Suppressed Undergrowth of Siberian Spruce (Picea obovata Ledeb.) in Early Ontogeny: One-Way Ticket or Survival Strategy?
Abstract
:1. Introduction
2. Materials and Methods
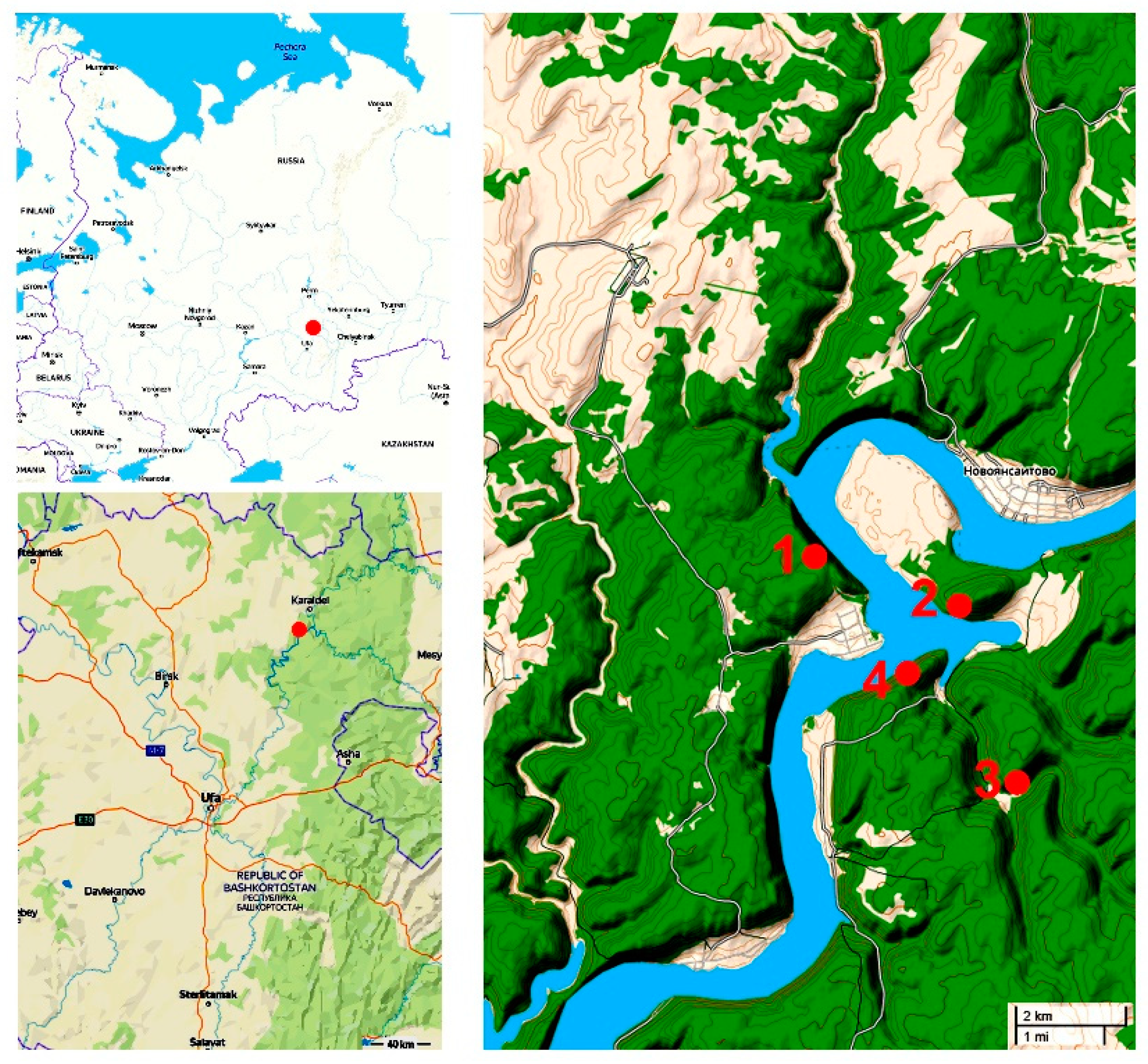
2.1. Site Descriptions
2.2. Sample Collection and Analysis
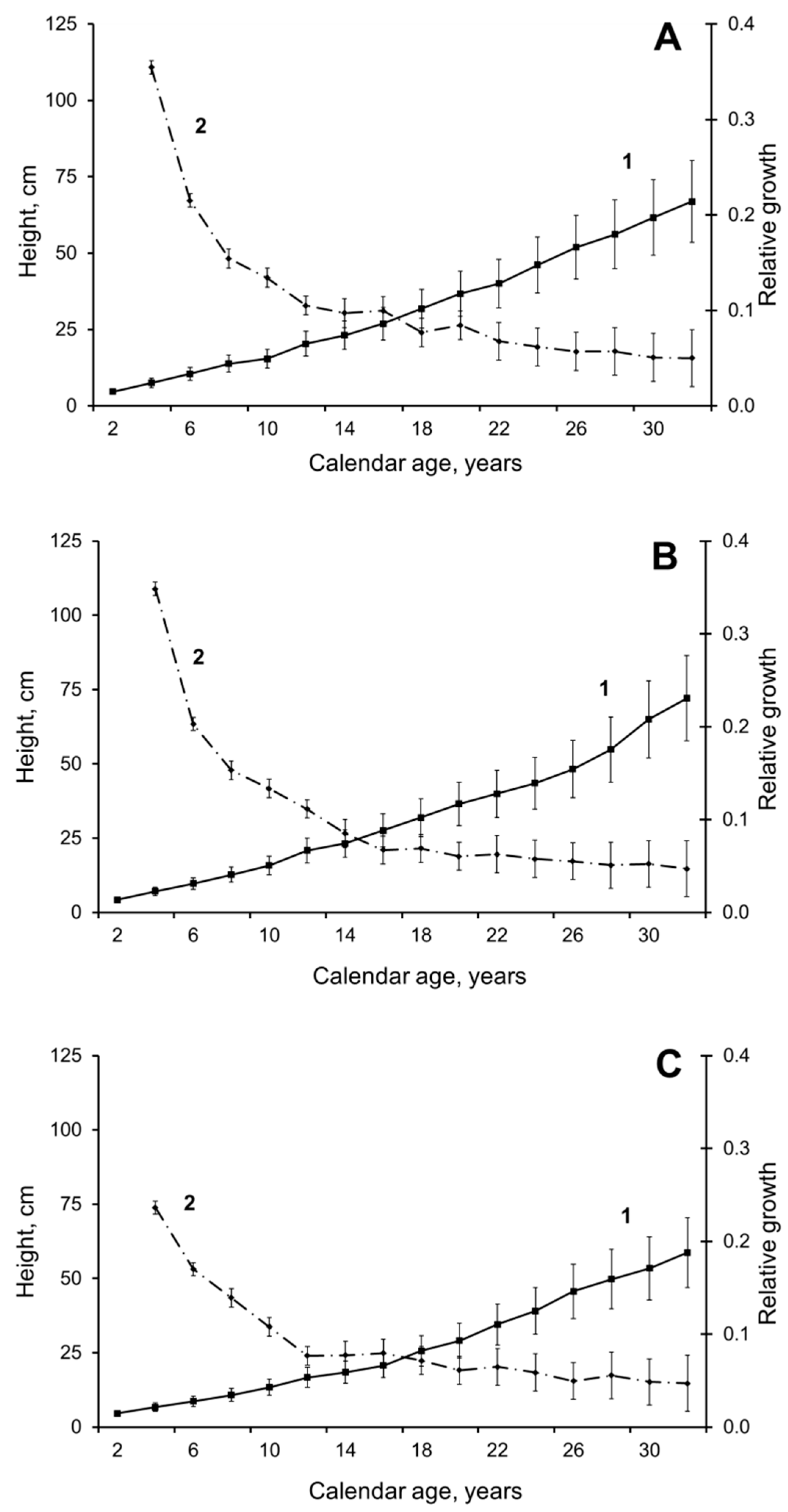
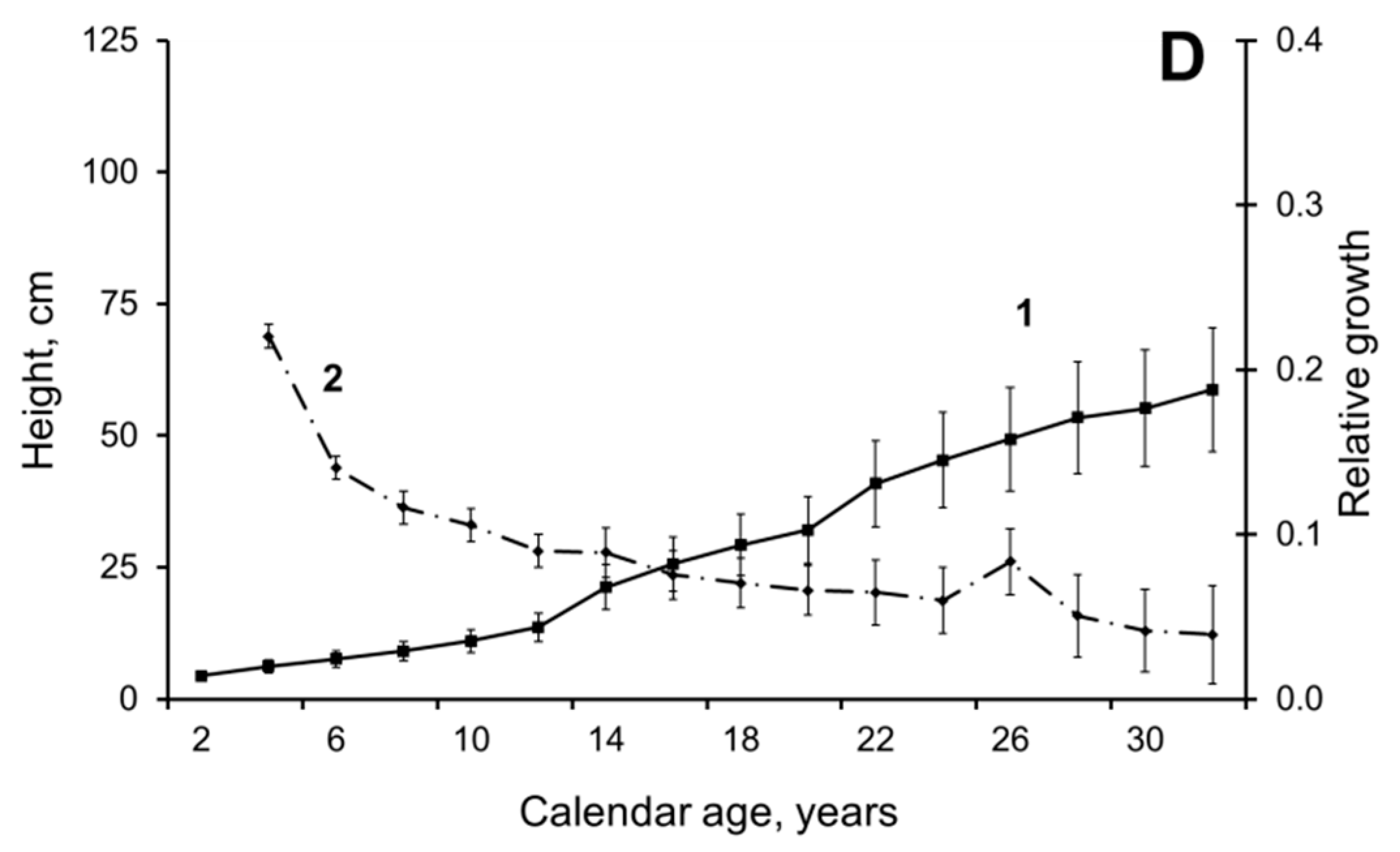
3. Results and Discussion
3.1. Pre-Juvenile Ontogeny Stage (p)
3.2. Juvenile Ontogeny Stage (j)
3.3. Immature Ontogeny Stage (im)
3.4. Virginile Ontogeny Stage (i)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piovesan, G.; Biondi, F. On tree longevity. New Phytol. 2021. [Google Scholar] [CrossRef]
- Usmanov, I.Y.; Rakhmankulova, Z.F.; Kulagin, A.Y. Ecological Physiology of Plants; Logos: Moscow, Russia, 2001; p. 224. (In Russian) [Google Scholar]
- Dobrowolska, D. Effect of stand density on oak regeneration in flood plain forests in Lower Silesia, Poland. Forestry 2008, 81, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Oberhauser, U. Secondary forest regeneration beneath pine (Pinus kesiya) plantations in the northern Thai highland: A chronosequence study. For. Ecol. Manag. 1997, 99, 171–183. [Google Scholar] [CrossRef]
- Emborg, J. Understorey light conditions and regeneration with respect to the structural dynamics of a near-natural temperate deciduous forest in Denmark. For. Ecol. Manag. 1998, 106, 83–95. [Google Scholar] [CrossRef]
- Yu, F.; Wang, D.; Shi, X.; Yi, X.; Huang, Q.; Hu, Y. Effects of environmental factors on tree seedling regeneration in a pine-oak mixed forest in the Qinling Mountains, China. J. Mount. Sci. 2013, 10, 845–853. [Google Scholar] [CrossRef]
- Hunziker, U.; Brang, P. Microsite patterns of conifer seedling establishment and growth in a mixed stand in the southern Alps. For. Ecol. Manag. 2005, 205, 67–79. [Google Scholar] [CrossRef]
- Peng, Y.Y.; Dang, Q.-L. Effects of soil temperature on biomass production and allocation in seedlings of four boreal tree species. For. Ecol. Manag. 2003, 180, 1–9. [Google Scholar] [CrossRef]
- Götmark, F.; Fridman, J.; Kempe, G.; Norden, B. Broadleaved tree species in conifer-dominated forestry: Regeneration and limitation of saplings in southern Sweden. For. Ecol. Manag. 2005, 214, 142–157. [Google Scholar] [CrossRef]
- Gabbasova, I.M.; Garipov, T.T.; Suleimanov, R.R.; Komissarov, M.A.; Khabirov, I.K.; Sidorova, L.V.; Nazyrova, F.I.; Prostyakova, Z.G.; Kotlugalyamova, E.Y. The influence of ground fires on the properties and erosion of forest soils in the Southern Urals (Bashkir State Nature Reserve). Eurasian Soil Sci. 2019, 52, 370–379. [Google Scholar] [CrossRef]
- Craine, J.; Dybzinski, R. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 2013, 27, 833–840. [Google Scholar] [CrossRef]
- Farjon, A. A Handbook of the World’s Conifers (2 Vols.); Brill: Leiden, The Netherlands, 2010; p. 1111. [Google Scholar] [CrossRef]
- Ammer, C. Konkurrenz um Licht—Zur Entwicklung der Naturverjüngung im Bergmischwald; Forstliche Forschungsberichte: München, Germany, 1996; Volume 151, p. 198. [Google Scholar]
- Knapp, A.; Smith, W. Factors influencing understory seedling establishment of Engelmann spruce (Picea engelmannii) and subalpine fir (Abies lasiocarpa) in southeast Wyoming. Can. J. Bot. 1982, 60, 2753–2761. [Google Scholar] [CrossRef]
- Nakamura, T. Effect of bryophytes on survival of conifer seedlings in subalpine forests of central Japan. Ecol. Res. 1992, 7, 155–162. [Google Scholar] [CrossRef]
- Lin, F.; Hao, Z.-Q.; Li, B.-H.; Ye, J.; Dai, G.-H.; Zhang, J.; Ni, W.-D. The relationship between the moss community characteristics and the regeneration in the dark coniferous forest of Changbai Mountain. Acta Ecol. Sin. 2007, 27, 1308–1314. [Google Scholar]
- Takahashi, M.; Sakai, Y.; Ootomo, R.; Shiozaki, M. Establishment of tree seedlings and water-soluble nutrients in coarse woody debris in an old-growth Picea-Abies forest in Hokkaido, northern Japan. Can. J. For. Res. 2000, 30, 1148–1155. [Google Scholar] [CrossRef]
- Iijima, H.; Shibuya, M.; Saito, H.; Takahashi, K. The effect of moss height on survival and growth of Picea jezoensis seedlings on fallen logs. J. For. Res. 2004, 86, 358–364. [Google Scholar] [CrossRef]
- Iijima, H.; Shibuya, M. Evaluation of suitable conditions for natural regeneration of Picea jezoensis on fallen logs. J. For. Res. 2010, 15, 46–54. [Google Scholar] [CrossRef]
- Narukawa, Y.; Iida, S.; Tanouchi, H.; Abe, S.; Yamamoto, S.-I. State of fallen logs and the occurrence of conifer seedlings and saplings in boreal and subalpine oldgrowth forests in Japan. Ecol. Res. 2003, 18, 267–277. [Google Scholar] [CrossRef]
- Kupferschmid, A.D.; Bugmann, H. Effect of microsites, logs and ungulate browsing on Picea abies regeneration in a mountain forest. For. Ecol. Manag. 2005, 205, 251–265. [Google Scholar] [CrossRef]
- Hisashi, S.; Nagaike, T. Microsites for seedling establishment of subalpine conifers in a forest with moss-type undergrowth on Mt. Fuji, central Honshu, Japan. Ecol. Res. 2005, 20, 678–685. [Google Scholar]
- Bače, R.; Svoboda, M.; Pouska, V.; Janda, P.; Červenka, J. Natural regeneration in Central-European subalpine spruce forests: Which logs are suitable for seedling recruitment? For. Ecol. Manag. 2015, 266, 254–262. [Google Scholar] [CrossRef]
- Alekseev, V.A. Forest Light Status; Nauka: Leningrad, Russia, 1975; p. 224. (In Russian) [Google Scholar]
- Rabotnov, T.A. Dynamics of plant coenotic popu1ations. In The Population Structure of Vegetation (Handbook of Vegetation Science); White, J., Ed.; Springer: Berlin/Heidelberg, Germany, 1985; Part 3, pp. 121–142. [Google Scholar]
- Belyaeva, N.; Danilov, D. Development of undergrowth phenological spruce forms in different species composition of forest stands. In Proceedings of the 24th Annual International Scientific Conference “Research for Rural Development 2018”, Jelgava, Latvia, 16–18 May 2018; pp. 117–124. [Google Scholar] [CrossRef]
- Black, R.A.; Bliss, L.C. Reproductive ecology of Picea mariana (Mill.) BSP., at tree line near Inuvik, Northwest Territories, Canada. Ecol. Monog. 1980, 50, 331–354. [Google Scholar] [CrossRef]
- Morneau, C.; Payette, S. Postfire lichen-spruce woodland recovery at the limit of the boreal forest in northern Quebec. Can. J. Bot. 1989, 67, 2770–2782. [Google Scholar] [CrossRef]
- Rubtsov, M.V.; Glazunov, Y.B.; Nikolaev, D.K. Regenerative and age dynamic of spruce population in pine plantations under conditions typical for spruce development. Contemp. Probl. Ecol. 2016, 9, 884–893. [Google Scholar] [CrossRef]
- Norton, D.A.; Ogden, J. Problems with the use of tree rings in the study of forest population dynamics. In Methods of Dendrochronology: Applications in the Environmental Sciences; Cook, E.R., Kairiukstis, L.A., Eds.; Kluwer Academic Publishers: Amsterdam, The Netherlands, 1990; pp. 284–288. [Google Scholar]
- DesRochers, A.; Gagnon, R. Is ring count at ground level a good estimation of black spruce age? Can. J. For. Res. 1997, 27, 1263–1267. [Google Scholar] [CrossRef]
- Niklasson, M. A comparison of three age determination methods for suppressed Norway spruce: Implications for age structure analysis. For. Ecol. Manag. 2002, 161, 279–288. [Google Scholar] [CrossRef]
- Kulagin, A.Y.; Davydychev, A.N.; Zaitsev, G.A. Specific features of the growth of Siberian spruce (Picea obovata Ledeb.) at early stages of ontogeny in broadleaf-conifer forests of the Ufa Plateau. Rus. J. Ecol. 2006, 37, 66–69. [Google Scholar] [CrossRef]
- Zaitsev, G.A.; Kulagin, A.Y.; Davydychev, A.N. The particularities of the growth of Siberian fir (Abies sibirica Ledeb.) in the first stages of ontogeny in conifer forests (Ufa plateau, Pre-Ural). Trees 2018, 32, 511–518. [Google Scholar] [CrossRef]
- Bellassen, V.; Luyssaert, S. Carbon sequestration: Managing forests in uncertain times. Nature 2014, 506, 153–155. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Zhou, M.; Lv, J.; Chen, K. Trends in global research in forest carbon sequestration: A bibliometric analysis. J. Clean. Prod. 2020, 252, 119908. [Google Scholar] [CrossRef]
- Daigneault, A.; Favero, A. Global forest management, carbon sequestration and bioenergy supply under alternative shared socioeconomic pathways. Land Use Policy 2021, 103, 105302. [Google Scholar] [CrossRef]
- Bastos, A.; Fleischer, K. Effects of rising CO2 levels on carbon sequestration are coordinated above and below ground. Nature 2021, 591, 532–534. [Google Scholar] [CrossRef]
- Serebryakov, I.G. Ecological Morphology of Plants; Vysshaja Shkola: Moscow, Russia, 1962; p. 378. (In Russian) [Google Scholar]
- Nukhimovskaya, Y.D. Ontogeny of Siberian fir (Abies sibirica Ledeb.) under the conditions of the Moscow region. Bull. Mosk. Obs. Ispyt. Prirody Otdel Biol. 1971, 76, 105–111. (In Russian) [Google Scholar]
- Makhatkov, I.D. Polivariancy of Siberian fir ontogeny. Bull. Mosk. Obs. Ispyt. Prirody Otdel Biol. 1991, 96, 79–88. (In Russian) [Google Scholar]
- Romanovskii, A.M. Polyvariation of Picea abies (Pinaceae) ontogeny in the Bryansk woodland. Bot. Zhurnal 2001, 86, 72–85. (In Russian) [Google Scholar]
- Ukhvatkina, O.N.; Komarova, T.A.; Trofimova, A.D. The features of ontogenesis Picea ajanensis (Lindl. et Gord.) Fisch. ex Carr. in the middle part of southern Sikhote-Alin mountains. Lesnoi Vestnik 2010, 3, 169–173. (In Russian) [Google Scholar]
- Nikolaeva, S.A.; Velisevich, S.N.; Savchuk, D.A. The ontogeny of Pinus sibirica in the southeast of the West Siberian Plain. Zhurnal Sib. Fed. Univ. Biol. 2011, 4, 3–12. (In Russian) [Google Scholar]
- Ivanova, N.S.; Yermakova, M.V.; Zolotova, E.S. The initial stage of ontogenesis of Picea obovata Ledeb on different soil. Izvestia Samara Sci. Cent. Russ. Acad. Sci. 2016, 18, 5–11. (In Russian) [Google Scholar]
- Stavrova, N.I.; Gorshkov, V.V.; Mishko, A.E. Ontogeny of Picea obovata (Pinaceae) in old-growth northern taiga dwarf shrub-green moss pine-spruce forests. Bot. Zhurnal 2017, 102, 163–185. (In Russian) [Google Scholar] [CrossRef]
- Mishko, A.E. Ontomorphogenesis of Siberian Spruce (Picea obovata Ledeb.) in the North Taiga Forests (on the Example of the Kola Peninsula). Ph.D. Thesis, The Komarov Botanical Institute of the Russian Academy of Sciences, Saint Petersburg, Russia, 2019. (In Russian). [Google Scholar]
- Kulagin, A.Y.; Shayakhmetov, I.F. Natural under-canopy regeneration and height-age structure of small-leaved linden (Tilia cordata Mill.) undergrowth in water-conservation forests around Pavlovskoe Reservoir, Ufa River. Rus. J. Ecol. 2007, 38, 247–252. [Google Scholar] [CrossRef]
- Kulagin, Y.Z. About perennial frozen soils in the Bashkir TransUral Region. Ecologia 1976, 2, 24–29. (In Russian) [Google Scholar]
- Mukatanov, A.H. Permafrost-affected soils of the Ufa Plateau. Pochvovedenie 1999, 7, 828–833. (In Russian) [Google Scholar]
- Kangas, A. Mensurational aspects. In Forest Inventory: Methodology and Applications; (Managing Forest Ecosystems, vol. 10); Kangas, A., Maltamo, M., Eds.; Springer: Dordrecht, Germany, 2006; Chapter 4; pp. 53–63. [Google Scholar] [CrossRef]
- Kershaw, J.A.; Ducey, M.J.; Beers, T.W.; Husch, B. Forest Mensuration, 5th ed.; Wiley: Chichester, UK, 2016; p. 613. [Google Scholar] [CrossRef]
- Sukachev, V.N. Biogeology Research Program and Methodology; Nauka: Moscow, Russia, 1966; p. 333. (In Russian) [Google Scholar]
- Yarmishko, V.T. Selection and establishment of permanent test areas in forest communities. In Methods of Studying Forest Communities; Andreeva, E.N., Bakkal, I.Y., Gorshkov, V.V., Lianguzova, I.V., Maznaya, E.A., Neshataev, V.U., Neshataeva, V.U., Stavrova, N.I., Yarmishko, V.T., Yarmishko, M.A., Eds.; Research Institute of Chemistry of St. Petersburg State University: Saint Petersburg, Russia, 2002; pp. 33–37. (In Russian) [Google Scholar]
- Kangas, A.; Maltamo, M. Forest Inventory: Methodology and Applications; (Managing Forest Ecosystems, vol. 10); Springer: Dordrecht, Germany, 2006; p. 362. [Google Scholar] [CrossRef]
- Newton, A.C. Forest Ecology and Conservation: A Handbook of Techniques; Oxford University Press: New York, NY, USA, 2007; p. 480. [Google Scholar] [CrossRef]
- Anuchin, N.P. Forest Inventory, 5th ed.; Forest industry: Moscow, Russia, 1982; p. 552. (In Russian) [Google Scholar]
- Pridnya, M.V. A trial in determining the age of Picea obovata advance growth with the help of pith nodes. Lesovedenie 1967, 5, 72–77. (In Russian) [Google Scholar]
- Sirén, G. Alikasvoskuusten biologiaa. Summary: On the biology of undergrown spruce. Acta. For. Fenn. 1950, 58, 1–90. [Google Scholar] [CrossRef] [Green Version]
- Chojnacki, W. O metodzie dokladnego okreslenia wieku podrostow i rocznych przyrostow u niektorych gatunkow drzew lesnych (About the method to determine accurately the age of saplings and the annual height growth of some forest tree species). Sylwan 1964, 1, 71–76. [Google Scholar]
- Ross, S.M. Introduction to Probability and Statistics for Engineers and Scientists, 4th ed.; Elsevier Academic Press: San Diego, CA, USA, 2009; p. 680. [Google Scholar] [CrossRef]
- Gatsuk, L.E.; Smirnova, O.V.; Vorontzova, L.I.; Zaugolnova, L.B.; Ztiukova, L.A. Age states of plants of various growth forms: A review. J. Ecol. 1980, 68, 675–696. [Google Scholar] [CrossRef]
- Schitt, P.G. Study of the Growth and Development of Fruit and Berryplants; Selkhozgiz: Moscow, Russia, 1958; p. 447. (In Russian) [Google Scholar]
- Rabotnov, T.A. The life cycle of perennial herbaceous plants in meadow coenoses. In Geobotany; Lavrenko, E.M., Sokolov, S.Y., Shennikov, A.P., Eds.; Nauka: Moscow, Russia, 1950; Volume 6, pp. 7–204. (In Russian) [Google Scholar]
- Uranov, A.A. Age spectrum of the phytocoenopopulation as a function of time and energetic wave processes. Biol. Nauki 1975, 2, 7–34. (In Russian) [Google Scholar]
- Smirnova, O.V.; Zaugolnova, L.B.; Ermakova, I.M. Plant Coenopopulations (Basic Concept and Structure); Nauka: Moscow, Russia, 1976; p. 217. (In Russian) [Google Scholar]
- Komarov, A.S.; Palenova, M.M.; Smirnova, O.V. The concept of discrete description of plant ontogenesis and cellular automata models of plant populations. Ecol. Model. 2003, 170, 427–439. [Google Scholar] [CrossRef]
- Smirnova, O.V.; Chistyakova, A.A.; Zaugolnova, L.B.; Evstigneev, O.I.; Popadiouk, R.V.; Romanovsky, A.M. Ontogeny of a tree. Bot. Zhurnal 1999, 84, 8–19. (In Russian) [Google Scholar]
- Evstigneev, O.; Korotkov, V.N. Ontogenetic stages of trees: An overview. Rus. J. Ecosys. Ecol. 2016, 1. [Google Scholar] [CrossRef] [Green Version]
- Dănescu, A.; Kohnle, U.; Bauhus, J.; Weiskittel, A.; Albrecht, A.T. Long-term development of natural regeneration in irregular, mixed stands of silver fir and Norway spruce. For. Ecol. Manag. 2018, 430, 105–116. [Google Scholar] [CrossRef]
- Nilsen, P. Selective Cutting in Mountain Spruce Forest—Regeneration and Production After Earlier Cuttings; Norwegian Forest Research Institute: As, Norway, 1984; p. 26. [Google Scholar]
- Lundqvist, L.; Nilson, K. Regeneration dynamics in an uneven-sized virgin Norway spruce forest in northern Sweden. Scand. J. For. Res. 2007, 22, 304–309. [Google Scholar] [CrossRef]
- Lin, C.J.; Laiho, O.; Lähde, E. Norway spruce (Picea abies L.) regeneration and growth of understory trees under single-tree selection silviculture in Finland. Eur. J. Forest. Res. 2012, 131, 683–691. [Google Scholar] [CrossRef]
- Zaugolnova, L.B. Age states in the ontogeny of ash (Fraxinus excelsior L.). In Problems of Morphogenesis of Flowering Plants and the Composition of Their Populations; Uranov, A.A., Ed.; Nauka: Moscow, Russia, 1968; pp. 81–102. (In Russian) [Google Scholar]
- Evstigneev, O.I.; Korotkova, N.V. Features of undergrowth development in Eastern European forests. Rus. J. Ecosys. Ecol. 2019, 4. [Google Scholar] [CrossRef]
- Benninghoff, W.S. Interaction of vegetation and soil frost phenomena. Arctic 1952, 5, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Ranade, S.S.; Delhomme, N.; García-Gil, M.R. Transcriptome analysis of shade avoidance and shade tolerance in conifers. Planta 2019, 250, 299–318. [Google Scholar] [CrossRef] [Green Version]
- Dinwoodie, J.M. Timber: Its Nature and Behavior, 2nd ed.; CRC Press: London, UK, 2000; p. 272. [Google Scholar] [CrossRef]
- Ishii, H.; McDowell, N. Age-related development of crown structure in coastal Douglas-fir trees. For. Ecol. Manag. 2002, 169, 257–270. [Google Scholar] [CrossRef]
- Ishii, H.T.; Ford, E.D.; Kennedy, M.C. Physiological and ecological implications of adaptive reiteration as a mechanism for crown maintenance and longevity. Tree Physiol. 2007, 27, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Pelt, R.V.; Sillett, S.C. Crown development of coastal Pseudotsuga menziesii, including a conceptual model for tall conifers. Ecol. Monogr. 2008, 78, 283–311. [Google Scholar] [CrossRef]
- Kramer, R.D.; Sillett, S.C.; Van Pelt, R.; Franklin, J.F. Neighborhood competition mediates crown development of Picea sitchensis in Olympic rainforests: Implications for restoration management. For. Ecol. Manag. 2019, 441, 127–143. [Google Scholar] [CrossRef]
- Tomlinson, P.B. Tree architecture: New approaches help to define the elusive biological property of tree form. Am. Sci. 1983, 71, 141–149. [Google Scholar]
- Canham, C.D. Growth and canopy architecture of shade-tolerant trees: Response to canopy gaps. Ecology 1988, 69, 786–795. [Google Scholar] [CrossRef]
- Pretzsch, H. The effect of tree crown allometry on community dynamics in mixed-species stands versus monocultures. a review and perspectives for modeling and silvicultural regulation. Forests 2019, 10, 810. [Google Scholar] [CrossRef] [Green Version]
- Bartkowicz, L.; Paluch, J. Co-occurrence of shade-tolerant and light-adapted tree species in uneven-aged deciduous forests of southern Poland. Eur. J. Forest. Res. 2019, 138, 15–30. [Google Scholar] [CrossRef] [Green Version]
- Leemans, R. Canopy gaps and establishment patterns of spruce (Picea abies (L.) Karst.) in two old-growth coniferous forests in central Sweden. Vegetatio 1991, 93, 157–165. [Google Scholar] [CrossRef]

| Stand Formula * | Age, Years | Stand Density | Growth Class | Tree Height, m | Tree Diameter, cm | Amount of Spruce Undergrowth, 1000 Stems Per Ha |
|---|---|---|---|---|---|---|
| Spruce–moss forest | ||||||
| 1 layer: 10S sngl: B, P, Ln | 130 | 0.40 | III | 28.2 | 24.2 | 8.6 (2.2 **) |
| 2 layer: 5S4B1Ln sngl. F | 70 | 0.10 | 9.5 | 11.5 | ||
| Fir–spruce–moss forest | ||||||
| 1 layer: 5F5S sngl. B, P | 90 | 0.79 | II | 21.6 | 20.4 | 15.0 (0.6) |
| 2 layer: 10F + S, Ln, B sngl. M, W | 70 | 0.06 | 9.9 | 8.7 | ||
| Pine–moss forest | ||||||
| 1 layer: 7P3S +B, sngl. Ln | 136 | 0.60 | III | 27.0 | 19.8 | 5.0 (1.1) |
| 2 layer: 5S3P2B + Ln sngl. F | 65 | 0.20 | 9.6 | 9.0 | ||
| Larch–moss forest | ||||||
| 1 layer: 5Lr4S1B sngl. P | 110 | 0.5 | V | 19.1 | 15.2 | 23.6 (0.4) |
| 2 layer: 6S3B1Lr + F | 80 | 0.5 | 9.1 | 9.2 | ||
| Age, Years | Forest Type | |||
|---|---|---|---|---|
| Fir–Spruce–Moss Forest | Spruce–Moss Forest | Larch–Moss Forest | Pine–Moss Forest | |
| 6–10 | 71 (40–94) | 75 (32–100) | 50 (27–79) | 54 (38–72) |
| 11–15 | 66 (40–93) | 51 (36–65) | 51 (34–75) | 50 (28–74) |
| 16–20 | 66 (32–83) | 66 (28–84) | 56 (38–82) | 46 (32–63) |
| 21–25 | 72 (39–94) | 69 (54–92) | 59 (22–91) | 41 (27–59) |
| 26–30 | 74 (56–88) | 70 (61–82) | 57 (40–74) | 58 (42–80) |
| 31–35 | 73 (66–79) | 74 (58–100) | 54 (36–77) | 42 (23–87) |
| Age Groups (Biological Age) | Fir–Spruce–Moss Forest | Spruce–Moss Forest | Larch–Moss Forest | Pine–Moss Forest | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| j | im | v | j | im | v | j | im | v | j | im | v | |
| 2–5 | 77 | 23 | - | 75 | 25 | - | 74 | 26 | - | 75 | 25 | - |
| 6–10 | 33 | 66 | 1 | 34 | 63 | 3 | 30 | 62 | 8 | 27 | 64 | 9 |
| 11–15 | 24 | 73 | 3 | 22 | 72 | 6 | 20 | 70 | 10 | 18 | 74 | 8 |
| 16–20 | 7 | 66 | 27 | 8 | 68 | 24 | - | 54 | 46 | - | 50 | 50 |
| 21–30 | - | 50 | 50 | - | 51 | 49 | - | 30 | 70 | - | 29 | 71 |
| 31–40 | - | 22 | 78 | - | 23 | 77 | - | 21 | 79 | - | 24 | 74 |
| 41–50 | - | 8 | 92 | - | 9 | 91 | - | - | 100 | - | - | 100 |
| 51 and older | - | - | 100 | - | - | 100 | - | - | 100 | - | - | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaitsev, G.; Davydychev, A.; Kulagin, A.; Giniyatullin, R.; Suleymanov, R.; Kulagin, A.; Egorova, N.; Komissarov, M.; Urazgildin, R.; Tagirova, O. Suppressed Undergrowth of Siberian Spruce (Picea obovata Ledeb.) in Early Ontogeny: One-Way Ticket or Survival Strategy? Forests 2021, 12, 851. https://doi.org/10.3390/f12070851
Zaitsev G, Davydychev A, Kulagin A, Giniyatullin R, Suleymanov R, Kulagin A, Egorova N, Komissarov M, Urazgildin R, Tagirova O. Suppressed Undergrowth of Siberian Spruce (Picea obovata Ledeb.) in Early Ontogeny: One-Way Ticket or Survival Strategy? Forests. 2021; 12(7):851. https://doi.org/10.3390/f12070851
Chicago/Turabian StyleZaitsev, Gleb, Alexander Davydychev, Alexey Kulagin, Rafak Giniyatullin, Ruslan Suleymanov, Andrey Kulagin, Natalya Egorova, Mikhail Komissarov, Ruslan Urazgildin, and Olesya Tagirova. 2021. "Suppressed Undergrowth of Siberian Spruce (Picea obovata Ledeb.) in Early Ontogeny: One-Way Ticket or Survival Strategy?" Forests 12, no. 7: 851. https://doi.org/10.3390/f12070851
APA StyleZaitsev, G., Davydychev, A., Kulagin, A., Giniyatullin, R., Suleymanov, R., Kulagin, A., Egorova, N., Komissarov, M., Urazgildin, R., & Tagirova, O. (2021). Suppressed Undergrowth of Siberian Spruce (Picea obovata Ledeb.) in Early Ontogeny: One-Way Ticket or Survival Strategy? Forests, 12(7), 851. https://doi.org/10.3390/f12070851









