The Effect of Seed Size on Germination and Seedling Growth in Sweet Chestnut (Castanea sativa Mill.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Experimental Design and Nursery Production
2.3. Germination and Seedling Data Collection
2.4. Data Analyses
3. Results and Discussion
4. Conclusions and Practical Implications
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gregorio, N.O.; Herbohn, J.L.; Harrison, S.R. The potential role of nurseries in improving access to high quality planting stock and promoting appropriate silvicultural systems to improve the productivity of smallholder tree farms in Leyte, Philippines. In ACIAR Smallholder Forestry Project–Redevelopment of a Timber Industry Following Extensive Land Clearing: Proceedings from the End-of-Project Workshop; Harrison, S.R., Herbohn, J.L., Suh, J., Mangaoang, E., Vanclay, J., Eds.; ACIAR Smallholder Forestry Project: Ormoc City, Phillipines, 2004; pp. 269–278. [Google Scholar]
- Allen, K.S.; Harper, R.W.; Bayer, A.; Brazee, N.J. A review of nursery production systems and their influence on urban tree survival. Urban For. Urban Green. 2017, 21, 183–191. [Google Scholar] [CrossRef]
- Pinchot, C.C.; Clark, S.L.; Schlarbaum, S.E.; Saxton, A.M.; Sung, S.S.; Hebard, F.V. Effects of temporal dynamics, nut weight and nut size on growth of American chestnut, Chinese chestnut and backcross generations in a commercial nursery. Forests 2015, 6, 1537–1556. [Google Scholar] [CrossRef]
- Prasetyo, L.B.; Mansur, I.; Djamhuri, E. Nursery establishment and management. In Survey on Silvicultural Techniques and Plantation Promoting Policies in Indonesia; Ando, K., Ed.; FORDA (Forestry Research and Development Agency) and JICA (Japan International Cooperation Agency): Ibaraki, Japan, 2003; pp. 51–68. [Google Scholar]
- Willan, R.L. Seed testing. In A Guide to Forest Seed Handling with Special Reference to Tropics; FAO: Rome, Italy, 1985. [Google Scholar]
- Bae Park, B.; Han, S.H.; Hernandez, J.O.; An, J.Y.; Nyam-Osor, B.; Jung, M.H.; Sang-Hoon Lee, P.; Lee, S.I. The use of deep container and heterogeneous substrate as potentially effective nursery practice to produce good quality nodal seedlings of Populus sibirica Tausch. Forests 2021, 12, 418. [Google Scholar] [CrossRef]
- Clark, S.L.; Schweitzer, C.J.; Schlarbaum, S.E.; Dimov, L.D.; Hebard, F.V. Nursery quality and first-year response of American chestnut (Castanea dentata) seedlings planted in the Southeastern United States. TPN 2010, 53, 13–21. [Google Scholar]
- Bhardwaj, R.L. Effect of growing media on seed germination and seedling growth of papaya cv. ’Red lady’. Afr. J. Plant Sci. 2014, 8, 178–184. [Google Scholar]
- Davis, A.S.; Jacobs, D.F. Quantifying root system quality of nursery seedlings and relationship to outplanting performance. New For. 2005, 30, 295–311. [Google Scholar] [CrossRef]
- Ivetić, V.; Davorija, Z.; Vilotić, D. Relationship between morphological and physiological attributes of hop hornbeam seedlings. Glas. Sumar. Fak. 2013, 108, 39. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; MacDonald, J.E. Why seedlings grow: Influence of plant attributes. New For. 2017, 49, 1–34. [Google Scholar] [CrossRef]
- Haase, D.; Davis, A.S. Developing and supporting quality nursery facilities and staff are necessary to meet global forest and landscape restoration needs. Reforesta 2017, 4, 69–93. [Google Scholar] [CrossRef]
- Bounous, G. Revival of chestnut culture in Mediterranean countries: Factors to improve the quality of productions. Adv. Hortic. Sci. 2006, 20, 7–15. [Google Scholar]
- Fernández-López, J.; Alía, R. Technical Guidelines for Genetic Conservation and Use for Chestnut (Castanea sativa Mill.); EUFORGEN International Plant Genetic Resources Institute: Rome, Italy, 2003. [Google Scholar]
- Mattioni, C.; Cherubini, M.; Micheli, E.; Villani, F.; Bucci, G. Role of domestication in shaping Castanea sativa genetic variation in Europe. Tree Genet. Genomes 2008, 4, 563–574. [Google Scholar] [CrossRef]
- Mattioni, C.; Ranzino, L.; Cherubini, M.; Leonardi, L.; la Mantia, T.; Castellana, S.; Villani, F.; Simeone, M.C. Monuments unveiled: Genetic characterization of large old chestnut (Castanea sativa Mill.) trees using comparative nuclear and chloroplast DNA analysis. Forests 2020, 11, 1118. [Google Scholar] [CrossRef]
- ISTA (International Seed Testing Association). Chapter 5: The Germination Test, International Rules for Seed Testing, 1st ed.; The International Seed Testing Association (ISTA): Bassersdorf, Switzerland, 2006. [Google Scholar]
- Takoutsing, B.; Tchoundjeu, Z.; Degrande, A.; Asaah, E.; Gyau, A.; Nkeumoe, F.; Tsobeng, A. Assessing the quality of seedlings in small-scale nurseries in the highlands of Cameroon: The use of growth characteristics and quality thresholds as indicators. Small-Scale For. 2014, 13, 65–77. [Google Scholar] [CrossRef]
- Gebretsadik, W. Assessing the role of quality thresholds on early performance of tree seedlings planted on degraded highlands. For. Res. 2018, 7, 220. [Google Scholar]
- Statistica (Data Analysis Software System), Version 13; TIBCO Software Inc.: Palo Alto, CA, USA, 2018. Available online: http://www.statsoft.com.
- R Core Team. R: A Language and Environment for Statistical Computing, v.3.4.3; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 4th ed.; W.H. Freeman and Co.: New York, NY, USA, 2012; p. 937. [Google Scholar]
- Idžojtić, M.; Zebec, M.; Poljak, I.; Medak, J. Variation of sweet chestnut (Castanea sativa Mill.) populations in Croatia according to the morphology of fruits. Sauteria 2009, 18, 323–333. [Google Scholar]
- Parsons, P.A.; Allard, R.W. Seasonal variation in lima bean seed size: An example of genotypic-environmental interaction. Heredity 1960, 14, 115–123. [Google Scholar] [CrossRef]
- Crochemore, M.L.; Huyghe, C.; Papineau, J.; Julier, B. Intra-plant variability in seed size and seed quality in Lupinus albus L. Agronomie 1994, 14, 5–13. [Google Scholar] [CrossRef]
- Parciak, W. Environmental variation in seed number, size and dispersal of fleshy-fruited plant. Ecology 2002, 83, 780–793. [Google Scholar] [CrossRef]
- Zas, R.; Sampedro, L. Heritability of seed weight in Maritime pine, a relevant trait in the transmission of environmental maternal effects. Heredity 2015, 114, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Gosling, P. Raising Trees and Shrubs from Seed; Forestry Commission Practice Guide, Forestry Commission: Edinburgh, UK, 2007; pp. 1–28. [Google Scholar]
- Young, J.A.; Young, C.G. Seeds of Woody Plants in North America; Dioscorides Press: Portland, OR, USA, 1992. [Google Scholar]
- Benedetti, S.; Gonzalez, M.; Garcia, E.; Quiroz, I. An analysis of the physical and germination parameters of the sweet chestnut (Castanea sativa). Cien. Inv. Agric. 2012, 39, 185–192. [Google Scholar] [CrossRef][Green Version]
- Donohue, K.; Dorn, L.; Griffith, C.; Kim, E.; Aguilera, A.; Polisetty, C.R.; Schmitt, J. Environmental and genetic influences on the germination of Arabidopsis thaliana in the field. Evolution 2005, 59, 740–757. [Google Scholar] [CrossRef]
- Tilki, F.; Alptekin, C.U. Germination and seedling growth of Quercus vulcanica: Effects of stratification, desiccation, radicle pruning, and season of sowing. New For. 2006, 32, 243–251. [Google Scholar] [CrossRef]
- Poljak, I.; Idžojtić, M.; Šatović, Z.; Ježić, M.; Ćurković-Perica, M.; Simovski, B.; Acevski, J.; Liber, Z. Genetic diversity of the sweet chestnut (Castanea sativa Mill.) in Central Europe and the western part of the Balkan Peninsula and evidence of marron genotype introgression into wild populations. Tree Genet. Genomes 2017, 13, 18. [Google Scholar] [CrossRef]
- Fredrick, C.; Muthuri, C.; Ngamau, K.; Sinclair, F. Provenance variation in seed morphological characteristics, germination and early seedling growth of Faidherbia albida. JHSF 2015, 7, 127–140. [Google Scholar]
- Calvo, L.; Hernández, V.; Valbuena, L.; Taboada, A. Provenance and seed mass determine seed tolerance to high temperatures associated to forest fires in Pinus pinaster. Ann. For. Sci. 2016, 73, 381–391. [Google Scholar] [CrossRef]
- Santelices Moya, R.; Espinoza Meza, S.; Magni Díaz, C.; Cabrera Ariza, A.; Donoso Calderón, S.; Peña-Rojas, K. Variability in seed germination and seedling growth at the intra- and inter- provenance levels of Nothofagus glauca (Lophozonia glauca), an endemic species of Central Chile. N. Z. J. For. Sci. 2017, 47, 10. [Google Scholar] [CrossRef]
- Doffou Akaffou, S.; Kouassi Kouame, A.; Boh Gore, N.B.; Yao Abessika, G.; Kouassi, H.K.; Hamon, P.; Sabatier, S.; Duminil, J. Effect of the seeds provenance and treatment on the germination rate and plants growth of four forest trees species of Côte d’Ivoire. J. For. Res. 2021, 32, 161–169. [Google Scholar] [CrossRef]
- Alptekin, C.; Tilki, F. Effects of stratification and pericarp removal on germination of Quercus libani acorns. Silva Balc. 2002, 2, 21–28. [Google Scholar]
- Garcia-De La Cruz, Y.; Lopez-Barrera, F.; Ramos-Prado, J.M. Germination and seedling emergence of four endangered oak species. Madera Bosques 2016, 22, 77–87. [Google Scholar] [CrossRef][Green Version]
- Purohit, V.K.; Tamta, S.; Nandi, S.K.; Rikhari, H.C.; Palni, L.M.S. Does acorn weight influence germination and subsequent seedling growth of central Himalayan oaks? J. Trop. For. Sci. 2003, 15, 483–492. [Google Scholar]
- Pandey, R.; Bagali, K.; Bargali, S.S. Does seed size affect water stress tolerance in Quercus leucotrichophora A. Camus at germination and early seedling growth stage? Biodiver. Int. J. 2017, 1, 24–30. [Google Scholar]
- Winn, A.A. Ecological and evolutionary consequences of seed mass in Prunella vulgaris. Ecology 1988, 69, 1537–1544. [Google Scholar] [CrossRef]
- Vera, M.L. Effects of altitude and seed mass on germination and seedling survival of heathland plants in north Spain. Plant Ecol. 1997, 133, 101–106. [Google Scholar] [CrossRef]
- Kaliniewicz, Z.; Tylek, P. Influence of scarification on the germination capacity of acorns harvested from uneven-aged stands of pedunculated oak (Quercus robur L.). Forests 2018, 9, 100. [Google Scholar] [CrossRef]
- Mao, P.; Guo, L.; Gao, Y.; Qi, L.; Cao, B. Effects of seed size and sand burial on germination and early growth of seedlings for coastal Pinus thunbergii Parl. in the Northern Shandong Peninsula, China. Forests 2019, 10, 281. [Google Scholar] [CrossRef]
- Martínez González, I.; Sanchez-Velazquez, L.R.; Ruiz-Guerra, B.; del Rosario Pineda-Lopez, M.; Velazquez-Rosas, N. The role of seed size in the emergence and survival of seedlings in contrasting environments: The case of Ceiba aesculifolia. New For. 2020, 52, 493–507. [Google Scholar] [CrossRef]
- Agboola, D.A. The effect of seed size on germination and seedling growth of three tropical tree species. J. Trop. For. Sci. 1996, 9, 44–51. [Google Scholar]
- Soylu, A.; Serdar, U. Rootstock selection on chestnut (Castanea sativa Mill.) in the middle of Black Sea region in Turkey. Acta Hortic. 2000, 538, 483–487. [Google Scholar] [CrossRef]
- Cicek, E.; Tilki, F. Seed size effects on germination, survival and seedling growth of Castanea sativa Mill. J. Biol. Sci. 2007, 7, 438–441. [Google Scholar] [CrossRef]
- Shepard, E.; Miller, D.D.; Miller, G.; Miller, D. Effect of weight on emergence and seedling vigor of Chinese chestnut. Hortic. Sci. 1989, 24, 516–519. [Google Scholar]
- Jarvis, P.G. The effects of acorn size and provenance on the growth of seedlings of sessile oaks. QJF 1963, 57, 11–19. [Google Scholar]
- Ke, G.; Werger, M.J.A. Different responses to shade of evergreen and deciduous oak seedlings and the effect of acorn size. Acta Oecol. 1999, 20, 579–586. [Google Scholar] [CrossRef]
- Seiwa, K. Effect of seed size and emergence time on tree seedling establishment; importance of developmental constraints. Oecologia 2000, 123, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.M. Bigger is not always better: Conflicting selective pressures on seed size in Quercus ilex. Evolution 2004, 58, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Navarro, F.B.; Jimenez, M.M.; Ripoll, M.A.; Ondono, E.; Gallego, E.; Simon, E. Direct sowing of holm oak acorns: Effects of acorn size and soil treatment. Ann. For. Sci. 2006, 63, 961–967. [Google Scholar] [CrossRef]
- González-Rodríquez, V.; Villar, R.; Navarro-Cerrillo, R.M. Maternal influences on seed mass effect and initial seedling growth in four Quercus species. Acta Oecol. 2011, 37, 1–9. [Google Scholar] [CrossRef]
- Pesendorfer, M.B. The effect of Seed Size Variation in Quercus pacifica on Seedling Establishment and Growth. General Technical Report PSW-GTR-251. In Proceedings of the 7th California Oak Symposium: Managing Oak Woodlands in a Dynamic World, Visalia, CA, USA, 3–6 November 2014. [Google Scholar]
- Bonfil, C. The effects of seed size, cotyledon reserves and herbivory on seedling survival and growth in Quercus rugosa and Q. laurina (Fagaceae). Am. J. Bot. 1998, 85, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Sage, R.D.; Koenig, W.D.; McLaughlin, B.C. Fitness consequences of seed size in the valley oak Quercus lobata Née (Fagaceae). Ann. For. Sci. 2011, 68, 477–484. [Google Scholar] [CrossRef]
- Burris, J.S.; Edje, O.T.; Wahab, A.H. Effects of seed size on seedling performance in soybeans: II. Seedling growth and photosynthesis and field performance. Crop Sci. 1973, 13, 207–210. [Google Scholar] [CrossRef]
- Adebisi, M.A.; Kehinde, T.O.; Salau, A.W.; Okesola, L.A.; Porbeni, J.B.O.; Esuruoso, A.O.; Oyekale, K.O. Influence of different seed size fractions on seed germination, seedling emergence and seed yield characters in tropical soybean (Glycine max L. Merrill). Int. J. Agric. Res. 2013, 8, 26–33. [Google Scholar] [CrossRef]
- Snider, J.L.; Collins, G.D.; Whitaker, J.; Chapman, K.D.; Horn, P. The impact of seed size and chemical composition on seedling vigor, yield and fiber quality of cotton in five production environments. Field Crop Res. 2016, 193, 186–195. [Google Scholar] [CrossRef]
- Clark, S.L.; Schlarbaum, S.E. Effects of acorn size and mass on seedling quality of northern red oak (Quercus rubra). New For. 2018, 49, 571–583. [Google Scholar] [CrossRef]
- Bounous, G.; Beccaro, G. Chestnut culture: Directions for establishing new orchards. Nucis-Newsletter 2002, 11, 30–34. [Google Scholar]
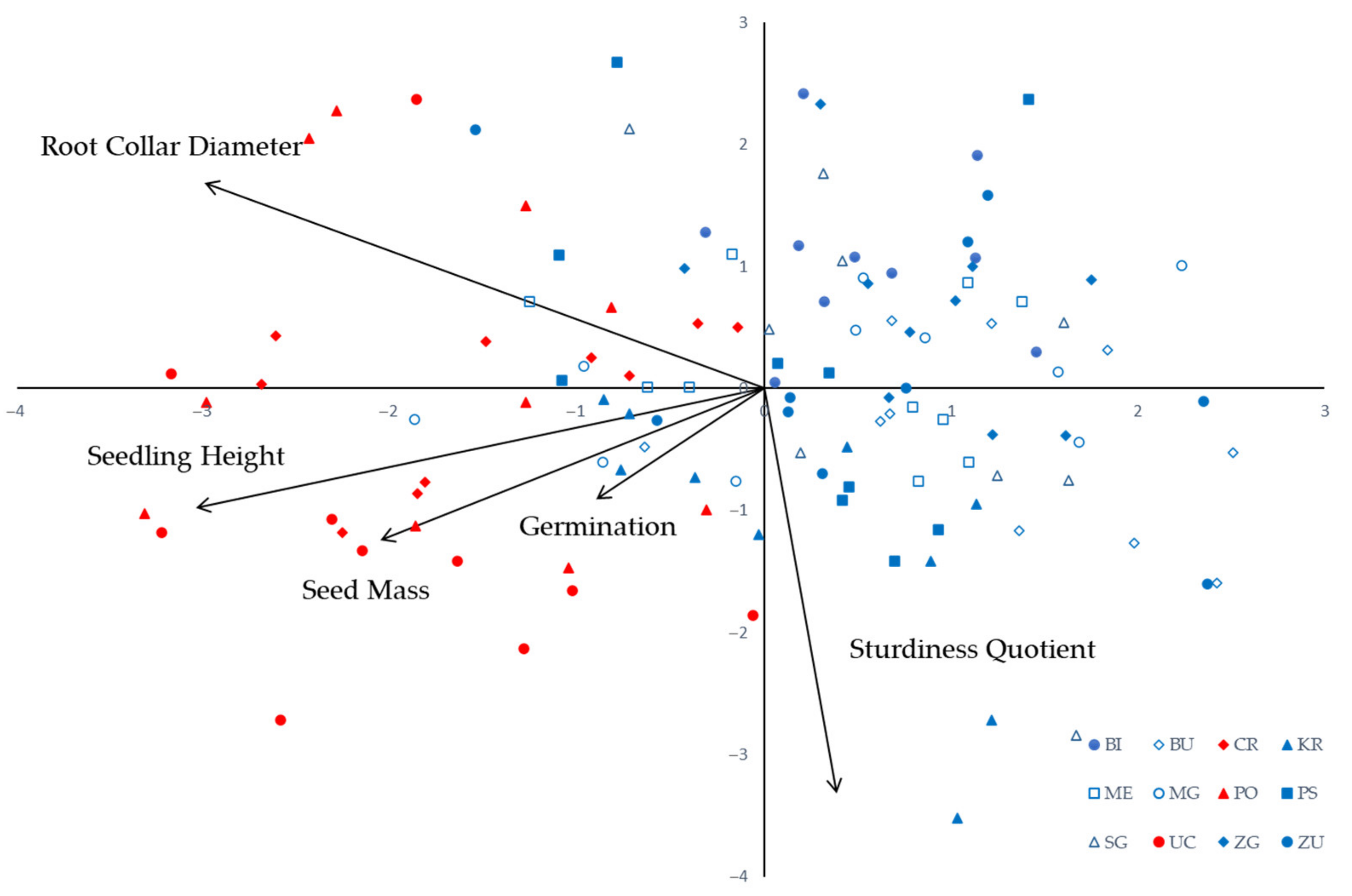
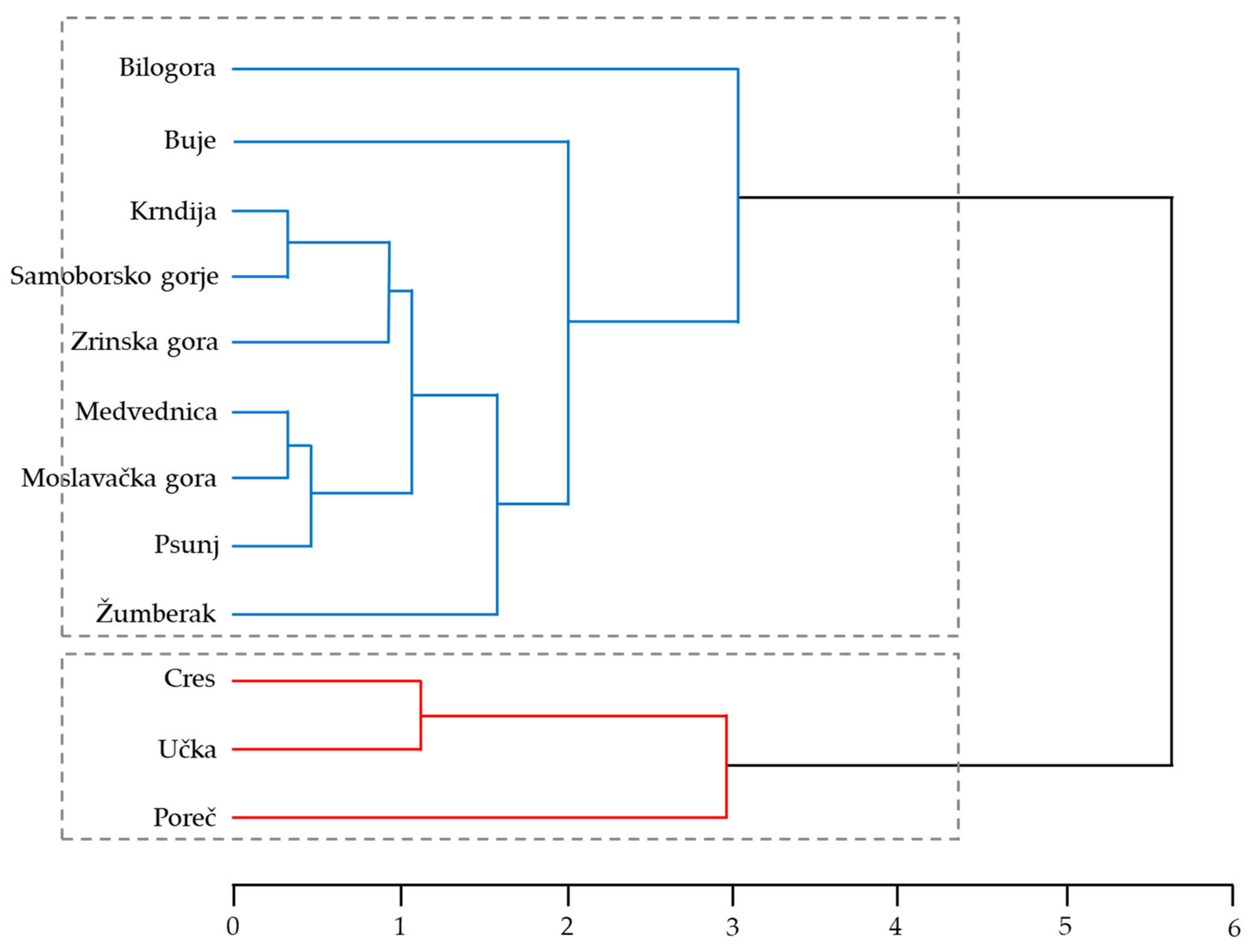
| Population ID | Seed Mass (g) | Germination (%) | Root Collar D. (mm) | Seedling Height (cm) | Sturdiness Quotient | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CV (%) | CV (%) | CV (%) | CV (%) | CV (%) | ||||||
| BU | 6.0 ± 1.6 ab | 25.7 | 57 ± 16.6 ab | 29.4 | 5.2 ± 1.2 a | 23.5 | 24.0 ± 4.5 ab | 18.5 | 4.7 ± 0.8 abc | 16.9 |
| CR | 10.5 ± 2.4 d | 22.6 | 67 ± 12.8 abc | 19.0 | 8.4 ± 1.1 bc | 12.8 | 32.3 ± 4.2 cd | 13.1 | 3.9 ± 0.3 abc | 8.1 |
| PO | 7.8 ± 1.1 ac | 14.4 | 58 ± 12.9 abc | 22.2 | 9.5 ± 1.9 c | 19.9 | 38.8 ± 6.2 de | 15.9 | 4.2 ± 1.1 abc | 24.9 |
| UC | 9.4 ± 2.8 cd | 30.2 | 69 ± 17.0 abc | 24.8 | 8.5 ± 1.9 bc | 22.3 | 40.5 ± 5.6 e | 13.8 | 4.9 ± 1.0 bc | 20.7 |
| BI | 5.0 ± 1.5 b | 31.0 | 60 ± 15.3 abc | 25.3 | 7.3 ± 1.1 abc | 14.9 | 25.5 ± 4.4 abc | 17.4 | 3.5 ± 0.6 a | 15.8 |
| KR | 7.0 ± 1.1 abc | 16.3 | 80 ± 12.1 c | 15.2 | 5.9 ± 1.5 a | 25.0 | 29.0 ± 4.0 bc | 13.8 | 5.1 ± 1.0 c | 19.6 |
| ME | 6.7 ± 2.3 abc | 34.0 | 63 ± 17.6 abc | 27.9 | 6.6 ± 1.5 ab | 23.0 | 26.5 ± 5.0 abc | 18.9 | 4.1 ± 0.5 abc | 11.0 |
| MG | 7.0 ± 2.2 abc | 30.8 | 62 ± 17.0 abc | 27.5 | 6.5 ± 1.4 ab | 21.7 | 26.5 ± 6.1 abc | 23.0 | 4.1 ± 0.5 abc | 12.1 |
| PS | 7.0 ± 0.7 abc | 9.8 | 70 ± 12.0 abc | 17.1 | 7.0 ± 1.9 ab | 26.7 | 26.2 ± 4.8 abc | 18.3 | 4.0 ± 1.1 abc | 27.1 |
| SG | 6.7 ± 1.4 ab | 21.2 | 77 ± 10.6 bc | 13.7 | 5.9 ± 2.0 a | 33.9 | 21.4 ± 3.7 a | 17.1 | 3.9 ± 1.2 abc | 29.6 |
| ZG | 6.2 ± 1.4 ab | 21.9 | 53 ± 17.5 a | 32.9 | 6.4 ± 1.3 ab | 21.0 | 23.6 ± 2.1 ab | 9.1 | 3.8 ± 0.5 ab | 12.4 |
| ZU | 7.8 ± 2.0 ac | 25.5 | 55 ± 13.9 a | 25.3 | 6.2 ± 2.0 ab | 33.0 | 23.6 ± 4.4 ab | 18.8 | 4.0 ± 0.9 abc | 21.6 |
| Trait | PC—Principal Component | ||
|---|---|---|---|
| PC1 | PC2 | PC3 | |
| Seed Mass | −0.587506 | −0.354325 | 0.183710 |
| Gremination | −0.257149 | −0.259451 | −0.930168 |
| Root Collar Diameter | −0.855226 | 0.481539 | 0.015402 |
| Seedling Height | −0.869198 | −0.278959 | 0.154842 |
| Sturdiness Quotient | 0.111337 | −0.947850 | 0.148190 |
| Eigenvalue | 1.91 | 1.40 | 0.95 |
| % of Variance | 38.21 | 28.02 | 18.90 |
| Trait | SM | G | RCD | SH | SQ |
|---|---|---|---|---|---|
| Seed Mass (SM) | ns | * | ** | ns | |
| Gremination (G) | 0.097 | ns | ns | ns | |
| Root Collar Diameter (RCD) | 0.295 | 0.075 | *** | *** | |
| Seedling Height (SH) | 0.381 | 0.138 | 0.657 | * | |
| Sturdiness Quotient (SQ) | 0.125 | 0.070 | −0.502 | 0.272 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tumpa, K.; Vidaković, A.; Drvodelić, D.; Šango, M.; Idžojtić, M.; Perković, I.; Poljak, I. The Effect of Seed Size on Germination and Seedling Growth in Sweet Chestnut (Castanea sativa Mill.). Forests 2021, 12, 858. https://doi.org/10.3390/f12070858
Tumpa K, Vidaković A, Drvodelić D, Šango M, Idžojtić M, Perković I, Poljak I. The Effect of Seed Size on Germination and Seedling Growth in Sweet Chestnut (Castanea sativa Mill.). Forests. 2021; 12(7):858. https://doi.org/10.3390/f12070858
Chicago/Turabian StyleTumpa, Katarina, Antonio Vidaković, Damir Drvodelić, Mario Šango, Marilena Idžojtić, Ivan Perković, and Igor Poljak. 2021. "The Effect of Seed Size on Germination and Seedling Growth in Sweet Chestnut (Castanea sativa Mill.)" Forests 12, no. 7: 858. https://doi.org/10.3390/f12070858
APA StyleTumpa, K., Vidaković, A., Drvodelić, D., Šango, M., Idžojtić, M., Perković, I., & Poljak, I. (2021). The Effect of Seed Size on Germination and Seedling Growth in Sweet Chestnut (Castanea sativa Mill.). Forests, 12(7), 858. https://doi.org/10.3390/f12070858







