Identification of miR397a and Its Functional Characterization in Callus Growth and Development by Regulating Its Target in Liriodendron
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Bioinformatics Analysis
2.3. Cloning and Transformation
2.4. Quantitative Real-Time PCR
2.5. Callus Proliferation and MTS Assays
2.6. Physiological Analyses
3. Results
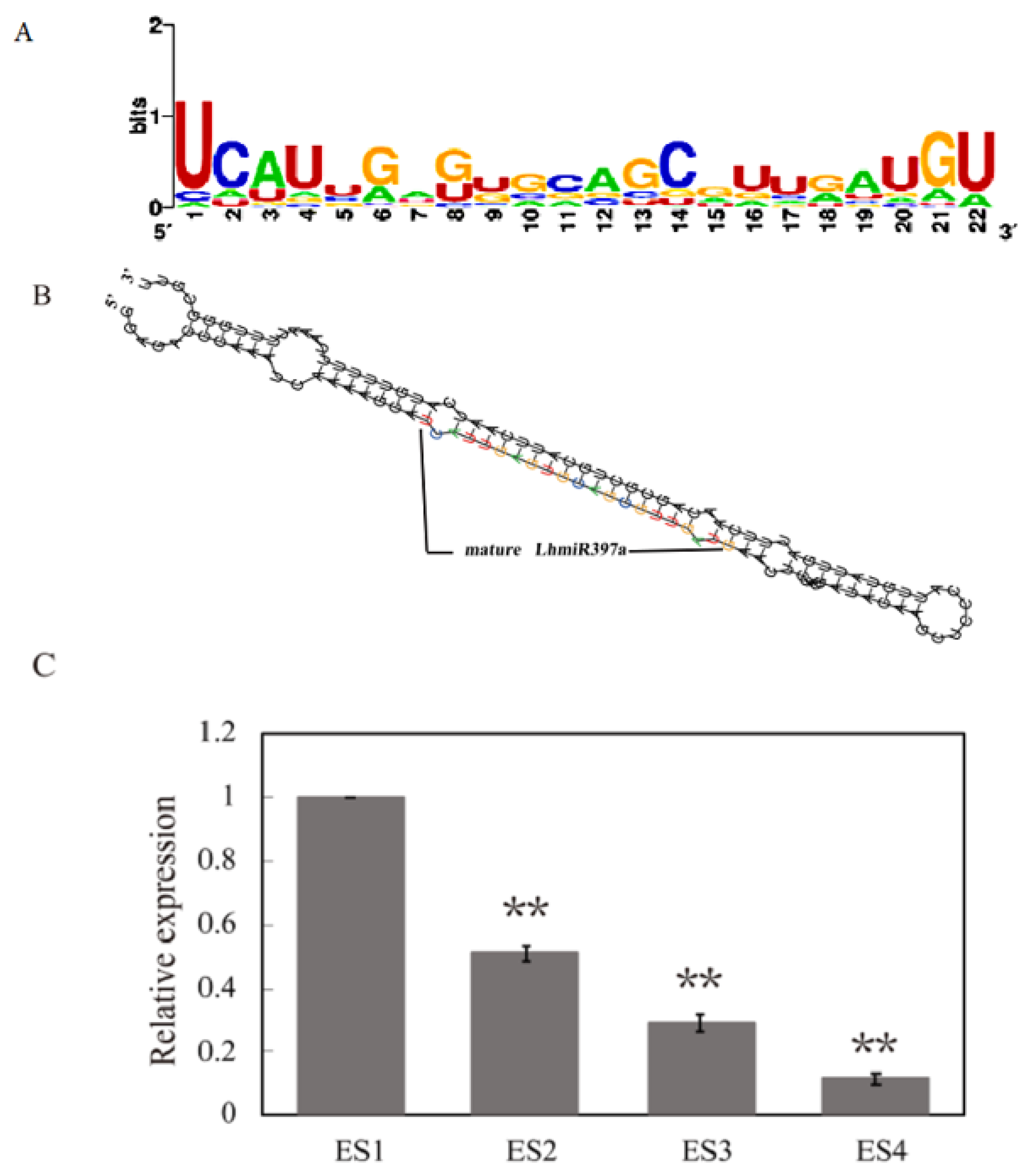
3.1. Identification of miR397 and LhmiR397a Differentially Expressed during Early SE
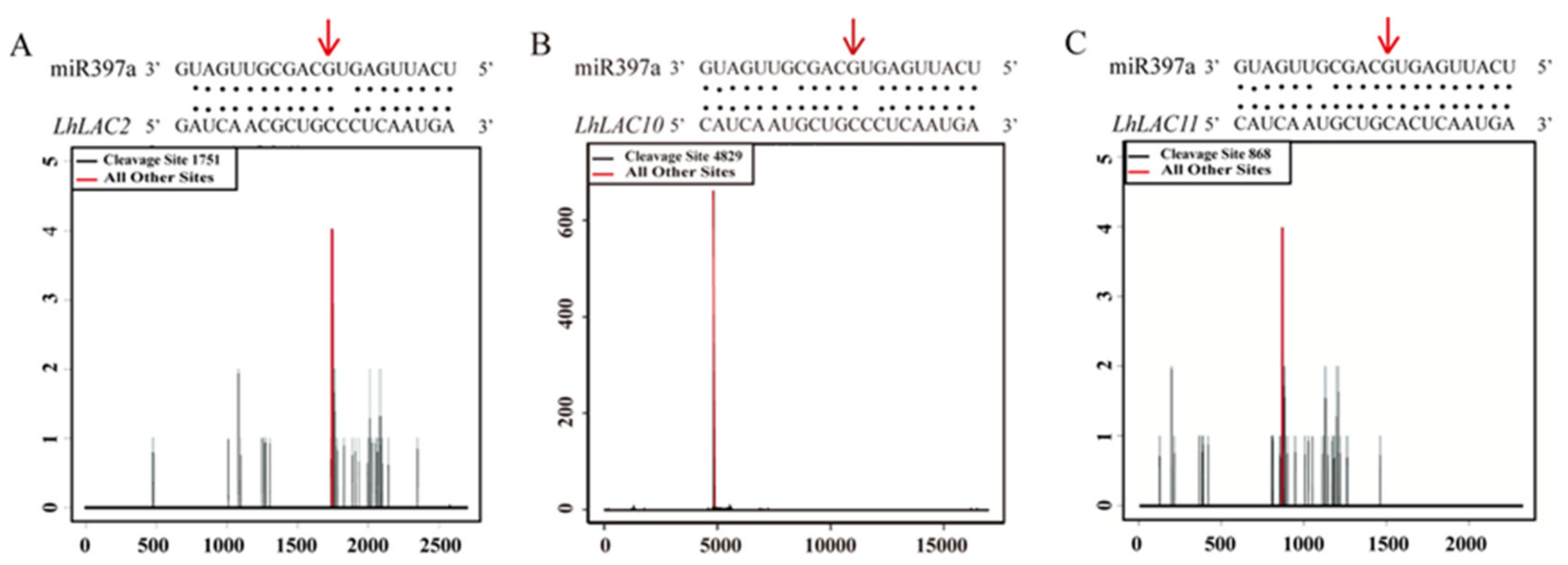
3.2. Three LAC Genes Are Targets of miR397a
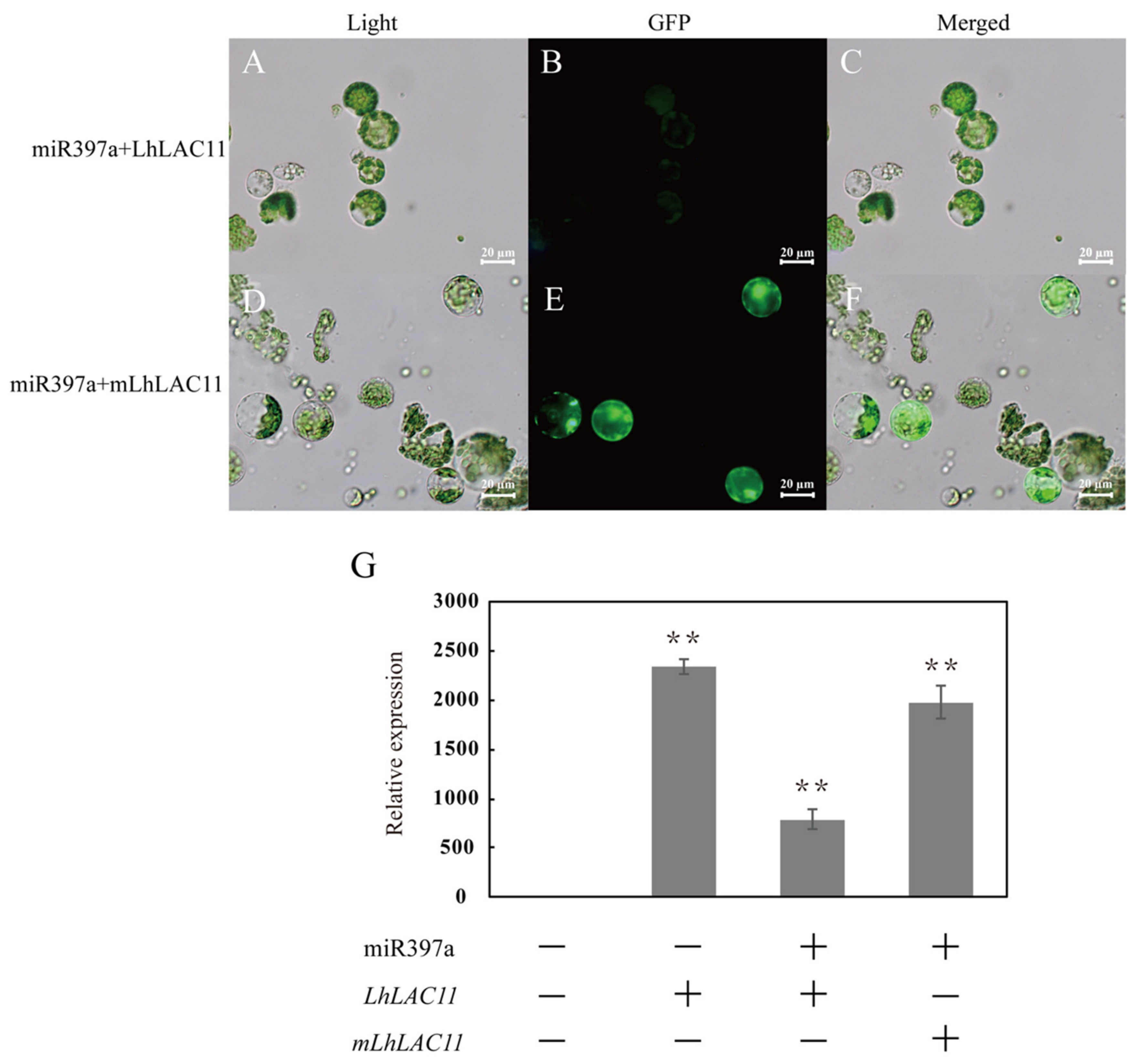
3.3. Downregulation of LAC Transcript Abundance by miR397a Overexpression
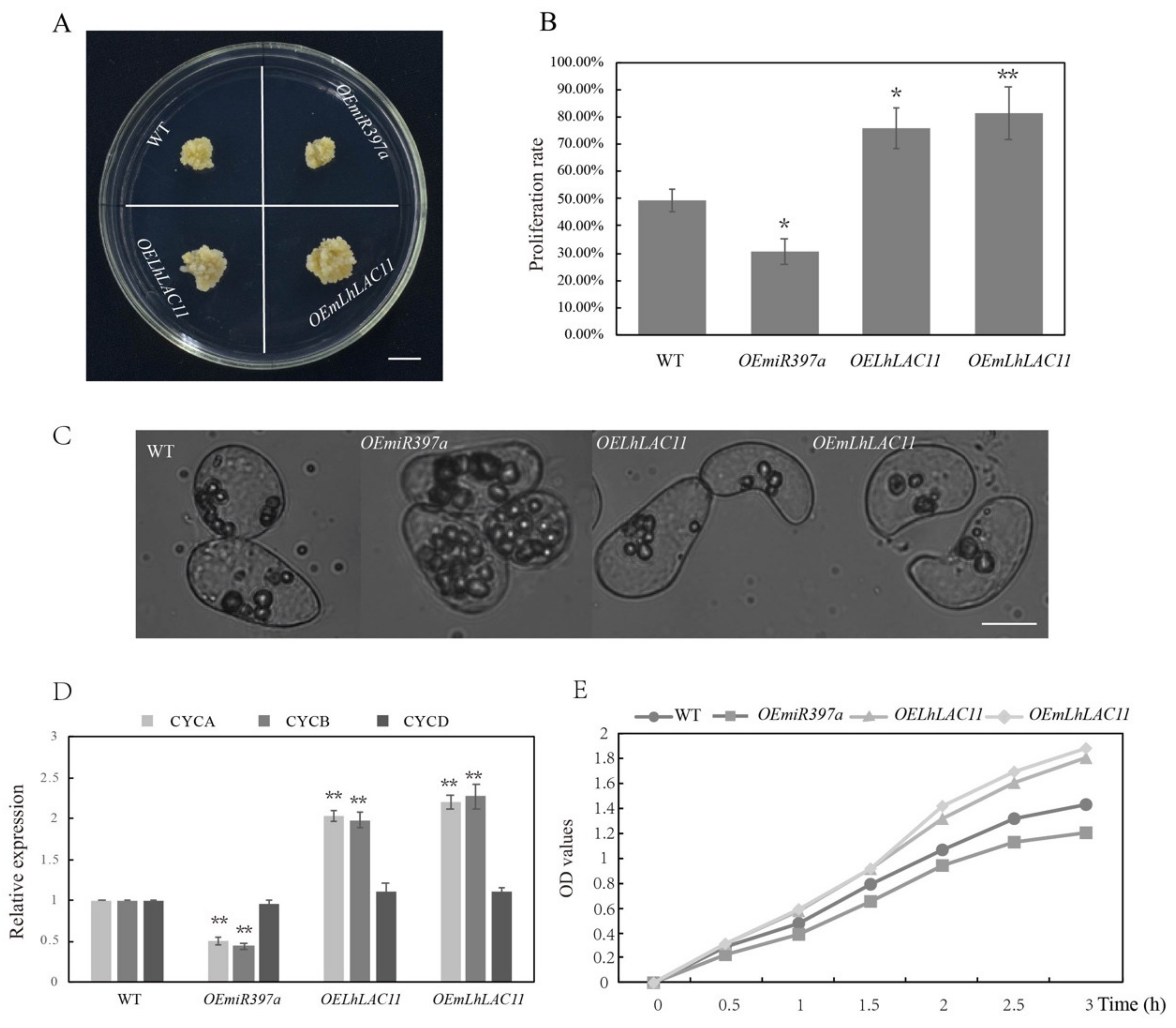
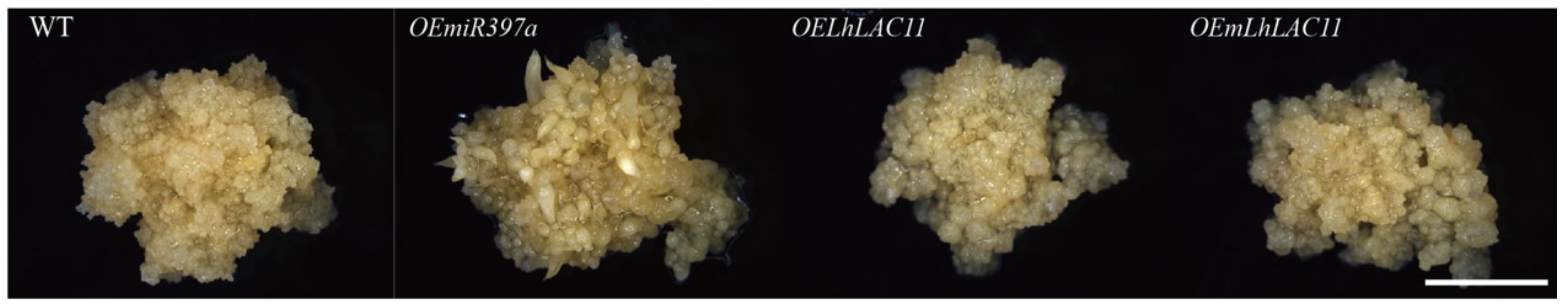
3.4. miR397a Downregulated LAC Transcript and Affected Callus Growth and Development
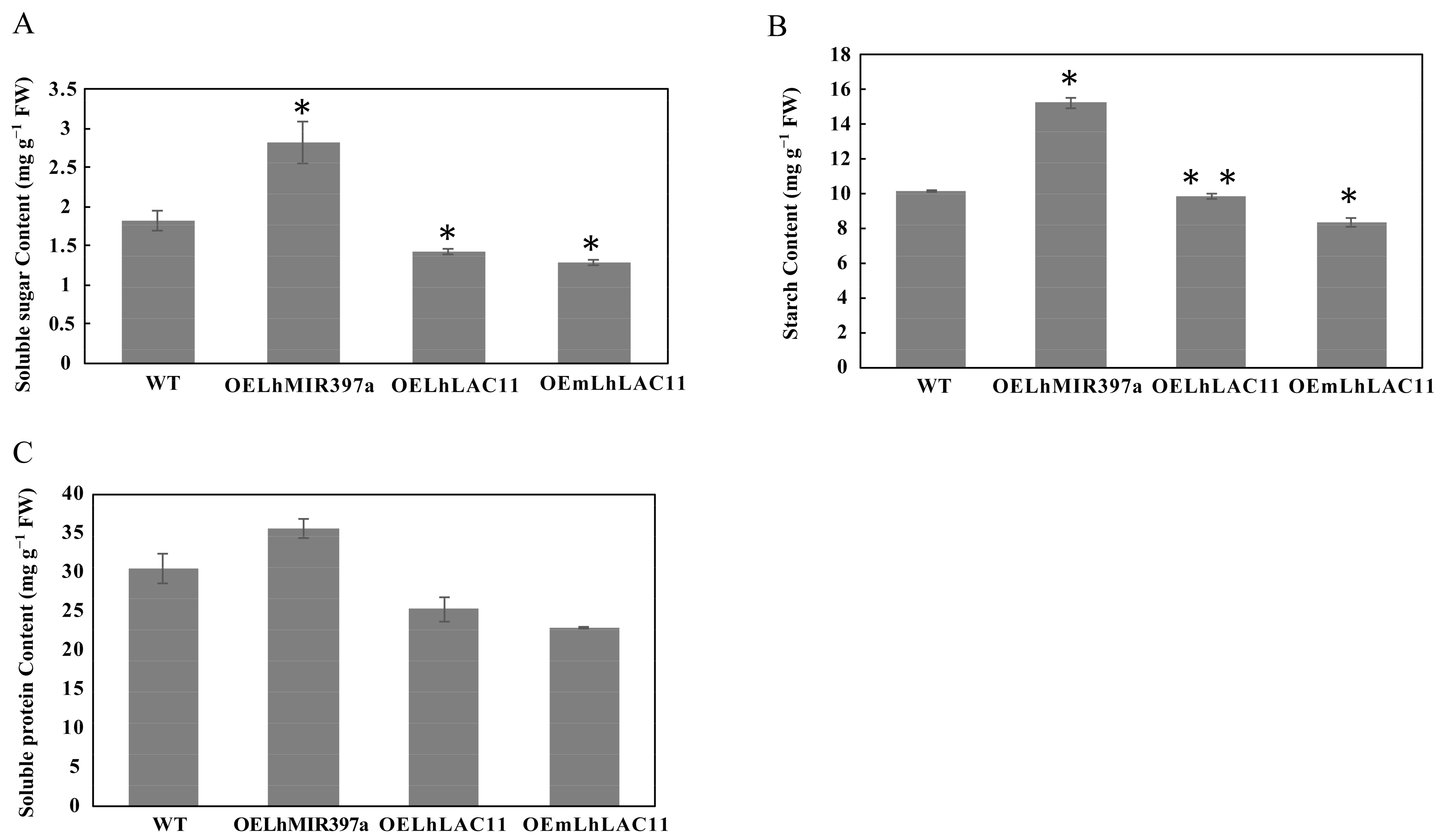
3.5. Accumulation of Soluble Sugar, Starch, and Soluble Protein in Callus-Overexpressing miR397a
4. Discussion
4.1. Characteristic Analysis of the miR397 Gene and Identification of the Target Transcript
4.2. miR397a-LAC Regulation is Involved in Callus Growth and Development
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thorpe, T. History of plant tissue culture. Mol. Biotechnol. 2007, 37, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Stobbe, H.; Schmitt, U.; Eckstein, D.; Dujesiefken, D. Developmental stages and fine structure of surface callus formed after debarking of living lime trees (Tilia sp.). Ann. Bot. 2002, 89, 773–782. [Google Scholar] [CrossRef] [Green Version]
- Cline, M.N. The histology and histochemistry of the wound-healing process in geranium cuttings in relationship to basal stem rot caused by pythium ultimum. Plant Dis. 1980, 67, 636–638. [Google Scholar] [CrossRef] [Green Version]
- Ikeuchi, M.; Favero, D.S.; Sakamoto, Y.; Iwase, A.; Coleman, D.; Rymen, B.; Sugimoto, K. Molecular mechanisms of plant regeneration. Annu. Rev. Plant Biol. 2019, 70, 377–406. [Google Scholar] [CrossRef] [PubMed]
- Skoog, F.; Miller, C.O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. Soc. Exp. Biol. 1957, 54, 118–130. [Google Scholar]
- Li, T.T.; Chen, J.H.; Qiu, S.; Zhang, Y.J.; Wang, P.K.; Yang, L.W.; Lu, Y.; Shi, J.S. Deep Sequencing and Microarray Hybridization Identify Conserved and Species-Specific MicroRNAs during Somatic Embryogenesis in Hybrid Yellow Poplar. PLoS ONE 2012, 7, e43451. [Google Scholar] [CrossRef]
- Steward, F.C.; Mapes, M.O.; Mears, K. Growth and organized development of cultured cells. II. Organization in cultures grown from freely suspended cells. Am. J. Bot. 1958, 45, 705–708. [Google Scholar] [CrossRef]
- Birnbaum, K.D.; Alvarado, A.S. Slicing across kingdoms: Regeneration in plants and animals. Cell 2008, 132, 697–710. [Google Scholar] [CrossRef] [Green Version]
- Valvekens, D.; Montagu, M.V.; Lijsebettens, M.V. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc. Natl. Acad. Sci. USA 1988, 85, 5536–5540. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Tu, L.L.; Tang, W.X.; Gao, W.H.; Lindsey, K.; Zhang, X.L. Small RNA and degradome profiling reveals a role for miRNAs and their targets in the developing fibers of Gossypium barbadense. Plant J. Cell Mol. Biol. 2015, 80, 331–344. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.L.; Wang, Q.L.; Sun, R.R.; Zhang, B.H. Deep sequencing reveals important roles of microRNAs in response to drought and salinity stress in cotton. J. Exp. Bot. 2015, 66, 789–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones-Rhoades, M.W.; Bartel, D.P.; Bartel, B. MicroRNAs and their regulatory roles in plants. Ann. Rev. Plant Biol. 2006, 57, 19–53. [Google Scholar] [CrossRef]
- Chen, X.M. Small RNAs and their roles in plant development. Ann. Rev. Cell Dev. Biol. 2009, 25, 21–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nodine, M.D.; Bartel, D.P. MicroRNAs prevent precocious gene expression and enable pattern formation during plant embryogenesis. Genes Dev. 2010, 24, 2678–2692. [Google Scholar] [CrossRef] [Green Version]
- Oh, T.J.; Wartell, R.M.; Cairney, J.; Pullman, G.S. Evidence for stage-specific modulation of specific microRNAs (miRNAs) and miRNA processing components in zygotic embryo and female gametophyte of loblolly pine (Pinus taeda). New Phytol. 2008, 179, 67–80. [Google Scholar] [CrossRef]
- Willmann, M.R.; Mehalick, A.J.; Packer, R.L.; Jenik, P.D. MicroRNAs regulate the timing of embryo maturation in Arabidopsis. Plant Physiol. 2011, 155, 1871–1884. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.M.; Liu, M.Y.; Ge, X.X.; Xu, Q.; Guo, W.W. Stage and tissue-specific modulation of ten conserved miRNAs and their targets during somatic embryogenesis of Valencia sweet orange. Planta 2011, 233, 495–505. [Google Scholar] [CrossRef]
- Zhang, J.H.; Zhang, S.G.; Han, S.Y.; Wu, T.; Li, X.M.; Li, W.F.; Qi, L.W. Genome-wide identification of microRNAs in larch and stage-specific modulation of 11 conserved microRNAs and their targets during somatic embryogenesis. Planta 2012, 236, 647–657. [Google Scholar] [CrossRef]
- Lin, Y.L.; Lai, Z.X. Comparative analysis reveals dynamic changes in miRNAs and their targets and expression during somatic embryogenesis in Longan (Dimocarpus longan Lour.). PLoS ONE 2013, 8, e60337. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.Y.; Wang, L.C.; Yuan, D.J.; Lindsey, K.; Zhang, X.L. Small RNA and degradome sequencing reveal complex miRNA regulation during cotton somatic embryogenesis. J. Exp. Bot. 2013, 64, 1521–1536. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.L.; Xu, L.; Wang, Y.; Huang, D.Q.; Yu, R.G.; Limera, C.; Gong, Y.; Liu, L. Genome-Wide Identification of Embryogenesis-Associated microRNAs in Radish (Raphanus sativus L.) by High-Throughput Sequencing. Plant Mol. Biol. Report. 2014, 32, 900–915. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, B.Y.; Gai, M.Z.; Song, S.L.; Jia, N.N.; Sun, H.M. Small RNA and transcriptome sequencing reveal a potential miRNA-mediated interaction network that functions during somatic embryogenesis in Lilium pumilum DC. Fisch. Front. Plant Sci. 2017, 8, 566. [Google Scholar] [CrossRef] [PubMed]
- Sunkar, R.; Zhu, J.K. Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 2004, 16, 2001–2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Ghany, S.E.; Pilon, M. MicroRNA-mediated systemic down-regulation of copper protein expression in response to low copper availability in Arabidopsis. J. Biol. Chem. 2008, 283, 15932–15945. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.F.; Yang, C.M.; Chiang, V.L. Conservation and diversity of microRNA-associated copper-regulatory networks in Populus trichocarpa. J. Integr. Plant Biol. 2011, 53, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; He, H.; Yu, D.Q. Identification of nitrogen starvation-responsive microRNAs in Arabidopsis thaliana. PLoS ONE 2012, 7, e48951. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.H.; Zhong, S.H.; Li, X.H.; Li, W.X.; Rothstein, S.J.; Zhang, S.H.; Bi, Y.M.; Xie, C.X. Genome-wide identification of microRNAs in response to low nitrate availability in maize leaves and roots. PLoS ONE 2011, 6, e28009. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.H.; Pei, H.X. Over-expression of miR397 improves plant tolerance to cold stress in Arabidopsis thaliana. J. Plant Biol. 2014, 57, 209–217. [Google Scholar] [CrossRef]
- Zhou, L.G.; Liu, Y.H.; Liu, Z.C.; Kong, D.Y.; Duan, M.; Luo, L.J. Genome-wide identification and analysis of drought-responsive microRNAs in Oryza sativa. J. Exp. Bot. 2010, 61, 4157–4168. [Google Scholar] [CrossRef]
- Luo, Y.C.; Zhou, H.; Li, Y.; Chen, J.Y.; Yang, J.H.; Chen, Y.Q.; Qu, L.H. Rice embryogenic calli express a unique set of microRNAs, suggesting regulatory roles of microRNAs in plant post-embryogenic development. FEBS Lett. 2006, 580, 5111–5116. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.Z.; Yu, Y.; Zhou, Y.F.; Yang, Y.W.; Lei, M.Q.; Lian, J.P.; He, H.; Zhang, Y.C.; Huang, W.; Chen, Y.Q. A natural variant of miR397 mediates a feedback loop in Circadian Rhythm. Plant Physiol. 2020, 182, 204–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.Y.; Zhang, S.C.; Yu, Y.; Luo, Y.C.; Liu, Q.; Ju, C.L.; Zhang, Y.C.; Qu, L.H.; Lucas, W.J.; Wang, X.J.; et al. MiR397b regulates both lignin content and seed number in Arabidopsis via modulating a laccase involved in lignin biosynthesis. Plant Biotechnol. J. 2014, 12, 1132–1142. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.C.; Yu, Y.; Wang, C.Y.; Li, Z.Y.; Liu, Q.; Xu, J.; Liao, J.Y.; Wang, X.J.; Qu, L.H.; Chen, F.; et al. Overexpression of microRNA OsmiR397 improves rice yield by increasing grain size and promoting panicle branching. Nat. Biotechnol. 2013, 31, 848. [Google Scholar] [CrossRef] [PubMed]
- Burklew, C.E.; Xie, F.; Ashlock, J.; Zhang, B. Expression of microRNAs and their targets regulates floral development in tobacco (Nicotiana tabacum). Funct. Integr. Genom. 2014, 14, 299–306. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, M.; Cheng, F.S.; Dai, C.; Sun, Y.F.; Lu, J.; Huang, Y.T.; Li, M.M.; He, Y.; Wang, F.Z.; et al. Identification of microRNAs correlated with citrus granulation based on bioinformatics and molecular biology analysis. Postharvest Biol. Technol. 2016, 118, 59–67. [Google Scholar] [CrossRef]
- Xue, C.; Yao, J.L.; Qin, M.F.; Zhang, M.Y.; Allan, A.C.; Wang, D.F.; Wu, J. PbrmiR397a regulates lignification during stone cell development in pear fruit. Plant Biotechnol. J. 2018, 17, 103–117. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.L.; Zhou, J.J.; Gao, L.; Tang, Y.L. Plant miR397 and its functions. Funct. Plant Biol. 2020, 48, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Crooks, G.; Hon, G.; Chandonia, J.; Brenner, S. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef] [Green Version]
- Zuker, M.; Stiegler, P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acid Res. 1981, 9, 133–148. [Google Scholar] [CrossRef]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server (2017 release). Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef] [Green Version]
- Huo, A.L.; Chen, Z.Y.; Wang, P.K.; Yang, L.M.; Wang, G.P.; Wang, D.D.; Liao, S.C.; Cheng, T.L.; Chen, J.H.; Shi, J.S. Establishment of transient gene expression systems in protoplasts from Liriodendron hybrid mesophyll cells. PLoS ONE 2017, 12, e0172475. [Google Scholar]
- Min, L.; Hu, Q.; Li, Y.; Xu, J.; Zhang, X. Leafy cotyledon1-casein kinase I-tcp15-phytochrome interacting factor4 network regulates somatic embryogenesis by regulating auxin homeostasis. Plant Physiol. 2015, 169, 2805–2821. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.X.; Gao, F.; Wang, H.; Shen, H.L.; Yang, L. Physiological and Biochemical Traits in Korean Pine Somatic Embryogenesis. Forests 2020, 11, 557. [Google Scholar] [CrossRef]
- Achkar, N.P.; Cambiagno, D.A.; Manavella, P.A. miRNA biogenesis: A dynamic pathway. Trends Plant Sci. 2016, 21, 1034–1044. [Google Scholar] [CrossRef]
- Ha, M.J.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rydén, L.G.; Hunt, L.T. Evolution of protein complexity: The blue copper-containing oxidases and related proteins. J. Mol. Evol. 1993, 36, 41–66. [Google Scholar] [CrossRef]
- Kato, N.; Esaka, M. Changes in ascorbate oxidase gene expression and ascorbate levels in cell division and cell elongation in tobacco cells. Physiol. Plant 2010, 105, 321–329. [Google Scholar] [CrossRef]
- Sedbrook, J.C.; Carroll, K.L.; Hung, K.F.; Masson, P.H.; Somerville, C.R. The Arabidopsis SKU5 gene encodes an extracellular glycosyl phosphatidylinositol-anchored glycoprotein involved in directional root growth. Plant Cell. 2002, 14, 1635–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Wang, F.; Liu, B.Y.; Xu, C.Z.; He, Q.X.; Cheng, W.; Zhao, X.Y.; Ding, Z.H.; Zhang, W.; Zhang, K.W.; et al. Zmsks13, a cupredoxin domain-containing protein, is required for maize kernel development via modulating redox homeostasis. New Phytol. 2020, 229, 2163–2178. [Google Scholar] [CrossRef] [PubMed]
- Inzeé, D.; De Veylder, L. Cell Cycle Regulation in Plant Development. Annu. Rev. Genet. 2006, 40, 77–105. [Google Scholar] [CrossRef] [PubMed]
- Veylder, L.D.; Beeckman, T.; Inze, D. The ins and outs of the plant cell cycle. Nat. Rev. Mol. Cell Biol. 2007, 8, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.Y.; Wei, X.L.; Wang, X.; Wei, Y. Changes in biochemistry and histochemical characteristics during somatic embryogenesis in Ormosia henryi Prain. Plant Cell Tissue Organ Cult. 2020, 144, 505–517. [Google Scholar] [CrossRef]
- Saeed, T.; Shahzad, A. High frequency plant regeneration in Indian Siris via cyclic somatic embryogenesis with biochemical, histological and SEM investigations. Ind. Crops Prod. 2015, 76, 623–637. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Lu, F.; Lu, Y.; Cheng, T.; Shi, J.; Chen, J.; Hao, Z. Identification of miR397a and Its Functional Characterization in Callus Growth and Development by Regulating Its Target in Liriodendron. Forests 2021, 12, 912. https://doi.org/10.3390/f12070912
Wang D, Lu F, Lu Y, Cheng T, Shi J, Chen J, Hao Z. Identification of miR397a and Its Functional Characterization in Callus Growth and Development by Regulating Its Target in Liriodendron. Forests. 2021; 12(7):912. https://doi.org/10.3390/f12070912
Chicago/Turabian StyleWang, Dan, Fengjuan Lu, Ye Lu, Tielong Cheng, Jisen Shi, Jinhui Chen, and Zhaodong Hao. 2021. "Identification of miR397a and Its Functional Characterization in Callus Growth and Development by Regulating Its Target in Liriodendron" Forests 12, no. 7: 912. https://doi.org/10.3390/f12070912
APA StyleWang, D., Lu, F., Lu, Y., Cheng, T., Shi, J., Chen, J., & Hao, Z. (2021). Identification of miR397a and Its Functional Characterization in Callus Growth and Development by Regulating Its Target in Liriodendron. Forests, 12(7), 912. https://doi.org/10.3390/f12070912





