The Role of Canopy Cover Dynamics over a Decade of Changes in the Understory of an Atlantic Beech-Oak Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Study Design and Variable Survey
2.3. Data Analysis
3. Results
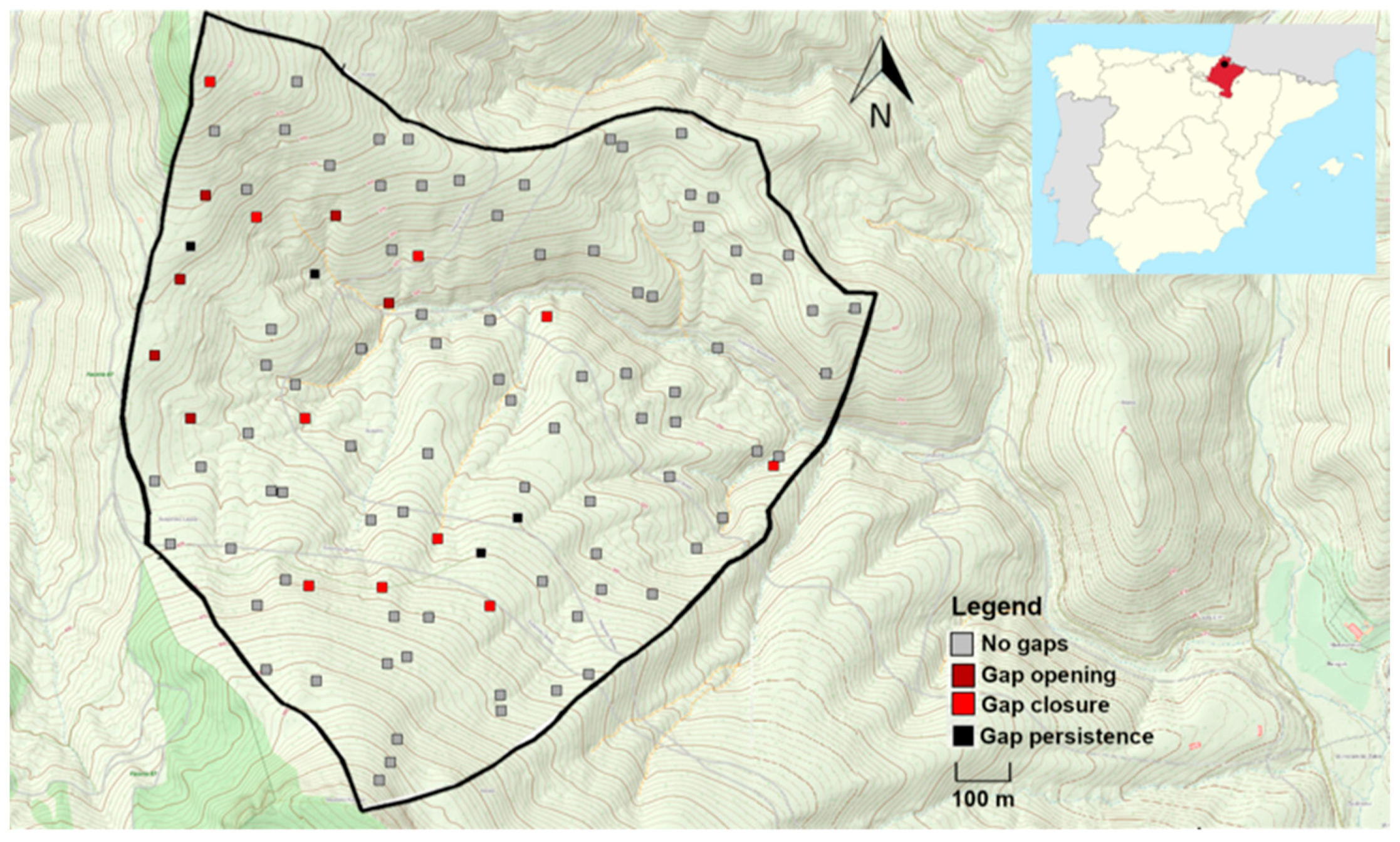
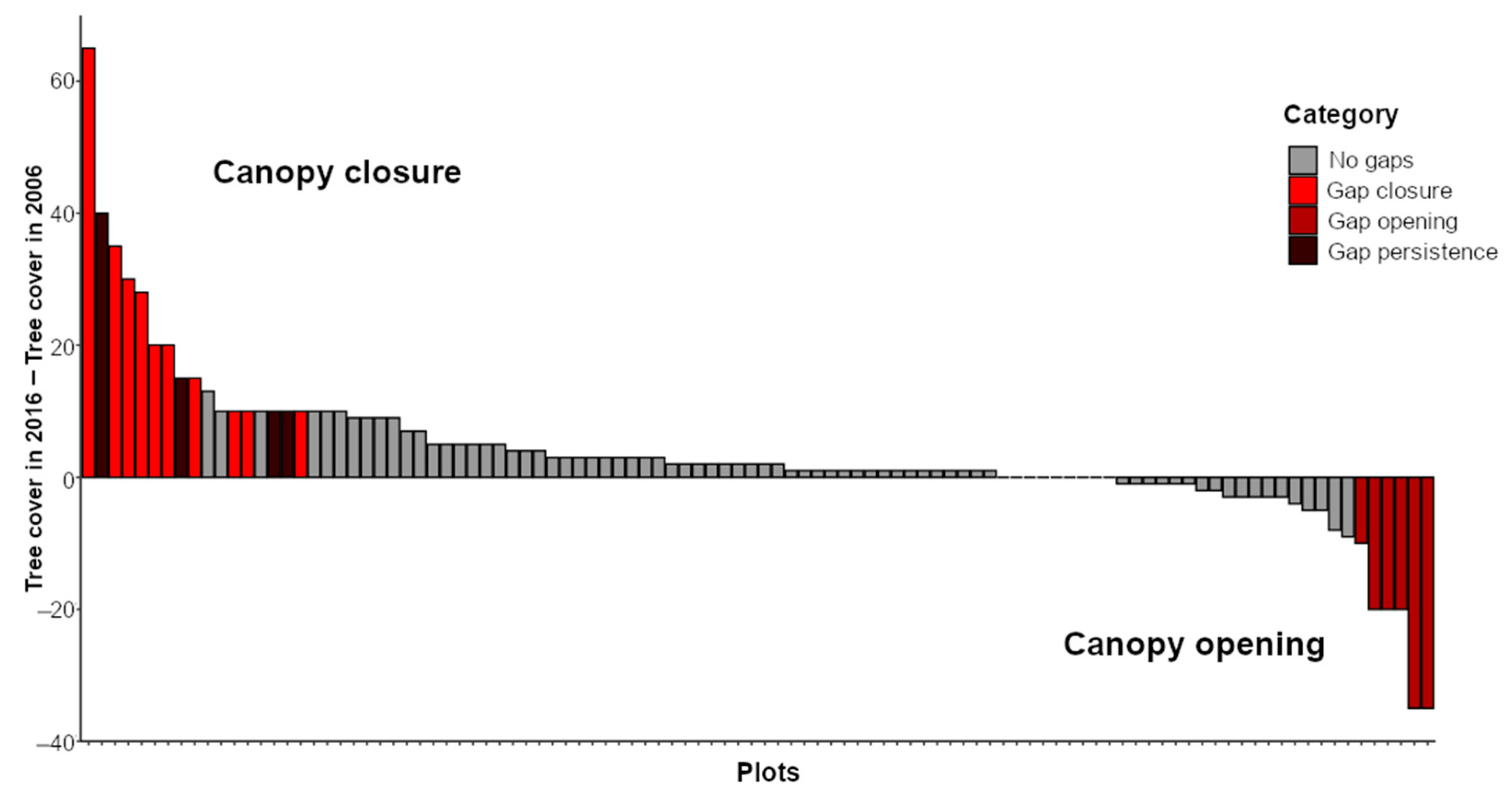
3.1. Frequency and Dynamism of Gaps
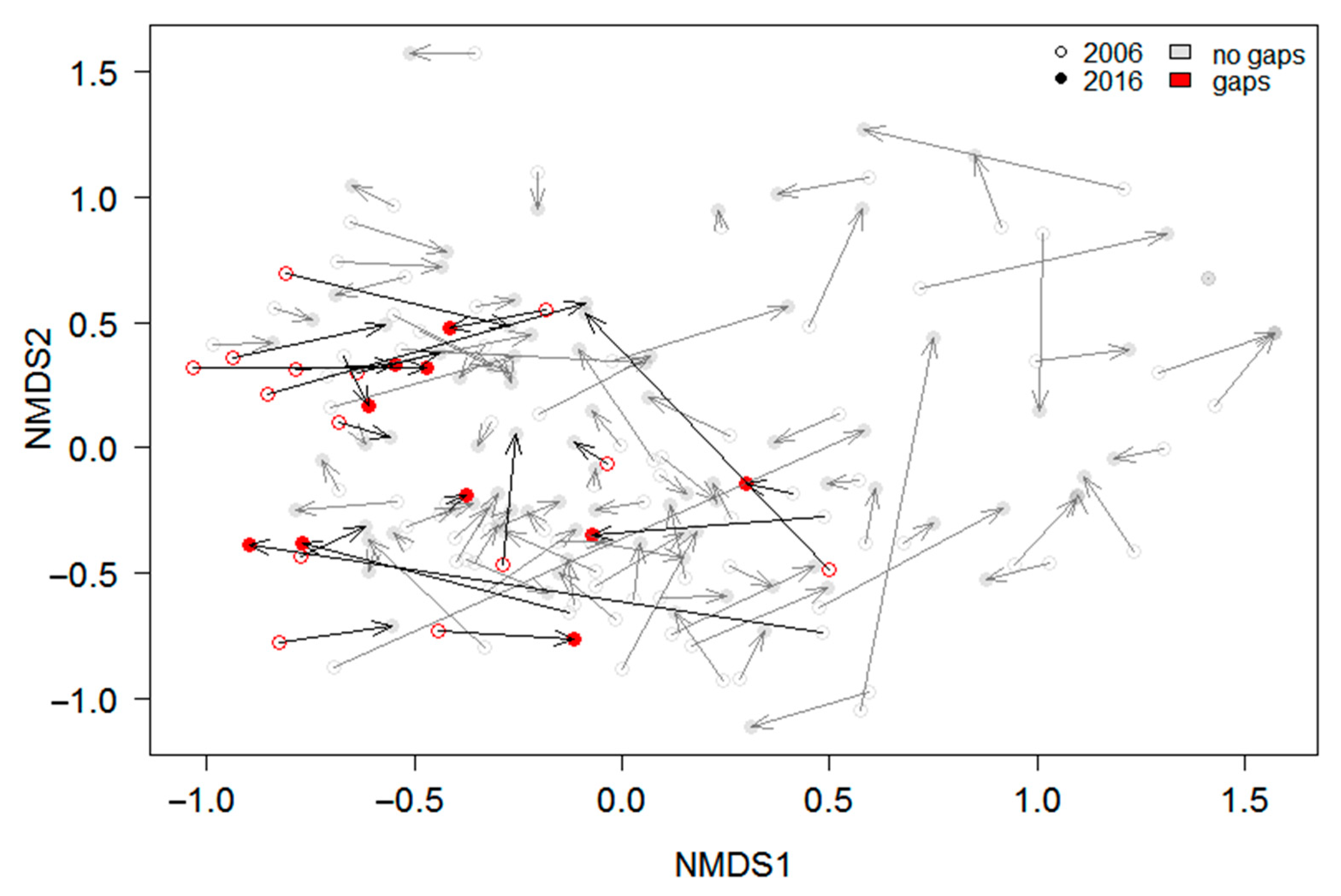
3.2. Effects of Gap Dynamics on the Understory Taxonomical and Functional Composition and Diversity
3.3. Indicator Species for Gap Dynamics Categories and Their Functional Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of the World’s Forests 2020. In Forests, Biodiversity and People; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Reich, P.B.; Frelich, L. Temperate deciduous forests. In The Earth System: Biological and Ecological Dimensions of Global Environmental Change. Encyclopedia of Global Environmental Change; Mooney, H.A., Canadell, J.G., Eds.; John Wiley & Sons: Chichester, UK, 2002; Volume 2, pp. 565–569. [Google Scholar]
- Gilliam, F.S. Forest ecosystems of temperate climatic regions: From ancient use to climate change. New Phytol. 2016, 212, 871–887. [Google Scholar] [CrossRef]
- Aude, E.; Lawesson, J.E. Vegetation in Danish beech forests: The importance of soil, microclimate and management factors, evaluated by variation partitioning. Plant Ecol. 1998, 134, 53–65. [Google Scholar] [CrossRef]
- Honnay, O.; Hermy, M.; Coppin, P. Effects of area, age and diversity of forest patches in Belgium on plant species richness, and implications for conservation and reforestation. Biol. Conserv. 1999, 87, 73–84. [Google Scholar] [CrossRef]
- Graae, B.J.; Okland, R.H.; Petersen, P.M.; Jensen, K.; Fritzboger, B. Influence of historical, geographical and environmental variables on understorey composition and richness in Danish forests. J. Veg. Sci. 2004, 15, 465–474. [Google Scholar] [CrossRef]
- Kolb, A.; Diekmann, M. Effects of environment, habitat configuration and forest continuity on the distribution of forest plant species. J. Veg. Sci. 2004, 15, 199–208. [Google Scholar] [CrossRef]
- Härdtle, W.; Oheimb, G.V.; Westphal, C. Relationships between the vegetation and soil conditions in beech and beech-oak forests of northern Germany. Plant Ecol. 2005, 177, 113–124. [Google Scholar] [CrossRef]
- Schuster, B.; Diekmann, M. Species richness and environmental correlates in deciduous forests of Northwest Germany. For. Ecol. Manag. 2005, 206, 197–205. [Google Scholar] [CrossRef]
- Degen, T.; Devillez, F.; Jacquemart, A.-L. Gaps promote plant diversity in beech forests (Luzulo-Fagetum), North Vosges, France. Ann. For. Sci. 2005, 62, 429–440. [Google Scholar] [CrossRef]
- Naaf, T.; Wulf, M. Effects of gap size, light and herbivory on the herb layer vegetation in European beech forest gaps. For. Ecol. Manag. 2007, 244, 141–149. [Google Scholar] [CrossRef]
- Burton, J.I.; Mladenoff, D.J.; Forrester, J.A.; Clayton, M.K. Experimentally linking disturbance, resources and productivity to diversity in forest ground-layer plant communities. J. Ecol. 2014, 102, 1634–1648. [Google Scholar] [CrossRef]
- Rozas, V. Structural heterogeneity and tree spatial patterns in an old-growth deciduous lowland forest in Cantabria, northern Spain. Plant Ecol. 2006, 185, 57–72. [Google Scholar] [CrossRef]
- Gazol, A.; Ibáñez, R. Different response to environmental factors and spatial variables of two attributes (cover and diversity) of the understorey layers. For. Ecol. Manag. 2009, 258, 1267–1274. [Google Scholar] [CrossRef]
- Gazol, A.; Ibáñez, R. Plant species composition in a temperate forest: Multi-scale patterns and determinants. Acta Oecol. 2010, 36, 634–644. [Google Scholar] [CrossRef]
- Muscolo, A.; Bagnato, S.; Sidari, M.; Mercurio, R. A review of the roles of forest canopy gaps. J. For. Res. 2014, 25, 725–736. [Google Scholar] [CrossRef]
- Feldmann, E.; Drößler, L.; Hauck, M.; Kucbel, S.; Pichler, V.; Leuschner, C. Canopy gap dynamics and tree understory release in a virgin beech forest, Slovakian Carpathians. For. Ecol. Manag. 2018, 415–416, 38–46. [Google Scholar] [CrossRef]
- Olano, J.M.; Caballero, I.; Laskurain, N.A.; Loidi, J.; Escudero, A. Seed bank spatial pattern in a temperate secondary forest. J. Veg. Sci. 2002, 13, 75–784. [Google Scholar] [CrossRef]
- Gálhidy, L.; Mihók, B.; Hagyó, A.; Rajkai, K.; Standovár, T. Effects of gap size and associated changes in light and soil moisture on the understory vegetation of a Hungarian beech forest. Plant Ecol. 2006, 183, 133–145. [Google Scholar] [CrossRef]
- Tinya, F.; Ódor, P. Congruence of the spatial pattern of light and understory vegetation in an old-growth, temperate mixed forest. For. Ecol. Manag. 2016, 381, 84–92. [Google Scholar] [CrossRef]
- Tinya, F.; Kovács, B.; Prättälä, A.; Farkas, P.; Aszalós, R.; Ódor, P. Initial understory response to experimental silvicultural treatments in a temperate oak-dominated forest. Eur. J. For. Res. 2019, 138, 65–77. [Google Scholar] [CrossRef]
- Augusto, L.; Pascal, B.; Ranger, J. Impact of tree species on forest soil acidification. For. Ecol. Manag. 1998, 105, 67–78. [Google Scholar] [CrossRef]
- Augusto, L.; Dupouey, J.-L.; Ranger, J. Effects of tree species on understory vegetation and environmental conditions in temperate forests. Ann. For. Sci. 2003, 60, 823–831. [Google Scholar] [CrossRef]
- Härdtle, W.; von Oheimb, G.; Westphal, C. The effects of light and soil conditions on the species richness of the ground vegetation of deciduous forests in northern Germany (Schleswig-Holstein). For. Ecol. Manag. 2003, 182, 327–338. [Google Scholar] [CrossRef]
- Kern, C.C.; Montgomery, R.A.; Reich, P.B.; Strong, T.F. Harvest-Created Canopy Gaps Increase Species and Functional Trait Diversity of the Forest Ground-Layer Community. For. Sci. 2014, 60, 335–344. [Google Scholar] [CrossRef]
- Watt, A.S. Pattern and process in the plant community. J. Ecol. 1947, 35, 1–22. [Google Scholar] [CrossRef]
- Nagel, T.A.; Svoboda, M.; Rugani, T.; Diaci, J. Gap regeneration and replacement patterns in an old-growth Fagus-Abies forest of Bosnia-Herzegovina. Plant Ecol. 2010, 208, 307–318. [Google Scholar] [CrossRef]
- Orman, O.; Wrzesiński, P.; Dobrowolska, D.; Szewczyk, J. Regeneration growth and crown architecture of European beech and silver fir depend on gap characteristics and light gradient in the mixed montane old-growth stands. For. Ecol. Manag. 2021, 482, 118866. [Google Scholar] [CrossRef]
- Rozas, V. Regeneration patterns, dendroecology, and forest-use history in an old-growth beech-oak lowland forest in Northern Spain. For. Ecol. Manag. 2003, 182, 175–194. [Google Scholar] [CrossRef]
- Gazol, A.; Ibáñez, R. Scale-specific determinants of a mixed beech and oak seedling-sapling bank under different environmental and biotic conditions. Plant Ecol. 2010, 211, 37–48. [Google Scholar] [CrossRef]
- Dobrowolska, D. Effect of stand density on oak regeneration in flood plain forests in Lower Silesia, Poland. Forestry 2008, 81, 511–523. [Google Scholar] [CrossRef]
- Schröter, M.; Härdtle, W.; von Oheimb, G. Crown plasticity and neighborhood interactions of European beech (Fagus sylvatica L.) in an old-growth forest. Eur. J. For. Res. 2012, 131, 787–798. [Google Scholar] [CrossRef]
- Orman, O.; Dobrowolska, D. Gap dynamics in the Western Carpathian mixed beech old-growth forests affected by spruce bark beetle outbreak. Eur. J. For. Res. 2017, 136, 571–581. [Google Scholar] [CrossRef]
- Kenderes, K.; Mihók, B.; Standovár, T. Thirty years of gap dynamics in a central european beech forest reserve. Forestry 2008, 81, 111–123. [Google Scholar] [CrossRef]
- Kern, C.C.; Montgomery, R.A.; Reich, P.B.; Strong, T.F. Canopy gap size influences niche partitioning of the ground-layer plant community in a northern temperate forest. J. Plant Ecol. 2013, 6, 101–112. [Google Scholar] [CrossRef]
- Canullo, R.; Simonetti, E.; Cervellini, M.; Chelli, S.; Bartha, S.; Wellstein, C.; Campetella, G. Unravelling mechanisms of short-term vegetation dynamics in complex coppice forest systems. Folia Geobot. 2017, 52, 71–81. [Google Scholar] [CrossRef]
- Castroviejo, S. Flora Iberica. Plantas Vasculares de la Península Ibérica e Islas Baleares; Real Jardín Botánico, CSIC: Madrid, Spain, 2020. [Google Scholar]
- Aizpuru, I.; Aseginolaza, C.; Uribe-Echebarría, P.M.; Urrutia, P.; Zorrakin, I. Claves Ilustradas de la Flora del País Vasco y Territorios Limítrofes; Servicio Central de Publicaciones del Gobierno Vasco: Vitoria-Gasteiz, Spain, 1999; pp. 1–831. [Google Scholar]
- Westhoff, V.; van der Maarel, E. The Braun-Blanquet approach. In Ordination and Classification of Communities. Handbook of Vegetation Science Part V; Whittaker, R.H., Ed.; Junk: The Hague, The Netherlands, 1973; pp. 617–726. [Google Scholar]
- Van der Maarel, E. Transformation of cover-abundance values for appropriate numerical treatment—Alternatives to the proposals by Podani. J. Veg. Sci. 2007, 18, 767–770. [Google Scholar] [CrossRef]
- Zeibig, A.; Diaci, J.; Wagner, S. Gap disturbance patterns of a Fagus sylvatica virgin forest remnant in the mountain vegetation belt of Slovenia. For. Snow Landsc. Res. 2005, 79, 69–80. [Google Scholar]
- Runkle, J.R. Patterns of Disturbance in Some Old-Growth Mesic Forests of Eastern North America. Ecology 1982, 63, 1533–1546. [Google Scholar] [CrossRef]
- Nagel, T.A.; Svoboda, M. Gap disturbance regime in an old-growth Fagus-Abies forest in the Dinaric Mountains, Bosnia-Herzegovina. Can. J. For. Res. 2008, 38, 2728–2737. [Google Scholar] [CrossRef]
- Gobierno de Navarra. Mapa Topográfico 1:5.000; Departamento de Obras Públicas, Transporte y Comunicaciones, Servicio de Publicaciones del Gobierno de Navarra: Navarra, Spain, 2004. [Google Scholar]
- Valladares, F. La disponibilidad de luz bajo el dosel de los bosques y matorrales ibéricos estimada mediante fotografía hemisférica. Ecología 2006, 20, 11–30. [Google Scholar]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Diaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; ter Steege, H.; Morgan, H.D.; van der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Gillison, A.N. Plant Functional Types and Traits at the Community, Ecosystem and World Level. In Vegetation Ecology, 2nd ed.; van der Maarel, E., Franklin, J., Eds.; John Wiley & Sons: Oxford, UK, 2013; pp. 347–386. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ; National Institute of Health: Bethesda, MD, USA, 1997–2019. Available online: https://imagej.nih.gov/ij/ (accessed on 30 May 2019).
- Kattge, J.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Bönisch, G.; Garnier, E.; Westoby, M.; Reich, P.B.; Wright, I.J.; et al. TRY—A global database of plant traits. Glob. Chang. Biol. 2011, 17, 2905–2935. [Google Scholar] [CrossRef]
- Garnier, E.; Cortez, J.; Billès, G.; Navas, M.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A.; et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New multidimensional functional diversity indices for a multifaced framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef]
- Rao, C.R. Diversity and dissimilarity coefficients: A unified approach. Theor. Popul. Biol. 1982, 21, 24–43. [Google Scholar] [CrossRef]
- Mouchet, M.A.; Villéger, S.; Mason, N.W.H.; Mouillot, D. Functional diversity measures: An overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol. 2010, 24, 867–876. [Google Scholar] [CrossRef]
- Carmona, C.P.; Mason, N.W.H.; Azcárate, F.M.; Peco, B. Inter-annual fluctuations in rainfall shift the functional structure of Mediterranean grasslands across gradients of productivity and disturbance. J. Veg. Sci. 2015, 26, 538–551. [Google Scholar] [CrossRef]
- Gotelli, N.J.; McCabe, D.J. Species Co-Occurrence: A Meta-Analysis of J. M. Diamond’s Assembly Rules Model. Ecology 2002, 83, 2091–2096. [Google Scholar] [CrossRef]
- Spasojevic, M.J.; Suding, K.N. Inferring community assembly mechanisms from functional diversity patterns: The importance of multiple assembly processes. J. Ecol. 2012, 100, 652–661. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Dunnett, C.W. Pairwise Multiple Comparisons in the Unequal Variance Case. J. Am. Stat. Assoc. 1980, 75, 796–800. [Google Scholar] [CrossRef]
- Lau, M.K. DTK: Dunnett-Tukey-Kramer Pairwise Multiple Comparison Test Adjusted for Unequal Variances and Unequal Sample Sizes. R Package Version 3.5. 2013. Available online: https://CRAN.R-project.org/package=DTK (accessed on 28 May 2021).
- Dufrene, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 28 May 2021).
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P.; Shipley, B. FD: Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology. R Package Version 1.0-12. 2014. Available online: https://cran.r-project.org/web/packages/FD/FD.pdf (accessed on 28 May 2021).
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 31 May 2021).
- Cáceres, M.D.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Von Oheimb, G.; Friedel, A.; Bertsch, A.; Härdtle, W. The effects of windthrow on plant species richness in a Central European beech forest. Plant Ecol. 2007, 191, 47–65. [Google Scholar] [CrossRef]
- Gazol, A.; Ibáñez, R. Variation of plant diversity in a temperate unmanaged forest in northern Spain: Behind the environmental and spatial explanation. Plant Ecol. 2009, 207, 1–11. [Google Scholar] [CrossRef]
- Hill, M.O.; Mountford, J.O.; Roy, D.B.; Bunce, R.G.H. Ellenberg’s Indicator Values for British Plants; Institute of Terrestrial Ecology: Huntingdon, UK, 1999. [Google Scholar]
- Givnish, T.J. Comparative Studies of Leaf Form: Assessing the Relative Roles of Selective Pressures and Phylogenetic Constraints. New Phytol. 1987, 106, 131–160. [Google Scholar] [CrossRef]
- Patry, C.; Kneeshaw, D.; Aubin, I.; Messier, C. Intensive forestry filters understory plant traits over time and space in boreal forests. Forestry 2017, 90, 436–444. [Google Scholar] [CrossRef]
- Eler, K.; Kermavnar, J.; Marinšek, A.; Kutnar, L. Short-term changes in plant functional traits and understory functional diversity after logging of different intensities: A temperate fir-beech forest experiment. Ann. For. Res. 2018, 61, 223–241. [Google Scholar] [CrossRef]
- Schumann, M.E.; White, A.S.; Witham, J.W. The effects of harvest-created gaps on plant species diversity, composition, and abundance in a Maine oak-pine forest. For. Ecol. Manag. 2003, 176, 543–561. [Google Scholar] [CrossRef]
- Bongjoh, C.A.; Mama, N. Early regeneration of commercial timber species in a logged-over forest of southern Cameroon. In Proceedings of the Seminar FORAFRI Libreville—Session 2: Knowledge Ecosystem, Montpellier, QC, Canada, 12–16 October 1998; pp. 1–9. [Google Scholar]
- Hautier, Y.; Niklaus, P.A.; Hector, A. Competition for light causes plant biodiversity loss after eutrophication. Science 2009, 324, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Navas, M.; Violle, C. Plant traits related to competition: How do they shape the functional diversity of communities? Community Ecol. 2009, 10, 131–137. [Google Scholar] [CrossRef]

| Variable | Gap Closure vs. No Gap | Gap Opening vs. No Gap | Gap Persistence vs. No Gap | Gap Closure vs. Gap Opening | Gap Persistence vs. Gap Closure | Gap Persistence vs. Gap Opening |
|---|---|---|---|---|---|---|
| dif.NMDS1 | 0.108 | −0.503 | 0.153 | 0.611 | 0.045 | 0.657 |
| dif.NMDS2 | 0.104 | −0.036 | −0.129 | 0.140 | −0.233 | −0.093 |
| dif.abundance | 0.863 | 5.380 | 5.213 | −4.517 | 4.35 | −0.167 |
| dif.richness | −2.010 | 5.224 | −1.610 | −7.233 | 0.4 | −6.833 |
| dif.shannon | −0.014 | 0.511 | −0.158 | −0.525 | −0.144 | −0.669 |
| dif.CWMLDMC | 5.757 | 12.018 | 28.618 | −6.261 | 22.861 | 16.600 |
| dif.CWMSLA | 0.667 | 1.461 | 0.899 | −0.794 | 0.232 | −0.562 |
| dif.CWMLSlog | 0.069 | 0.039 | 0.105 | 0.030 | 0.036 | 0.066 |
| dif.CWMPHlog | 0.082 | 0.091 | 0.119 | −0.009 | 0.036 | 0.028 |
| dif.CWMSMlog | 0.292 | 0.047 | 0.412 | 0.245 | 0.120 | 0.365 |
| dif.SESFRicLDMC | −0.025 | −0.219 | −0.489 | 0.193 | −0.464 | −0.270 |
| dif.SESFRicSLA | −0.175 | −0.381 | −0.179 | 0.205 | −0.004 | 0.202 |
| dif.SESFRicLSlog | −0.058 | −0.134 | −0.073 | 0.076 | −0.015 | 0.060 |
| dif.SESFRicPHlog | −0.403 | −0.540 | 1.002 | 0.138 | 1.404 | 1.542 |
| dif.SESFRicSMlog | −1.058 | −0.287 | −0.132 | −0.771 | 0.926 | 0.156 |
| dif.SESRaoLDMC | −0.003 | 0.158 | 0.837 | −0.162 | 0.840 | 0.679 |
| dif.SESRaoSLA | −0.417 | 0.063 | −0.444 | −0.480 | −0.027 | −0.507 |
| dif.SESRaoLSlog | −0.463 | −0.134 | −0.649 | −0.330 | −0.186 | −0.516 |
| dif.SESRaoPHlog | 0.467 | 0.577 | −0.400 | −0.110 | −0.867 | −0.977 |
| dif.SESRaoSMlog | 0.376 | 0.152 | −0.068 | 0.225 | −0.444 | −0.220 |
| Canopy Dynamics | Species | Statistic | p Value |
|---|---|---|---|
| Gap closure | Athyrium filix-femina | 0.628 | 0.041 * |
| Campanula patula | 0.517 | 0.04 * | |
| Gap opening | Eupatorium cannabinum | 0.577 | 0.006 ** |
| Taraxacum gr. officinale | 0.567 | 0.018 * | |
| Luzula multiflora subsp. multiflora | 0.547 | 0.046 * | |
| Gap persistence | Juncus gr. effusus | 0.684 | 0.007 ** |
| Dryopteris dilatata | 0.683 | 0.007 ** | |
| Pilosella sp. | 0.500 | 0.039 * | |
| Urtica dioica | 0.500 | 0.045 * | |
| Veronica officinalis | 0.500 | 0.041 * |
| Canopy Dynamics | Indicator Species | LDMC | SLA | logLS | logHeight | logSM |
|---|---|---|---|---|---|---|
| Gap closure | Athyrium filix−femina | −0.626 | 1.326 | 1.619 | 0.332 | |
| Campanula patula | −1.514 | |||||
| Gap opening | Eupatorium cannabinum | −0.377 | ||||
| Taraxacum gr. officinale | 0.676 | 1.376 | 0.469 | −1.004 | ||
| Luzula multiflora subsp. multiflora | −0.485 | −0.097 | −0.132 | −0.664 | −0.257 | |
| Gap persistence | Juncus gr. effusus | −0.107 | −1.392 | −2.445 | 1.087 | |
| Urtica dioica | −0.779 | |||||
| Veronica officinalis | 0.069 | −0.269 | −0.756 | −1.671 | −0.691 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valerio, M.; Ibáñez, R.; Gazol, A. The Role of Canopy Cover Dynamics over a Decade of Changes in the Understory of an Atlantic Beech-Oak Forest. Forests 2021, 12, 938. https://doi.org/10.3390/f12070938
Valerio M, Ibáñez R, Gazol A. The Role of Canopy Cover Dynamics over a Decade of Changes in the Understory of an Atlantic Beech-Oak Forest. Forests. 2021; 12(7):938. https://doi.org/10.3390/f12070938
Chicago/Turabian StyleValerio, Mercedes, Ricardo Ibáñez, and Antonio Gazol. 2021. "The Role of Canopy Cover Dynamics over a Decade of Changes in the Understory of an Atlantic Beech-Oak Forest" Forests 12, no. 7: 938. https://doi.org/10.3390/f12070938
APA StyleValerio, M., Ibáñez, R., & Gazol, A. (2021). The Role of Canopy Cover Dynamics over a Decade of Changes in the Understory of an Atlantic Beech-Oak Forest. Forests, 12(7), 938. https://doi.org/10.3390/f12070938






