Changes in Long-Term Light Properties of a Mixed Conifer—Broadleaf Forest in Southwestern Europe
Abstract
:1. Introduction
2. Materials and Methods
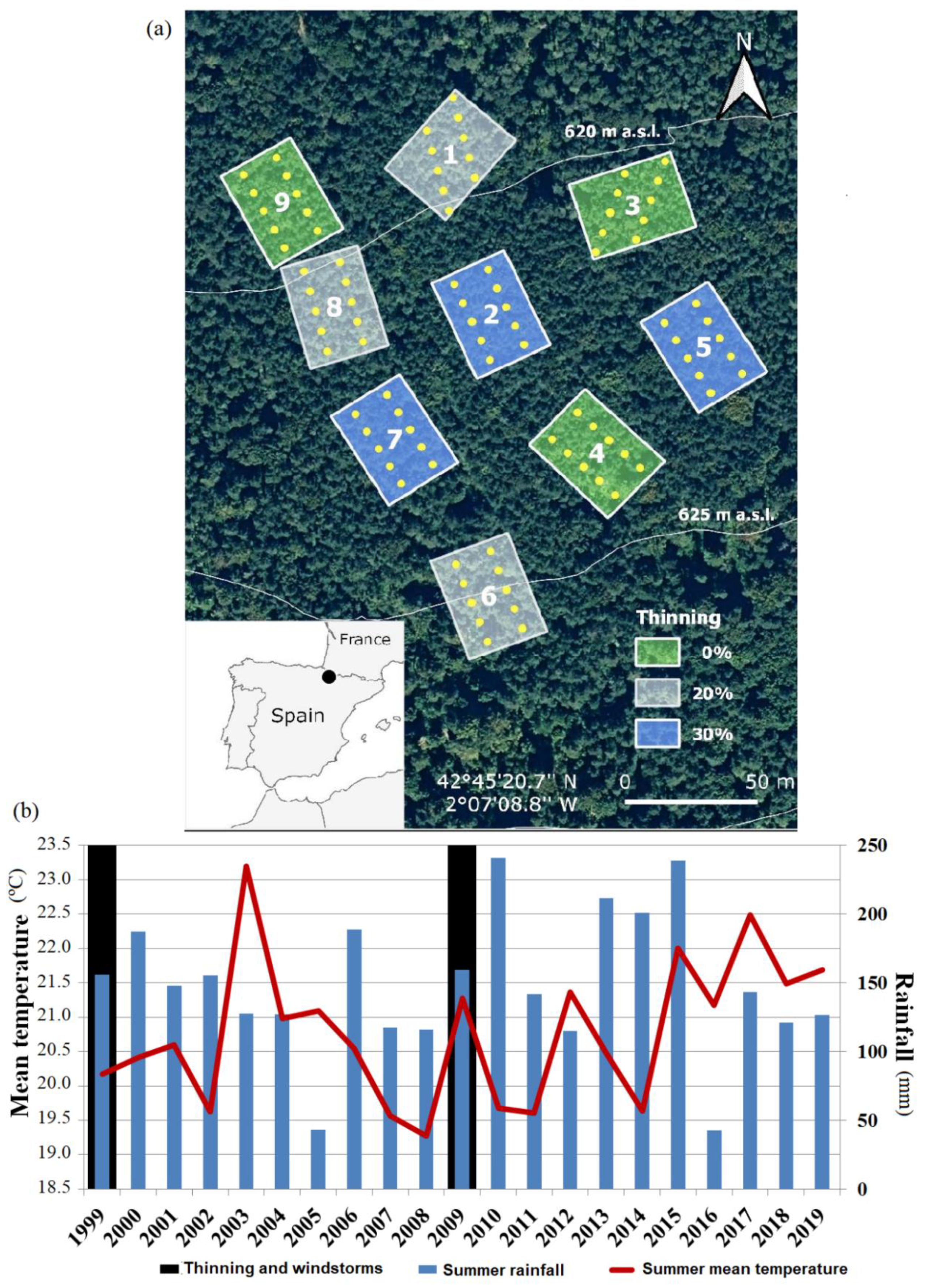
2.1. Study Area
2.2. Experimental Design and Tree and Recruit Samplings
2.3. Image Acquisition and Hemispherical Photographs
2.4. Light Variables from Hemispherical Pictures
2.5. Canopy Richness and Broadleaf Cover
2.6. Data Analyses
2.6.1. Correlation between Light Variables and Data Filters
2.6.2. Differences between Years and Plots in Light Variables
2.6.3. Time-Series Analyses of Light Variables
2.6.4. Relationships between Light Properties and Forest Canopy Composition
3. Results
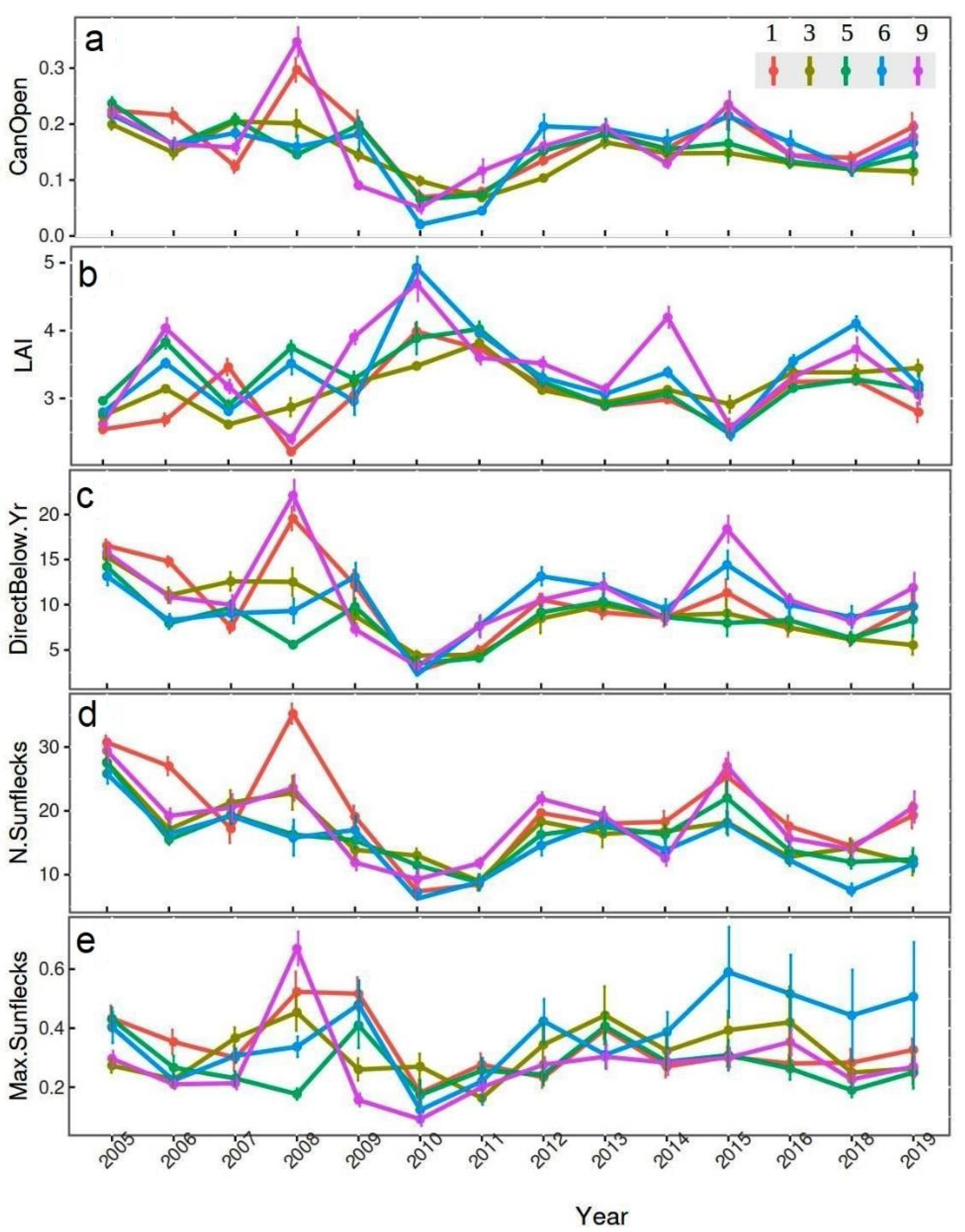
3.1. Temporal Changes in Light Variables
3.2. Spatial Differences in Light Variables
3.3. Time-Dependent Effects on Light Variables
3.4. Forest Canopy Structure as Driver of Light Variables
4. Discussion
4.1. Light Variables and Their Temporal Autocorrelation
4.2. Relationships between Forest Canopy Structure and Understory Light Variables
4.3. Management Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mátyás, C. Forests and Climate Change in Eastern Europe and Central Asia; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2010; Volume 8. [Google Scholar]
- Dale, V.L.; Joyce, L.A.; McNulty, S.; Neilson, R.P.; Ayres, M.P.; Flannigan, M.D.; Hanson, P.J.; Irland, L.C.; Lugo, A.E.; Peterson, C.J.; et al. Climate change and forest disturbances. Bioscience 2001, 59, 723–734. [Google Scholar] [CrossRef] [Green Version]
- Dubrovskis, E.; Donis, J.; Racenis, E.; Kitenberga, M.; Jansons, A. Wind-induced stem breakage height effect on potentially recovered timber value: Case study of the Scots pine (Pinus sylvestris L.) in Latvia. For. Stud. 2018, 69, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, S.-I. Forest gap dynamics and tree regeneration. J. For. Res. 2000, 5, 223–229. [Google Scholar] [CrossRef]
- Quine, C.P.; Gardiner, B.A. Understanding how the interaction of wind and trees results in windthrow, stem breakage, and canopy gap formation. In Plant Disturbance Ecology; Elsevier: Amsterdam, The Netherlands, 2007; pp. 103–155. [Google Scholar]
- Ulanova, N.G. The effects of windthrow on forests at different spatial scales: A review. For. Ecol. Manag. 2000, 135, 155–167. [Google Scholar] [CrossRef]
- Meng, S.X.; Rudnicki, M.; Lieffers, V.; Reid, D.E.B.; Silins, U. Preventing crown collisions increases the crown cover and leaf area of maturing lodgepole pine. J. Ecol. 2006, 94, 681–686. [Google Scholar] [CrossRef]
- Blanco, J.A.; Imbert, J.B.; Castillo, F.J. Nutrient return via litterfall in two contrasting Pinus sylvestris forests in the Pyrenees under different thinning intensities. For. Ecol. Manag. 2008, 256, 1840–1852. [Google Scholar] [CrossRef]
- Clark, J.S.; Iverson, L.; Woodall, C.W.; Allen, C.D.; Bell, D.; Bragg, D.C.; D’Amato, A.W.; Davis, F.; Hersh, M.; Ibanez, I.; et al. The impacts of increasing drought on forest dynamics, structure, and biodiversity in the United States. Glob. Chang. Biol. 2016, 22, 2329–2352. [Google Scholar] [CrossRef] [Green Version]
- Spinoni, J.; Vogt, J.V.; Naumann, G.; Barbosa, P.; Dosio, A. Will drought events become more frequent and severe in Europe? Int. J. Climatol. 2017, 38, 1718–1736. [Google Scholar] [CrossRef] [Green Version]
- Flower, C.E.; Gonzalez-Meler, M.A. Responses of temperate forest productivity to insect and pathogen disturbances. Ann. Rev. Plant Biol. 2015, 66, 547–569. [Google Scholar] [CrossRef]
- Anaya-Romero, M.; Muñoz-Rojas, M.; Ibáñez, B.; Marañón, T. Evaluation of forest ecosystem services in Mediterranean areas. A regional case study in South Spain. Ecosyst. Serv. 2016, 20, 82–90. [Google Scholar] [CrossRef]
- Oliver, C.D.; Larson, B.A. Forest Stand Dynamics; John Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- Côté, I.M.; Darling, E.; Brown, C. Interactions among ecosystem stressors and their importance in conservation. Proc. R. Soc. B Boil. Sci. 2016, 283, 20152592. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; Baessler, C.; Schubert, H.; Klotz, S. Long-Term Ecological Research; Springer: Berlin/Heidelberg, Germany, 2010; pp. 978–990. [Google Scholar]
- Lindenmayer, D.B.; Likens, G.E.; Andersen, A.; Bowman, D.; Bull, C.M.; Burns, E.; Dickman, C.R.; Hoffmann, A.A.; Keith, D.A.; Liddell, M.J.; et al. Value of long-term ecological studies. Austral. Ecol. 2012, 37, 745–757. [Google Scholar] [CrossRef]
- Lieffers, V.J.; Messier, C.; Stadt, K.J.; Gendron, E.; Comeau, P.G. Predicting and managing light in the understory of boreal forests. Can. J. For. Res. 1999, 29, 796–811. [Google Scholar] [CrossRef]
- Weiner, J. Asymmetric competition in plant populations. Trends Ecol. Evol. 1990, 5, 360–364. [Google Scholar] [CrossRef]
- Modrý, M.; Hubený, D.; Rejšek, K. Differential response of naturally regenerated European shade tolerant tree species to soil type and light availability. For. Ecol. Manag. 2004, 188, 185–195. [Google Scholar] [CrossRef]
- Berentsen, W.H.; East, W.G.; Poulsen, T.M.; Windley, B.F. Europe. Encyclopedia Britannica. 2020. Available online: https://www.britannica.com/place/Europe (accessed on 28 October 2021).
- Tsai, H.-C.; Chiang, J.-M.; McEwan, R.W.; Lin, T.-C. Decadal effects of thinning on understory light environments and plant community structure in a subtropical forest. Ecosphere 2018, 9, e02464. [Google Scholar] [CrossRef] [Green Version]
- Lelli, C.; Bruun, H.H.; Chiarucci, A.; Donati, D.; Frascaroli, F.; Fritz, Ö.; Heilmann-Clausen, J. Biodiversity response to forest structure and management: Comparing species richness, conservation relevant species and functional diversity as metrics in forest conservation. For. Ecol. Manag. 2019, 432, 707–717. [Google Scholar] [CrossRef]
- Lhotka, J.M.; Loewenstein, E.F. Effect of midstory removal on understory light availability and the 2–year response of underplanted Cherrybark oak seedlings. South. J. Appl. For. 2009, 33, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Naaf, T.; Wulf, M. Effects of gap size, light and herbivory on the herb layer vegetation in European beech forest gaps. For. Ecol. Manag. 2007, 244, 141–149. [Google Scholar] [CrossRef]
- Pretzsch, H.; Biber, P. Tree species mixing can increase maximum stand density. Can. J. For. Res. 2016, 46, 1179–1193. [Google Scholar] [CrossRef] [Green Version]
- Pretzsch, H.; del Río, M.; Schütze, G.; Ammer, C.; Annighöfer, P.; Avdagic, A.; Barbeito, I.; Bielak, K.; Brazaitis, G.; Coll, L.; et al. Mixing of Scots pine (Pinus sylvestris L.) and European beech (Fagus sylvatica L.) enhances structural heterogeneity, and the effect increases with water availability. For. Ecol. Manag. 2016, 373, 149–166. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Castro, E.; Casado González, M.Á.; Costa Tenorio, M.; Escribano Bombín, R.; García Antón, M.; Génova Fuster, M.; Gómez Manzaneque, A.; Gómez Manzaneque, F.; Moreno Saiz, J.C.; Morla Juaristi, C.; et al. Los Bosques Ibéricos: Una Interpretación Geobotánica; Planeta: Barcelona, Spain, 1997. (In Spanish) [Google Scholar]
- De Andrés, E.G.; Seely, B.; Blanco, J.A.; Imbert, J.B.; Lo, Y.-H.; Castillo, F.J. Increased complementarity in water-limited environments in Scots pine and European beech mixtures under climate change. Ecohydrology 2017, 10, e1810. [Google Scholar] [CrossRef] [Green Version]
- González de Andrés, E.; Camarero, J.J.; Blanco, J.A.; Imbert, J.B.; Lo, Y.H.; Sangüesa-Barreda, G.; Castillo, F.J. Tree-to-tree competition in mixed European beech–Scots pine forests has different impacts on growth and water-use efficiency depending on site conditions. J. Ecol. 2018, 106, 59–75. [Google Scholar] [CrossRef] [Green Version]
- Seidel, D.; Fleck, S.; Leuschner, C.; Hammett, T. Review of ground-based methods to measure the distribution of biomass in forest canopies. Ann. For. Sci. 2011, 68, 225–244. [Google Scholar] [CrossRef] [Green Version]
- Fournier, R.A.; Hall, R.J. Hemispherical Photography in Forest Science: Theory, Methods, Applications; Springer: The Netherlands, 2017. [Google Scholar]
- Chianucci, F.; Cutini, A. Digital hemispherical photography for estimating forest canopy properties: Current controversies and opportunities. Ifor. -Biogeosci. For. 2012, 5, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Jonckheere, I.; Fleck, S.; Nackaerts, K.; Muys, B.; Coppin, P.; Weiss, M.; Baret, F. Review of methods for in situ leaf area index determination: Part, I. Theories, sensors and hemispherical photography. Agric. For. Meteorol. 2004, 121, 19–35. [Google Scholar] [CrossRef]
- Meteo-Navarra. Meteorología y Climatología de Navarra: Navascués. 2021. Available online: http://meteo.navarra.es/estaciones/estacion.cfm?IDestacion=178 (accessed on 9 June 2021). (In Spanish).
- AEMET—Spanish Meteorological Agency. Ciclogenesis Explosiva Enero. 2009. Available online: http://www.aemet.es/en/noticias/2009/03/ciclogenesisexplosiva (accessed on 16 February 2021). (In Spanish).
- Pozueta, F. Hacia un Modelo Conceptual de la Biodiversidad en un Bosque Mixto de Pino-Haya. Master’s Thesis, Universidad Pública de Navarra, Pamplona, Spain, 2014. (In Spanish). [Google Scholar]
- García-Sancet, M.A. Influencia de los Parámetros de Dosel y Lumínicos Sobre la Regeneración de Pinus sylvestris L. En un Bosque Mixto con Tres Intensidades de Clara. Master’s Thesis, Universidad Pública de Navarra, Pamplona, Spain, 2017. (In Spanish). [Google Scholar]
- Ter Steege, H. Hemiphot.R: Free R Scripts to Analyse Hemispherical Photographs for Canopy Openness, Leaf Area Index and Photosynthetic Active Radiation under Forest Canopies. Naturalis Biodiversity Center, Leiden, The Netherlands. Available online: https://github.com/naturalis/Hemiphot (accessed on 3 August 2021).
- Welles, J.M.; Norman, J.M. Instrument for indirect measurement of canopy architecture. Agron. J. 1991, 83, 818–825. [Google Scholar] [CrossRef]
- Chazdon, R.L. Sunflecks and their importance to forest understorey plants. Adv. Ecol. Res. 1988, 18, 1–63. [Google Scholar] [CrossRef]
- Arozarena, I. Influencia de la Gestión Forestal en la Evolución del Dosel Arbóreo en un Rodal Mixto del Prepirineo Navarro en Aspurz. Master’s Thesis, Universidad Pública de Navarra, Pamplina, Spain, 2020. (In Spanish). [Google Scholar]
- Venables, W.N.; Ripley, B.D. Package MASS. Available online: http://www.r-project.org (accessed on 17 October 2012).
- Lenth, R. Estimated Marginal Means, Aka Least-Squares Means. 1.4.8; R Package. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 28 October 2021).
- Hyndman, R.J.; Athanasopoulos, G. Forecasting: Principles and Practice; OTexts: Melbourne, VI, Australia, 2018. [Google Scholar]
- Trapletti, A.; Hornik, K. Tseries: Time Series Analysis and Computational Finance. R Package Version 0.10-48. Available online: https://cran.r-project.org/web/packages/tseries/index.html (accessed on 3 August 2021).
- Douglas, B.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar]
- Sakamoto, Y.; Ishiguro, M.; Kitagawa, G. Akaike Information Criterion Statistics; Reidel: Dordrecht, The Netherlands, 1986. [Google Scholar]
- Hu, S. Akaike Information Criterion. 2007. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.353.4237&rep=rep1&type=pdf (accessed on 3 August 2021).
- Vrieze, S.I. Model selection and psychological theory: A discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol. Methods 2012, 17, 228–243. [Google Scholar] [CrossRef] [Green Version]
- Barton, K. MuMIn: Multi-Model Inference. 2020. Available online: http://CRAN.R-project.org/package=MuMIn (accessed on 28 October 2021).
- Costilow, K.; Knight, K.; Flower, C. Disturbance severity and canopy position control the radial growth response of maple trees (Acer spp.) in forests of northwest Ohio impacted by emerald ash borer (Agrilus planipennis). Ann. For. Sci. 2017, 74, 10. [Google Scholar] [CrossRef] [Green Version]
- Poulson, T.L.; Platt, W.J. Gap light regimes influence canopy tree diversity. Ecology 1989, 70, 553–555. [Google Scholar] [CrossRef]
- Jonckheere, I.; Muys, B.; Coppin, P. Allometry and evaluation of in situ optical LAI determination in Scots pine: A case study in Belgium. Tree Physiol. 2005, 25, 723–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montes, F.; Pita, P.; Rubio, A.; Canellas, I. Leaf area index estimation in mountain even-aged Pinus silvestris L. stands from hemispherical photographs. Agric. For. Meteorol. 2007, 145, 215–228. [Google Scholar] [CrossRef]
- Davi, H.; Baret, F.; Huc, R.; Dufrêne, E. Effect of thinning on LAI variance in heterogeneous forests. For. Ecol. Manag. 2008, 256, 890–899. [Google Scholar] [CrossRef]
- Aussenac, G. Interactions between forest stands and microclimate: Ecophysiological aspects and consequences for silviculture. Ann. For. Sci. 2000, 57, 287–301. [Google Scholar] [CrossRef]
- Kramer, K.; Brang, P.; Bachofen, H.; Bugmann, H.; Wohlgemuth, T. Site factors are more important than salvage logging for tree regeneration after wind disturbance in Central European forests. For. Ecol. Manag. 2014, 331, 116–128. [Google Scholar] [CrossRef]
- Rodríguez-Calcerrada, J.; Pérez-Ramos, I.M.; Ourcival, J.M.; Limousin, J.M.; Joffre, R.; Rambal, S. Is selective thinning an adequate practice for adapting Quercus ilex coppices to climate change? Ann. For. Sci. 2011, 68, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Tinya, F.; Márialigeti, S.; Bidló, A.; Ódor, P. Environmental drivers of the forest regeneration in temperate mixed forests. For. Ecol. Manag. 2019, 433, 720–728. [Google Scholar] [CrossRef] [Green Version]
- Schelhaas, M.-J.; Nabuurs, G.-J.; Schuck, A. Natural disturbances in the European forests in the 19th and 20th centuries. Glob. Chang. Biol. 2003, 9, 1620–1633. [Google Scholar] [CrossRef]
- Lain, E.J.; Haney, A.; Burris, J.M.; Burton, J. Response of vegetation and birds to severe wind disturbance and salvage logging in a southern boreal forest. For. Ecol. Manag. 2008, 256, 863–871. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Fisk, J.P.; Holm, J.A.; Bailey, V.; Bohrer, G.; Gough, C.M. Moderate forest disturbance as a stringent test for gap and big-leaf models. Biogeosciences 2015, 12, 513–526. [Google Scholar] [CrossRef] [Green Version]
- Valladares, F.; Guzmán, B. Canopy structure and spatial heterogeneity of understory light in an abandoned Holm oak woodland. Ann. For. Sci. 2006, 63, 749–761. [Google Scholar] [CrossRef] [Green Version]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef] [Green Version]
- Bigler, C.; Bräker, O.U.; Bugmann, H.; Dobbertin, M.; Rigling, A. Drought as an inciting mortality factor in Scots pine stands of the Valais, Switzerland. Ecosystems 2006, 9, 330–343. [Google Scholar] [CrossRef] [Green Version]
- Rubio-Cuadrado, Á.; Camarero, J.J.; del Río, M.; Sánchez-González, M.; Ruiz-Peinado, R.; Bravo-Oviedo, A.; Gil, L.; Montes, F. Long-term impacts of drought on growth and forest dynamics in a temperate beech-oak-birch forest. Agric. For. Meteorol. 2018, 259, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Candel-Pérez, D.; Lo, Y.-H.; Blanco, J.A.; Chiu, C.-M.; Camarero, J.J.; de Andrés, E.G.; Imbert, J.B.; Castillo, F.J. Drought-induced changes in wood density are not prevented by thinning in Scots pine stands. Forests 2018, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- De Andrés, E.G.; Blanco, J.A.; Imbert, J.B.; Guan, B.T.; Lo, Y.; Castillo, F.J. ENSO and NAO affect long-term leaf litter dynamics and stoichiometry of Scots pine and European beech mixedwoods. Glob. Chang. Biol. 2019, 25, 3070–3090. [Google Scholar] [CrossRef] [PubMed]
- Messier, C.; Parent, S.; Bergeron, Y. Effects of overstory and understory vegetation on the understory light environment in mixed boreal forests. J. Veg. Sci. 1998, 9, 511–520. [Google Scholar] [CrossRef]
- Rohner, B.; Kumar, S.; Liechti, K.; Gessler, A.; Ferretti, M. Tree vitality indicators revealed a rapid response of beech forests to the 2018 drought. Ecol. Indic. 2021, 120, 106903. [Google Scholar] [CrossRef]
- Petriţan, A.M.; von Lüpke, B.; Petriţan, I.C. Influence of light availability on growth, leaf morphology and plant architecture of beech (Fagus sylvatica L.), maple (Acer pseudoplatanus L.) and ash (Fraxinus excelsior L.) saplings. Eur. J. For. Res. 2009, 128, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Sevillano, I.; Short, I.; Grant, J.; O’Reilly, C. Effects of light availability on morphology, growth and biomass allocation of Fagus sylvatica and Quercus robur seedlings. For. Ecol. Manag. 2016, 374, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Morin, X.; Fahse, L.; Scherer-Lorenzen, M.; Bugmann, H. Tree species richness promotes productivity in temperate forests through strong complementarity between species. Ecol. Lett. 2011, 14, 1211–1219. [Google Scholar] [CrossRef]
- Zimmermann, A.; Zimmermann, B. Requirements for throughfall monitoring: The roles of temporal scale and canopy complexity. Agric. For. Meteorol. 2014, 189–190, 125–139. [Google Scholar] [CrossRef]
- Seidel, D.; Leuschner, C.; Scherber, C.; Beyer, F.; Wommelsdorf, T.; Cashman, M.; Fehrmann, L. The relationship between tree species richness, canopy space exploration and productivity in a temperate broad-leaf mixed forest. For. Ecol. Manag. 2013, 310, 366–374. [Google Scholar] [CrossRef]
- Fotis, A.T.; Morin, T.H.; Fahey, R.T.; Hardiman, B.; Bohrer, G.; Curtis, P.S. Forest structure in space and time: Biotic and abiotic determinants of canopy complexity and their effects on net primary productivity. Agric. For. Meteorol. 2018, 250-251, 181–191. [Google Scholar] [CrossRef]
- Schütz, J.-P.; Götz, M.; Schmid, W.; Mandallaz, D. Vulnerability of spruce (Picea abies) and beech (Fagus sylvatica) forest stands to storms and consequences for silviculture. Eur. J. For. Res. 2006, 125, 291–302. [Google Scholar] [CrossRef]
- Leuschner, C.; Voß, S.; Foetzki, A.; Clases, Y. Variation in leaf area index and stand leaf mass of European beech across gradients of soil acidity and precipitation. Plant Ecol. 2006, 186, 247–258. [Google Scholar] [CrossRef]
- Bianchi, S.; Cahalan, C.; Hale, S.; Gibbons, J.M. Rapid assessment of forest canopy and light regime using smartphone hemispherical photography. Ecol. Evol. 2017, 7, 10556–10566. [Google Scholar] [CrossRef] [Green Version]

| Plot | Pine Density | Beech Density | Pine Basal Area | Pine Average Tree Height | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trees ha−1 | Trees ha−1 | m2 ha−1 | m | |||||||
| 1999 | 2009 | 2018 | 2009 | 2018 | 1999 | 2009 | 2018 | 1999 | 2018 | |
| Control | ||||||||||
| Plot 3 | 2467 | 1650 | 1224 | 392 | 283 | 44.0 | 47.2 | 47.4 | 16.3 | 19.8 |
| Plot 4 | 4792 | 2650 | 1383 | 942 | 767 | 44.6 | 48.2 | 37.1 | 12.2 | 17.6 |
| Plot 9 | 3292 | 1883 | 1599 | 408 | 442 | 38.3 | 43.8 | 47.9 | 12.6 | 19.9 |
| Average | 3517 | 2061 | 1402 | 581 | 497 | 42.3 | 46.4 | 44.1 | 13.7 | 19.1 |
| Light thinning | ||||||||||
| Plot 1 | 2350 | 2150 | 1258 | 175 | 167 | 30.5 | 43.9 | 42.0 | 12.2 | 17.7 |
| Plot 6 | 2392 | 2042 | 899 | 208 | 517 | 34.7 | 44.9 | 29.0 | 12.7 | 18.9 |
| Plot 8 | 1842 | 1650 | 999 | 475 | 817 | 30.4 | 41.3 | 39.5 | 13.7 | 19.0 |
| Average | 2195 | 1947 | 1052 | 286 | 500 | 31.9 | 43.4 | 36.8 | 12.9 | 18.5 |
| Heavy thinning | ||||||||||
| Plot 2 | 2575 | 2500 | 1266 | 383 | 517 | 28.5 | 41.2 | 35.8 | 11.7 | 17.0 |
| Plot 5 | 2275 | 2192 | 1216 | 192 | 300 | 28.1 | 41.6 | 36.0 | 12.3 | 18.9 |
| Plot 7 | 1750 | 1650 | 833 | 292 | 325 | 30.5 | 41.1 | 34.9 | 13.4 | 20.1 |
| Average | 2200 | 2114 | 1105 | 289 | 381 | 29.0 | 41.3 | 35.6 | 12.5 | 18.7 |
| Site average | 2637 | 2041 | 1186 | 385 | 459 | 34.4 | 43.7 | 38.8 | 13.0 | 18.8 |
| Plot | CanOpen % | LAI m2 m−2 | Direct Below µmol m−2 s−1 | Direct Below.Yr µmol m−2 s−1 | Diff Below µmol m−2 s−1 | Diff Below.Yr µmol m−2 s−1 | N.Sunflecs Number | Max. Sunflecks Minutes | Canopy Cover % | Canopy Richness # Species |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | ||||||||||
| Plot 3 | 14.5 ± 1.6 | 3.16 ± 0.11 | 11.67 ± 1.74 | 8.92 ± 1.18 | 050 ± 0.04 | 0.43 ± 0.04 | 16.60 ± 1.95 | 0.32 ± 0.06 | 0.67 ± 0.59 | 2.80 ± 0.73 |
| Plot 4 | 14.4 ± 2.0 | 3.54 ± 0.15 | 11.51 ± 2.14 | 7.82 ± 1.33 | 0.46 ± 0.05 | 0.40 ± 0.05 | 14.03 ± 1.85 | 0.39 ± 0.11 | 0.64 ± 0.47 | 2.17 ± 1.09 |
| Plot 9 | 16.5 ± 2.2 | 3.42 ± 0.19 | 12.78 ± 1.83 | 11.21 ± 1.45 | 0.51 ± 0.06 | 0.44 ± 0.05 | 18.54 ± 2.04 | 0.28 ± 0.04 | 0.33 ± 0.43 | 1.90 ± 1.11 |
| average | 15.1 ± 1.9 | 3.37 ± 0.15 | 11.99 ± 1.90 | 9.32 ± 1.32 | 0.49 ± 0.05 | 0.42 ± 0.04 | 16.39 ± 1.95 | 0.33 ± 0.07 | 0.55 ± 0.50 | 2.29 ± 0.98 |
| Light thinning | ||||||||||
| Plot 1 | 17.2 ± 2.1 | 3.03 ± 0.14 | 13.99 ± 1.98 | 10.07 ± 1.44 | 0.57 ± 0.06 | 0.49 ± 0.05 | 20.05 ± 2.32 | 0.34 ± 0.05 | 0.50 ± 0.51 | 2.23 ± 1.19 |
| Plot 6 | 15.6 ± 2.2 | 3.33 ± 0.17 | 11.07 ± 1.91 | 9.96 ± 1.34 | 0.51 ± 0.06 | 0.45 ± 0.05 | 15.28 ± 1.90 | 0.38 ± 0.10 | 0.56 ± 0.38 | 2.93 ± 1.22 |
| Plot 8 | 16.0 ± 2.0 | 3.43 ± 0.19 | 12.47 ± 2.06 | 9.03 ± 1.35 | 0.49 ± 0.05 | 0.42 ± 0.05 | 17.54 ± 2.11 | 0.30 ± 0.05 | 0.55 ± 0.49 | 3.03 ± 1.10 |
| average | 16.3 ± 2.1 | 3.27 ± 0.17 | 12.51 ± 1.99 | 9.68 ± 1.38 | 0.52 ± 0.06 | 0.45 ± 0.05 | 17.62 ± 2.11 | 0.34 ± 0.06 | 0.54 ± 0.46 | 2.73 ± 1.17 |
| Heavy thinning | ||||||||||
| Plot 2 | 18.4 ± 2.2 | 3.33 ± 0.17 | 12.72 ± 1.94 | 10.63 ± 1.47 | 0.57 ± 0.06 | 0.50 ± 0.05 | 18.36 ± 2.05 | 0.30 ± 0.05 | 0.64 ± 0.66 | 2.90 ± 1.82 |
| Plot 5 | 15.3 ± 1.7 | 3.31 ± 0.13 | 10.59 ± 1.49 | 8.12 ± 1.08 | 0.48 ± 0.04 | 0.42 ± 0.04 | 15.92 ± 1.63 | 0.28 ± 0.05 | 0.62 ± 0.53 | 2.27 ± 1.10 |
| Plot 7 | 16.3 ± 2.0 | 3.49 ± 0.16 | 12.47 ± 1.83 | 8.86 ± 1.38 | 0.51 ± 0.05 | 0.44 ± 0.05 | 17.37 ± 1.92 | 0.35 ± 0.07 | 0.51 ± 0.48 | 3.03 ± 1.22 |
| average | 16.7 ± 2.0 | 3.38 ± 0.15 | 11.93 ± 1.75 | 9.20 ± 1.31 | 0.52 ± 0.05 | 0.45 ± 0.05 | 17.22 ± 1.87 | 0.31 ± 0.05 | 0.59 ± 0.56 | 2.73 ± 1.38 |
| Site average | 16.0 ± 2.0 | 3.34 ± 0.16 | 12.14 ± 1.88 | 9.40 ± 1.34 | 0.51 ± 0.05 | 0.44 ± 0.05 | 17.08 ± 1.98 | 0.33 ± 0.06 | 0.56 ± 0.50 | 2.59 ± 1.17 |
| Variables | Estimate | Std. Error | df | t Value | p Value |
|---|---|---|---|---|---|
| CanOpen | |||||
| (Intercept) | 0.265 | 0.009 | 210.796 | 29.453 | 0.000 |
| PropCov | −0.110 | 0.014 | 149.936 | −7.824 | 0.000 |
| Year2008 | 0.027 | 0.009 | 189.448 | 2.835 | 0.005 |
| Year2016 | −0.032 | 0.011 | 252.260 | −2.828 | 0.005 |
| LAI | |||||
| (Intercept) | 2.865 | 0.096 | 12.183 | 29.972 | 0.000 |
| PropCov | −0.296 | 0.060 | 259.631 | −4.953 | 0.000 |
| Year2008 | 0.255 | 0.064 | 258.033 | 4.016 | 0.000 |
| Year2016 | 0.824 | 0.068 | 258.261 | 12.035 | 0.000 |
| DirectBelow.Yr | |||||
| (Intercept) | 14.689 | 1.164 | 47.474 | 12.623 | 0.000 |
| PropCov | −3.670 | 1.834 | 252.590 | −2.001 | 0.047 |
| Richness | 0.653 | 0.275 | 232.342 | 2.379 | 0.018 |
| Year2008 | 0.462 | 1.211 | 184.759 | 0.382 | 0.703 |
| Year2016 | −5.843 | 1.463 | 217.527 | −3.995 | 0.000 |
| PropCov:Year2008 | −1.899 | 2.202 | 183.835 | −0.863 | 0.390 |
| PropCov:Year2016 | 0.556 | 2.130 | 204.093 | 0.261 | 0.794 |
| N.Sunflecks | |||||
| (Intercept) | 30.477 | 2.083 | 92.589 | 14.629 | 0.000 |
| PropCov | −3.532 | 2.656 | 253.321 | −1.330 | 0.185 |
| Richness | −0.859 | 0.763 | 255.344 | −1.126 | 0.261 |
| Year2008 | −3.787 | 2.461 | 191.487 | −1.539 | 0.125 |
| Year2016 | −17.027 | 2.848 | 208.860 | −5.979 | 0.000 |
| PropCov:Year2008 | −7.120 | 3.384 | 185.216 | −2.104 | 0.037 |
| PropCov:Year2016 | 1.229 | 3.210 | 203.560 | 0.383 | 0.702 |
| Richness:Year2008 | 1.787 | 0.939 | 204.944 | 1.904 | 0.058 |
| Richness:Year2016 | 1.764 | 0.895 | 223.855 | 1.971 | 0.050 |
| Max. Sunflecks | |||||
| (Intercept) | 0.467 | 0.033 | 152.423 | 14.319 | 0.000 |
| PropCov | −0.132 | 0.047 | 195.165 | −2.804 | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz de la Cuesta, I.; Blanco, J.A.; Imbert, J.B.; Peralta, J.; Rodríguez-Pérez, J. Changes in Long-Term Light Properties of a Mixed Conifer—Broadleaf Forest in Southwestern Europe. Forests 2021, 12, 1485. https://doi.org/10.3390/f12111485
Ruiz de la Cuesta I, Blanco JA, Imbert JB, Peralta J, Rodríguez-Pérez J. Changes in Long-Term Light Properties of a Mixed Conifer—Broadleaf Forest in Southwestern Europe. Forests. 2021; 12(11):1485. https://doi.org/10.3390/f12111485
Chicago/Turabian StyleRuiz de la Cuesta, Ignacio, Juan A. Blanco, J. Bosco Imbert, Javier Peralta, and Javier Rodríguez-Pérez. 2021. "Changes in Long-Term Light Properties of a Mixed Conifer—Broadleaf Forest in Southwestern Europe" Forests 12, no. 11: 1485. https://doi.org/10.3390/f12111485
APA StyleRuiz de la Cuesta, I., Blanco, J. A., Imbert, J. B., Peralta, J., & Rodríguez-Pérez, J. (2021). Changes in Long-Term Light Properties of a Mixed Conifer—Broadleaf Forest in Southwestern Europe. Forests, 12(11), 1485. https://doi.org/10.3390/f12111485








