Effect of Defoliation on the Defense Reactions of Silver Birch (Betula pendula) Infected with Phytophthora plurivora
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experiment Design
- Control—uninfected and non-defoliated trees
- P. plurivora—plants infected with pathogen P. plurivora without defoliation;
- P. plurivora + defoliation 30%—pathogen P. plurivora infected plants slightly stressed caused by removing 30% of the leaves;
- P. plurivora + defoliation 60%—pathogen P. plurivora infected plants with severe stress caused by removing 60% of the leaves;
- Defoliation 30%—uninfected plants and slightly stressed caused by removing 30% of the leaves, and
- Defoliation 60%—uninfected plants and severely stressed caused by removing 60% of the leaves.
2.2. Verification of the Infection of the Seedlings
2.3. Biometric Parameters
2.4. Measurement of the Root Systems
- TRL—total root length;
- MRL—length of the mother roots (2–5 mm);
- FRL—length of fine roots (0–2 mm), and
- FRSA—area of fine roots.
2.5. Chlorophyll Fluorescence
2.6. Analysis of Heat Shock Proteins Gene Expression
2.7. Observation and Identification of Parasitic Organisms on Birch Leaves
- 0—healthy leaves (no symptoms),
- 1—little disease (25% of leaf surface),
- 2—medium disease (26% to 50% of leaf surface),
- 3—strong disease (51% to 75% of the leaf surface),
- 4—severe disease (more than 76% of the leaf surface).
2.8. Chemical Analysis of Birch Shoots Extracts
2.9. Statistical Analysis
3. Results
3.1. Detection of Phytophthora plurivora
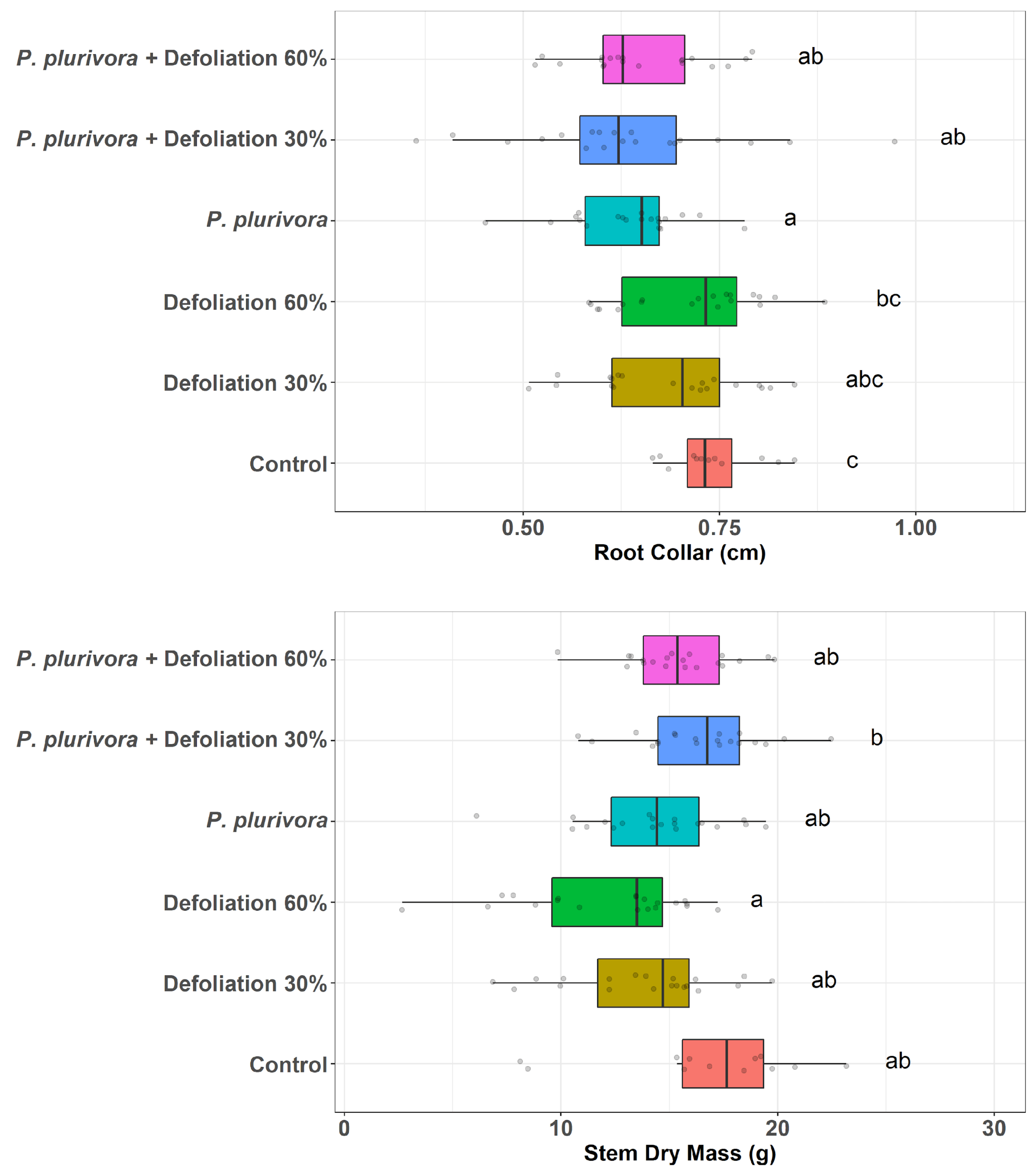
3.2. Biometric Parameters
3.3. Morphological Parameters of the Root System
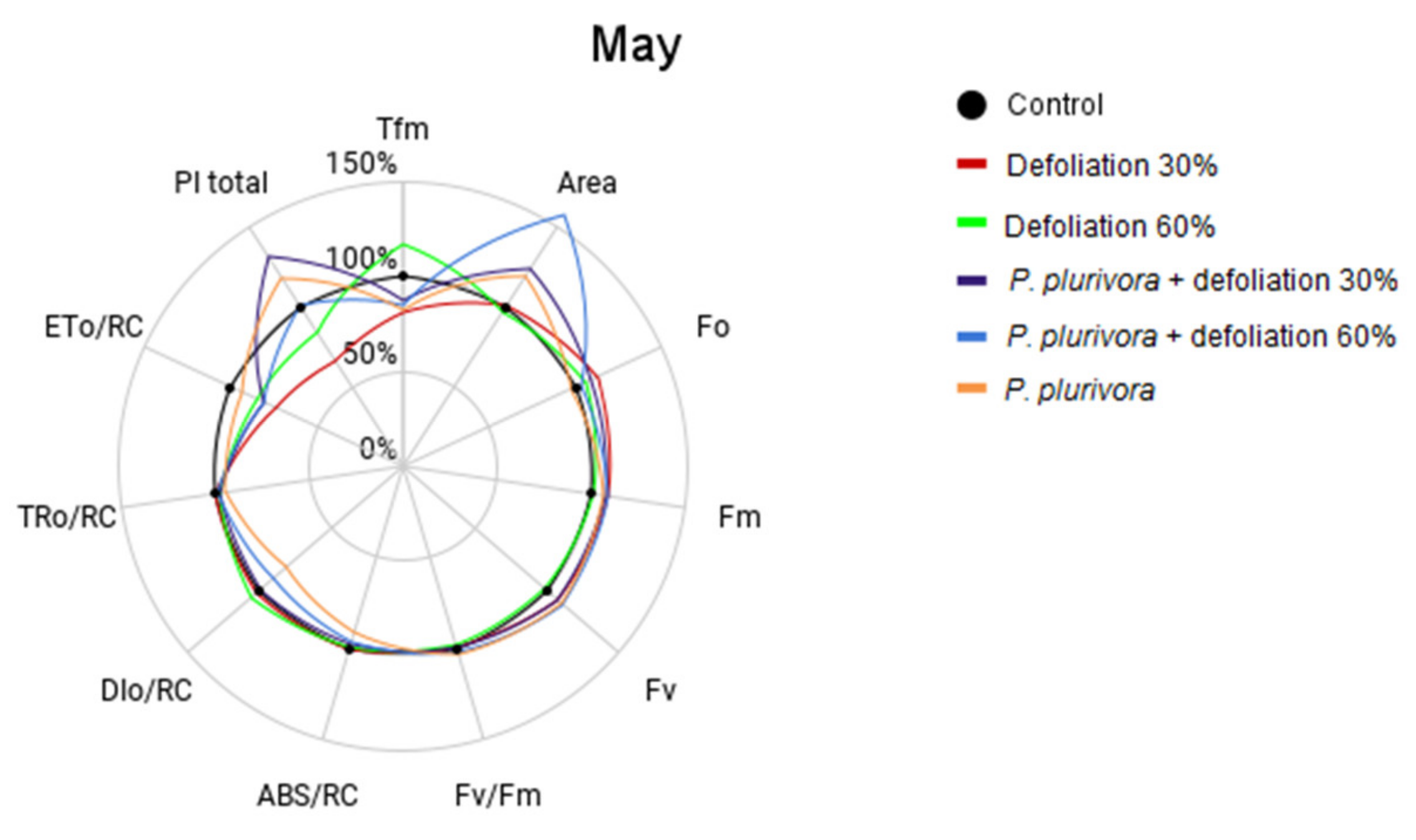
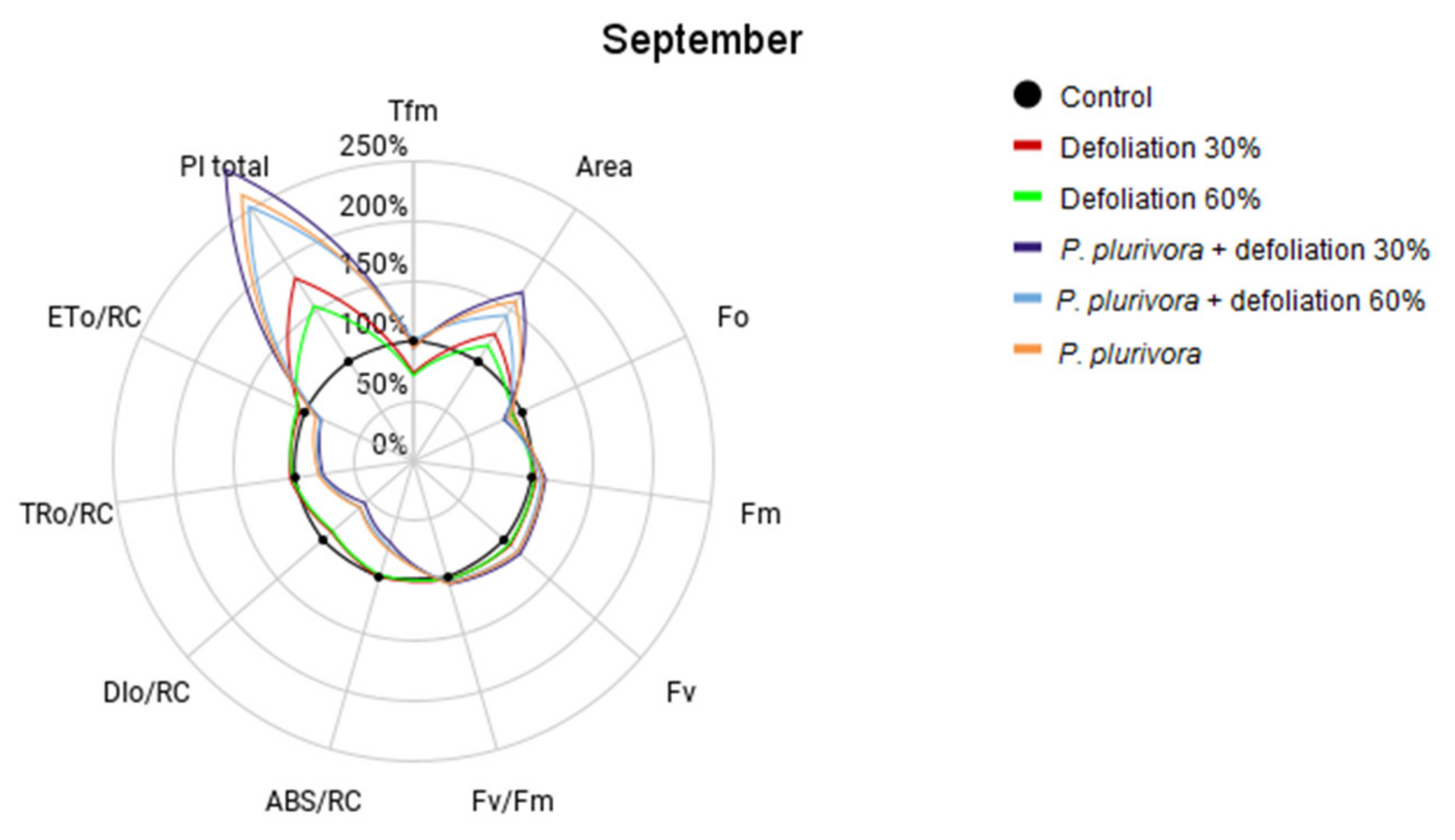
3.4. Chlorophyll-a Fluorescence Measurements
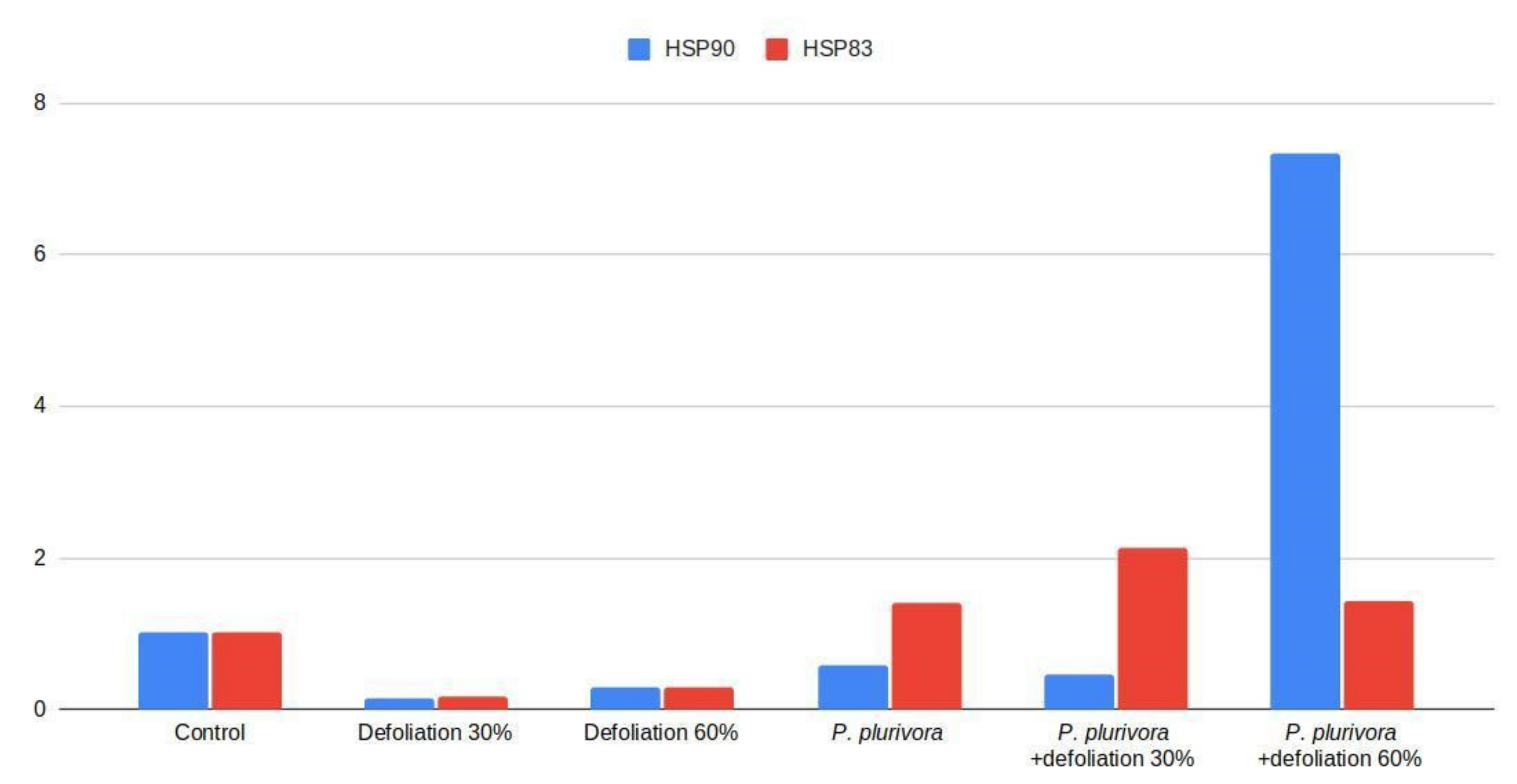
3.5. Heat Shock Protein Gene Expression Analysis
3.6. Interactions with Other Species of Pathogens on Birch Leaves
3.7. Chemical Composition of Birch Shoots Extracts in the Different Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hynynen, J.; Niemistö, P.; Viherä-Aarnio, A.; Brunner, A.; Hein, S.; Velling, P. Silviculture of Birch (Betula pendula Roth and Betula pubescens Ehrh.) in Northern Europe. For. Int. J. For. Res. 2010, 83, 103–119. [Google Scholar] [CrossRef]
- Silfver, T.H.; Roininen, H.; Oksanen, E.; Rousi, M. Genetic and Environmental Determinants of Silver Birch Growth and Herbivore Resistance. For. Ecol. Manag. 2009, 257, 2145–2149. [Google Scholar] [CrossRef]
- Green, S. Fungi Associated with Shoots of Silver Birch (Betula pendula) in Scotland. Mycol. Res. 2004, 108, 1327–1336. [Google Scholar] [CrossRef]
- Hansen, E.M.; Reeser, P.W.; Sutton, W. Phytophthora Beyond Agriculture. Annu. Rev. Phytopathol. 2012, 50, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Stukely, M.J.C.; Hardy, G.E.S.J.; White, D.; Paap, T.; Dunstan, W.A.; Burgess, T.I. Multiple New Phytophthora Species from ITS Clade 6 Associated with Natural Ecosystems in Australia: Evolutionary and Ecological Implications. Persoonia 2011, 26, 13–39. [Google Scholar] [CrossRef]
- Jung, T.; Burgess, T.I. Re-Evaluation of Phytophthora Citricola Isolates from Multiple Woody Hosts in Europe and North America Reveals a New Species, Phytophthora plurivora Sp. Nov. Persoonia 2009, 22, 95–110. [Google Scholar] [CrossRef]
- Malewski, T.; Brzezińska, B.; Belbahri, L.; Oszako, T. Role of Avian Vectors in the Spread of Phytophthora Species in Poland. Eur. J. Plant. Pathol. 2019, 155, 1363–1366. [Google Scholar] [CrossRef]
- Rytkönen, A.; Lilja, A.; Vercauteren, A.; Sirkiä, S.; Parikka, P.; Soukainen, M.; Hantula, J. Identity and Potential Pathogenicity of Phytophthora Species Found on Symptomatic Rhododendron Plants in a Finnish Nursery. Can. J. Plant Pathol. 2012, 34, 255–267. [Google Scholar] [CrossRef]
- Jankowiak, R.; Stępniewska, H.; Bilański, P.; Kolařík, M. Occurrence of Phytophthora plurivora and Other Phytophthora Species in Oak Forests of Southern Poland and Their Association with Site Conditions and the Health Status of Trees. Folia Microbiol. 2014, 59, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Orlikowski, L.B.; Trzewik, A.; Ptaszek, M.; Orlikowska, T. Relationship between Source of Water, Occurrence, and Pathogenicity of Phytophthora plurivora. Acta Mycol. 2012, 47, 3–9. [Google Scholar] [CrossRef][Green Version]
- Corcobado, T.; Cech, T.L.; Brandstetter, M.; Daxer, A.; Hüttler, C.; Kudláček, T.; Jung, M.H.; Jung, T. Decline of European Beech in Austria: Involvement of Phytophthora spp. and Contributing biotic and Abiotic factors. Forests 2020, 11, 895. [Google Scholar] [CrossRef]
- Michalska, Z.; Myssura, M.; Walczak, U. Owady Minujące Gór Polski/Mining Insects of Polish Mountains. Wiadomości Entomol. 2010, 29, 73–82. [Google Scholar]
- Koltunov, E.V. Насекoмые-Фитoфаги Лесных Биoгеoценoзoв в Услoвиях Антрoпoгеннoгo Вoздействия. Available online: https://www.elibrary.ru/item.asp?id=26002068 (accessed on 9 May 2021).
- Foyer, C.H.; Rasool, B.; Davey, J.W.; Hancock, R.D. Cross-Tolerance to Biotic and Abiotic Stresses in Plants: A Focus on Resistance to Aphid Infestation. J. Exp. Bot. 2016, 67, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Scharf, K.-D.; Berberich, T.; Ebersberger, I.; Nover, L. The Plant Heat Stress Transcription Factor (Hsf) Family: Structure, Function and Evolution. Biochim. Biophys. Acta Gene Regul. Mech. 2012, 1819, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of Plant Heat-Shock Proteins and Molecular Chaperones in the Abiotic Stress Response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef]
- Kotak, S.; Larkindale, J.; Lee, U.; von Koskull-Döring, P.; Vierling, E.; Scharf, K.-D. Complexity of the Heat Stress Response in Plants. Curr. Opin. Plant Biol. 2007, 10, 310–316. [Google Scholar] [CrossRef]
- Haq, S.U.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.-X.; Zhang, H.-X.; Wei, A.-M.; Gong, Z.-H. Heat Shock Proteins: Dynamic Biomolecules to Counter Plant Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, X.; Cobb, G.P.; Anderson, T.A. Plant MicroRNA: A Small Regulatory Molecule with Big Impact. Dev. Biol. 2006, 289, 3–16. [Google Scholar] [CrossRef]
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular Chaperones in Protein Folding and Proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef]
- Khateeb, W.A.; Muhaidat, R.; Alahmed, S.; Zoubi, M.S.A.; Al-Batayneh, K.M.; El-Oqlah, A.; Gamar, M.A.; Hussein, E.; Aljabali, A.A.; Alkaraki, A.K. Heat Shock Proteins Gene Expression and Physiological Responses in Durum Wheat (Triticum durum) under Salt Stress. Physiol. Mol. Biol. Plants 2020, 26, 1599–1608. [Google Scholar] [CrossRef]
- Chaudhary, R.; Baranwal, V.K.; Kumar, R.; Sircar, D.; Chauhan, H. Genome-Wide Identification and Expression Analysis of Hsp70, Hsp90, and Hsp100 Heat Shock Protein Genes in Barley under Stress Conditions and Reproductive Development. Funct. Integr. Genom. 2019, 19, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Wang, J.; Zhang, D.; Wang, Y. A Gene Regulatory Network Controlled by BpERF2 and BpMYB102 in Birch under Drought Conditions. Int. J. Mol. Sci. 2019, 20, 3071. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, M.H.; Goltsev, V.N.; Żuk-Gołaszewska, K.; Zivcak, M.; Brestic, M. Chlorophyll Fluorescence: Understanding Crop Performance—Basics and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781315153605. [Google Scholar]
- Jung, T.; Blaschke, H.; Neumann, P. Isolation, Identification and Pathogenicity of Phytophthora Species from Declining Oak Stands. For. Pathol. 1996, 26, 253–272. [Google Scholar] [CrossRef]
- Nowakowska, J.A.; Malewski, T.; Tereba, A.; Oszako, T. Rapid Diagnosis of Pathogenic Phytophthora Species in Soil by Real-Time PCR. For. Pathol. 2017, 47, e12303. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. ISBN 9780123721808. [Google Scholar]
- Caligiani, A.; Tonelli, L.; Palla, G.; Marseglia, A.; Rossi, D.; Bruni, R. Looking beyond Sugars: Phytochemical Profiling and Standardization of Manna Exudates from Sicilian Fraxinus excelsior L. Fitoterapia 2013, 90, 65–72. [Google Scholar] [CrossRef]
- Banks, J.M. Continuous Excitation Chlorophyll Fluorescence Parameters: A Review for Practitioners. Tree Physiol. 2017, 37, 1128–1136. [Google Scholar] [CrossRef]
- Cetner, M.; Dąbrowski, P.; Samborska, I.; Łukasik, I.; Swoczyna, T.; Pietkiewicz, S.; Bąba, W.; Kalaji, H. Chlorophyll fluorescence measurements in environmental studies (Zastosowanie pomiarów fluorescencji chlorofilu w badaniach środowiskowych). Kosmos 2016, 65, 197–205. [Google Scholar]
- Živčák, M.; Brestič, M.; Olšovská, K.; Slamka, P. Performance Index as a Sensitive Indicator of Water Stress in Triticum aestivum L. Plant Soil Environ. 2008, 54, 133–139. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a Fluorescence as a Tool to Monitor Physiological Status of Plants under Abiotic Stress Conditions. Acta Physiol. Plant 2016, 38, 1–11. [Google Scholar] [CrossRef]
- Armenteros, J.J.A.; Sønderby, C.K.; Sønderby, S.K.; Nielsen, H.; Winther, O. DeepLoc: Prediction of Protein Subcellular Localization Using Deep Learning. Bioinformatics 2017, 33, 3387–3395. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Fernald, R.D. Comprehensive Algorithm for Quantitative Real-Time Polymerase Chain Reaction. J. Comput. Biol. 2005, 12, 1047–1064. [Google Scholar] [CrossRef]
- Khvasko, A.V. Осoбеннoсти Развития Мучнистoй Рoсы Дуба в Услoвиях Беларуси и Усoвершенствoвание Защитных Мерoприятий[Peculiarities of Oak Powdery Mildew in the Conditions of Belarus and Improvement of Protective Measures]. Ph.D. Thesis, Belarusian State Technological University, Minsk, Belorusia.
- Kraska, S.A.P.; Dziki, D.; Stocki, M.; Stocka, N.; Różyło, K. Green Grain of Spelt (Triticum aestivum Ssp. Spelta) Harvested at the Stage of Milk-Dough as a Rich Source of Valuable Nutrients. Emir. J. Food Agric. 2019. [Google Scholar] [CrossRef]
- Łaska, G.; Sieniawska, E.; Świątek, Ł.; Zjawiony, J.; Khan, S.; Boguszewska, A.; Stocki, M.; Angielczyk, M.; Polz-Dacewicz, M. Phytochemistry and Biological Activities of Polemonium Caeruleum L. Phytochem. Lett. 2019, 30, 314–323. [Google Scholar] [CrossRef]
- Keča, N.; Tkaczyk, M.; Żółciak, A.; Stocki, M.; Kalaji, H.; Nowakowska, J.; Oszako, T. Survival of European Ash Seedlings Treated with Phosphite after Infection with the Hymenoscyphus fraxineus and Phytophthora Species. Forests 2018, 9, 442. [Google Scholar] [CrossRef]
- Sadowska, A.; Zapora, E.; Sawicka, D.; Niemirowicz-Laskowska, K.; Surażyński, A.; Sułkowska-Ziaja, K.; Kała, K.; Stocki, M.; Wołkowycki, M.; Bakier, S.; et al. Heterobasidion Annosum Induces Apoptosis in DLD-1 Cells and Decreases Colon Cancer Growth in In Vivo Model. Int. J. Mol. Sci. 2020, 21, 3447. [Google Scholar] [CrossRef]
- Sajkowska-Kozielewicz, J.J.; Kozielewicz, P.; Makarova, K.; Stocki, M.; Barnes, N.M.; Paradowska, K. Geissospermiculatine, a New Alkaloid from Geissospermum Reticulatum Bark. Molecules 2020, 26, 143. [Google Scholar] [CrossRef]
- Agresti, A. Categorical Data Analysis; Wiley Series in Probability and Statistics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; ISBN 9780471360933. [Google Scholar]
- Westfall, P.H.; Wolfinger, R.D. Multiple Tests with Discrete Distributions. Am. Stat. 1997, 51, 3–8. [Google Scholar] [CrossRef]
- Kramer, C.Y. Extension of Multiple Range Tests to Group Means with Unequal Numbers of Replications. Biometrics 1956, 12, 307. [Google Scholar] [CrossRef]
- Mollie, E.B.; Kristensen, K.; Koen, J.; Magnusson, A.; Casper, W.B.; Nielsen, A.; Hans, J.S.; Mächler, M.; Benjamin, M.B. GlmmTMB Balances Speed and Flexibility Among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S.; Statistics and Computing; Springer: New York, NY, USA, 2002; ISBN 9781441930088. [Google Scholar]
- Lenth, R.V. Estimated Marginal Means, Aka Least-Squares Means [R Package Emmeans Version 1.6.1]. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 10 June 2021).
- Strouts, R.G. Phytophthora diseases of trees and shrubs. In Arboricultural Leaflet; HMSO: London, UK, 1981; Volume 8, p. 16. [Google Scholar]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; The American Phytopathological Society: St Paul, MA, USA, 1996; p. 562. ISBN 0890542120. [Google Scholar]
- Akhler, M.; Tobocka, M.; Oszako, T. Pathogenic Oomycetes of Phytophthora Genus—A New Threat to Forests in Europe. Sylwan 2017, 161, 870–880. [Google Scholar]
- Barry, K.M.; Pinkard, E.A. Growth and Photosynthetic Responses Following Defoliation and Bud Removal in Eucalypts. For. Ecol. Manag. 2013, 293, 9–16. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Bosa, K.; Kościelniak, J.; Hossain, Z. Chlorophyll a Fluorescence—A Useful Tool for the Early Detection of Temperature Stress in Spring Barley (Hordeum vulgare L.). OMICS 2011, 15, 925–934. [Google Scholar] [CrossRef]
- Garab, G.; Cseh, Z.; Kovács, L.; Rajagopal, S.; Várkonyi, Z.; Wentworth, M.; Mustárdy, L.; Dér, A.; Ruban, A.V.; Papp, E.; et al. Light-Induced Trimer to Monomer Transition in the Main Light-Harvesting Antenna Complex of Plants: Thermo-Optic Mechanism. Biochemistry 2002, 41, 15121–15129. [Google Scholar] [CrossRef]
- Aldea, M.; Hamilton, J.G.; Resti, J.P.; Zangerl, A.R.; Berenbaum, M.R.; Frank, T.D.; DeLucia, E.H. Comparison of Photosynthetic Damage from Arthropod Herbivory and Pathogen Infection in Understory Hardwood Saplings. Oecologia 2006, 149, 221–232. [Google Scholar] [CrossRef]
- Swindell, W.R.; Huebner, M.; Weber, A.P. Transcriptional Profiling of Arabidopsis Heat Shock Proteins and Transcription Factors Reveals Extensive Overlap between Heat and Non-Heat Stress Response Pathways. BMC Genom. 2007, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Liu, J.-H.; Ma, X.; Luo, D.-X.; Gong, Z.-H.; Lu, M.-H. The Plant Heat Stress Transcription Factors (HSFs): Structure, Regulation, and Function in Response to Abiotic Stresses. Front. Plant Sci. 2016, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Kubienová, L.; Sedlářová, M.; Vítečková-Wünschová, A.; Piterková, J.; Luhová, L.; Mieslerová, B.; Lebeda, A.; Navrátil, M.; Petřivalský, M. Effect of Extreme Temperatures on Powdery Mildew Development and Hsp70 Induction in Tomato and Wild solanum spp. Plant Prot. Sci. 2013, 49, S41–S54. [Google Scholar] [CrossRef]
- Liu, J.; Pang, X.; Cheng, Y.; Yin, Y.; Zhang, Q.; Su, W.; Hu, B.; Guo, Q.; Ha, S.; Zhang, J.; et al. The Hsp70 Gene Family in Solanum tuberosum: Genome-Wide Identification, Phylogeny, and Expression Patterns. Sci. Rep. 2018, 8, 16628. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.E.; Oh, S.; Seo, E.; Choi, D. HSP70s Enhance a Phytophthora infestans Effector-Induced Cell Death via an MAPK Cascade in Nicotiana benthamiana. Mol. Plant Microbe Interact. 2018, 31, 356–362. [Google Scholar] [CrossRef]
- Acosta-Muñiz, C.H.; Escobar-Tovar, L.; Valdes-Rodríguez, S.; Fernández-Pavia, S.; Arias-Saucedo, L.J.; de la Cruz Espindola Barquera, M.; Lim, M.Á.G. Identification of Avocado (Persea americana) Root Proteins Induced by Infection with the Oomycete Phytophthora Cinnamomi Using a Proteomic Approach. Physiol. Plant. 2012, 144, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Kadota, Y.; Shirasu, K. The HSP90 Complex of Plants. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.I.B.; Kanneganti, T.-D.; Young, C.; Cakir, C.; Huitema, E.; Win, J.; Armstrong, M.R.; Birch, P.R.J.; Kamoun, S. The C-Terminal Half of Phytophthora infestans RXLR Effector AVR3a Is Sufficient to Trigger R3a-Mediated Hypersensitivity and Suppress INF1-Induced Cell Death in Nicotiana benthamiana. Plant J. 2006, 48, 165–176. [Google Scholar] [CrossRef]
- Kanneganti, T.-D.; Huitema, E.; Cakir, C.; Kamoun, S. Synergistic Interactions of the Plant Cell Death Pathways Induced by Phytophthora infestans Nep1-Like Protein PiNPP1.1 and INF1 Elicitin. Mol. Plant Microbe Interact. 2006, 19, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Haukioja, E.; Neuvonen, S. Induced Long-Term Resistance of Birch Foliage against Defoliators: Defensive or Incidental? Ecology 1985, 66, 1303–1308. [Google Scholar] [CrossRef]
- Stocki, M. Recovering Biologically Active Compounds from Logging Residue of Birch (Betula spp.) with Supercritical Carbon Dioxide. Chem. Rev. 2018, 1, 120–124. [Google Scholar] [CrossRef]
- Stocki, M. Study on Extraction of Biologically Active Compounds from Birch (Betula) Buds with Supercritical Carbon Dioxide. Chem. Rev. 2019, 1, 154–157. [Google Scholar] [CrossRef]
- Szoka, Ł.; Isidorov, V.; Nazaruk, J.; Stocki, M.; Siergiejczyk, L. Cytotoxicity of Triterpene Seco-Acids from Betula pubescens Buds. Molecules 2019, 24, 4060. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Stocki, M.; Vetchinikova, L. Inheritance of Specific Secondary Volatile Metabolites in Buds of White Birch Betula pendula and Betula pubescens Hybrids. Trees 2019, 33, 1329–1344. [Google Scholar] [CrossRef]
- Stocki, M.; Banaszczak, P.; Stocka, N.; Borowik, T.; Zapora, E.; Isidorov, V. Taxonomic Implications of Volatile Secondary Metabolites Emitted from Birch (Betula L.) Buds. Biochem. Syst. Ecol. 2020, 92, 104132. [Google Scholar] [CrossRef]
- Loulier, J.; Lefort, F.; Stocki, M.; Asztemborska, M.; Szmigielski, R.; Siwek, K.; Grzywacz, T.; Hsiang, T.; Ślusarski, S.; Oszako, T.; et al. Detection of Fungi and Oomycetes by Volatiles Using E-Nose and SPME-GC/MS Platforms. Molecules 2020, 25, 5749. [Google Scholar] [CrossRef]
- Oszako, T.; Żółciak, A.; Tulik, M.; Tkaczyk, M.; Stocki, M.; Nowakowska, J.A. Wpływ Bacillus subtilis i Trichoderma asperellum na rozwój sadzonek brzóz zainfekowanych patogenem drobnych korzeni Phytophthora plurivora. Sylwan 2019, 163, 1006–1015. [Google Scholar] [CrossRef]
- Nowakowska, J.A.; Stocki, M.; Stocka, N.; Ślusarski, S.; Tkaczyk, M.; Caetano, J.M.; Tulik, M.; Hsiang, T.; Oszako, T. Interactions between Phytophthora cactorum, Armillaria gallica and Betula pendula Roth. Seedlings Subjected to Defoliation. Forests 2020, 11, 1107. [Google Scholar] [CrossRef]
- Braun, U. A Monograph of the Erysiphales (Powdery Mildews), 89th ed.; Beihefte Zur Nova Hedwigia: Cramer, Weinheim, 1987. [Google Scholar]



| Primers and Probes Name | Sequence | Reference |
|---|---|---|
| P. plurivora-f | CCG TAT CAA CCC TTT TAG | [26] |
| P. plurivora-r | GCT GAA AGT TGC TAT CTA | [26] |
| P. plurivora-probe | [JOE]CCC AGA CCG AAG TCC AAA CAT[NFQ-MGB] | [26] |
| BpHsp90-f | GACAGAGGAGATCGCGAAGG | (new design) |
| BpHsp90-r | GCCCTCAACTGAGAAGTGCT | (new design) |
| BpHsp83-f | AGGATTGCTCCTGACAAGGC | (new design) |
| BpHsp83-r | CAACGCCTCCATGAACTCCT | (new design) |
| ITS1 | TCCGTAGGTGAACCTGCGG | [27] |
| ITS4 | TCCTCCGCTTATTGATATGC | [27] |
| BpTub-f | ACAGTGCATTCGAGCCATCA | (new design) |
| BpTub-r | CACCTCAGCCACACACTGGTAG | (new design) |
| Compound | Content (%) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Flavonoids, among others: | 0.48 | 0.75 | - | 0.54 | 0.42 | 4.78 |
| Catechine | 0.48 | 0.15 | - | - | - | 0.48 |
| Kempheride | - | 0.6 | trace * | 0.54 | 0.42 | 1.83 |
| 3-Methylluteolin | - | - | - | - | - | 0.28 |
| 3,7-Dimethylquercetin | - | - | - | - | - | 0.42 |
| Santin | - | - | - | - | - | 0.64 |
| Cirsimaritin | - | - | - | - | - | 0.77 |
| Other phenols, including: | 3.08 | 4.22 | 1.57 | 5.98 | 3.54 | 0.3 |
| Eugenol | - | 0.17 | - | 0.19 | - | - |
| Tyrosol | 0.38 | 0.59 | trace * | 1.05 | 0.63 | - |
| 3-Hydroxybenzoic acid | - | - | 0.21 | - | - | - |
| Betuligenol | 1.61 | 2.41 | 0.21 | 3.29 | 2.54 | 0.3 |
| Protocatechuic acid | - | - | 0.58 | 0.28 | - | - |
| 2-(4-Hydroxyphenyl)propionate | 0.54 | 0.25 | trace * | 0.71 | 0.19 | - |
| Rhododendrin | - | 0.26 | - | - | - | - |
| 1-Docosyl p-coumarate | 0.54 | 0.54 | 0.56 | 0.45 | 0.19 | trace * |
| Triterpenes, among others: | 49.88 | 48.2 | 18.08 | 44.04 | 51.11 | 64.9 |
| Squalene | - | 0.23 | - | - | - | 0.23 |
| 3,4-seco-Dammara-4(29),20(21),24(25)-trien-3-oic acid | 27.03 | 32.5 | 7.35 | 29.31 | 32.31 | 33.86 |
| Dammaradien-3-one | 1.67 | 2.7 | 0.6 | 2.1 | 2.18 | 4.01 |
| 3,4-seco-Olean-4(24)-en-19-on-3-oic acid | 0.63 | 0.44 | 0.45 | 0.26 | 0.47 | 0.35 |
| 3,4-seco-Urs-4(23),20(30)-dien-3-oic acid | 2.86 | 2.16 | 0.43 | 1.78 | 2.64 | 3.49 |
| 20-Hydroxy-3,4-seco-dammara-4(28),24-dien-3-oic acid | 2.26 | 0.86 | 2.17 | 0.79 | 1.21 | 2.05 |
| Dipterocarpol, isomer 20R | 0.97 | 0.56 | trace * | 0.72 | 0.71 | 1 |
| 3,4-seco-Urs-4(23),20(30)-dien-19-ol-3-oic acid | - | - | - | - | 0.27 | 0.6 |
| Dipterocarpol, isomer 20S | 2.5 | 0.66 | 0.36 | 0.96 | 1.35 | 1.37 |
| Betulin | 0.53 | 0.25 | 0.81 | 0.27 | 0.37 | 1.18 |
| Oleanolic acid | 0.61 | 0.22 | 0.49 | 0.43 | 0.21 | 0.34 |
| Betulinic acid | 0.79 | 0.35 | 0.73 | 0.53 | 0.34 | 0.7 |
| Kabraleon | 0.67 | 0.33 | 0.41 | 0.36 | 0.49 | 1.18 |
| Methyl acetylbetulinate | 0.45 | 0.32 | 1.88 | 0.41 | 0.39 | 0.48 |
| Sterols, including: | 5.19 | 5.02 | 12.35 | 3.93 | 3.89 | 5.69 |
| Campesterol | - | - | 0.75 | 0.25 | 0.25 | 0.59 |
| β-Sitosterol | 4.8 | 4.58 | 9.8 | 3.42 | 3.37 | 4.74 |
| Stigmastanol | 0.4 | 0.44 | 1.8 | 0.26 | 0.27 | 0.36 |
| Fatty acids + fatty acids esters | 19.36 | 20.94 | 39.73 | 21.92 | 19.89 | 13.79 |
| Fatty alcohols | 7.25 | 6.9 | 12.83 | 8.41 | 7.25 | 5.44 |
| Alkanes | 6.5 | 7.78 | 6.12 | 7.84 | 9.02 | 3.8 |
| Other compounds, including: | 7.6 | 5.41 | 8.96 | 6.11 | 4.09 | 1.3 |
| meso-2,3-Butanediol | 0.97 | 0.33 | 0.5 | 0.47 | - | - |
| 3-Hydroksyheptane | - | - | 0.28 | - | - | - |
| threo-2,3-Butanediol | 0.65 | 0.34 | 0.7 | 0.61 | - | - |
| Lactic acid | 0.68 | 0.34 | 0.45 | 0.38 | 0.37 | - |
| 2-Methylpentane-2,4-diol | 2.92 | 1.78 | 5.89 | 1.49 | 0.95 | 0.47 |
| 2-Phenylethanol | - | 0.12 | - | 0.14 | - | - |
| Linalool | - | 0.15 | trace * | 0.4 | 0.19 | - |
| Glycerol | 0.8 | 0.67 | 0.7 | 0.7 | 0.73 | 0.19 |
| Phytol | 1.57 | 1.7 | 0.44 | 1.93 | 1.85 | 0.64 |
| Unidentified compounds | 0.67 | 0.77 | 0.36 | 1.24 | 0.81 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berezovska, D.; Oszako, T.; Malewski, T.; Stocki, M.; Marozau, A.; Stocka, N.; Moser, W.K.; Baggett, L.S.; Belbahri, L.; Nowakowska, J.A. Effect of Defoliation on the Defense Reactions of Silver Birch (Betula pendula) Infected with Phytophthora plurivora. Forests 2021, 12, 910. https://doi.org/10.3390/f12070910
Berezovska D, Oszako T, Malewski T, Stocki M, Marozau A, Stocka N, Moser WK, Baggett LS, Belbahri L, Nowakowska JA. Effect of Defoliation on the Defense Reactions of Silver Birch (Betula pendula) Infected with Phytophthora plurivora. Forests. 2021; 12(7):910. https://doi.org/10.3390/f12070910
Chicago/Turabian StyleBerezovska, Daria, Tomasz Oszako, Tadeusz Malewski, Marcin Stocki, Aleh Marozau, Natalia Stocka, Warren Keith Moser, Larry Scott Baggett, Lassaad Belbahri, and Justyna Anna Nowakowska. 2021. "Effect of Defoliation on the Defense Reactions of Silver Birch (Betula pendula) Infected with Phytophthora plurivora" Forests 12, no. 7: 910. https://doi.org/10.3390/f12070910
APA StyleBerezovska, D., Oszako, T., Malewski, T., Stocki, M., Marozau, A., Stocka, N., Moser, W. K., Baggett, L. S., Belbahri, L., & Nowakowska, J. A. (2021). Effect of Defoliation on the Defense Reactions of Silver Birch (Betula pendula) Infected with Phytophthora plurivora. Forests, 12(7), 910. https://doi.org/10.3390/f12070910









