29 ± 7.64 | 18.10 ± 7.74 | |
| Overall height, m | Min | 3.00 | 1.70 | 1.70 |
| Max | 50.00 | 47.37 | 50.00 | |
| Median | 19.00 | 15.00 | 16.43 | |
| Mode | 20.00 | 10.00 | 15.00 |
| TF | VZ | Total | ||
|---|---|---|---|---|
| Spp. | Trees (N/A, %) | 576 (4.08) | 466 (4.98) | 847 (4.54) |
| Hemi-ep. (N/A, %) | 3 (0.00) | 9 (5.00) | 11 (4.00) | |
| Palms (N/A, %) | 7 (4.81) | 5 (0.00) | 9 (1.82) | |
| Lianas (N/A, %) | 41 (29.80) | 58 (21.74) | 79 (24.44) | |
| Total (N/A, %) | 625 (5.63) | 526 (6.42) | 931 (6.05) | |
| Unique (%) | 405 (43.50) | 306 (32.87) | 711 (76.37) | |
| Singleton (%) | 285 (45.60) | 222 (42.21) | 314 (33.73) | |
| Gen. | Trees (N/A, %) | 214 (2.50) | 188 (1.00) | 273 (1.73) |
| Hemi-ep. (N/A, %) | 2 (0.00) | 2 (0.00) | 2 (0.00) | |
| Palms (N/A, %) | 7 (3.85) | 4 (0.00) | 7 (1.46) | |
| Lianas (N/A, %) | 31 (23.18) | 45 (14.72) | 54 (17.56) | |
| Total (N/A, %) | 247 (3.78) | 226 (2.35) | 317 (3.02) | |
| Unique (%) | 91 (28.71) | 70 (22.08) | 161 (50.79) | |
| Singleton (%) | 66 (26.72) | 42 (18.58) | 56 (17.67) | |
| Fam. | Trees (N/A, %) | 63 (1.97) | 53 (0.58) | 67 (1.26) |
| Hemi-ep. (N/A, %) | 2 (0.00) | 2 (0.00) | 2 (0.00) | |
| Palms (N/A, %) | 1 (0.00) | 1 (0.00) | 1 (0.00) | |
| Lianas (N/A, %) | 17 (17.88) | 23 (12.71) | 28 (14.44) | |
| Total (N/A, %) | 69 (2.83) | 63 (1.79) | 77 (2.28) | |
| Unique (%) | 14 (18.18) | 8 (10.39) | 22 (28.57) | |
| Singleton (%) | 9 (13.04) | 4 (6.35) | 6 (7.79) |
| Family | No. Inds. | BA (m2) | No. Spp. | Rel. Den. | Rel. Dom. | Rel. Div. | FIV |
|---|---|---|---|---|---|---|---|
| Leguminosae | 286 | 16.12 | 98 | 11.58 | 13.67 | 15.68 | 40.93 |
| Lecythidaceae | 383 | 20.70 | 30 | 15.51 | 17.55 | 4.80 | 37.86 |
| Sapotaceae | 163 | 9.54 | 47 | 6.60 | 8.09 | 7.52 | 22.21 |
| Chrysobalanaceae | 186 | 9.61 | 33 | 7.53 | 8.15 | 5.28 | 20.96 |
| Myristicaceae | 203 | 8.49 | 21 | 8.22 | 7.20 | 3.36 | 18.78 |
| Moraceae | 134 | 8.68 | 31 | 5.43 | 7.36 | 4.96 | 17.75 |
| Lauraceae | 91 | 6.50 | 30 | 3.68 | 5.51 | 4.80 | 14.00 |
| Burseraceae | 114 | 3.03 | 34 | 4.62 | 2.57 | 5.44 | 12.62 |
| Urticaceae | 73 | 4.35 | 17 | 2.96 | 3.69 | 2.72 | 9.36 |
| Malvaceae | 89 | 2.26 | 21 | 3.60 | 1.92 | 3.36 | 8.88 |
| Subtotal | 1722 | 89.26 | 362 | 69.72 | 75.70 | 57.92 | 203.34 |
| Remaining | 748 | 28.65 | 263 | 30.28 | 24.30 | 42.08 | 96.66 |
| Total | 2470 | 117.91 | 625 | 100 | 100 | 100 | 300 |
| Family | No. Inds. | BA (m2) | No. Spp. | Rel. Den. | Rel. Dom. | Rel. Div. | FIV |
|---|---|---|---|---|---|---|---|
| Leguminosae | 357 | 20.46 | 73 | 12.55 | 12.55 | 13.88 | 38.98 |
| Lecythidaceae | 201 | 19.05 | 22 | 7.07 | 11.69 | 4.18 | 22.94 |
| Sapotaceae | 201 | 13.66 | 38 | 7.07 | 8.38 | 7.22 | 22.67 |
| Annonaceae | 279 | 8.70 | 35 | 9.81 | 5.34 | 6.65 | 21.80 |
| Euphorbiaceae | 138 | 17.05 | 22 | 4.85 | 10.46 | 4.18 | 19.50 |
| Malvaceae | 134 | 9.16 | 24 | 4.71 | 5.62 | 4.56 | 14.89 |
| Arecaceae | 170 | 7.64 | 5 | 5.98 | 4.69 | 0.95 | 11.61 |
| Urticaceae | 65 | 8.21 | 15 | 2.28 | 5.04 | 2.85 | 10.18 |
| Myristicaceae | 107 | 7.66 | 8 | 3.76 | 4.70 | 1.52 | 9.98 |
| Moraceae | 64 | 4.15 | 22 | 2.25 | 2.55 | 4.18 | 8.98 |
| Subtotal | 1716 | 115.75 | 264 | 60.32 | 71.01 | 50.19 | 181.51 |
| Remaining | 1129 | 47.26 | 262 | 39.68 | 28.99 | 49.81 | 118.49 |
| Total | 2845 | 163.01 | 526 | 100 | 100 | 100 | 300 |
| No. | Species | Family | G.F. | No. Inds. | BA (m2) | Plot occ. | Rel. Den. | Rel. Dom. | Rel. Freq. | IVI |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Eschweilera coriacea (DC.) S.A.Mori | Lecythidaceae | t, l | 87 | 4.13 | 33 | 3.63 | 3.58 | 1.79 | 8.99 |
| 2 | Eschweilera wachenheimii (Benoist) Sandwith | Lecythidaceae | t | 103 | 3.11 | 36 | 4.29 | 2.69 | 1.95 | 8.94 |
| 3 | Eschweilera truncata A.C.Sm. | Lecythidaceae | t | 59 | 3.20 | 18 | 2.46 | 2.77 | 0.98 | 6.21 |
| 4 | Euterpe precatoria Mart. | Arecaceae | p | 55 | 1.07 | 17 | 2.29 | 0.93 | 0.92 | 4.14 |
| 5 | Eschweilera grandiflora (Aubl.) Sandwith | Lecythidaceae | t | 37 | 1.80 | 18 | 1.54 | 1.56 | 0.98 | 4.08 |
| 6 | Osteophloeum platyspermum (Spruce ex A.DC.) Warb. | Myristicaceae | t | 23 | 2.34 | 17 | 0.96 | 2.03 | 0.92 | 3.91 |
| 7 | Pouteria guianensis Aubl. | Sapotaceae | t | 30 | 1.74 | 20 | 1.25 | 1.50 | 1.09 | 3.84 |
| 8 | Iryanthera hostmannii (Benth.) Warb. | Myristicaceae | t | 36 | 1.34 | 21 | 1.50 | 1.16 | 1.14 | 3.80 |
| 9 | Cariniana micrantha Ducke | Lecythidaceae | t | 10 | 3.27 | 9 | 0.42 | 2.83 | 0.49 | 3.73 |
| 10 | Brosimum rubescens Taub. | Moraceae | t | 15 | 2.26 | 13 | 0.63 | 1.95 | 0.71 | 3.29 |
| 10 | Subtotal | - | - | 455 | 24.26 | 202 | 18.97 | 20.99 | 10.97 | 50.92 |
| 615 | Remaining | - | - | 1944 | 91.30 | 1640 | 81.03 | 79.01 | 89.03 | 249.08 |
| 625 | Grand total | - | - | 2399 | 115.56 | 1842 | 100 | 100 | 100 | 300 |
| No. | Species | Family | G.F. | No. Inds. | BA (m2) | Plot occ. | Rel. Den. | Rel. Dom. | Rel. Freq. | IVI |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Hura crepitans L. | Euphorbiaceae | t | 14 | 11.68 | 9 | 0.52 | 7.47 | 0.50 | 8.49 |
| 2 | Virola surinamensis (Rol. ex Rottb.) Warb. | Myristicaceae | t | 56 | 5.98 | 26 | 2.07 | 3.83 | 1.45 | 7.34 |
| 3 | Eschweilera ovalifolia (DC.) Nied. | Lecythidaceae | t | 50 | 5.75 | 22 | 1.84 | 3.68 | 1.23 | 6.75 |
| 4 | Astrocaryum jauari Mart. | Arecaceae | p | 59 | 2.88 | 11 | 2.18 | 1.85 | 0.61 | 4.64 |
| 5 | Garcinia madruno (Kunth) Hammel | Clusiaceae | t | 57 | 1.61 | 20 | 2.10 | 1.03 | 1.12 | 4.25 |
| 6 | Tapura juruana (Ule) Rizzini | Dichapetalaceae | t | 32 | 2.66 | 22 | 1.18 | 1.70 | 1.23 | 4.11 |
| 7 | Leonia glycycarpa Ruiz & Pav. | Violaceae | t | 44 | 1.60 | 25 | 1.62 | 1.02 | 1.39 | 4.04 |
| 8 | Eschweilera parviflora (Aubl.) Miers | Lecythidaceae | t | 35 | 2.65 | 18 | 1.29 | 1.70 | 1.00 | 3.99 |
| 9 | Pouteria glomerata (Miq.) Radlk. | Sapotaceae | t | 39 | 2.08 | 19 | 1.44 | 1.33 | 1.06 | 3.83 |
| 10 | Himatanthus sucuuba (Spruce ex Müll.Arg.) Woodson | Apocynaceae | t | 38 | 2.12 | 19 | 1.40 | 1.36 | 1.06 | 3.82 |
| 10 | Subtotal | - | - | 424 | 39.02 | 191 | 15.64 | 24.96 | 10.65 | 51.26 |
| 516 | Remaining | - | - | 2287 | 117.29 | 1602 | 84.36 | 75.04 | 89.35 | 248.74 |
| 526 | Grand total | - | - | 2711 | 156.31 | 1793 | 100 | 100 | 100 | 300 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bredin, Y.K.; Hawes, J.E.; Peres, C.A.; Haugaasen, T. Structure and Composition of Terra Firme and Seasonally Flooded Várzea Forests in the Western Brazilian Amazon. Forests 2020, 11, 1361. https://doi.org/10.3390/f11121361
Bredin YK, Hawes JE, Peres CA, Haugaasen T. Structure and Composition of Terra Firme and Seasonally Flooded Várzea Forests in the Western Brazilian Amazon. Forests. 2020; 11(12):1361. https://doi.org/10.3390/f11121361
Chicago/Turabian StyleBredin, Yennie K., Joseph E. Hawes, Carlos A. Peres, and Torbjørn Haugaasen. 2020. "Structure and Composition of Terra Firme and Seasonally Flooded Várzea Forests in the Western Brazilian Amazon" Forests 11, no. 12: 1361. https://doi.org/10.3390/f11121361
APA StyleBredin, Y. K., Hawes, J. E., Peres, C. A., & Haugaasen, T. (2020). Structure and Composition of Terra Firme and Seasonally Flooded Várzea Forests in the Western Brazilian Amazon. Forests, 11(12), 1361. https://doi.org/10.3390/f11121361





