Nitrogen Fertilization, Stand Age, and Overstory Tree Species Impact the Herbaceous Layer in a Central Appalachian Hardwood Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Data Collection and Analysis
2.4. Statistical Analysis
3. Results
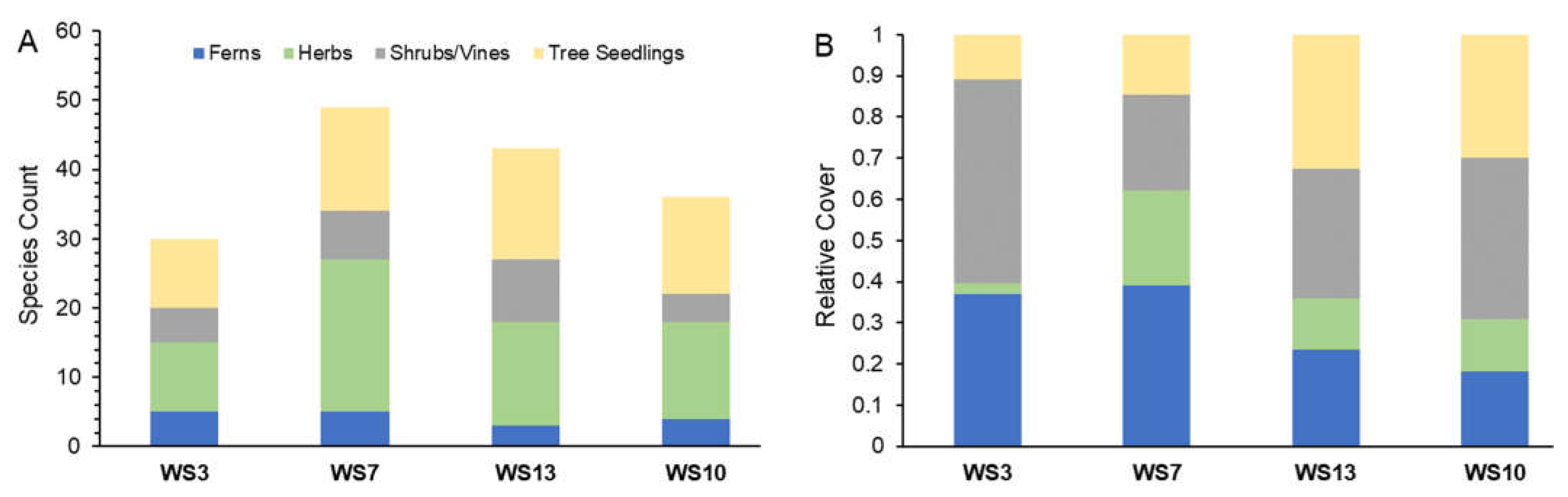
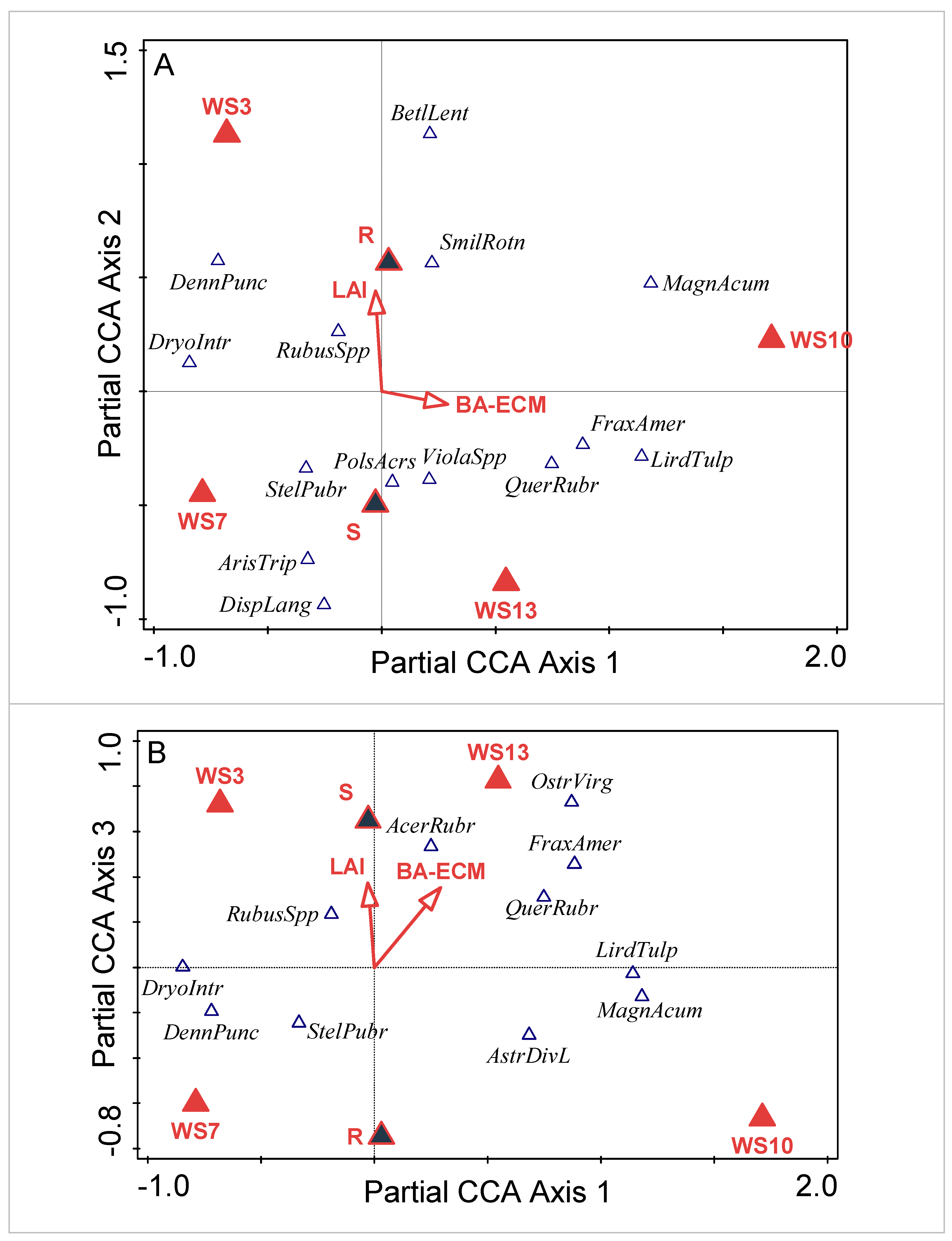
3.1. Community Composition
3.1.1. Watershed-Level Herb-Layer Community Composition
3.1.2. Plot-Level Herb-Layer Community Composition
3.2. Herb-Layer Indices: Cover, S, H, and J
3.2.1. WS3 vs. WS7: Fertilized vs. Unfertilized 50-Year-Old Stands
3.2.2. WS7 vs. WS13: Stand Age of 50 Years vs. 110 Years
3.2.3. WS13 vs. WS10: Northerly vs. Southerly Watershed Aspect in 110-Year-Old Stands
4. Discussion
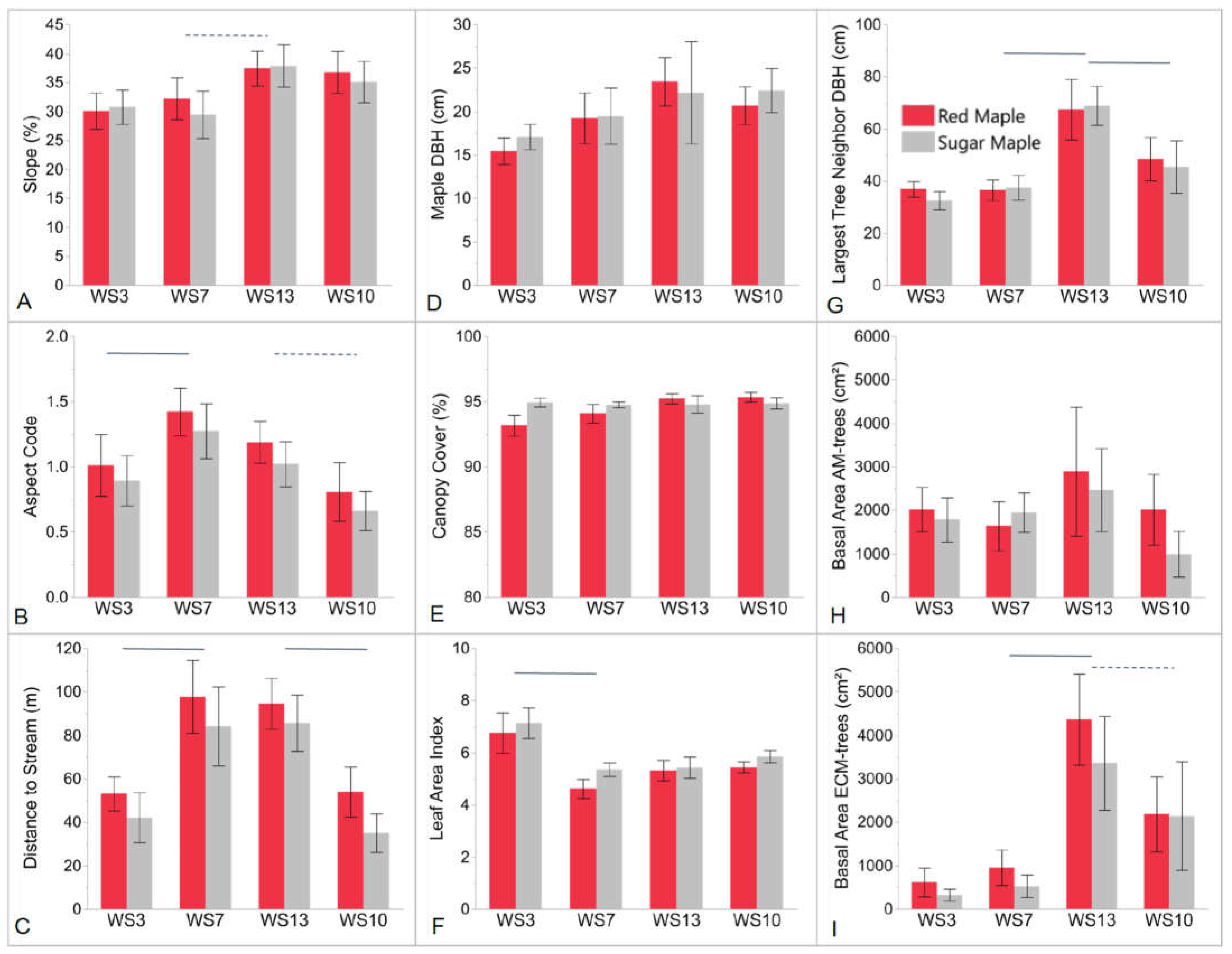
4.1. Influence of Abiotic Environmental Factors
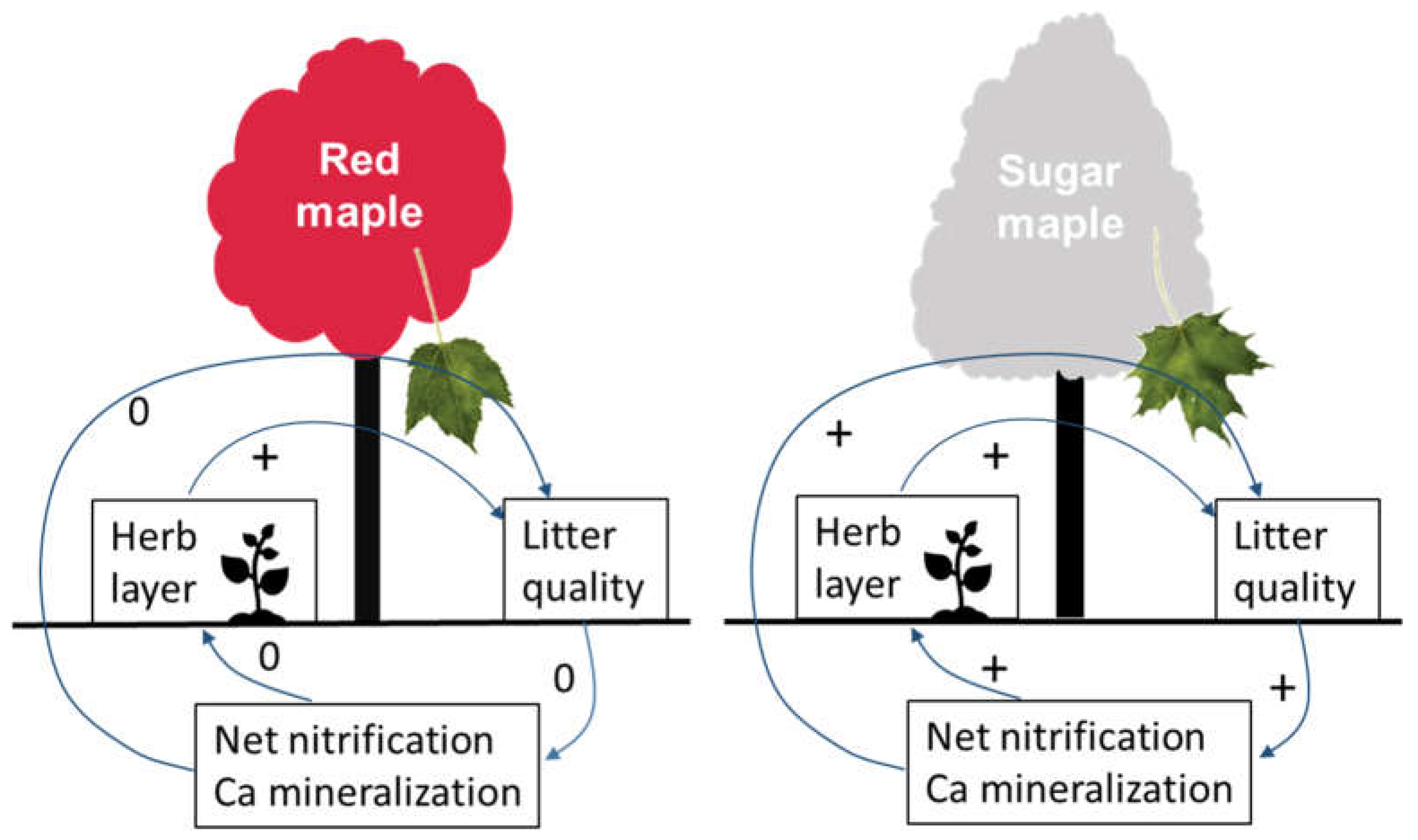
4.2. Herb-Layer Responses to Overstory Red Maple vs. Sugar Maple
4.3. Herb-Layer Responses to Fertilization: WS3 vs. WS7
4.4. Herb-Layer Responses to Land Use History (Stand Age): WS7 vs. WS13
4.5. Herb-Layer Responses to Watershed Aspect: WS13 vs. WS10
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilliam, F. The ecological significance of the herbaceous layer in temperate forest ecosystems. Bioscience 2007, 57. [Google Scholar] [CrossRef]
- Muller, R.N. Nutrient relations of the herbaceous layer in deciduous forest ecosystems. In The Herbaceous Layer in Forests of Eastern North America; Gilliam, F.S., Roberts, M.R., Eds.; Oxford University Press: Oxford, UK, 2014; pp. 13–34. [Google Scholar]
- Hooper, D.; Chapin, F.S., III; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Allan, E.; Weisser, W.W.; Fischer, M.; Schulze, E.-D.; Weigelt, A.; Roscher, C.; Baade, J.; Barnard, R.L.; Beßler, H.; Buchmann, N.; et al. A comparison of the strength of biodiversity effects across multiple functions. Oecologia 2013, 173, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Knops, J.; Wedin, D.; Reich, P.; Ritchie, M.; Siemann, E. The influence of functional diversity and composition on ecosystem processes. Science 1997, 277, 1300–1302. [Google Scholar] [CrossRef] [Green Version]
- Muller, R.N.; Bormann, F.H. Role of Erythronium americanum Ker. in energy flow and nutrient dynamics of a northern hardwood forest ecosystem. Science 1976, 193, 1126–1128. [Google Scholar] [CrossRef] [PubMed]
- Melillo, J.M.; Aber, J.D.; Linkins, A.E.; Ricca, A.; Fry, B.; Nadelhoffer, K.J. Carbon and nitrogen dynamics along the decay continuum: Plant litter to soil organic matter. Plant Soil 1989, 115, 189–198. [Google Scholar] [CrossRef]
- Elliott, K.J.; Vose, J.; Knoepp, J.; Clinton, B.; Kloeppel, B. Functional role of the herbaceous layer in eastern deciduous forest ecosystems. Ecosystems 2015, 18, 221–236. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Z.; Wang, X.; Zhou, L.; Lin, Y.; Fu, S. Effects of understory removal and tree girdling on soil microbial community composition and litter decomposition in two Eucalyptus plantations in South China. Funct. Ecol. 2011, 25, 921–931. [Google Scholar] [CrossRef]
- Maguire, D.; Forman, R.T.T. Herb cover effects on tree seedling patterns in a mature hemlock-hardwood forest. Ecology 1983, 64, 1347–1380. [Google Scholar] [CrossRef]
- Horsley, S.B. Role of allelopathy in hay-scented fern interference with black cherry regeneration. J. Chem. Ecol. 1993, 19, 2737–2755. [Google Scholar] [CrossRef]
- Gilliam, F. The Herbaceous Layer in Forests of Eastern North America; Oxford University Press: Oxford, UK, 2014; pp. 1–688. [Google Scholar]
- Bennie, J.; Huntley, B.; Wiltshire, A.; Hill, M.; Baxter, R. Slope, aspect and climate: Spatially explicit and implicit models of topographic microclimate in chalk grassland. Ecol. Model. 2008, 216, 47–59. [Google Scholar] [CrossRef]
- Cantlon, J.E. Vegetation and microclimates on north and south slopes of Cushetunk Mountain, New Jersey. Ecol. Monogr. 1953, 23, 241–270. [Google Scholar] [CrossRef]
- Måren, I.E.; Karki, S.; Prajapati, C.; Yadav, R.K.; Shrestha, B.B. Facing north or south: Does slope aspect impact forest stand characteristics and soil properties in a semiarid trans-Himalayan valley? J. Arid Environ. 2015, 121, 112–123. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.; Kang, S.; Lee, D. Variations of soil enzyme activities in a temperate forest soil. Ecol. Res. 2009, 24, 1137–1143. [Google Scholar] [CrossRef]
- Mudrick, D.A.; Hoosein, M.; Hicks, R.R.; Townsend, E.C. Decomposition of leaf litter in an Appalachian forest: Effects of leaf species, aspect, slope position and time. For. Ecol. Manag. 1994, 68, 231–250. [Google Scholar] [CrossRef]
- Peterjohn, W.T.; Harlacher, M.A.; Christ, M.J.; Adams, M.B. Testing associations between tree species and nitrate availability: Do consistent patterns exist across spatial scales? For. Ecol. Manag. 2015, 358, 335–343. [Google Scholar] [CrossRef]
- Gilliam, F.; Roberts, M. Interactions between the Herbaceous Layer and Overstory Canopy of Eastern Forests. In The Herbaceous Layer in Forests of Eastern North America; Oxford University Press: Oxford, UK, 2014; pp. 233–254. [Google Scholar]
- Carlisle, A.; Brown, A.H.F.; White, E.J. The nutrient content of tree stem flow and ground flora litter and leachates in a sessile oak (Quercus petraea) woodland. J. Ecol. 1967, 55, 615–627. [Google Scholar] [CrossRef]
- Carl, R.C.; Ralph, E.J.B. Correlations of understory herb distribution patterns with microhabitats under different tree species in a mixed mesophytic forest. Oecologia 1984, 62, 337–343. [Google Scholar]
- Finzi, A.C.; Breemen, N.V.; Canham, C.D. Canopy tree-soil interactions within temperate forests: Species effects on soil carbon and nitrogen. Ecol. Appl. 1998, 8, 440–446. [Google Scholar] [CrossRef]
- Bigelow, S.; Canham, C. Litterfall as a niche construction process in a northern hardwood forest. Ecosphere 2015, 6, 117. [Google Scholar] [CrossRef] [Green Version]
- Cornelissen, J.; Aerts, R.; Cerabolini, B.; Werger, M.; van der Heijden, M. Carbon cycling traits of plant species are linked with mycorrhizal strategy. Oecologia 2001, 129, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, J. An experimental comparison of leaf decomposition rates in a wide range of temperate plant species and types. J. Ecol. 1996, 84, 573–582. [Google Scholar] [CrossRef]
- Lovett, G.M.; Weathers, K.C.; Arthur, M.A. Control of nitrogen loss from forested watersheds by soil carbon:nitrogen ratio and tree species composition. Ecosystems 2002, 5, 712–718. [Google Scholar] [CrossRef]
- Lovett, G.M.; Mitchell, M.J. Sugar maple and nitrogen cycling in the forests of eastern North America. Front. Ecol. Environ. 2004, 2, 81–88. [Google Scholar] [CrossRef]
- Driscoll, C.; Whitall, D.; Aber, J.; Boyer, E.; Castro, M.; Cronan, C.; Goodale, C.; Groffman, P.; Hopkinson, C.; Lambert, K.; et al. Nitrogen pollution in the northeastern United States: Sources, effects, and management options. Bioscience 2003, 53. [Google Scholar] [CrossRef]
- Gilliam, F. Response of the herbaceous layer of forest ecosystems to excess nitrogen deposition. J. Ecol. 2006, 94, 1176–1191. [Google Scholar] [CrossRef] [Green Version]
- Rajaniemi, T.K. Why does fertilization reduce plant species diversity? Testing three competition-based hypotheses. J. Ecol. 2002, 90, 316–324. [Google Scholar] [CrossRef] [Green Version]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, C.A.; Raiff, D.T.; Burnham, M.B.; Gilliam, F.S.; Adams, M.B.; Peterjohn, W.T. Nitrogen fertilization interacts with light to increase Rubus spp. cover in a temperate forest. Plant Ecol. 2016, 217, 421–430. [Google Scholar] [CrossRef]
- Gilliam, F.; Billmyer, J.; Walter, C.; Peterjohn, W. Effects of excess nitrogen on biogeochemistry of a temperate hardwood forest: Evidence of nutrient redistribution by a forest understory species. Atmos. Environ. 2016, 146, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Halpern, C.B. Early successional patterns of forest species: Interactions of life history traits and disturbance. Ecology 1989, 70, 704–720. [Google Scholar] [CrossRef]
- Kermavnar, J.; Eler, K.; Marinšek, A.; Kutnar, L. Post-harvest forest herb layer demography: General patterns are driven by pre-disturbance conditions. For. Ecol. Manag. 2021, 491, 119121. [Google Scholar] [CrossRef]
- Götmark, F.; Paltto, H.; Nordén, B.; Götmark, E. Evaluating partial cutting in broadleaved temperate forest under strong experimental control: Short-term effects on herbaceous plants. For. Ecol. Manag. 2005, 214, 124–141. [Google Scholar] [CrossRef]
- Roberts, M.R.; Zhu, L. Early response of the herbaceous layer to harvesting in a mixed coniferous–deciduous forest in New Brunswick, Canada. For. Ecol. Manag. 2002, 155, 17–31. [Google Scholar] [CrossRef]
- Gilliam, F. Effects of harvesting on herbaceous layer diversity of a Central Appalachian Hardwood forest in West Virginia, USA. For. Ecol. Manag. 2002, 155, 33–43. [Google Scholar] [CrossRef]
- Duffy, D.; Meier, A. Do Appalachian herbaceous understories ever recover from clearcutting? Conserv. Biol. 1992, 6, 196–201. [Google Scholar] [CrossRef]
- Adams, M.B.; Edwards, P.J.; Wood, F.; Kochenderfer, J.N. Artificial watershed acidification on the Fernow Experimental Forest, USA. J. Hydrol. 1993, 150, 505–519. [Google Scholar] [CrossRef]
- Adams, M.B.; Kochenderfer, J.N.; Wood, F.; Angradi, T.R.; Edwards, P. Forty Years of Hydrometeorological Data from the Fernow Experiment Forest, West Virginia; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Radnor, PA, USA, 1994; p. 24.
- Gilliam, F.; Turrill, N.; Aulick, S.; Evans, D.; Adams, M.B. Herbaceous layer and soil response to experimental acidification in a central Appalachian hardwood forest. J. Environ. Qual. 1994, 23, 835–844. [Google Scholar] [CrossRef] [Green Version]
- Adams, M.B.; Edwards, P.J.; Ford, W.M.; Schuler, T.M.; Thomas-Van Gundy, M.; Wood, F. Fernow Experimental Forest: Research History and Opportunities. In Experimental Forests and Ranges EFR-2; USDA Forest Service: Washington, DC, USA, 2012. [Google Scholar]
- Peterjohn, W.T. Fernow Experimental Forest LTREB. Soil Chemistry 2011. Available online: http://www.as.wvu.edu/fernow/data.html (accessed on 29 May 2021).
- Adams, M.B.; Kochenderfer, J.; Edwards, P. The Fernow Watershed Acidification Study: Ecosystem Acidification, Nitrogen Saturation and Base Cation Leaching; Springer: Dordrecht, The Netherlands, 2007; Volume 7, pp. 267–273. [Google Scholar]
- Trimble, G.R., Jr.; Tryon, E.H.; Smith, H.C.; Hillier, J.D. Age and Stem Origin of Appalachian Hardwood Reproduction Following a Clearcut-Herbicide Treatment; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Upper Darby, PA, USA, 1986; Volume 589.
- Kochenderfer, J.N. Fernow and the Appalachian Hardwood Region. In The Fernow Watershed Acidification Study; Adams, M.B., DeWalle, D.R., Hom, J.L., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 17–19. [Google Scholar]
- Aubertin, G.M.; Patric, J.H. Water quality after clearcutting a small watershed in West Virginia. J. Environ. Qual. 1974, 3, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Adams, M.B.; DeWalle, D.R.; Peterjohn, W.T.; Gilliam, F.S.; Sharpe, W.E.; Williard, K.W.J. Soil Chemical Response to Experimental Acidification Treatments. In The Fernow Watershed Acidification Study; Adams, M.B., DeWalle, D.R., Hom, J.L., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 41–69. [Google Scholar]
- Peterjohn, W.T. Fernow Experimental Forest LTREB. Tree Survey Data 2000. Available online: http://www.as.wvu.edu/fernow/data.html (accessed on 10 June 2021).
- Walter, C.; Burnham, M.; Gilliam, F.; Peterjohn, W. A reference-based approach for estimating leaf area and cover in the forest herbaceous layer. Environ. Monit. Assess. 2015, 187, 657. [Google Scholar] [CrossRef] [PubMed]
- Begon, M.; Harper, J.L.; Townsend, C.R. Ecology: Individuals, Populations, and Communities, 3rd ed.; Blackwell Publishing: Berlin, Germany, 1996. [Google Scholar]
- Pielou, E.C. Ecological Diversity; Wiley: New York, NY, USA, 1975. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1949; p. 117. [Google Scholar]
- Eisenhut, S.; Stephan, K. The role of tree species, the herb layer, and watershed characteristics on nitrogen cycling in a central Appalachian hardwood forest. 2021; (Unpublished; Manuscript in Preparation). [Google Scholar]
- Beers, T.W.; Dress, P.E.; Wensel, L.C. Notes and observations: Aspect transformation in site productivity research. J. For. 1966, 64, 691–692. [Google Scholar]
- Brundrett, M.; Murase, G.; Kendrick, B. Comparative anatomy of roots and mycorrhizae of common Ontario trees. Can. J. Bot. 1990, 68, 551–578. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, Y.L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef]
- ter Braak, C.; Smilauer, P.N. Canoco Reference Manual and User’s Guide: Software for Ordination (Version 5.10); Microcomputer Power: Ithaca, NY, USA, 2018; p. 536. [Google Scholar]
- McDonald, J.H. Handbook of Biological Statistics, 2nd ed.; Sparky House Publishing: Baltimore, MD, USA, 2014. [Google Scholar]
- ter Braak, C.; Smilauer, P.N. Canoco Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: Ithaca, NY, USA, 2002; p. 500. [Google Scholar]
- Leps, J.; Smilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003; Volume 3, p. 282. [Google Scholar]
- Hobbie, S.E. Effects of plant species on nutrient cycling. Trends Ecol. Evol. 1992, 7, 336–339. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Gosz, J.R.; Grier, C.C.; Melillo, J.M.; Reiners, W.A. A comparative analysis of potential nitrification and nitrate mobility in forest ecosystems. Ecol. Monogr. 1982, 52, 155–177. [Google Scholar] [CrossRef] [Green Version]
- Dijkstra, F.A. Calcium mineralization in the forest floor and surface soil beneath different tree species in the northeastern US. For. Ecol. Manag. 2003, 175, 185–194. [Google Scholar] [CrossRef]
- Boudsocq, S.; Niboyet, A.; Lata, J.-C.; Raynaud, X.; Loeuille, N.; Mathieu, J.; Blouin, M.; Abbadie, L.; Barot, S. Plant preference for ammonium versus nitrate: A neglected determinant of ecosystem functioning? Am. Nat. 2012, 180, 60–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterjohn, W.T.; Adams, M.B.; Gilliam, F.S. Symptoms of nitrogen saturation in two central Appalachian hardwood forest ecosystems. Biogeochemistry 1996, 35, 507–522. [Google Scholar] [CrossRef]
- Brundrett, M.; Kendrick, B. The roots and mycorrhizas of herbaceous woodland plants. I. Quantitative aspects of morphology. New Phytol. 1990, 114, 457–468. [Google Scholar] [CrossRef]
- Chapman, S.K.; Langley, J.A.; Hart, S.C.; Koch, G.W. Plants actively control nitrogen cycling: Uncorking the microbial bottleneck. New Phytol. 2006, 169, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.; Kendrick, B. The mycorrhizal status, root anatomy, and phenology of plants in a sugar maple forest. Can. J. Bot. 1988, 66, 1153–1173. [Google Scholar] [CrossRef]
- Jakobsen, I. Hyphal fusion to plant species connections–Giant mycelia and community nutrient flow. New Phytol. 2004, 164, 4–7, 961–972. [Google Scholar] [CrossRef]
- St Clair, S.B.; Lynch, J.P. Base cation stimulation of mycorrhization and photosynthesis of sugar maple on acid soils are coupled by foliar nutrient dynamics. New Phytol. 2005, 165, 581–590. [Google Scholar] [CrossRef]
- Gilliam, F.; Adams, M.B.; Peterjohn, W. Response of soil fertility to 25 years of experimental acidification in a temperate hardwood forest. J. Environ. Qual. 2020, 49. [Google Scholar] [CrossRef]
- Edwards, P.; Wood, F. Fernow Experimental Forest Stream Chemistry. Available online: https://doi.org/10.2737/RDS-2011-0017 (accessed on 29 May 2021).
- Atkins, J.W.; Bond-Lamberty, B.; Fahey, R.T.; Haber, L.T.; Stuart-Haëntjens, E.; Hardiman, B.S.; LaRue, E.; McNeil, B.E.; Orwig, D.A.; Stovall, A.E.L.; et al. Application of multidimensional structural characterization to detect and describe moderate forest disturbance. Ecosphere 2020, 11, e03156. [Google Scholar] [CrossRef]
- Bormann, F.H.; Likens, G.E. Pattern and Process in a Forested Ecosystem: Disturbance, Development and the Steady State Based on the Hubbard Brook Ecosystem Study; Springer: New York, NY, USA, 1979. [Google Scholar]
- Roberts, M.; Gilliam, F. Response of the Herbaceous Layer to Disturbance in Eastern Forests. In The Herbaceous Layer in Forests of Eastern North America; Oxford University Press: Oxford, UK, 2014; pp. 320–339. [Google Scholar]
- Small, C.; McCarthy, B. Spatial and temporal variation in the response of understory vegetation to disturbance in a central Appalachian oak forest. J. Torrey Bot. Soc. 2002, 129, 136. [Google Scholar] [CrossRef]
- Melillo, J.M.; Aber, J.D.; Muratore, J.F. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 1982, 63, 621–626. [Google Scholar] [CrossRef]
- Gilliam, F. Response of herbaceous layer species to canopy and soil variables in a central Appalachian hardwood forest ecosystem. Plant Ecol. 2019, 220, 1131–1138. [Google Scholar] [CrossRef]
- Fei, S.; Steiner, K. Evidence for increasing red maple abundance in the eastern United States. For. Sci. 2007, 53, 473–477. [Google Scholar]
- Gilliam, F.; Burns, D.; Driscoll, C.; Frey, S.; Lovett, G.; Watmough, S. Decreased atmospheric nitrogen deposition in eastern North America: Predicted responses of forest ecosystems. Environ. Pollut. 2018, 244, 560–574. [Google Scholar] [CrossRef] [PubMed]
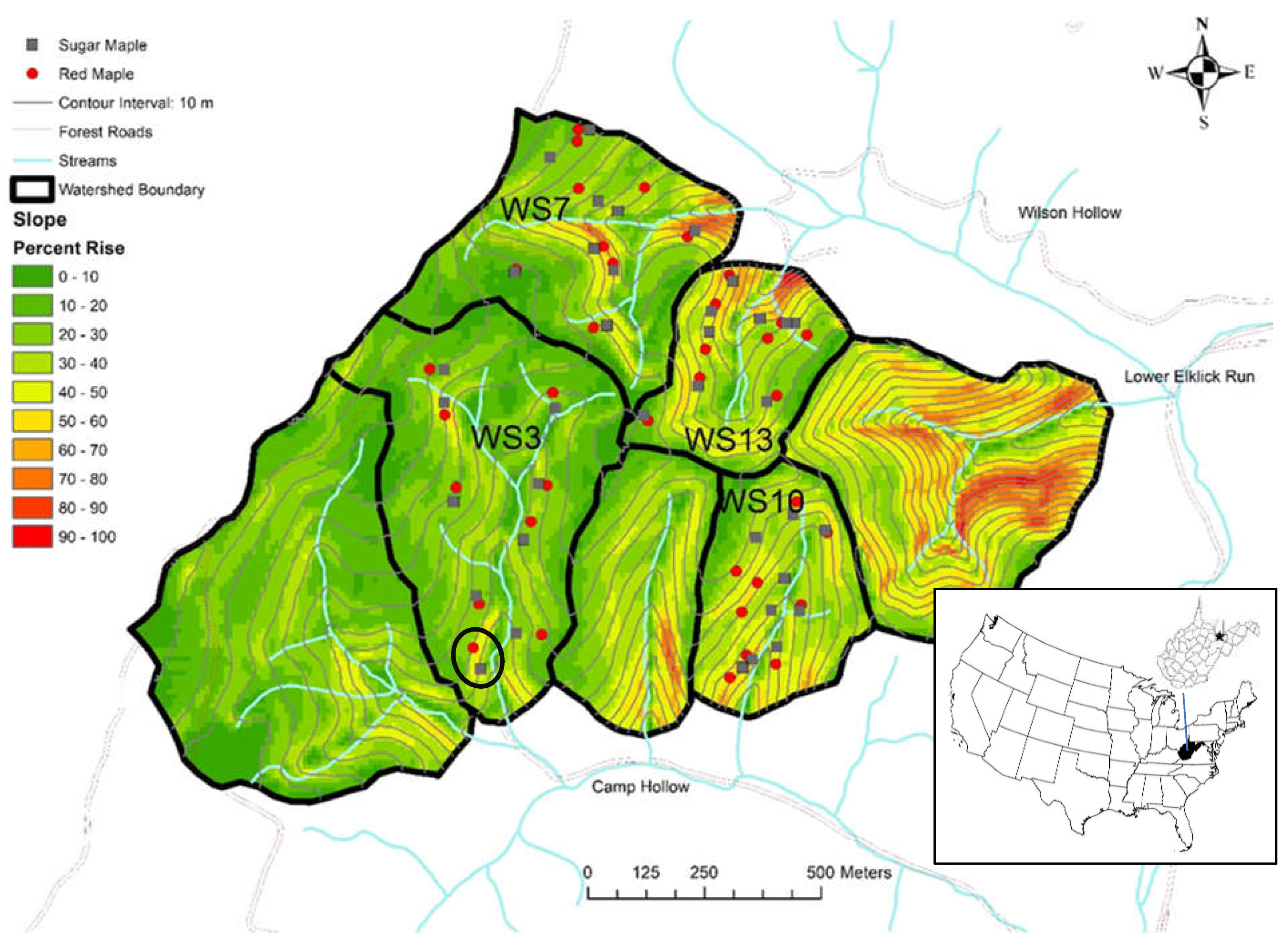



| Watershed ID | Location * | Area (ha) | Elevation (m) | Average Slope (%) (Min–Max) | Dominant Tree Species ** | Aspect | Stand Age (year) in 2020 | Fertilization Treatment |
|---|---|---|---|---|---|---|---|---|
| WS3 | 39.05413 N 79.68625 W | 34.3 | 730–870 | 20.6 (0–60) | Black cherry, red maple, sweet birch northern red oak | S | ~50 | Fertilized |
| WS7 | 39.06388 N 79.68029 W | 24.2 | 705–860 | 25.8 (0–90) | Tulip-poplar, black cherry, sweet birch, red maple | E | ~50 | Not fertilized |
| WS10 | 39.05411 N 79.68029 W | 15.2 | 700–805 | 33.4 (0–70) | Chestnut oak, northern red oak, red maple, blackgum | S | ~110 | Not fertilized |
| WS13 | 39.06280 N 79.67917 W | 14.2 | 695–810 | 35.2 (0–100) | Northern red oak, sugar maple, red maple, tulip-poplar | N | ~110 | Not fertilized |
| Dependent Variable | Predictor | Description |
|---|---|---|
| Cover Species richness (S) Shannon index (H) Evenness (J) | Watershed (WS) | Watershed-level treatment effect; 2 factor levels; factor levels (treatments) varied by watershed pair:
|
| Plot-center maple (M) | 2 factor levels: red maple, sugar maple | |
| WS × M | Watershed (treatment)—maple species interaction | |
| Slope | Plot-level slope in % | |
| Aspect code | Plot-level aspect code A′ | |
| Distance to stream | Slope distance from plot center to perennial stream | |
| DBH | DBH of plot-center tree | |
| Large neighbor DBH | DBH of the largest of the five nearest neighbor trees to the plot center | |
| Canopy cover | Percent of sky covered by canopy | |
| Large Neighbor Myc. | Mycorrhizal association of the largest of the five nearest neighbor trees to the plot center; 3 factor levels: AM, ECM, both AM and ECM | |
| Leaf Area Index | LAI, total leaf area (m2) found over 1 m2 of ground | |
| BA of ECM trees | Basal area of ectomycorrhizal trees among the five nearest neighbors of the plot center (BA-ECM) | |
| BA of AM trees | Basal area of arbuscular mycorrhizal trees among the five nearest neighbors of the plot center (BA-AM) |
| Cover | S | H | J | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| July 2018 | May 2019 | July 2019 | July 2018 | May 2019 | July 2019 | July 2018 | May 2019 | July 2019 | July 2018 | May 2019 | July 2019 | |
| WS3 | 32.6 | 12.4 | 32.5 | 30 | 32 | 31 | 2.0 | 2.2 | 1.8 | 0.59 | 0.62 | 0.53 |
| WS7 | 36.4 | 18.6 | 31.7 | 48 | 46 | 46 | 2.7 | 2.8 | 2.6 | 0.69 | 0.74 | 0.69 |
| WS10 | 16.8 | 10.0 | 11.3 | 43 | 42 | 45 | 2.8 | 3.1 | 2.9 | 0.74 | 0.82 | 0.77 |
| WS13 | 19.5 | 10.9 | 15.4 | 36 | 35 | 40 | 2.5 | 2.6 | 2.5 | 0.69 | 0.72 | 0.68 |
| Watershed ID | Stream Water Ca2+ (mg/L) * | SD | Stream Water NO3− (mg/L) * | SD | Treatment | Stand Age in 2020 (year) |
|---|---|---|---|---|---|---|
| WS3 | 2.28 | 0.21 | 8.55 | 1.14 | Fertilization | ~50 |
| WS7 | 2.07 | 0.14 | 4.64 | 0.67 | No fertilization | ~50 |
| WS13 | 1.76 | 0.18 | 1.96 | 0.63 | No fertilization, north aspect | ~110 |
| WS10 | 1.60 | 0.20 | 0.83 | 0.32 | No fertilization, south aspect | ~110 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, L.J.; Stephan, K. Nitrogen Fertilization, Stand Age, and Overstory Tree Species Impact the Herbaceous Layer in a Central Appalachian Hardwood Forest. Forests 2021, 12, 829. https://doi.org/10.3390/f12070829
Smith LJ, Stephan K. Nitrogen Fertilization, Stand Age, and Overstory Tree Species Impact the Herbaceous Layer in a Central Appalachian Hardwood Forest. Forests. 2021; 12(7):829. https://doi.org/10.3390/f12070829
Chicago/Turabian StyleSmith, Lacey J., and Kirsten Stephan. 2021. "Nitrogen Fertilization, Stand Age, and Overstory Tree Species Impact the Herbaceous Layer in a Central Appalachian Hardwood Forest" Forests 12, no. 7: 829. https://doi.org/10.3390/f12070829
APA StyleSmith, L. J., & Stephan, K. (2021). Nitrogen Fertilization, Stand Age, and Overstory Tree Species Impact the Herbaceous Layer in a Central Appalachian Hardwood Forest. Forests, 12(7), 829. https://doi.org/10.3390/f12070829





