1. Introduction
The oaks (
Quercus, Fagaceae) comprise more than 435 species distributed across temperate and tropical regions of the northern hemisphere [
1,
2,
3]. They are among the best-known and most ecologically significant forest trees in North America (including Mexico) and Eurasia, shaping forest and savanna ecosystems [
4] and the diversity of urban forests [
5,
6], and molding the development of human civilization and mythology [
7,
8,
9,
10]. Oaks are united by a suite of distinctive floral traits—in particular, pendant male catkins and tricarpellate female flowers with linear styles and expanded stigmas—and a signature fruit trait: the circular single fruit or acorn seated within a cup-shaped extra-floral accessory structure, a specialized involucre called a cupule. The cupule is the defining feature of Fagaceae. While other genera of the family feature acorns and valveless rounded cupules, the combination of floral character states shared by oaks is unique within the family [
11,
12].
The most recent classification system of
Quercus recognizes eight sections within two subgenera, subgenus
Quercus (ca. 295 spp.) and subg.
Cerris (ca. 140 spp. [
3]; see
Table 1). This old dichotomy between oak subgenera, which dates to the early Eocene, was recognized in early molecular studies of the genus and family [
13,
14] and is robustly supported by an extensive RAD-seq (next-generation DNA sequencing) phylogenomic study of a comprehensive worldwide sample of species [
15]. The two subgenera represent a deep biogeographic split among modern taxa: subg.
Cerris is restricted to Europe, Asia, and northern Africa; and subg.
Quercus, the American oak clade, is largely restricted to the Americas except for two dispersals back to Eurasia. Eight sections of the genus are also supported by a combination of RAD-seq data and macro- and microscopic characters, most notably patterns of pollen ornamentation viewed under SEM [
3]. These subtle yet well-established differences in pollen exine, and their correlation with phylogenomic data, give us high confidence in the basic split between subgenera and the pollen subtypes that correspond to each of the recognized sections [
3,
16].
Fossilized pollen has been used to pin the modern sections in time and space, serving as an invaluable resource to interpret paleobiogeography and to time-calibrate the global oak phylogeny [
15,
16]. Time-calibrated phylogenomic analyses, combined with fossil and modern oak species distributions, support an initial split in the American oak clade between the red oaks, sect.
Lobatae, and the remainder of the clade between ca. 54 and 48 Ma (= million years ago). The non-
Lobatae American oak clade sections exhibit high ecological diversity, with several striking contrasts between three early-diverging clades—sects.
Protobalanus, Ponticae and
Virentes—and the main radiation of white oaks, sect.
Quercus. These early-branching lineages are species-poor, two to seven species each, restricted in distribution, and evergreen to wintergreen in habit. By contrast, sect.
Quercus, with approximately 150 species of white oak, has the broadest distribution of all forest trees in the Northern Hemisphere [
3]. This may be owing to the evolution of deciduousness and other traits associated with a range of continental climates, an exceptionally high range of functional diversity that sets the American oaks up as a model clade for understanding tree diversification and ecology [
17,
18]. The other main branch of the American oaks and the sister group to these lineages, sect.
Lobatae, comprises the second largest clade next to the white oaks. It forms a remarkably parallel radiation to the white oaks with respect to distribution, habit, and morphology [
17,
19]. There is, however, an important point of ecological distinction between these two large oak clades:
Lobatae show lower levels of diversification in western North America, especially in the California Floristic Province (CA-FP) and xeric woodlands of the American southwest [
20]. The reasons for this ecological distinction between the clades may be key to understanding coexistence of these sections in the Americas, and the consequent interaction between diversification and tree diversity in oaks [
17,
21,
22]
The sectional classification for
Quercus [
3] is solid and unlikely to change. In this paper, we present a subsectional classification for the American oak clade based largely on our phylogenomic work of the past five years [
15,
19,
23,
24,
25]. Our current sampling of species for RAD-seq analysis stands at roughly 250 taxa: A total of 177 from subg.
Quercus, the American oak clade, and 73 from subg.
Cerris, the Eurasian oak clade. Within subg.
Quercus, our coverage is nearly complete for the 92 species treated within the
Flora of North America (FNA north of Mexico [
1]). This phylogenomic work reveals compelling continental patterns of oak diversity at the broadest and narrowest phylogenetic scales. At the scale of entire clades, the early-branching clades as well as many other lineages within the two largest sections of subg.
Quercus, sects.
Lobatae (red oaks) and
Quercus (white oaks), are distributed exclusively within North America north of Mexico (hereafter, “the FNA region”). It is also clear that the number of species within the FNA region with relationships to species outside the region is relatively low, limited to a single species of sect.
Ponticae and a single clade in sect.
Quercus, as discussed below. Thus, the oak flora of the FNA region is nearly a self-contained unit for study. At the same time, within regional clades, multiple radiations of taxa have been sampled at the population level for both our global phylogeny and as part of earlier stand-alone studies targeting the fine-scale systematics of challenging species complexes [
23,
25,
26,
27,
28]. These enable the same dataset that informs classification to provide insight into species taxonomy. With this framework in place, we are well positioned to provide a phylogenetically-driven and timely bookend on the systematics of the taxa treated in the FNA region.
Our goals with this review are: (1) To describe the biogeographic patterns of oak diversity within North America; (2) to formally classify 62 species into nine well-sampled monophyletic subsections; and (3) to review the impact of phylogenomic data on the systematics of multiple species complexes and distill the results of hypothesis testing involving hybridization within the flora.
1.1. Phylogeny and Biogeography of the Oak Flora in North America
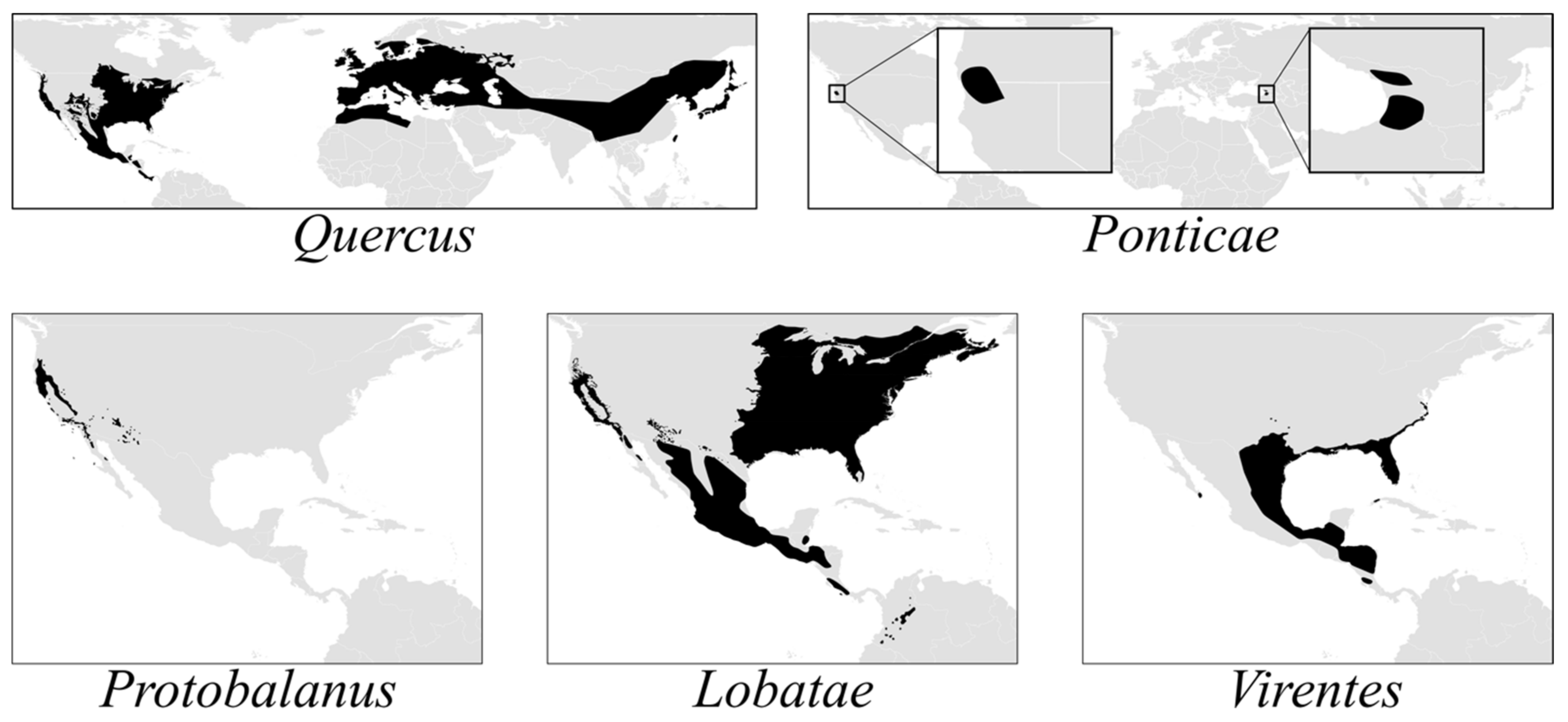
The American oak clade (
Quercus subg.
Quercus) comprises three endemic lineages—sects.
Lobatae,
Protobalanus and
Virentes—and two Holarctic or transcontinental lineages—sects.
Ponticae and
Quercus (
Figure 1). Sections
Lobatae,
Protobalanus, and
Quercus likely arose in what is now the boreal zone. Middle Eocene pollen records from Axel Heiberg Island (79°55‘N, 88°58‘ W) indicate the presence of modern oak lineages at high latitudes in North America by c. 45 Ma [
29]. As temperatures at high latitudes decreased by ca. 3–5 °C during the Eocene–Oligocene climate transition 34 Ma [
30],
Lobatae and
Quercus were pushed southward toward their modern-day distributions (
Figure 1). A parallel pattern of vicariance in the red oaks and the white oaks is evident from the phylogeny, with each section diverging into a western clade centered in the California Floristic Province (CA-FP) sister to an eastern North American clade. The red oaks and white oaks then radiated simultaneously in the CA-FP and eastern North America before diverging from an eastern North American ancestor to diversify in Mexico and Central America.
The oak flora of the FNA region contains a high level of global phylogenetic diversity—all five sections of subg.
Quercus, including the relictual sects.
Ponticae (
Q. sadleriana only) and
Protobalanus (five species), which are restricted to the CA-FP or, in the case of
Q. chrysolepis and
Q. palmeri of sect.
Protobalanus, range from the CA-FP to the adjacent southwest. Our work suggests that older crown group ages [
15] and plastome divergences [
13,
33] are associated with many of these deeply divergent subclades, a pattern consistently in contrast to the more recently derived subclades containing mostly Mexican and Central American species. These nested clades contain three unrelated red oak species and two distinct groups of white oak species in the FNA region, which suggests multiple recent dispersal events or range extensions from Mexico back into the southwestern edge of the FNA region [
19].
1.2. Patterns of Ecological Diversity
A broad overview of North American species diversity and community assembly published in 2018, based on tree species within U.S. Forest Service Forest Inventory and Analysis (FIA) plots [
17,
18], shows that FNA oak diversity peaks in the southeastern piedmont and coastal plain regions [
18]. While these analyses did not include ca. 30 oak shrubs and small tree species, they demonstrate the broad distribution of oak diversity in the FNA region. What they miss most significantly is the impressive level of shrub and small tree diversity in the CA-FP and adjacent desert biomes of the southwest that accounts for about 18 species across four sections, or roughly two-thirds of the species of smaller stature within the flora. While the addition of these species changes little to the initial observation that the uplands of eastern and central North America are the least diverse areas for oaks, this diversity is important to understanding the role of oak phylogenetic diversity in a range of ecological and evolutionary contexts, including community assembly and ecosystem functions of non-forested oak-dominated ecosystems.
The broad geographic and ecological distribution of oaks across at least eight distinct North American biomes has generated a complex array of foliar adaptations, especially within the two main species-rich sections,
Lobatae and
Quercus. While each of the sections covers a broad ecological and geographic range, these ranges begin to subdivide and segregate regionally. Eastern North American subclades
Stellatae (sect.
Quercus) and
Phellos (sect.
Lobatae), here treated as subsections, centered in the piedmont and coastal plain, highlight previously unsuspected close relationships between lobe-leaved and non-lobe-leaved species. In the southeast, the added presence of generally non-lobe-leaved species of sect.
Virentes (live oaks) with already diverse communities of sympatric oak species revealed a clear pattern of phylogenetic sorting and convergent evolution among species from the three sections along moisture and fire gradients [
26,
34].
For species placed within western clades of red and white oaks and centered on the CA-FP—subclades
Dumosae (sect.
Quercus) and
Agrifoliae (sect.
Lobatae), here treated as subsections—a combination of sclerophylly and deciduousness has presumably evolved in response to drought. In each case, phylogenies resolved the lobe-leaved deciduous species sister to more diverse clades of mostly sclerophyllous shrub species [
23,
27,
35]. This parallel distinction between leaf habit and species diversity in the white and red oaks of the CA-FP suggests an increase in diversification rate in response to decreasing summer precipitation and general support for the hypothesis of convergent patterns of foliar evolution. Additional complexity in the evolution of character states of leaf persistence is observed within the most diverse clades of red and white oaks along abiotic gradients southward into Mexico and Central America, demonstrating sympatric and strongly parallel diversification in climatic niche and leaf habit increasing in rate with the move into Mexico [
19].
1.3. Classification of the American Oak Clade: Species Level Systematics
The ecological and biogeographic segregation of species within sections suggests a biological rationale for naming subsections, beyond the practical ramifications of any classification system for the writing of keys and identification of species. At the species level, phylogenetic relationships among oaks species remain difficult to resolve with confidence, especially for a group well known for hybridization [
24,
36,
37]. But recent studies using a range of DNA markers suggest that hybridization does not pose an insurmountable barrier to achieving that goal [
24,
26,
36,
38,
39,
40,
41]. Population-level sampling is critical to establishing that oak species show genetic cohesion, and the use of reduced-representation next-generation sequencing data on exemplars across species ranges has provided robust evidence for the existence of species boundaries even among closely related species occurring in sympatry (e.g., [
42,
43,
44,
45,
46]. A species level analysis of sect.
Virentes stands out as the first oak study with complete phylogenomic sampling of any oak clade, using RAD-seq data and including multiple accessions throughout the range of each species, to generate a phylogeny and provide the context needed to guide a range of comparative investigations [
26]. Since then, two more clades, treated here as subsections
Dumosae and
Agrifoliae, have been comprehensively sampled and analyzed [
23,
27,
35,
47].
Throughout this paper, our work rests on a concept of oak species as populations of individuals that cohere genomically in a subset of the genome that is likely shared across all members of the species, barring early-generation hybrids [
48,
49]. While the mechanisms maintaining species boundaries in oaks very likely differ across the range of even a single species, and while those mechanisms maintained across a species’ range may vary in strength across the range, it remains the case that we are able to recognize most oak species ecologically and morphologically [
50,
51,
52]. In addition, as individual cases of species pairs and species clusters are investigated with increasing amounts of molecular data, sampled from across the genome, they converge with very few exceptions on genomic clusters that accord with recognizable species [
23,
38,
53,
54,
55,
56]. Exceptions will no doubt result from incomplete divergence, as time since speciation is likely a key factor in resolving oak species, even with the application of multiple criteria [
48]. Our work thus far has shown that clades from the FNA region often vary in their depth of relative species divergence, with several examples that suggest we are capturing various stages of diversification, ranging from isolation by distance on the California Channel Islands (e.g., sect.
Protobalanus, subsect.
Dumosae) to differentiation in ecological niche space in both coastal and continental regions (e.g., sect.
Virentes, subsect.
Prinoideae).
Analogous to the argument that has been made for plant species as a whole [
56], the correlation between morphological patterns of similarity and genomic evidence for reproductive barriers suggests that our use of the species category in oaks is both meaningful and based on an integrative approach using multiple criteria [
48]. Where studies have found difficulty distinguishing morphological and ecological species using molecular data (e.g., [
57,
58]), returning with higher numbers of markers generally recovers clusters that cleanly separate the species (e.g., [
43,
59]). This suggests that there may be genomic heterogeneity in
where and
how much of the genome is shared within species vs. among species, even if the regions of the genome that distinguish recently diverged oak species may be few and far between [
60] and interspersed with regions that exchange relatively freely between species. At the same time, it gives us good reason to expect that species we are able to recognize ecologically and morphologically will also cohere genomically, so long as we look closely enough.
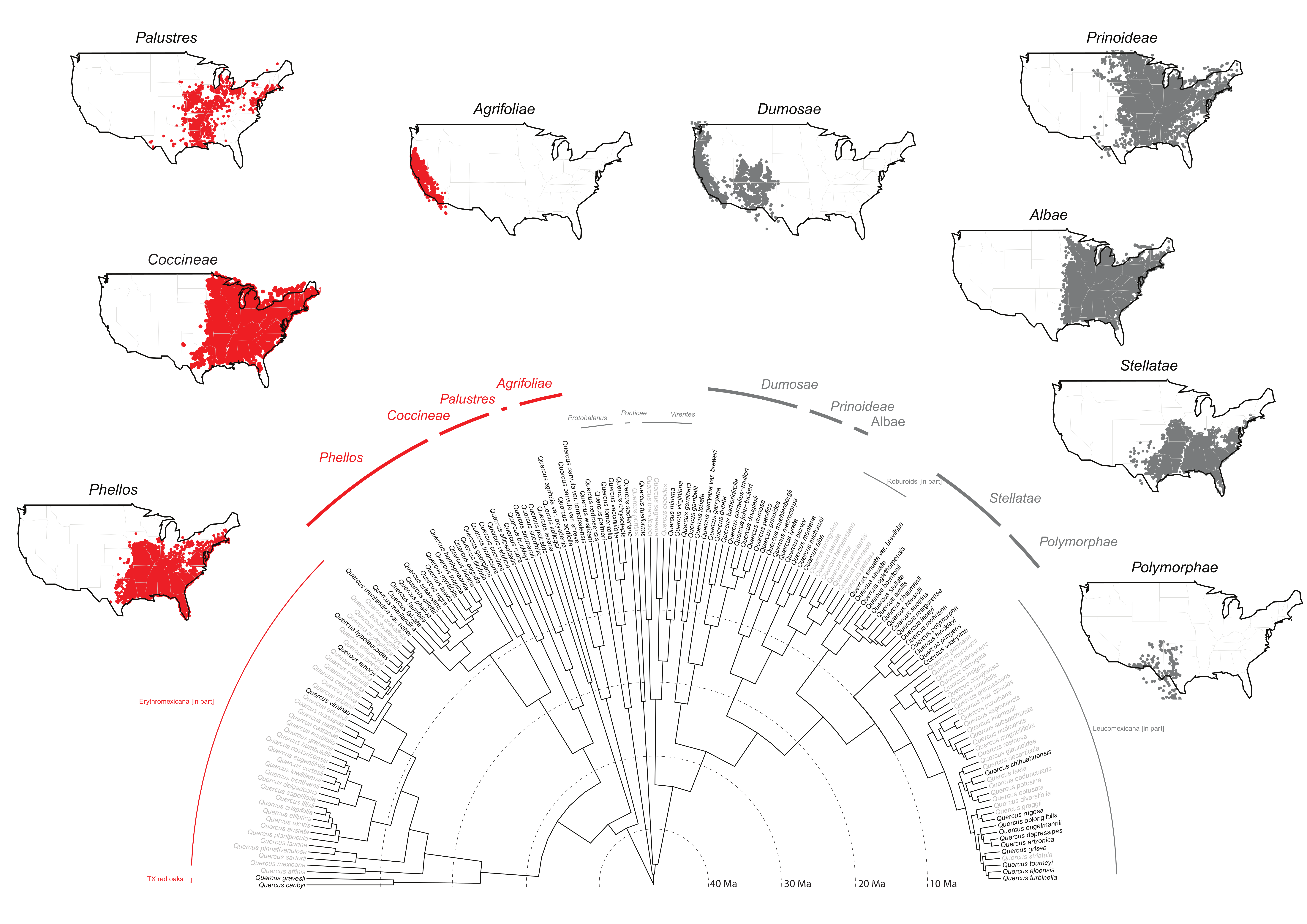
Moreover, in spite of taxonomic difficulties at the species level, our RAD-seq work already cited and ongoing work based on sequence-capture data [
37,
61] strongly support the clades we recognize as subsections in the current work (
Figure 2). Given our sampling to date and the strong phylogenetic support, the timing is reasonable to recognize subsections for the FNA region. To complete the American oak clade (
Quercus subg.
Quercus), better sampling of the Mexican and Central American taxa is needed and ongoing.
3.4. Section Lobatae–(37/ca. 120)
Lobatae occupy a range of habitats throughout the Americas, with just one species,
Q. humboldtii Bonpl., extending into northern Colombia [
83,
84]; this is the only oak species of any section to range into South America, where it is ecologically important in a diverse array of forest types [
85,
86].
Lobatae are defined by long styles encircled at their base by a perigon, a skirt-like perianth structure [
3,
87]. Most
Lobatae species have biennial fruit, but three unrelated species within the FNA region bear annual fruit (
Q. agrifolia, Q. emoryi, and Q. elliottii), and a fourth unrelated species,
Q. hypoleucoides, is polymorphic [
88].
The earliest branch within
Lobatae is a clade we treat here as subsect.
Agrifoliae, distributed largely within the CA-FP. It is sister to a succession of subclades distributed from eastern North America to well south of the FNA region. Four of these clades are densely sampled in our phylogenetic work and treated below as subsects., but a fifth group within the FNA region, informally known as the Texas red oaks (e.g.,
Q. canbyi Cory and Parks =
Q. graciliformis C.H. Muller; =
Q. gravesii Sudw.), may or may not contain other rare, unsampled species that occur in Texas (e.g.,
Q. rubusta C.H. Muller and
Q. tardifolia C.H. Muller). The placement of this group between the grade formed by temperate subsects. and the large clade formed by all other
Lobatae south of the FNA region seems to geographically parallel the white oak biogeographic patterns (
Figure 2). However, additional studies are needed to evaluate the possibility that the taxonomic complexities in the Trans-Pecos Region represent relictual hybrid populations or rare taxa with relationships to species within subsect.
Coccineae or to Mexican taxa [
1,
72,
89,
90]. With our limited sample, the Texas Red oak group is the provisional sister to the clade formed by all other
Lobatae south of the FNA region, informally called
Erythromexicana, whose distributions suggest at least two major hotspots of species richness: The Northern Sierra Madre Orientale and Serranías Meridionales of Jalisco [
91]. Three additional species within the FNA region,
Q. emoryi Torr.,
Q. hypoleucoides A. Camus, and
Q. viminea Trel., are clearly related to three different subclades within
Erythromexicana.
Reconstruction of the timing and biogeography of the
Lobatae phylogeny suggests an initial diversification of red oaks within the FNA region, followed by movement south into Mexico about 15 million years ago, associated with an increased net diversification rate most likely attributable to increased speciation rate [
19]. This increase in species diversification rate was likely a response to the combination of tectonic movement, volcanism, and climatic oscillations that generated a mosaic of seasonal montane habitats in Mexico [
92,
93]. One major barrier to the
Lobatae diversification was the Nicaraguan Depression [
93]. As with the parallel and equally rapid diversification of white oaks, species diversity is considerably lower southward to Costa Rica.
In this subsectional treatment, we re-classify a total of 29 species of sect. Lobatae recovered in our global analysis, and resolved with high confidence into four clades.
3.4.1. Quercus subsect. Agrifoliae (Trel.) A. Camus, Monogr. Genre Quercus 3: 46. 1952
Q. subsect. Californicae A. Camus, Monogr. Genre Quercus 3: 426. 1952.
Shrubs and trees to 30 m; Bark gray, dark brown to black, smooth or with wrinkled rings perpendicular to trunk axis or shallowly sinuous-furrowed to deeply furrowed, occasionally cross-checked into islands; Twigs slender, pubescent to glabrous; Buds light brown to reddish brown, ovoid to conic 3–9 mm; Leaves deciduous to evergreen, petiole 2–40 mm; Leaf blade circular to elliptic, ovate, obovate to oblong or narrowly lanceolate; base obtuse, rounded to cordate; margin entire or spinose with up to 30 awns or with 7–11 acute lobes and 13–30 awns; apex acute, attenuate, blunt to rounded; surfaces abaxially glabrous to densely pubescent with multiradiate trichomes or with small axillary tufts of 4–8 rayed fasciculate trichomes; Acorns biennial or annual, cup thin to thick, sometimes proximally tuberculate, deeply and narrowly cup-shaped, turbinate or U-shaped to deeply bowl-shaped, scales sometimes obtuse proximally to more acute distally, tips ragged or loose especially along distal cup edge, nut ovoid, oblong, conic to broadly ellipsoid; cotyledons distinct.
Distribution: California, north to OR, south to Mexico (B.C.) (see
Figure 2)
Includes:Q. agrifolia Née (the type), Q. kelloggii Newb., * Q. parvula Greene, * Q. wislizeni A.DC.
The distinct nature of the red oaks of the CA-FP was recognized by Trelease, who viewed them as an isolated lineage and treated them as series
Agrifoliae (pages 205, 206 in [
70]). His treatment was mostly followed by Camus, although she classified the deciduous species
Q. kelloggii into a monotypic subsection. Despite the contrast in leaf habit, species of
Agrifoliae share a suite of diagnostic features including a deep cup and oblong nut and with cup scale tips generally appearing ragged or loose and occasionally inrolled.
Agrifoliae have been the subject of recent morphometric and genetic studies, resulting in intense scrutiny of the eleven named taxa, which include four species, four varieties, and three named hybrids [
22,
47,
94]. These studies all demonstrate that the lobe-leaved deciduous species
Q. kelloggii is sister to the remaining subevergreen species, in support of Camus’ recognition that it is taxonomically distinct. Tests for introgression based on the molecular genetic data also demonstrate that
Q. kelloggii hybridizes with every species in the complex, but there is yet no evidence that F
1 hybrids backcross to parental species in nature [
23,
95]. This potential barrier to introgression, with F
1 hybrids an apparent dead-end between
Q. kelloggii and the remainder of the section, is of broad interest: Hybridization between oak species with distinctly different leaf habits is striking on its own [
96,
97], and biologically, the limits on introgression within any oak section is poorly understood. We also note that the divergence time estimate between
Q. kelloggii and the remainder of the subsection is roughly 20 Ma, among the oldest splitting times inferred within crown groups among the major oak groups within subg.
Quercus. The deep divergence separating
Q. kelloggii from the other California red oaks may contribute to the maintenance of reproductive isolation in the subsection. Nonetheless, at finer phylogenetic scales, lingering taxonomic problems at the varietal level in
Agrifoliae appear to be mainly due to hybridization (see references above).
Within the subevergreen complex,
Q. agrifolia is sister to
Q. parvula +
Q. wislizeni. Within each of these species, recognized varieties correspond to north-south extremes in species distribution and values in sets of continuous morphological traits [
94]. For the coastally distributed
Q. parvula, there is strong support for var.
shrevei (C.H. Muller) Nixon as the only valid variety whereas both var.
tamalpaisensis and var.
parvula have complex genetic backgrounds that likely involve gene flow from different combinations of subevergreen species [
47]. While more study is needed, our findings indicate that the narrowly endemic
Q. parvula var.
tamalpaisensis S.K. Langer represents a hybrid population that is roughly 60%
Q. wislizeni and 40%
Q. parvula var.
shrevei. In this case, leaf size is notably larger in the introgressed populations, well outside the range of the parental species [
23].
Disentangling
Q. parvula var.
shrevei from
Q. wislizeni demonstrates that two main taxa span coastal and interior areas of the CA-FP. Molecular dating analysis [
23] is consistent with the hypothesis proposed by Axelrod in 1983 [
98] that
Q. parvula and
Q. wislizeni split from a common ancestor approximately 10–12 Ma. The fossil record shows that the
Q. wislizeni +
Q. parvula clade likely originated within what is now the Great Basin, east of the Sierra Nevada [
99,
100], with the lineage that would become modern
Q. parvula moving westward in response to the drying climate following uplift of the Sierra Nevada.
Quercus wislizeni is the more drought-tolerant taxon that also later moved west, following the westward movement of
Q. parvula. As the climate continued to dry in the region,
Q. wislizeni continued to expand westward with
Q. parvula contracting even farther west, eventually resulting in its current coastal distribution.
One other coastal red oak,
Q. agrifolia, is well supported except that the southernly distributed var.
oxyadenia (Torr.) J.T. Howell, while morphologically and elevationally distinct (>750 m), is weakly supported by molecular data [
23,
95]. In this case, it’s possible that the var.
oxyadenia morphology has arisen multiple times in response to climatological differences in the drier, higher elevation and more southerly environments in which it is found. Future studies should include disjunct Baja populations of
Q. agrifolia to test the hypothesis that a distinct genetic entity with the morphology of var.
oxyadenia inhabits the more isolated areas farther south, and that widespread interbreeding between the var.
agrifolia and var.
oxyadenia forms has confounded that distinction just north within the FNA region.
3.4.2. Quercus subsect. Palustres (Trel.) A. Camus, Monogr. Genre Quercus 3: 360. 1952
Large trees to 25 m; Bark gray, brown, with flat ridges and shallow to broad fissures; Twigs slender, glabrous; Buds gray to reddish brown, ovoid 3–7 mm; Leaves deciduous, petiole 20–60 mm; Leaf blade ovate, elliptic, obovate; base cuneate to truncate; margin with 5–11 lobes, and 9–30 awns, lobes acute to distally expanded, apex acute to acuminate; surfaces abaxially glabrous except for conspicuous axillary tufts of fasciculate trichomes; Acorns biennial, cup thin, scale bases visible on inner surface, saucer to deeply goblet shaped with minor to pronounced constriction at the base, scale tips appressed, nut globose to broadly ovoid; cotyledons distinct.
Distribution: Northern and Atlantic States, north to Canada (ONT), west to OK, KS, south to eastern TX, LA (see
Figure 2)
Includes:Q. palustris Muenchh. (the type), Q. texana Buckley
Palustres include two largely allopatric bottomland species with highly similar vegetative features that were recognized by Camus to be distinct among lobe-leaved red oaks within North America. However, both Camus and Trelease thought that
Q. palustris and
Q. georgiana were closely related, while our analyses place
Q. georgiana within subsect.
Phellos. Although apomorphies are not obvious, phenetic studies using morphological data from leaves and fruit closely clustered
Q. palustris and
Q. texana (=
Q. nuttallii E.J. Palmer) to the exclusion of other lobe-leaved species of
Lobatae [
101].
The first genetic result to suggest that at least
Q. palustris was divergent from other eastern North American lobe-leaved species was obtained by allozyme analyses [
102,
103]. Phylogenomic studies confirm the early genetic work and resolve
Palustres as the first branch among the eastern North American
Lobatae, sister to the remainder of the section. For the two taxa of
Palustres, dating analysis also shows a deep split, ca. 20 Ma, a pattern consistent with the branching structure within four of the five early-diverging red oak lineages within the FNA region.
3.4.3. Quercus subsect. Coccineae (Trel.) A. Camus, Monogr. Genre Quercus 3: 386. 1952
Q. subsect. Velutinae (Trel.) A. Camus, Monogr. Genre Quercus 3: 376. 1952.
Shrubs and medium to large trees to 30 m; Bark dark brown to black, gray-brown, furrowed, ridges wide, shiny or with scaly fissures; Twigs somewhat thick, glabrous or sparsely pubescent; Buds reddish brown, tawny, gray, or silver, conic to ovoid-ellipsoid 3–12 mm; Leaves deciduous, petiole 20–60 mm; Leaf blade circular, ovate, elliptic, ovate to obovate; base cuneate, obtuse to truncate; margin with 5–11 lobes, and 12–55 awns, lobes acute, ovate-oblong, to distally expanded, apex obtuse, acute, to acuminate; surfaces abaxially glabrous or pubescent, trichomes multiradiate or stipitate fasciculate in conspicuous axillary tufts; Acorns biennial, cup thin to thick, cup to saucer-shaped, hemispheric to turbinate, scales smooth to tuberculate, scale tips appressed to loose at the margin, nut ovoid, subglobose to oblong; cotyledons distinct.
Distribution: Northern and Atlantic States, north to Canada (N.B., N.S., ONT, QUE, P.E.I.), west to KS, OK, TX, south to FL (see
Figure 2)
Includes:Q. acerifolia (Palmer) Stoynoff and Hess, Q. buckleyi Nixon and Dorr, Q. coccinea Muenchh. (the type), Q. ellipsoidalis E.J. Hill, Q. rubra L., Q. shumardii Buckland, Q. velutina Lam.
The circumscription of this group of eastern North American lobe-leaved tree species by Trelease is nearly fully supported by our analyses. The few exceptions include
Q. velutina which had been either aligned with
Q. marilandica or treated as a monotypic [
72,
75,
90], and the inclusion of
Q. gravesii (=
Q. texana var.
chesosensis Sarg.) by Trelease, which we resolved as sister lineage to
Erythromexicana insofar as we have sampled it to date (see above).
Coccineae are defined by a certain phenetic similarity and regional proximity. One distinction shared by many of these species (e.g.,
Q. rubra, Q. shumardii) is wide geographic range, which promotes differentiation in morphological, physiological and ecological traits, and patterns of variation that have been variously treated as species and varieties [
104,
105]. We resolved two subclades that correspond to the main morphological groups that have been generally recognized: 1) (
Q. acerifolia—Q. buckleyi—Q. shumardii) +
Q. rubra); and 2) (
Q. velutina + Q. coccinea) +
Q. ellipsoidalis).
The first group includes the
Q. shumardii complex, a cluster of continuously varying morphological forms that have been recognized as varieties and species (e.g.,
Q. acerifolia) [
103]. Edaphic specialization appears to be an important driver of differentiation within this complex as
Q. buckleyi (on limestone in TX and OK),
Q. shumardii var.
schneckii (Britton) Sarg. (uplands west of the Blue Ridge), and
Q. acerifolia (on sandstone, shales) are all endemic to the south-central Midwest. Additional range extensions of
Q. acerifolia-like populations on limestone into east central Alabama are likely (W. Finch, pers. comm.), and fine-scale molecular and morphological studies of the complex are underway (Y. Wu, pers. comm.). The taxonomic history of
Q. rubra involves an intraspecific taxon often associated with the higher elevations in the Appalachians, ca. above 1000 m (e.g., var.
ambigua (A. Gray) Fernald = var.
borealis (Michx. f.) Farw.). These populations have been quantitatively distinguished by phenetic analysis of morphometric data [
101] and by flavonoid profiles [
106], but intergradation between the two morphotypes precludes taxonomic recognition [
107]. Additional work is needed to better understand the nature of this clinal pattern of variation.
The second group within subsect.
Coccineae includes two widespread species,
Q. coccinea and
Q. velutina, and the north-central endemic
Q. ellipsoidalis [
108,
109], which has the distinction of being our northernmost member of sect.
Lobatae [
110,
111] and, historically, a locus of substantial taxonomic confusion in the upper Midwest (e.g., [
111,
112,
113,
114,
115,
116,
117]). The question of whether
Q. ellipsoidalis is distinct from
Q. coccinea has been studied with AFLP markers [
43,
118], microsatellites [
59], and RAD-seq data [
15] using population-level sampling, including many of the morphologically similar red oaks (including
Q. palustris) that co-occur in the northern part of the FNA region. There is strong evidence for recognizing
Q. ellipsoidalis as a distinct species based on this fairly broad sampling. Further, there is little evidence for gene flow between
Q. ellipsoidalis and
Q. coccinea, which resolve as largely allopatric sister species, but a low level of ongoing gene flow between
Q. ellipsoidalis and both
Q. velutina and
Q. rubra [
43,
119,
120,
121]. The more distantly related
Q. rubra and
Q. ellipsoidalis also show evidence of genomically heterogeneous introgression driven by selection: alleles that distinguish the two species at a gene associated with flowering phenology segregate along moisture gradients and are preferentially shared between species in intermediate environments [
122,
123]. Genomic study of this species is underway (O. Gailing, pers. comm.). This western Great Lakes species may turn out to be a gateway to broader understanding of speciation in red oaks.
3.4.4. Quercus subsect. Phellos (G. Don) A. Camus, Monogr. Genre Quercus 3: 293. 1952
Q. subsect. Myrtifoliae (Trel.) A. Camus, Monogr. Genre Quercus 3: 280. 1952; Q. subsect. Nigrae (G.Don) A. Camus, Monogr. Genre Quercus 3: 322. 1952; Q. subsect. Marilandicae (Trel.) A. Camus, Monogr. Genre Quercus 3: 329. 1952; Q. subsect. Laevis A. Camus, Monogr. Genre Quercus 3: 338. 1952; Q. subsect. Pagodifoliae (Trel.) A. Camus (as Pagodaefoliae), Monogr. Genre Quercus 3: 343. 1952; Q. subsect. Ilicifoliae (Oersted) Trel. A. Camus, Monogr. Genre Quercus 3: 353. 1952.
Shrubs or large trees to 40 m; Bark hard, black, roughly furrowed separated by smooth furrows or with rectangular blocks; Twigs slender to thick, glabrous or glabrescent or pubescent; Buds light brown, reddish-brown to purplish, conic to ovoid 2–10 mm; Leaves deciduous or subevergreen, petiole 1.5–60 mm; Leaf blade elliptic, ovate, obovate, circular to rhombic; base cuneate, rounded or cordate; margin entire, revolute or with 3–11 lobes, and 1–25 awns, lobes acute, attenuate to falcate; surfaces abaxially glabrous to tomentulous to uniformly pubescent, trichomes multiradiate, rosulate or stipitate fasciculate in axillary tufts; Acorns biennial and annual, cup thin to involute, shallow goblet-shaped, saucer to cup-shaped, scale tips appressed to loose at the margin, nut ovoid to subglobose; cotyledons distinct.
Distribution: Atlantic and Gulf region States, north to IA, MN, west to OK, TX (see
Figure 2)
Includes: Q. arkansana Sarg., * Q. elliottii Wilbur, Q. falcata Michx., Q. georgiana M. A. Curtis, Q. hemisphaerica Bartram ex Willd., * Q. ilicifolia Wangen., Q. imbricaria Michx., Q. incana Bartram, * Q. inopina Ashe, Q. laevis Walter, Q. laurifolia Michx., Q. marilandica Muenchh.,*Q. myrtifolia Willd., Q. nigra L., Q. pagoda Raf., Q. phellos L. (the type)
The center of species diversity for
Phellos falls within the North Atlantic Coastal Plain (NACP), a recently recognized global biodiversity hotspot [
124]. The combined distributions of eleven of the 16 species conform to the broad outline of the NACP, while the ranges of three of the 11 species,
Q. hemisphaerica, Q. phellos and
Q. nigra, have expanded from the southeastern coastal plain into adjacent piedmont, likely due to a combination of ecological disturbance and escape from cultivation [
107].
Resolution of these 16 species aligns a heterogeneous and variously treated set of species into subsect.
Phellos, making it the largest subsection within the FNA region. It is clear from previous treatments that a subset of this diversity was recognized as a natural group based on sharing the non-lobed laurel-like leaf type, small acorns, cups covering less than half of the nut, with scales closely appressed (subsect.
Phellos sensu Camus:
Q. imbricaria, Q. incana, Q. laurifolia, Q. phellos, Q. pumila Walter (=
Q. elliottii). Other potentially related but phenetically dissimilar taxa with lobed leaves (e.g.,
Q. falcata, Q. ilicifolia, Q. marilandica) instead were segregated into series and subsects, often consisting of one or two species ([
72,
75]; see [
125] for review).
Our results are generally consistent with more recent studies of flavonoid profiles and leaf architecture across these taxa: the systematics of the eight species treated by Trelease and later by Camus within their concepts
Laurifoliae,
Marilandicae, and
Nigrae cannot be divorced from other taxa within the southeastern FNA region, e.g.,
Q. laevis,
Q. myrtifolia, and
Q. inopina [
125]. The suggestion of a hybrid origin for many of these taxa [
125] does not appear to be supported by phylogenomic study [
15,
19], which clearly resolves these species, with the caveat that this radiation arose relatively recently, within the last 10 Ma. More intensive and targeted sampling is generally needed to test specific cases of reticulate evolution, and this clade has yet to be investigated so closely.
Based on population level sampling, we found that several of the lobe-leaved species show a close relationship to species with unlobed leaves. Additionally, although the five subclades resolved in our analyses are not strongly supported, the following preliminary patterns of species relationship suggest that diversification may be traced along several underlying ecological trajectories. For example, the group formed by (
Q. hemisphaerica—Q. incana—Q. myrtifolia—Q. inopina) share unlobed leaves and occupy core NACP habitats of mostly sandhills; by contrast, the group formed by (
Q. ilicifolia—Q. georgiana—Q. imbricaria) tend to occur in upland areas outside of the NACP in various habitats and substrates, including ravines and granitic outcrops, except that Q.
ilicifolia ranges onto the northern edge of the NACP from New Jersey to Maine. Two other sets of species span sandhills to bottomlands. The larger of the two comprises (
Q. elliottii—Q. pagoda—Q. phellos—Q. laurifolia—Q. nigra—Q. arkansana), which includes only three taxa that Camus placed with her concept of
Phellos. A close relationship between
Q. arkansana and
Q. nigra is supported here [
125]. The final group ((
Q. laevis + Q. marilandica)
—Q. falcata) tends to occupy sandy well-drained poor soils spanning coastal areas to adjacent uplands. Two of the tree species often resolved as sister taxa,
Q. laevis and
Q. marilandica, share a combination of bark, twig, and foliar characteristics. We also sampled
Q. marilandica var.
ashei Sudw. from the type location (Scott Mt.) in the Cross Timbers Region of Oklahoma. The placement of two accessions as sister to var.
marilandica supports previous evidence to recognize this taxon as distinct [
88].
3.5. Section Quercus–(45/ca. 150)
The white oaks stand out as the most species rich and most broadly distributed of all oak clades (
Figure 1). The center of species diversity, ca. 100 spp., occurs in Mexico [
84], but expansion of white oaks into the driest shrublands of the southwest (e.g.,
Q. turbinella, Q. cornelius-mulleri) and Intermountain west (
Q. gambelii), and their extension into the coldest woodlands of the northern Midwest (
Q. macrocarpa) demonstrate a physiological breadth not seen in the
Lobatae [
17]. Although white oaks share diagnostic features such as basal abortive ovules, glabrous endocarp, and annual fruit maturation with sects.
Virentes and
Ponticae, they are easily distinguished from
Virentes and to a lesser extent
Ponticae [
3].
As with the
Lobatae, our analyses reveal that the earliest branch of white oaks, which we treat here as subsect.
Dumosae, is distributed largely within the CA-FP. It is sister to two major clades: One comprises three clades of mostly north temperate species that range from eastern North America to Eurasia, the other, a succession of three clades: the predominantly eastern subsect.
Stellatae; a predominantly Texan group we treat here as subsect.
Polymorphae; and the more recently derived Mexican and Central American clade, informally called
Leucomexicana pending more detailed study. For the FNA region, we sampled every species except for
Q. intricata Trel. (Trans-Pecos Region; Chihuahuan Desert Region) and
Q. carmenensis C.H. Muller (Chisos Mts, Sierra del Carmen Region, Coahuila, Mexico); both of these unsampled species are likely to be placed into the groups we have recognized in this treatment. We also place ten additional FNA species distributed along the southwestern edge of the FNA region into at least three subclades within
Leucomexicana. These species, a mix of shrubs and trees ranging from open chaparral, woodlands, and slopes of the Chihuahuan Desert Region west to the southern CA-FP, had been placed into many different series and subsections by Trelease and Camus. While additional sampling is needed for formal recognition, eight of these species, including
Q. engelmannii from the CA-FP [
35,
126,
127] and
Q. turbinella from the Sonoran Desert, fall into one clade, suggesting a distinct east-west regional radiation that straddles the southwestern border of the FNA region that extends into Mexico.
Where the ranges of distantly related white oaks narrowly intersect, species have formed hybrid zones in areas of sympatry. One case study includes the tree species
Q. engelmannii and two shrub species,
Q. cornelius-mulleri and
Q. berberidifolia, both placed here in subsect.
Dumosae. Morphological observations of hybridization remain anecdotal, but recent molecular analyses using phylogenetic methods to estimate whether allele sharing is due to gene flow conclude that ancient introgression had shuttled alleles from
Q. cornelius-mulleri into
Q. engelmannii [
127]. In another case study,
Q. gambelli, the only white oak of the Intermountain west, and here placed in subsect.
Dumosae (see below), has long been recognized for hybridizing with white oak species of at least four distinct subclades that we recognize, including
Q. turbinella and
Q. grisea of
Leucomexicana (e.g., [
128,
129]) and
Q. macrocarpa of subsect.
Prinoideae [
130]. While recent genetic analyses show that morphologically typical
Q. gambelii is genetically homogeneous (samples from AZ, CO, NM, UT), there is strong evidence that ancient introgression with
Q. macrocarpa causes it to be unstable in phylogenomic analyses [
25]. The fact that secondary contact zones exist between well separated white oak lineages makes them ideal case studies for ancient introgression and the development of more complete understanding of the nature of the mosaic structure of the oak genome [
15,
127,
131].
Reconstruction of the timing and biogeography of the
Quercus phylogeny also suggests an initial diversification of white oaks within the FNA region, followed by simultaneous movement south in a pattern closely parallel to
Lobatae, then across the entire geographic range of Eurasia. The direction of movement, i.e., west through the Beringian Land Bridge or east through the North Atlantic Land Bridges, into Eurasia remains ambiguous, however, recent analyses based on whole plastome genome data also suggest a clear origin of the roburoids within the Americas (B. Wang, P.S. Manos et al. in prep.). The two relatively young and nearly contemporaneous radiations,
Leucomexicana and roburoids, starting about 15 Ma [
15], together account for roughly 75% of the diversity of the section (see
Figure 2).
In this subsectional treatment, we re-classify a total of 33 species of sect. Quercus recovered in our global analysis, and resolved with high confidence into five clades.
3.5.1. Quercus subsect. Dumosae (Trel.) A. Camus, Monogr. Genre Quercus 2: 462. 1936
Q. subsect. Douglasieae (Trel.) Camus, Monogr. Genre Quercus 3: 666. 1936; Q. subsect. Lobatae (Trel.) Camus, Monogr. Genre Quercus 3: 681. 1936; Q. subsect. Gambelieae (Trel.) Camus, Monogr. Genre Quercus 3: 694. 1936.
Shrubs, small to large trees to 30 m; Bark light to dark gray, dark brown, scaly or deeply checkered in age; Twigs slender, yellowish, gray, brown or reddish brown, densely puberulent to tomentulose with spreading hairs to glabrate; Buds yellowish, brown to reddish brown, ovate, globose, ovoid to fusiform, 1–12 mm; Leaves deciduous or subevergreen, petiole 1–20 mm; Leaf blade broadly obovate, elliptic, subrotund to ovate or oblong to oblanceolate, deeply to shallowly 4–8 lobed; base truncate, rounded-attenuate, cuneate or cordate; margins entire, irregularly shallowly toothed, coarsely toothed to spinose-toothed or lobed, lobes oblong to spatulate, obtuse, rounded, subacute or blunt; surfaces abaxially grayish to whitish, dull green, yellowish to light green, blue-green to glaucous, waxy or glossy, densely velvety with erect 4–8 rayed fasciculate trichomes or dense to sparsely appressed to semi-erect 8–12 rayed stellate trichomes, sometimes with interlocking or fused rays, becoming glabrous; Acorns annual, 1–3 subsessile or pedunculate, cup saucer-shaped, hemispheric, deeply cup-shaped or turbinate, base flat or rounded, scales closely appressed, ovate, weakly to strongly tuberculate, scale tips acute, mostly free often reflexed, nut conic, ovoid to globose, ellipsoid, oblong to fusiform, cylindric or barrel-shaped; cotyledons distinct.
Distribution: Pacific States, north to Canada (B.C.), south to Mexico (B.C.) (see
Figure 2)
Includes: * Q. berberidifolia Liebm., * Q. cornelius-mulleri Nixon and K. P. Steele, Q. douglasii Hook. and Arn., * Q. dumosa Nutt. (the type), * Q. durata Jepson, * Q. gambelii Nutt., Q. garryana Douglas ex Hook, * Q. john-tuckeri Nixon and C. H. Muller, Q. lobata Née, * Q. pacifica Nixon and C. H. Muller
Dumosae form the sister group to all white oaks, diverging by at least 40 Ma likely from within the FNA region. Two clades of basically sclerophyllous shrubby species are nested within an early-branching grade of the two deciduous lobe-leaved species,
Q. lobata and
Q. garryana [
27]. Fossil leaf compressions attributed to precursors of
Q. lobata (e.g.,
Q. prelobata) occur well north of the current distribution, indicating a broader ancestral distribution of the stem group going back to the early Miocene, 16–23.5 Ma [
99,
100].
In addition to the nine
Dumosae taxa that occur in the Pacific States, we expand the subsection to include
Q. gambelii, consistent with the high degree of leaf and trichome similarity between
Q. gambelii and
Q. garryana sensu lato [
132].
Quercus gambelii is a curiously widespread species that consists of many isolated but weakly differentiated populations [
77]. It was the eponymous center of the 11-species Treleasian series
Gambelieae, but all those taxa have subsequently been subsumed into
Q. gambelii or are otherwise not recognized. It was never classified with other lobed-leaved species [
72,
74,
90]. Its uncertain systematic affinities may have been partly a consequence of its isolated geography. In the northern part of its range, it is often the only oak species within the Intermountain west. But in the southwest,
Q. gambelii is surrounded by and often sympatric with multiple species representing at least four subsects. of white oaks, resulting in a rich taxonomic and ecological literature describing patterns of natural hybridization [
128,
129,
133,
134].
However, its uncertain position may be due to its cryptic morphology, a lobedness that may derive in part from an ancient history of introgression with eastern lobed white oaks. Our phylogenomic studies detected a primary signal of phylogenetic history between
Q. gambelii and
Q. lobata as the branches leading to accessions of both species formed a clade [
25]. The position of this clade was determined to be unstable in the phylogenetic space between
Dumosae and
Prinoideae. Our work, based on tests designed to detect secondary phylogenetic signals due to introgression, suggests that ancient hybridization between
Q. gambelii and
Q. macrocarpa is the likely cause of phylogenetic uncertainty in the placement of this species. Our inference of historical rather than contemporary gene flow is further supported by the fact that
Q. gambelii,
Q. macrocarpa, and
Q. lobata are strongly monophyletic across analyses. If ongoing gene flow were the cause of phylogenetic instability, we would expect some individuals of these species to switch positions between species or become dragged to phylogenetically intermediate positions themselves. Instead, all individuals of these species form separate clades by species.
Thus
Q. gambelii seems to have arisen from allopatric speciation within the formerly widespread range of
Q. lobata or the ancestral complex formed by
Q. lobata and the named varieties of
Q. garryana [
20,
25]. In this scenario, uplift of the Sierra Nevada, increasing aridity, and other developing north-south barriers isolated populations of ancestral
Q. gambelii during the Miocene. The aridity of the Great Basin would have forced the nascent species eastward, into mesic woodlands, and to the southeast, along the foothills of the Rocky Mountains into its current elevational range of 1000 to 3000 m. While
Q. gambelii does not currently overlap in range with
Q. lobata,
Q. garryana, or
Q. macrocarpa, periods of range expansion during warm and wet periods over the last 5 million years likely generated extensive secondary contact zones between
Q. gambelii and
Q. macrocarpa [
99]. In addition to our genetic results, support for historical range extension and hybridization comes from morphological evidence observed in outlier populations of
Q. gambelii in the Black Hills of South Dakota and
Q. macrocarpa in northeastern New Mexico [
130].
The main diversification within
Dumosae involves an array of mostly shrub species, including
Q. pacifica, the second taxon endemic to the California Channel Islands, that have vexed systematists for well over a century [
20,
132,
135]. It was suggested that all of the California shrub species, except for
Q. turbinella are derived from a lobe-leaved ancestor similar to
Q. lobata. Nixon [
122] outlined anatomical evidence for this relationship, noting the venation pattern shared between sclerophyllous shrub species and lobe-leaved species, specifically branched secondary veins, multiple and irregularly spaced teeth, and an often coarse pattern of lobation. This pattern contrasts with the condition of secondary veins that rarely branch, generally resulting in one tooth per secondary vein and regularly spaced teeth, like those of
Q. turbinella and other shrub species in the southwestern FNA region and Mexico. Our analyses confirm this hypothesis, while in-depth studies have generated additional patterns of species relationships within the shrub diversity of
Dumosae.
By ca. 15 Ma, the nested crown lineage of
Dumosae diverged into two clades, one clade comprising
Q. pacifica—
Q. dumosa and a distinct well supported subclade of (
Q. cornelius-mulleri- (
Q. john-tuckeri +
Q. douglasii)), and the other clade formed by a grade of population samples representing the widespread
Q. berberidifolia, within which are nested two separate subclades of
Q. durata var.
durata and var.
gabrielensis Nixon and C.H. Muller [
27,
35,
127]. This overall pattern and taxonomy are consistent with previous morphological work that segregated the narrow endemic
Q. dumosa from
Q. berberidifolia and recognized the parapatrically distributed
Q. cornelius-mulleri and
Q. john-tuckeri to be distinct from previous taxonomic treatments (see [
132] for review). The nested position of the tree species
Q. douglasii within the shrub clade formed by
Q. cornelius-mulleri and
Q. john-tuckeri is an interesting example of an evolutionary reversal back to the tree habit. The timing of divergence of the nested crown clade of shrubby species, from
Q. pacifica through the
Q. berberidifolia clade, is consistent with the hypothesis of Kim et al. (2018) [
127]: Diversification of the shrub species coincides with mid-Miocene development of the Mediterranean climate in the CA-FP [
136]. The onset of this climate regime would have increased the frequency of fire, potentially favoring shrubs over trees. The evolution of
Q. douglasii, a tree with shrub-like traits, may have been enabled by ecological opportunity in the relatively mesic foothills of the Sierra Nevada.
Recent demographic studies of gene flow among different combinations of shrub species suggest that hybridization continues to promote taxonomic uncertainty while ecology and climate reinforce genomic cohesiveness and identity [
35,
127,
137]. The full complement of genetic analyses confirms that most of the morphologically-defined species of
Dumosae are cohesive and often ecologically isolated to some degree. One notable exception stands out from studies of the
Q. berberidifolia—
Q. durata complex, which have revealed the most challenging case to date.
Quercus durata appears to have arisen twice from within
Q. berberidifolia, despite the generally clear taxonomic distinctions between
Q. berberidifolia and
Q. durata. While
Q durata var.
durata is well separated, albeit nested, within
Q. berberidifolia, var.
gabrielensis is only weakly distinguished [
27,
127]. This distinction likely involves ecological isolation, as
Q. durata var.
durata mostly occurs on serpentine soils. Such cases may be more widespread in oaks than we suspect and bear closer investigation.
3.5.2. Quercus subsect. Prinoideae (Trel.) A. Camus, Monogr. Genre Quercus 2: 434. 1936
Q. subsect. Lyratae (Oersted) A. Camus, Monogr. Genre Quercus 2: 723. 1936; Q. subsect. Macrocarpae (Trel.) A. Camus, Monogr. Genre Quercus 2: 738. 1936.
Shrubs or large trees to 30–50 m; Bark dark gray to light gray, thin flaky to papery, scaly, flat-ridged or with thick plates underlying scales; Twigs coarse, slender to moderate, gray, tan, reddish brown, fine-pubescent to villous becoming glabrate or with round to radiating flat corky wings; Buds gray, brown, red-brown, subrotund to broadly ovoid 1–4 mm; Leaves deciduous, deciduous or persistent stipules, petiole 4–30 mm; Leaf blade moderate to large, lanceolate, obovate to narrowly elliptic or broadly obovate; base rounded, truncate, cuneate to acute; margins regularly undulate, toothed or shallow-lobed to deeply lobed; surfaces abaxially glaucous to light green, tomentose, trichomes erect long-rayed fasciculate and small stellate; Acorns annual, 1–3 on thin to stout peduncle, cup hemispheric, cup-shaped, turbinate to goblet-shaped or spheroid, base rounded, scales closely appressed to laterally connate, moderately to prominently keeled, tuberculate or coarsely thickened and fringed at the margin with short awns, nut ovoid-ellipsoid or oblong; cotyledons distinct.
Distribution: Atlantic States and Mississippi Valley, north to Canada (MAN, N.B., ONT, QUE, SASK), south to the mountains of western TX, NM, and northeastern Mexico (COAH, N.L., HGO, TAMPS) (see
Figure 2)
Includes:Q. bicolor Willd, Q. lyrata Walter, Q. macrocarpa Michx., Q. muehlenbergii Engelm., * Q. prinomides Willd. (the type)
Prinoideae form the sister to a moderately sized temperate clade that spans eastern North America and Eurasia. The five species we recognize here share a common set of trichomes and similar cup scale morphology. Camus classified
Q. montana and
Q. michauxii in her concept of subsect.
Prinoideae, while recognizing
Q. lyrata and
Q. macrocarpa in separate monotypic groups. Our analyses placed the former species pair with
Q. alba, consistent with their lack of the trichome types observed across all
Prinoideae [
138,
139].
Extensive population sampling and analysis resolved a deep split within the crown group, ca. 20 Ma, pairing tree species
Q. muehlenbergii and shrub
Q. prinoides sister to a well-supported group of (
Q. macrocarpa- (
Q. lyrata +
Q. bicolor)). As with morphology, our data are unable to detect a clean separation between the widespread and ecologically distinct “species-pair,”
Q. muehlenbergii (limestone) and
Q. prinoides (sands, shales). Further studies are needed to understand the ecological processes behind the weak genetic separation of taxa, despite the heritable differences observed in
Q. prinoides, such as the shrub habit and early maturation to flower and fruit [
132]. It may be that the situation in
Q. muehlenbergii mirrors that of
Q. berberidifolia and
Q. durata, in which the same phenotype has arisen more than once due to parallel selective processes across the range of the more widespread species.
3.5.3. Quercus subsect. Albae (G. Don) A. Camus, Monogr. Genre Quercus 2: 728. 1936
Large trees to 30 m; Bark dark gray or brown with V-shaped furrows or light brown to gray, scaly; Twigs moderate, green, brown to reddish brown, glabrous or fine-pubescent to sparse spreading hairs, becoming glabrous; Buds gray, light brown, reddish-brown, ovoid 3–6 mm; Leaves deciduous, petiole 5–30 mm; Leaf blade moderate, broadly obovate to narrowly elliptic or narrowly obovate; base cuneate to acute or rounded acuminate to broadly cuneate; margins regularly toothed or moderately to deeply lobed; surfaces abaxially light green or yellowish; trichomes solitary, appressed, and erect or appressed, 1–2 rayed fasciculate, persistent to quickly shed; Acorns annual, 1–3 sessile or pedunculate, cup shallowly cup-shaped to hemispheric or deeply goblet shaped, base rounded, rim thin to moderate, scales concentric to laterally connate or loosely to closely appressed, slightly keeled to prominently tuberculate, nut ovoid-ellipsoid, cylindric or oblong; cotyledons distinct.
Distribution: Atlantic States and Mississippi Valley, north to Canada (ONT, QUE), south to eastern TX, northern FL (see
Figure 2)
Includes:Q. alba L. (the type), Q. michauxii Nutt., Q. montana Willd.
The three
Albae species are quite divergent with respect to morphology, especially features of the bark and cup scales. Although the leaves of
Q. alba are generally described as glabrous and frequently are at maturity [
130], fasciculate trichomes are present in the early stages of leaf development, and simple trichomes may persist as the leaf expands.
Quercus michauxii and
Q. montana also share fasciculate trichomes, but these are persistent, and erect with long rays in the former and appressed with short rays in the latter.
Albae, as delimited here, lack the stellate trichomes observed in
Prinoideae [
138,
139]. Our analyses break-up the so-called chestnut group of the FNA region (
Q. michauxii, Q. montana, Q. muhlenbergii,
Q. prinoides, Q. sadleriana), leading to the distribution species with the ‘prinoid’ or ‘castaneoid’ leaf shape into three distinct temperate clades of white oaks:
Albae, Ponticae, and
Prinoideae.
Albae combine morphological elements of the prinoids and roburoids to form one of the most genetically cohesive oak subclades of the FNA region. The relationships among the three species (
Q. montana—(
Q. michauxii +
Q. alba)) are well supported and based on strong sampling. The sister group relationship of
Albae to the roburoids extends the signature pattern of early-diverging deciduous shallowly and deeply lobe-leaved species from the FNA region to Eurasia. This biogeographic connection has been difficult to estimate phylogenetically as a result of ancient hybridization between the
Q. pontica sublineage of
Ponticae and ancestral roburoids in Eurasia [
24].
3.5.4. Quercus subsect. Stellatae (Trel.) A. Camus, Monogr. Genre Quercus 2: 710. 1936
Q. subsect Confusae (Trel.) A. Camus, Monogr. Genre Quercus 2: 671. 1936; Q. subsect Durandieae (Trel.) A. Camus, Monogr. Genre Quercus 2: 676. 1936.
Shrubs, often rhizomatous, moderate to large trees to 30 m; Bark light gray or whitish, light brown, scaly or flaky to papery and exfoliating; Twigs slender to moderately thick,, densely tomentose, sparsely pubescent, glabrate; Buds gray to reddish-brown, ovoid to globose to acute at the apex, 1–6 mm; Leaves deciduous to wintergreen, petiole 1–15 mm; Leaf blade small to moderately large, obovate to narrowly elliptic, oblong to oblanceolate or rounded 3-dentate to obtriangular; base rounded-attenuate, acute to cordate, cuneate or obtuse; margins undulated, revolute, entire or shallow, moderate to deeply lobed, 3–5 lobes rounded, spatulate or sinuate; surfaces abaxially silvery, grayish, dull to yellowish green or dark green, trichomes dense to scattered, 3–10-rayed stipitate fasciculate, stellate, often becoming glabrate; Acorns annual, 1–3 subsessile or pedunculate, cup thin, deeply cup-shaped, goblet-shaped, turbinate, hemispheric, base rounded or constricted, cup scales gray to brown, thin, tightly appressed, flattened to only slightly tuberculate, scale tips acute, nut ovoid, globose, barrel-shaped to elliptic; cotyledons distinct.
Distribution: Atlantic States west to eastern NM, north to southern IA, southeastern NY (see
Figure 2).
Includes:Q. austrina Small, * Q. boyntonii Beadle, * Q. chapmanii Sarg., * Q. havardii Rydberg, Q. margarettae Ashe, Q. oglethorpensis W.H. Duncan, Q. similis Ashe, * Q. sinuata Walter, Q. stellata Wangenh. (the type)
Stellatae comprise a balanced mix of trees of various size and low-growing shrubs that occupy mainly lowlands including deep sands, alluvial flatwoods, and bottomlands. Species of this subsection are more or less distributed in the southeast of the FNA region, strongly overlapping in range and habitat type with species of the red oak clade Phellos. The placement of Q. havardii within this subsection expands the range farther west to eastern New Mexico.
In addition to the five species Camus recognized in her subsect.
Stellatae (
Q. boyntonii, Q. chapmanii, Q. margarettae, Q. similis, Q. stellata), our expanded delimitation of the group to nine species includes three species—
Q. austrina,
Q. havardii [
66], and
Q. sinuata—that had been treated in small subsections of one or two species each, and the unclassified and most recently described oak species east of the Mississippi,
Q. oglethorpensis. Duncan (1950) [
140] had suggested a close affinity of
Q. oglethorpensis to many of the species placed here based on similarities in cup scales, bark, and leaf epidermal features. Strong similarities in cup shape and well-defined cup scales that are thin, generally narrow, and flat and only slightly tuberculate also support the expansion of the historically recognized post oak subgroup sensu Camus to include four additional species.
Stellatae diversified within the last 15 Ma, around the same time as subsect. Phellos and clades within Leucomexicana. We resolved Q. sinuata and Q. oglethorpensis as early-branching species, consistent with Duncan’s (1950) view that both are somewhat isolated evolutionarily. The remaining seven species are resolved into three moderately supported subclades: Q. margarettae—Q. austrina; Q. chapmanii—Q. havardii; and Q. stellata, Q. similis, and Q. boyntonii. Additional population sampling, especially in the southern Gulf states and west into Texas, is needed to better understand the relationship of geography and potential hybridization to this initial estimate of phylogeny.
3.5.5. Quercus subsect. Polymorphae (Trel.) A. Camus, Monogr. Genre Quercus 2: 561. 1936
Shrubs, often rhizomatous, small to moderate trees to 20 m; Bark light gray to brown, papery, scaly or deeply furrowed, sometimes exfoliating in long strips; Twigs slender, gray, yellowish to light brown, reddish brown, pubescent to tomentose or soon glabrate; Buds gray, brown, reddish-brown, round to ovoid or with apex acute, 0.5–10 mm; Leaves subevergreen, deciduous, petiole 2–25 mm; Leaf blade small to moderate, elliptic, narrowly lanceolate to oblong, ovate, obovate, subrotund or rotund; base rounded, cordate, cordulate or cuneate; margins thick or thin, flat, revolute to regularly undulate-crisped, entire to toothed, shallowly lobed sometimes with 2–3 spinescent teeth on each side; surfaces abaxially light green, blue-green, dark green, glaucous or whitish, glabrous, floccose or tomentose, trichomes dense to scattered, flat, curly or stiffened stellate, with erect or appressed simple hairs, sometimes becoming glabrous; Acorns annual, 1–2 subsessile or pedunculate, cup thin, shallowly to deeply cup-shaped, turbinate, hemispheric or funnel-shaped, cup scales appressed, thickened basally, moderately to strongly tuberculate, scale tips appressed; nut ovoid-ellipsoid, oblong or barrel-shaped, sometimes flattened at both ends; cotyledons distinct or connate.
Distribution: Southwestern States AZ, NM, OK, TX, northeastern Mexico south to Guatemala (see
Figure 2)
Includes: * Q. hinckleyi C.H. Muller, Q. laceyi Small, Q. mohriana Buckley, Q. polymorpha Schltdl. and Cham. (the type), Q. pungens Liebm., Q. vaseyana Buckley
Polymorphae form a small and variable clade that diverged from
Leucomexicana more than 15 Ma. Of the six recognized taxa, only
Q. hinckleyi and
Q. polymorpha share the same Bioregion climate cluster with
Leucomexicana taxa based on climatic ranges estimated from Worldclim data [
77], while the other four species cluster with Eastern North American taxa [
19]. The clade is remarkably heterogeneous in morphology and habit, with a patchy distribution in the Chihuahuan Desert Region and adjacent Edwards Plateau except for
Q. polymorpha, which ranges from southwest Texas, south along the east slope of the Sierra Madre Oriental to Guatemala [
83,
132].
One well supported subgroup of taxa comprises
Q. hinckleyi—Q. pungens—Q. vaseyana.
Quercus pungens and
Q. vaseyana have been linked taxonomically, the latter initially treated as a variety of the former, and both classified by Muller (1951) [
90] in his series
Vaseyanae. Muller described and placed
Q. hinckleyi within series
Glaucoideae, a group he delimited to include the FNA taxa
Q. laceyi and
Q. depressipes Trel. and the widespread Mexican species
Q. glaucoides M. Martins and Galeotti. Our analyses instead support a regional radiation of taxa, including
Q. laceyi, while placing the FNA taxa associated with the historical concept of series
Glaucoideae (e.g.,
Q. depressipes,
Q. engelmannii Greene,
Q. arizonica Sarg.) within
Leucomexicana (
Figure 2).
Except for
Q. pungens and
Q. vaseyana, none of the taxa treated here within
Polymorphae have been considered closely related in previous classifications. For example,
Q. laceyi, Q. polymorpha, and
Q. mohriana were placed in different sets of mostly Mexican taxa: (1)
Q. mohriana with
Q. arizonica in subsect.
Arizonicae sensu Camus [
74]; (2)
Q. polymorpha with
Q. porphyrogenita Trel. and a few other Mexican taxa of doubtful validity within subsect.
Polymorphae sensu Camus [
83]; and (3)
Q. laceyi within series
Glaucoideae sensu Muller, and as noted later [
141], with putative affinities to either
Q. porphyrogenita or eastern North American white oaks.
Our current sampling and high level of resolution and support within section
Quercus are sufficient to conclude that most of the previous speculation on the disparate affinities of these taxa is not supported. We sampled two accessions of the widespread
Q. polymorpha, one from the northernmost population in Texas (Dolan Falls, Val Verde Co.) the other from south of Monterrey (Nuevo Leon; Mexico), about 600 km apart, and they fell sister to one another with strong support [
15]. Similarly, each taxon with the exception of
Q. hinckleyi has been sampled from two separate populations, with all individuals of these species forming tight, cohesive clades.
While our sampling in Mexico is preliminary, analyses suggest at least one compelling scenario for the biogeographic history of this clade. Aside from
Q. polymorpha, these taxa currently form the most narrowly distributed radiation of the North American oak clades, and the timing of the clade’s crown diversification coincides closely with the mid-Miocene expansion of arid zones [
142]. Species of this clade generally occur on limestone and desert slopes of the southwestern states (AZ, NM, TX, OK) and three north-central states of Mexico (CHIH, COAH, N.L.). The more mesophytic and broadly distributed
Q. polymorpha sublineage occurs to the south along the east slope of the Sierra Madre Oriental. Current patterns of distribution suggest a concentrated distribution of relictual species of various habit and climatic niche breadth, with a notable biogeographic disjunction between the
Q. polymorpha and
Q. mohriana sublineages by the end of the Pliocene, ca. 3–5 Ma, well after the diversification of main radiation of
Leucomexicanae [
19]. It is likely that the historical range of
Polymorphae was more widespread farther north and west into the FNA region and south into Mexico during wetter and more mild climates during the late Wisconsin glaciation episode (22–11 thousand years ago). The most distinctive of these taxa,
Q. hinckleyi, is a narrow endemic of the Trans-Pecos Region, with spinescent, subrotund leaves. It has been called a “climate relict” based on leaf fossil evidence from packrat middens throughout the Pleistocene ([
143], for discussion see [
144]).