Chemical Control of Corythucha arcuata (Say, 1832), an Invasive Alien Species, in Oak Forests
Abstract
1. Introduction
2. Materials and Methods
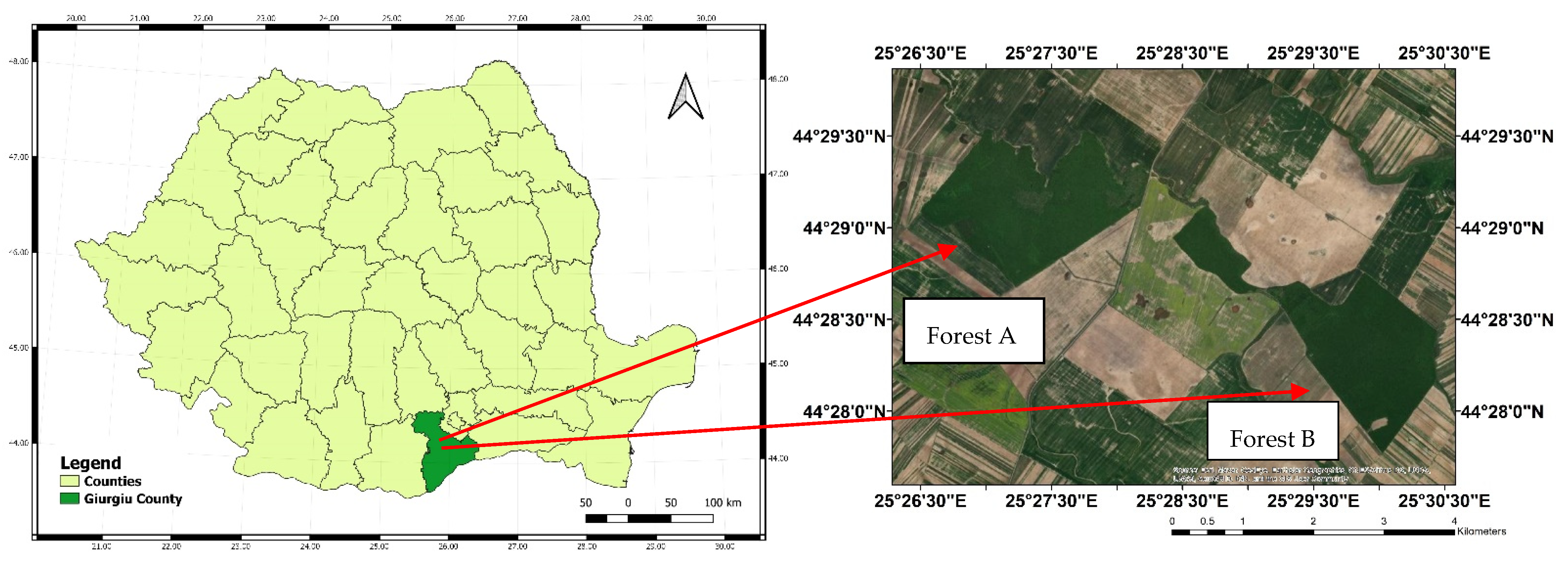
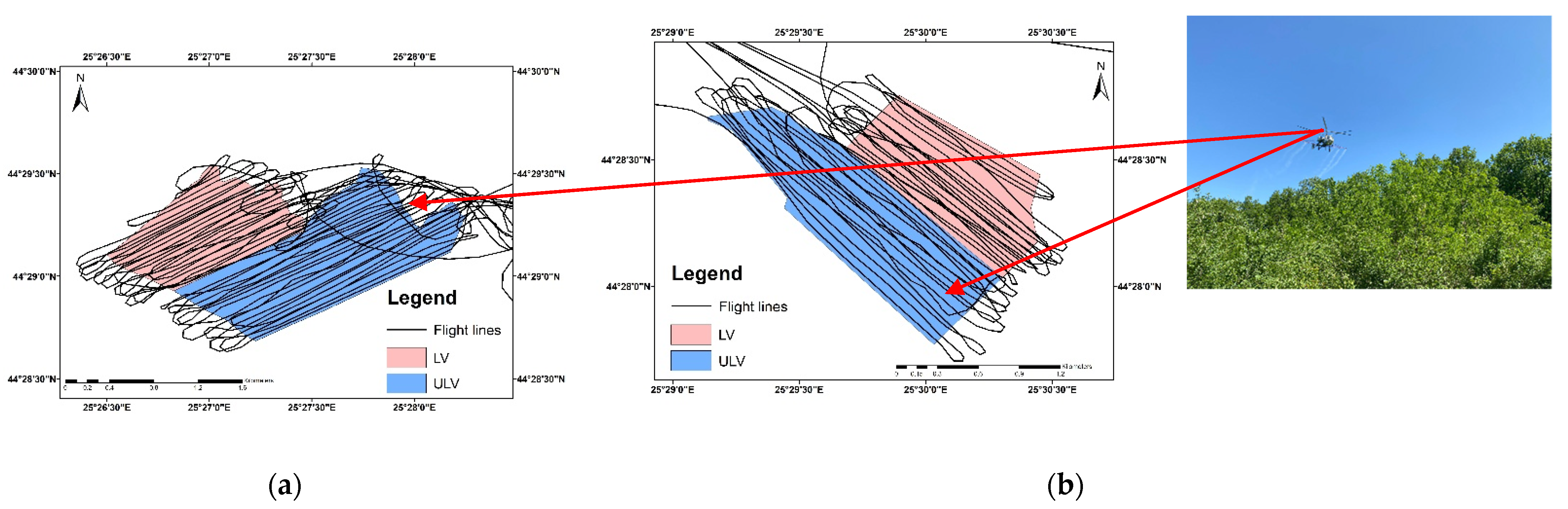
2.1. Study Site and Data Collection
- Forest A was sprayed with a contact insecticide (Alfametrin 10CE) over an area of 190 ha. Both LV (0.1 L/ha commercial product in 29.9 L/ha water) and ULV (0.1 L/ha commercial product in 2.9 L/ha water) treatments were applied. Alfametrin 10CE is a synthetic pyrethroid insecticide that acts on harmful insects by contact and ingestion, and it is based on the active substance alpha-cypermethrin.
- Forest B was sprayed with a systemic insecticide (APIS 200 SE) over an area of 160 ha. Both LV (0.2 L/ha commercial product in 29.8 L/ha water) and ULV (0.2 L/ha commercial product in 2.8 L/ha water) treatments were applied. APIS 200 EC is a neonicotinoid insecticide that acts on harmful insects by systemic action, penetrating rapidly into the plants’ leaves and acting on all stages of insect development, and it is based on the active substance acetamiprid.
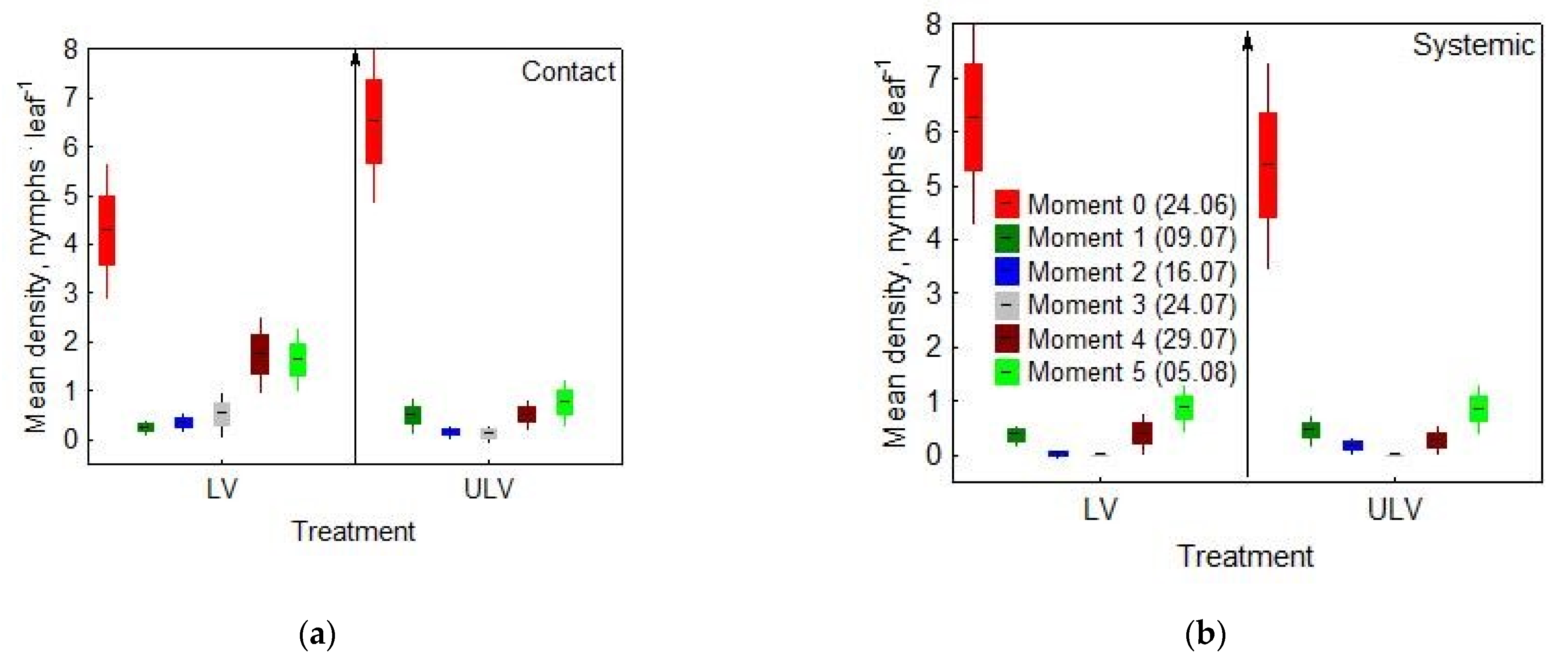
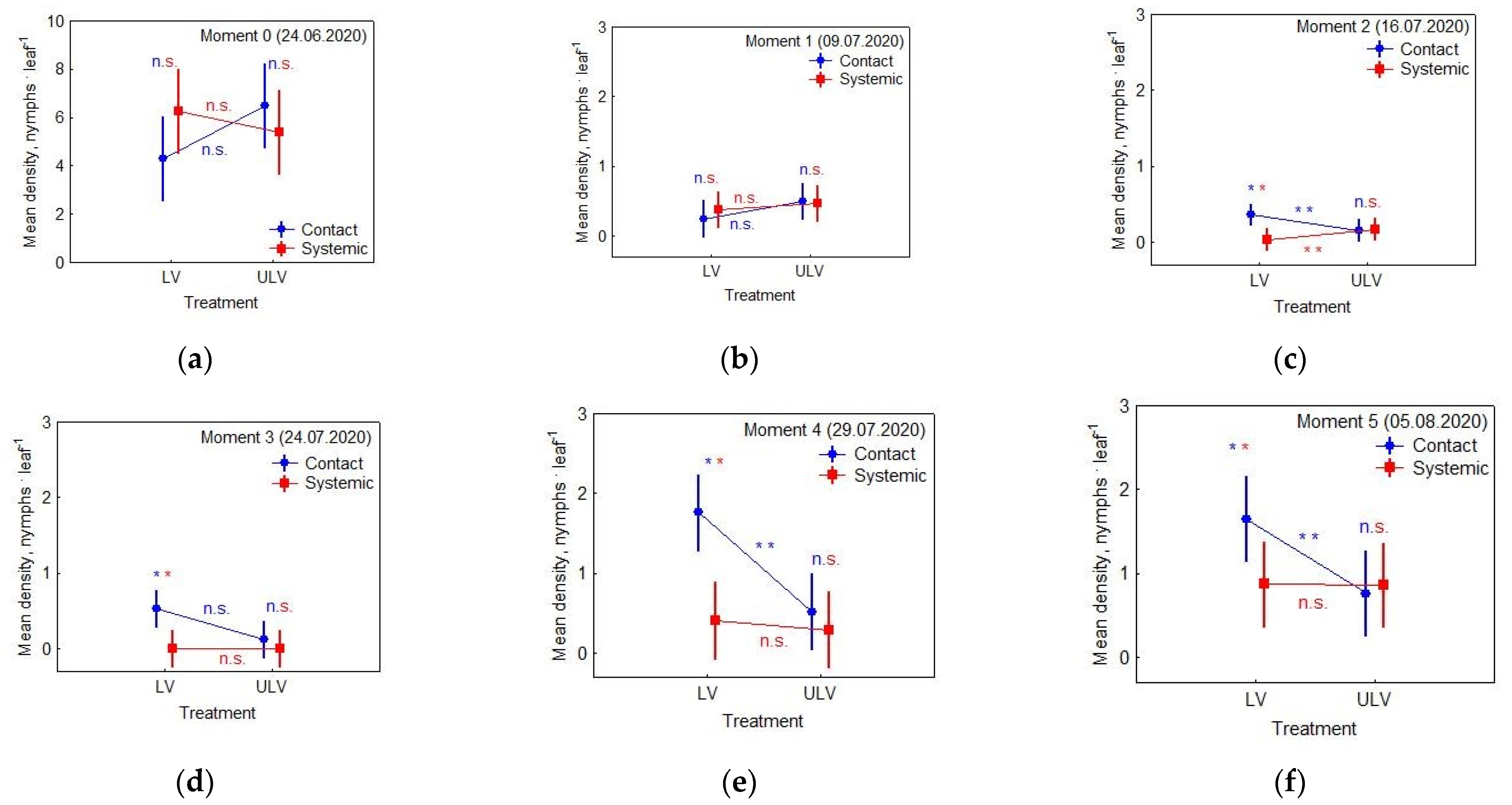
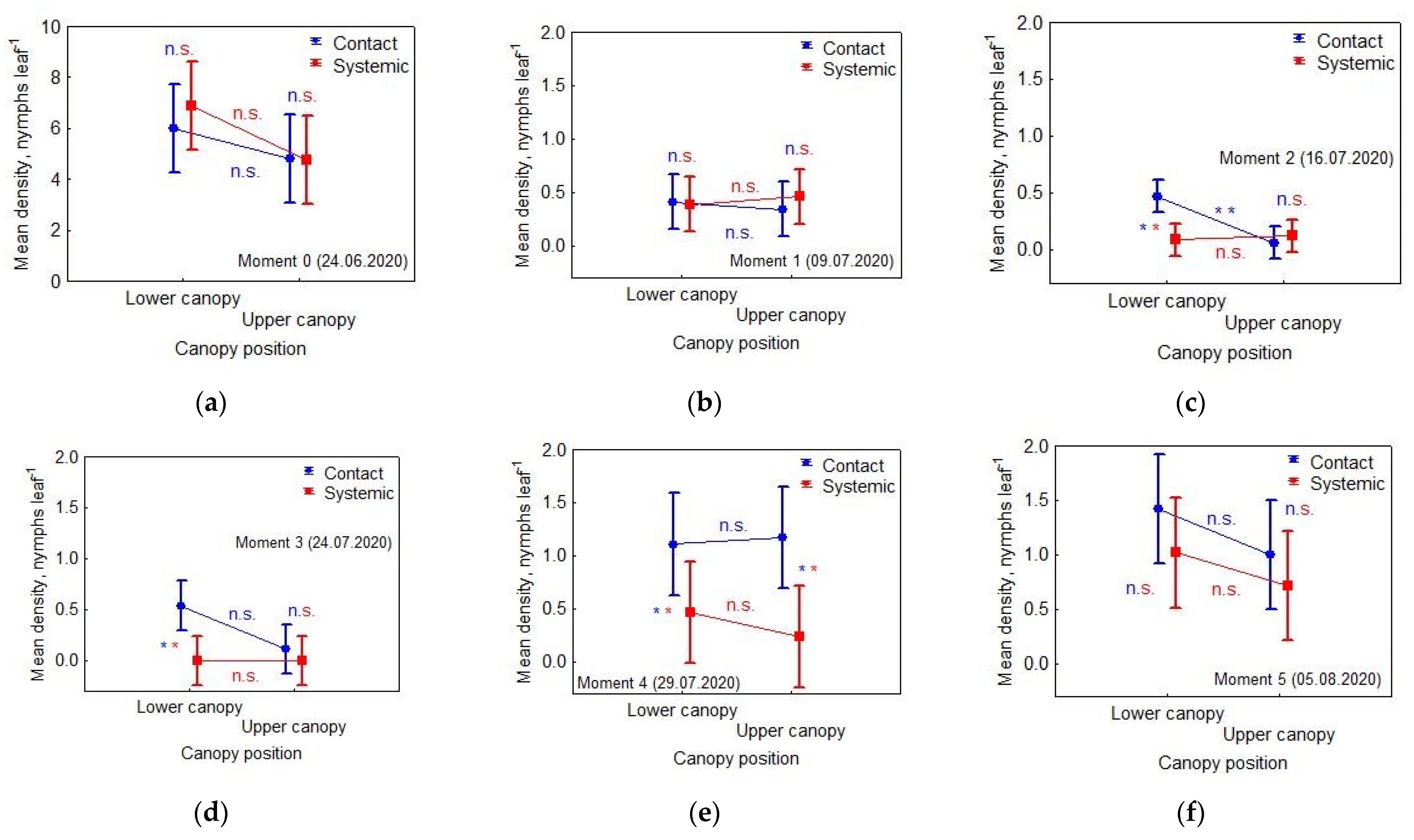
- Moment 0 (24 June 2020): the day of spraying
- Moment 1 (9 July 2020): checking the effectiveness of control methods
- Moment 2 (16 July 2020): checking re-infestations
- Moment 3 (24 July 2020): checking re-infestations
- Moment 4 (29 July 2020): checking re-infestations
- Moment 5 (5 August 2020): checking re-infestations
- 0–10%—very weak discoloration indicated by yellow (
)
- 11–25%—weak discoloration, indicated by pink (
)
- 26–50%—medium discoloration, indicated by green (
)
- 51–75%—strong discoloration, indicated by light blue (
)
- 76–100%—very strong discoloration, indicated by dark blue (
)
2.2. Data Analysis
3. Results
3.1. Effects of Applied Insecticide and Treatment on Nymphs’ Density
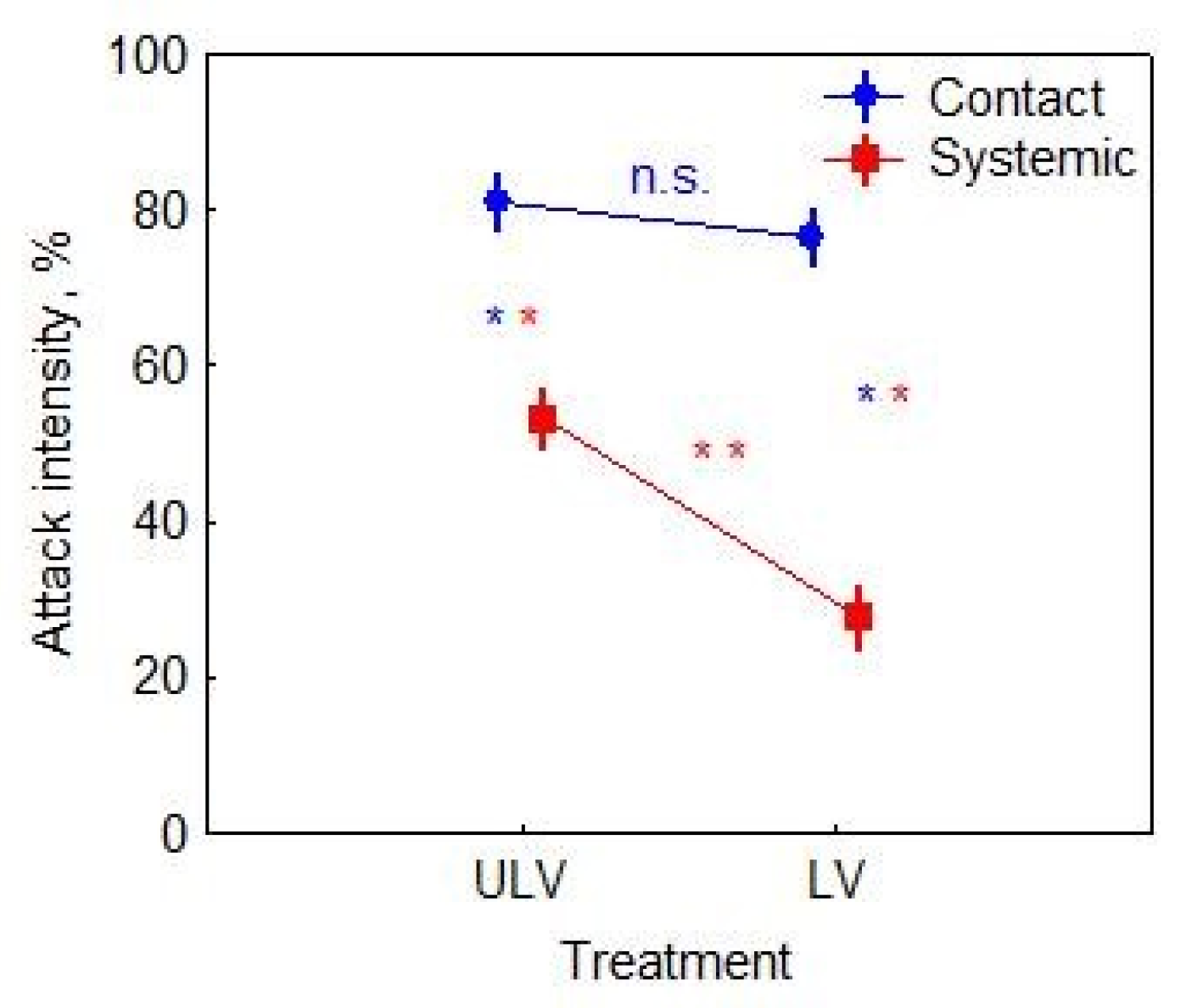
3.2. Attack Intensity Assessment at the End of Growing Season
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trumbore, S.; Brando, P.; Hartmann, H. Forest health and global change. Science 2015, 349, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Williamson, M.; Fitter, A. The varying succes of invaders. Ecology 1996, 77, 1661–1666. [Google Scholar] [CrossRef]
- Juliano, S.A.; Lounibos, L.P. Ecology of invasive mosquitoes: Effects on resident species and on human health. Ecol. Lett. 2005, 8, 558–574. [Google Scholar] [CrossRef]
- European Environment Agency. Invasive Alien Species: A Growing Problem for Environment and Health. 2013. Available online: https://www.eea.europa.eu/highlights/invasive-alien-species-a-growing (accessed on 26 April 2021).
- Davis, M.A.; Grime, J.P.; Thompson, K. Fluctuating resources in plant communities: A general theory of invasibility. J. Ecol. 2000, 88, 528–534. [Google Scholar] [CrossRef]
- Lounibos, L.P. Invasions by insect vectors of human disease. Annu. Rev. Entomol. 2002, 47, 233–266. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D.; Martin, J.L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; Garcia-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D.; Lach, L.; Zuniga, R.; Morrison, D. Environmental and economic costs of nonindigenous species in the United States. BioScience 2000, 50, 53–65. [Google Scholar] [CrossRef]
- Lovell, S.J.; Stone, S.F.; Fernandez, L. The economic impacts of aquatic invasive species: A review of the literature. Agric. Resour. Econ. Rev. 2006, 35, 195–208. [Google Scholar] [CrossRef]
- Meyerson, L.A.; Mooney, H.A. Invasive alien species in an era of globalization. Front. Ecol. Environ. 2007, 5, 199–208. [Google Scholar] [CrossRef]
- Vilà, M.; Basnou, C.; Pyšek, P.; Josefsson, M.; Genovesi, P.; Gollasch, S.; Nentwig, W.; Olenin, S.; Roques, A.; Roy, D.; et al. How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Front. Ecol. Environ. 2010, 8, 135–144. [Google Scholar] [CrossRef]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, J.M.; Keesing, F.; Ostfeld, R.S. Novel organisms: Comparing invasive species, GMOs, and emerging pathogens. Ambio 2013, 42, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, T.M.; Essl, F.; Evans, T.; Hulme, P.E.; Jeschke, J.M.; Kühn, I.; Kumschick, S.; Marková, Z.; Mrugała, A.; Nentwig, A.; et al. A unified classification of alien species based on the magnitude of their environmental impacts. PLoS Biol. 2014, 12, e1001850. [Google Scholar] [CrossRef] [PubMed]
- Hulme, P.E. Invasive species challenge the global response to emerging diseases. Trends Parasitol. 2014, 30, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Schindler, S.; Staska, B.; Adam, M.; Rabitsch, W.; Essl, F. Alien species and public health impacts in Europe: A literature review. NeoBiota 2015, 27, 1–23. [Google Scholar] [CrossRef]
- Bernardinelli, I.; Zandigiacomo, P. Prima segnalazione di Corythucha arcuata (Say) (Heteroptera, Tingidae) in Europa. Informatore Fitopatologico 2000, 50, 47–49. [Google Scholar]
- Forster, B.; Giacalone, I.; Moretti, M.; Dioli, P.; Wermelinger, B. Die Amerikanishe Eichennetzwaanze Corythucha arcuata (Say) (Heteroptera, Tingidae) hat die Südschweitz erreicht. Mitteilungen-Schweiz. Entomol. Ges. 2005, 78, 317–323. [Google Scholar]
- Mutun, S. First report of the oak lace bug, Corythucha arcuata (Say, 1832) (Heteroptera: Tingidae) from Bolu, Turkey. Isr. J. Zool. 2003, 49, 323–324. [Google Scholar]
- Dobreva, M.; Simov, N.; Georgiev, G.; Mirchev, P.; Georgieva, M. First record of Corythucha arcuata (Say) (Heteroptera: Tingidae) on the Balkan Peninsula. Acta Zool. Bulg. 2013, 65, 409–412. [Google Scholar]
- Csóka, G.; Hirka, A.; Somlyai, M. A tölgy csipkéspoloska (Corythucha arcuata Say, 1832—Hemiptera, Tingidae) els”o észlelése Magyarországon. Növényvédelem 2013, 49, 293–296. [Google Scholar]
- Hrašovec, B.; Posarić, D.; Lukić, I.; Pernek, M. Prvi nalaz hrastove mrežaste stjenice Corythucha arcuata u Hrvatskoj. Šumar. List 2013, 9, 499–503. [Google Scholar]
- Poljakovic-Pajnik, L.; Drekic, M.; Pilipovic, A.; Nikolic, N.; Pap, P.; Vasic, V.; Markovic, M. Pojava velikih šteta od Corythucha arcuata (Say) (Heteroptera: Tingidae) u šumama hrasta u Vojvodini. In Proceedings of the XIII savetovanje o zaštiti bilja, Zlatibor, Serbia, 23–26 November 2015. [Google Scholar]
- Pap, P.; Drekic, M.; Poljakovic-Pajnik, L.; Markovic, M.; Vasic, V. Monitoring zdravstvenog stanja šuma na teritoriji Vojvodine u 2015. God. Topola 2015, 195–196, 117–133. [Google Scholar]
- Glavendekić, M. Fauna iekologija insekata koji naseljavaju invazivne i nativne ukrasne biljke. In Ukrasne i Invazivne Biljke u Uslovima Klimatskih Promena—Uticaji i Adaptacije; Obratov-Petkovic, D., Ed.; Univerzitet u Beogradu-Šumarski fakultet: Beograd, Serbia, 2017; pp. 240–264. [Google Scholar] [CrossRef]
- Neimorovets, V.V.; Shchurov, V.I.; Bondarenko, A.S.; Skvortsov, M.M.; Konstantinov, F.V. First documented outbreak and new data on the distribution of Corythucha arcuata (Say, 1832) (Hemiptera: Tingidae) in Russia. Acta Zool. Bulg. 2017, 9, 139–142. [Google Scholar]
- Don, I.; Don, C.D.; Sasu, L.R.; Vidrean, D.; Brad, M.L. Insect Pests on the Trees and Shrubs from the Macea Botanical Garden; Studia Universitatis ‘Vasile Goldiş’ Arad Engineering Sciences and Agrotourism Series; 2016; Volume 11, pp. 23–28. Available online: http://www.facultateadeinginerie.ro/studia/studia112/112-don.pdf (accessed on 6 May 2021).
- Chireceanu, C.; Teodoru, A.; Chiriloaie, A. First record of oak lace bug Corythucha arcuata (Tingidae: Heteroptera) in Romania. In Proceedings of the 7th ESENIAS Workshop with Scientific Conference Networking and Regional Cooperation towards Invasive Alien Species Prevention and Management in Europe, Sofia, Bulgaria, 28–30 March 2017. [Google Scholar]
- Csóka, G.; Hirka, A.; Mutun, S.; Glavendekic, M.; Mikó, Á.; Szőcs, L.; Paulin, P.; Eötvös, C.B.; Gáspár, C.; Csepelényi, M.; et al. Spread and potential host range of the invasive oak lace bug [Corythucha arcuata (Say, 1832)—Heteroptera: Tingidae] in Eurasia. Agric. For. Entomol. 2019, 22, 61–74. [Google Scholar] [CrossRef]
- Jurc, M.; Jurc, D. The first record and the beginning the spread of Oak lace bug, Corythucha arcuata (Say, 1832) (Heteroptera: Tingidae), in Slovenia. Šumar. List 2017, 141, 485–488. [Google Scholar] [CrossRef][Green Version]
- Glavendekic, M.; Vukovic-Bojanovic, V. Prvi nalaz hrastove mrežaste stenice Corythucha arcuata (Say) (Hemiptera: Tingidae) u Bosni i Hercegovini i novi nalazi u Srbiji. In Book of Abstracts of XI Symposium of Entomologists of Serbia, Goč, Serbia; Glavendekic, M., Ed.; pp. 70–71. Available online: https://knlitd.pan.pl/index.php/en/news-events/357-xi-symposium-of-serbian-entomologists-17-21-09-2017-serbia (accessed on 6 May 2021).
- Dautbašic, M.; Zahirovic, K.; Mujezinovic, O.; Margaletic, J. Prvi nalaz hrastove mrežaste stjenice (Corythucha arcuata) u Bosni i Hercegovini. Šumar. List 2018, 142, 179–181. [Google Scholar] [CrossRef]
- Streito, J.C.; Balmès, V.; Aversenq, P.; Weill, P.; Chapin, E.; Clément, M.; Piednoir, F. Corythucha arcuata (Say, 1832) et Stephanitis lauri Rietschel, 2014, deux espèces invasives Nouvelles pour la faune de France (Hemiptera, Tingidae). L. Entomol. 2018, 74, 133–136. [Google Scholar]
- Zúbrik, M.; Gubka, A.; Rell, S.; Kunca, A.; Vakula, J.; Galko, J.; Nikolov, C.; Leontovyc, R. First record of Corythucha arcuata in Slovakia. Short Communication. Plant Protect. Sci. 2019, 55, 129–133. [Google Scholar] [CrossRef]
- Sallmannshofer, M.; Ette, M.S.; Hinterstoisser, W.; Cech, T.L.; Hoch, G. Erstnachweis der Eichennetzwanze, Corythucha arcuata, in Österreich. Forsch. Aktuell 2019, 66, 1–6. [Google Scholar]
- Mutun, S.; Ceyhan, Z.; Sözen, C. Invasion by the oak lace bug, Corythucha arcuata (Say) (Heteroptera: Tingidae) in Turkey. Turk. J. Zool. 2009, 33, 263–268. [Google Scholar] [CrossRef]
- Csepelényi, M.; Hirka, A.; Szénási, Á.; Mikó, Á.; Szőcs, L.; Csóka, G. Az inváziós tölgycsipkéspoloska (Corythucha arcuata (Say, 1832)) gyors terjeszkedése és tömeges fellépése Magyarországon. Erdtud. Közlemények 2017, 7, 127–134. [Google Scholar] [CrossRef]
- Simov, N.; Grozeva, S.; Langourov, M.; Georgieva, M.; Mirchev, P.; Georgiev, G. Rapid expansion of the Oak lace bug Corythucha arcuata (Say, 1832) (Hemiptera: Tingidae) in Bulgaria. Hist. Nat. Bulg. 2018, 27, 51–55. [Google Scholar]
- Tomescu, R.; Olenici, N.; Nețoiu, C.; Balacenoiu, F.; Buzatu, A. Invasion of the oak lace bug Corythucha arcuata (Say.) in Romania: A first extended reporting. Ann. For. Res. 2018, 61, 161–170. [Google Scholar] [CrossRef]
- Bernardinelli, I. Potential host plants of Corythucha arcuata (Het., Tingidae) in Europe: A laboratory study. J. Appl. Ecol. 2006, 130, 480–484. [Google Scholar] [CrossRef]
- Paulin, M.; Hirka, A.; Eötvös, C.S.; Gáspár, C.; Fürjes-Mikó, A.; Csóka, G. Known and predicted impacts of the invasive oak lace bug (Corythucha arcuata) in European oak ecosystems—A review. Folia Oecol. 2020, 47, 131–139. [Google Scholar] [CrossRef]
- Connell, W.A.; Beacher, J.H. Life history and control of the oak lace bug. Bull. Univ. Del. Agric. Exp. Stn. 1947, 265, 28. [Google Scholar]
- Wheeler, A.G.; Benjamin, J.R.; Stinner, R.; Henry, T.J. Biology and nymphal stages of Deraeocoris nebulosus (Hemiptera: Miridae), a predator of arthropod pests on ornamentals. Ann. Entomol. Soc. Am. 1975, 68, 1063–1068. [Google Scholar] [CrossRef]
- Puttler, B.; Bailey, W.C.; Triapitsyn, S.V. Notes on distribution, host associations, and bionomics of Erythmelus klopomor Triapitsyn (Hymenoptera, Mymaridae), an egg parasitoid of lace bugs in Missouri, USA, with particular reference to its primary host Corythucha arcuata (Say) (Hemiptera, Tingidae). J. Entomol. Acarol. Res. 2014, 46, 30–34. [Google Scholar] [CrossRef]
- Dreistadt, S.H.; Perry, E.J. Lace Bugs—Integrated Pest Management for Home Gardeners and Landscape Professionals. Pest Notes Publication. University of Calfornia, Agriculture and Natural Resources, Statewide Integrated Pest Management Program. 2014. Available online: http://ipm.ucanr.edu/PMG/PESTNOTES/pn7428.html (accessed on 1 May 2021).
- Boggs, J. More Lace Bugs. Ohio State University Extension. Available online: https://bygl.osu.edu/node/1073 (accessed on 5 May 2021).
- Shetlar, D.J. Lace Bugs. Ohio State University Extension. Available online: https://ohioline.osu.edu/factsheet/HYG-2150-10 (accessed on 5 May 2021).
- Drekić, M.; Pajnik, L.P.; Pilipović, A.; Nikolić, N. Suppression of Oak Lace Bug Corythucha Arcuata Say; UDK: Berlin, Germany, 2019; pp. 215–223. Available online: https://www.researchgate.net/publication/350176869_SUPPRESSION_OF_OAK_LACE_BUG_Corythucha_arcuata_Say (accessed on 6 May 2021).
- Berniardeli, I.; Zandigiacomo, P. Corythucha arcuata (Say): A new pest for European oaks. In Methodology of Forest Insect and Disease Survey in Central Europe. Proceedings of the IUFRO WP 7.03.10 Workshop, Bușteni, Romania, 24–28 Septemer 2000; Knizek, M., Ed.; IUFRO: Bușteni, Romania, 2000. [Google Scholar]
- Jin, H.; Webster, G.R.B. Persistence, Penetration, and Surface Availability of Cypermethrin and Its Major Degradation Products in Elm Bark. J. Agric. Food Chem. 1998, 46, 2851–2857. [Google Scholar] [CrossRef]
- Carrillo, D.; Crane, J.H.; Peña, J.E. Potential of Contact Insecticides to Control Xyleborus glabratus (Coleoptera: Curculionidae), a Vector of Laurel Wilt Disease in Avocados. J. Econ. Entomol. 2013, 106, 2286–2295. [Google Scholar] [CrossRef]
- Szőnyi, L. Adatok néhány fafaj vastagsági növekedéséhez. Az Erdő 1962, 97, 289–300. [Google Scholar]
- Járó, Z.; Tátraaljai, E. A fák éves növekedése. Erd. Kut. 1985, 76–77, 221–234. [Google Scholar]
- Hirka, A. Bükk, luc és kocsánytalan tölgy éves kerületnövekedési menetének vizsgálata. Erd. Kut. 1991, 82–83, 15–23. [Google Scholar]
- Euphresco Project—‘Corythucha arcuata (Heteroptera, Tingidae): Evaluation of the Pest Status in Europe and Development of Survey, Control and Management Strategies’; Unpublished work; 2020; Available online: https://zenodo.org/record/4898795#.YMLV9kwRWUk (accessed on 6 May 2021).
- Japelj, A.; Veenvliet, J.K.; Malovrh, J.; Verlič, A.; de Groot, M. Public preferences for the management of different invasive alien forest taxa. Biol. Invasions 2019, 21, 3349–3382. [Google Scholar] [CrossRef]

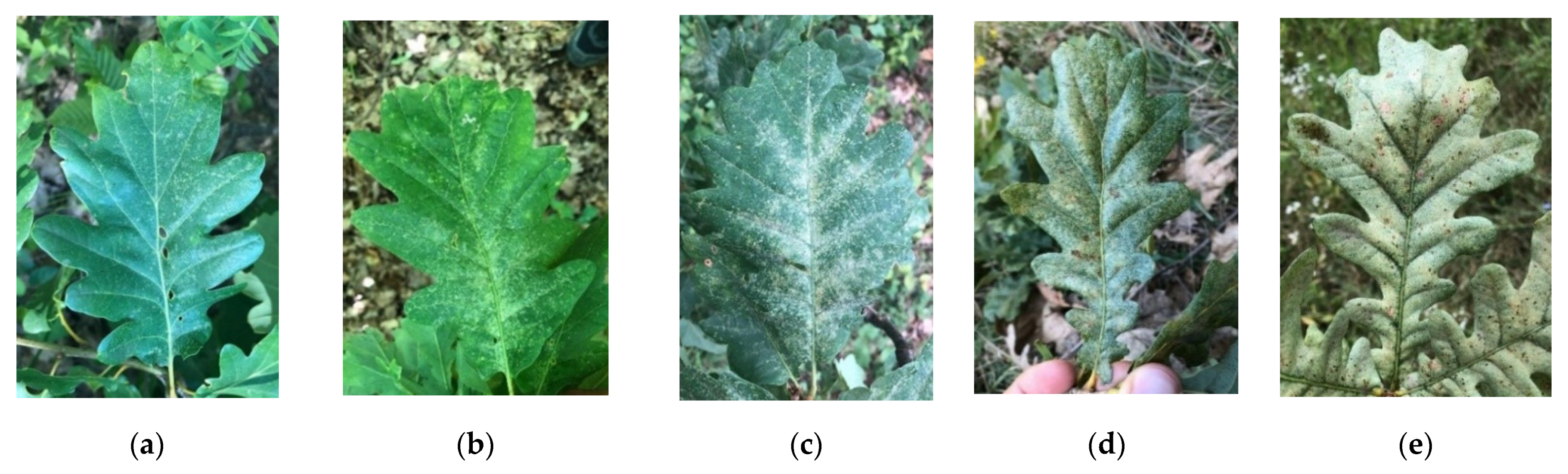
 very weak discoloration (0–10%);
very weak discoloration (0–10%);  weak discoloration (11–25%);
weak discoloration (11–25%);  medium discoloration (26–50%);
medium discoloration (26–50%);  strong discoloration (51–75%); and
strong discoloration (51–75%); and  very strong discoloration (76–100%).
very strong discoloration (76–100%).
 very weak discoloration (0–10%);
very weak discoloration (0–10%);  weak discoloration (11–25%);
weak discoloration (11–25%);  medium discoloration (26–50%);
medium discoloration (26–50%);  strong discoloration (51–75%); and
strong discoloration (51–75%); and  very strong discoloration (76–100%).
very strong discoloration (76–100%).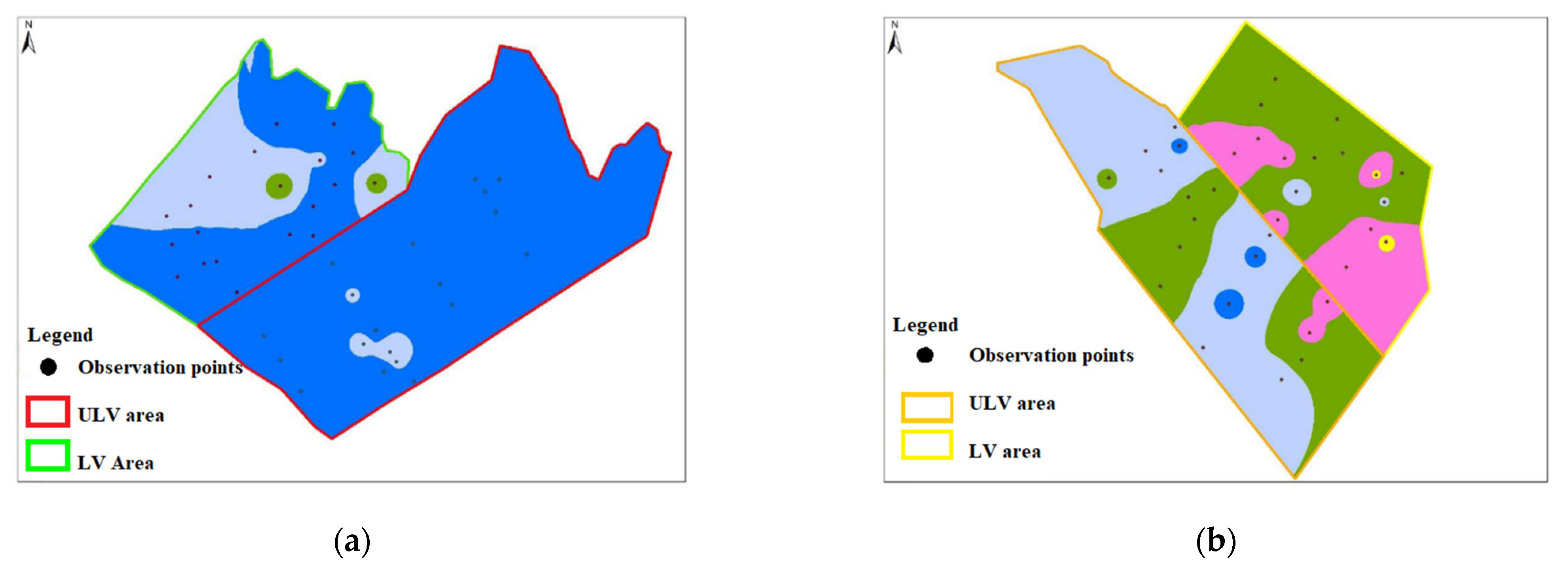
| Characteristics | Forest A | Forest B |
|---|---|---|
| Coordinates | 44°29′06″ N 25°27′16″ E | 44°28′24″ N 25°29′53″ E |
| Area (ha) | 190 | 160 |
| Dominant forest species | Quercus cerris, Q. frainetto, Q. robur | Quercus cerris, Q. frainetto, Q. robur |
| Secondary forest species | Acer platanoides, A. campestre | Acer platanoides, A. campestre |
| Regeneration mode | natural regeneration (sprout) | natural regeneration (sprout) |
| Age (years) | 60 | 60 |
| Average diameter (cm) | 23 | 25 |
| Average height (m) | 19 | 20 |
| Crown density of the stand (%) | 70 | 90 |
| Standing volume (m3 ha−1) | 150 | 170 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bălăcenoiu, F.; Nețoiu, C.; Tomescu, R.; Simon, D.C.; Buzatu, A.; Toma, D.; Petrițan, I.C. Chemical Control of Corythucha arcuata (Say, 1832), an Invasive Alien Species, in Oak Forests. Forests 2021, 12, 770. https://doi.org/10.3390/f12060770
Bălăcenoiu F, Nețoiu C, Tomescu R, Simon DC, Buzatu A, Toma D, Petrițan IC. Chemical Control of Corythucha arcuata (Say, 1832), an Invasive Alien Species, in Oak Forests. Forests. 2021; 12(6):770. https://doi.org/10.3390/f12060770
Chicago/Turabian StyleBălăcenoiu, Flavius, Constantin Nețoiu, Romică Tomescu, Dieter Carol Simon, Andrei Buzatu, Dragoș Toma, and Ion Cătălin Petrițan. 2021. "Chemical Control of Corythucha arcuata (Say, 1832), an Invasive Alien Species, in Oak Forests" Forests 12, no. 6: 770. https://doi.org/10.3390/f12060770
APA StyleBălăcenoiu, F., Nețoiu, C., Tomescu, R., Simon, D. C., Buzatu, A., Toma, D., & Petrițan, I. C. (2021). Chemical Control of Corythucha arcuata (Say, 1832), an Invasive Alien Species, in Oak Forests. Forests, 12(6), 770. https://doi.org/10.3390/f12060770











