1. Introduction
Commercial extraction of economically important plants from natural populations is a significant factor leading to the decline of many species across the globe [
1,
2,
3]. Wild harvesting has a variety of ecological consequences and impacts on forest resources, including loss of habitat, isolation and reduction in population size [
4]. Plant species confined to small and fragmented populations are particularly susceptible to loss of genetic variability as a result of overharvesting due to interrupted gene flow, inbreeding, and genetic drift, which ultimately influence the potential for long-term survival [
3]. In addition, the life-history strategy of species in disturbance-prone environments influences persistence under stressful conditions [
5]. While vegetative reproduction is common among many plant species [
2,
5,
6,
7], the population dynamics and genetic features of clonal growth have rarely been studied in systems of frequent disturbance, such as the fire-prone tropical savannas.
The sandalwood genus (
Santalum L. Santalaceae) is a broadly distributed group of plants with 16 extant species that occur throughout the Asia-Pacific region [
8]. A number of sandalwood species are endangered due to overharvesting of wild populations, as the timber is highly sought after due to the commercial value of fragrant oil derived from mature heartwood [
9]. Whole trees are harvested from natural populations to extract the oil-rich heartwood and roots, which are widely used in aromatherapy, cosmetics, perfumes, carvings and production of incense sticks [
10]. Slow growth rates and low levels of natural regeneration of valuable sandalwood species have exacerbated declines of natural populations subject to commercial harvesting [
11]. Increasing demand and a global decline of wild sources has made sandalwood one of the most expensive wood products in the world [
12]. As a consequence, there is significant pressure on remaining natural stands of sandalwood from unsustainable harvesting, habitat loss and disease [
13].
India has historically been the leading producer of sandalwood [
14]. However, there is an emerging global shortage of Indian sandalwood (
Santalum album) stocks due to reductions in wild harvested populations [
15]. The shortage of
S. album supplies has generated demand for other sandalwood species throughout Papua New Guinea, Australia and the Pacific Islands [
9]. In Australia,
S. spicatum and
S. lanceolatum have also been extensively wild harvested [
16,
17] and are currently threatened by grazing and habitat fragmentation [
18,
19].
Santalum lanceolatum R. Br. Sensu stricto, commonly referred to as northern sandalwood, is a small to medium tree endemic to the monsoonal dry tropics of northern Australia and is of emerging conservation and economic significance. Northern sandalwood was widely exploited in Far North Queensland (QLD), Australia, including the Northern Peninsula Area (NPA) between the late 1800s and 1930s. By around 1940, the wild resources in the region were exhausted, effectively ending the brief export industry [
20]. Growing up to 17 m in height and 25 cm in diameter at breast height (dbh) [
10], it is the largest and second most widely distributed of the seven sandalwood species native to Australia, which include
S. acuminatum,
S. album,
S. murrayanum,
S. leptocladum,
S. spicatum and
S. obtusifolium [
16]. Once recorded to inhabit a range of environments in all states of Australia [
4], a taxonomic review of the species in 2007 revised the range to a narrower area, generally contained within the northern humid and sub-humid areas north of 20° S latitude [
16].
Current populations of
S. lanceolatum at its northern distributional limit in the NPA are threatened by a range of human impacts, including previous overharvesting, grazing and fires [
1]. These factors, combined with poor natural recruitment, have led to a decline of northern sandalwood in the NPA. Furthermore, even though wild harvesting from the region ceased more than 70 years ago, there has been no research on the genetic structure of remaining natural stands in the area. The most recent survey of northern sandalwood observed a 19% decrease in known individuals in the NPA over a ten-year period [
10]. Consequently, northern sandalwood populations in the region are small and fragmented and considered to be locally threatened, with few large trees remaining [
10]. These isolated, small populations may become genetically segregated by chance events or selection pressures [
21]. As such, we might expect to see some level of differentiation between the most geographically isolated populations in the NPA. Furthermore, restricted gene flow may have significant consequences in plant populations that utilise clonal reproduction to maintain persistence and are found in clusters comprised of high numbers of genetically identical individuals [
4,
7]. However, there is no current research available that provides information of sufficient detail regarding the genetic architecture of the species and in particular of these isolated NPA populations.
Sandalwood reproduces both vegetatively and sexually with genetically identical individuals (clonality) observed in wild populations of
S. album [
22],
S. insulare [
2] and
S. leptocladum [
4]. Whilst we may observe increased somatic mutations across the life-span of persistent, clonal individuals, we still expect high levels of relatedness amongst parental (genets) and mutated ramets [
23]. Clonal reproduction can increase the degree of pollination between flowers within the same clone, i.e., geitonogamy [
24], leading to inbreeding depression in self-compatible plants or a decline in genetic diversity [
24] and low reproductive success in self-incompatible species [
4]. Research suggests a number of sandalwood species are self-incompatible [
4,
25,
26] with clonal populations of
S. leptocladum from southern Australia demonstrating pollen to pistil incompatibility, pollen sterility or pistil dysfunction, which contributes to poor reproductive success [
4]. Additionally, although poor seed production was reported for
S. lanceolatum by [
26], highlighting that just 20% of trees produced fruit from self-pollination experiments, this suggests northern sandalwood are not exclusively self-incompatible. Although early reports suggest
S. lanceolatum produces large amounts of seeds [
1], there is little evidence of seed-sourced natural recruitment from the remnant NPA populations, with most regeneration believed to be from asexual, vegetative reproduction through root suckering [
10,
19]. This suggests that
S. lanceolatum in the NPA may have significant levels of clonal structuring, which may exacerbate conservation concerns of
S. lanceolatum within the region and potentially for the taxon as a whole.
Northern sandalwood also remains a species of commercial interest due to the discovery of individuals with high quality oil profiles [
19]. This finding has sparked interest in developing a managed plantation led by traditional communities as a means to take advantage of the growing niche market for sandalwood products. However, because northern sandalwood in the NPA appears to be in decline [
10], conservation of the taxa in the region needs to be addressed as a priority. Understanding the levels of genetic variation and population structure are therefore key components to the development of a successful conservation strategy [
27,
28].
In this study, we investigated the genetic diversity, population genetic structure and levels of relatedness of natural stands of S. lanceolatum using twelve microsatellite markers. Our objectives were to: (i) quantify the genetic diversity within six natural populations of S. lanceolatum from the NPA; (ii) determine levels of genetic differentiation between the populations; (iii) assess genetic change and clonality of the populations and between age classes; (iv) identify genetic relationships of regionally distinct S. lanceolatum populations, and (v) to utilise the population genetic data to identify threats to S. lanceolatum in the study region and propose conservation measures to guide effective management of the species.
2. Materials and Methods
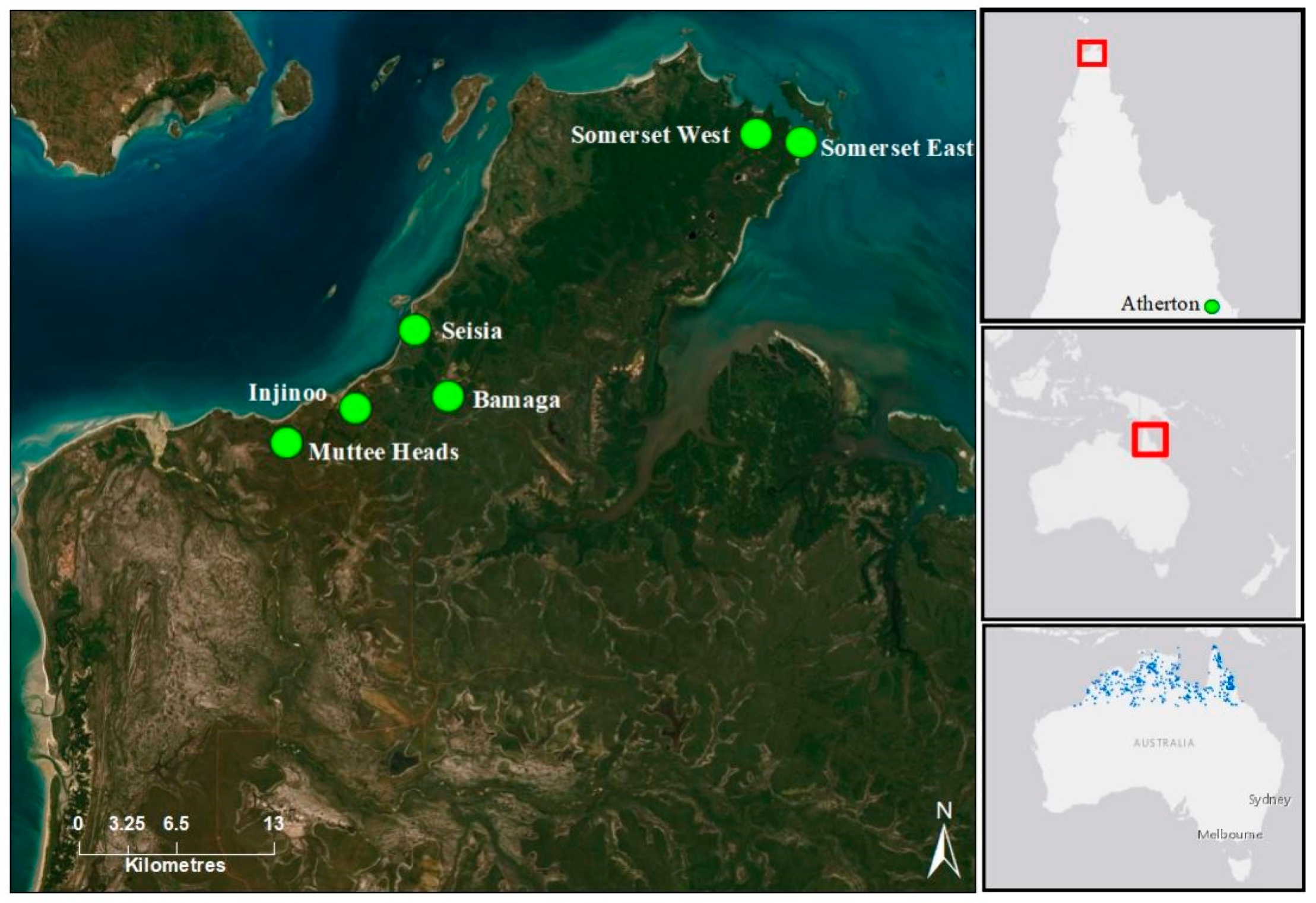
A total of 200 Santalum lanceolatum individuals were sampled from eight populations covering the geographic range of the species in the tropical savanna woodlands of the Northern Peninsula Area (NPA) and two geographic outlier populations from the Atherton region of Cape York, Queensland, Australia (
Figure 1). Rainfall in the NPA region is concentrated over the summer wet season, which is from December to February, with a yearly average rainfall of 1791.6 mm and an average of 94.9 days of rain annually [
29]. Average yearly temperatures range from a minimum of 24.7 °C to a maximum of 30.4 °C.
Though widely distributed across northern Australia, current populations of northern sandalwood in the NPA are small and fragmented, with low levels of natural recruitment [
10]. An exhaustive sampling approach was used to analyse clonality of
S. lanceolatum populations. In October 2018, leaf tissue was collected from between 12 and 46 individuals from each of these six populations, with a total of 177 individuals sampled from the NPA region. Three ‘central’ populations (Seisia, Bamaga and Injinoo) are found on the western coast of the NPA covering an area of approximately 80 km
2 (
Figure 1). The two Somerset populations (Somerset East and Somerset West) are approximately 5.5 km apart at the north-eastern tip of the Cape York and more than 28 km from the central cluster of populations. The Muttee Heads population is approximately 9 km to the south of the central cluster and is potentially isolated by a tidal estuary. Individual trees were assessed for evidence of reproductive structures, tree height and dbh. Trees with evidence of flowers and/or fruit, >2 m high and with >2 cm dbh were categorised as adults. Individuals < 2 m in height, with a dbh < 2 cm and no evidence of flowers and/or fruit were categorised as juveniles. For an age-class genetic diversity and structural analyses, a total of 60 trees classified as juveniles were collected from Bamaga (19 individuals), Seisia (21 individuals) and Injinoo (20 individuals). Material was collected under guidance and with permission from the traditional owners of the region, the Gudang/Yadhaykenu people.
Additional leaf tissue was collected from 23 individuals from two S. lanceolatum populations (Rocky Creek and Price Creek, 27.5 km apart; ~748 km SSE from the closest NPA population) on the Atherton tablelands, in southern Cape York. These populations were collected to provide comparison and perspective of genetic features to the NPA populations. We also collected two leaf samples to use as outgroups for hierarchal clustering analysis between S. lanceolatum populations that were sourced from five Santalum congeners (S. album, S. austrocaledonicum, S. leptocladum, S. macgregorii, S. yasi) grown at research facilities near Gympie, Queensland, Australia.
Leaf samples were stored in zip lock plastic bags with silica gel at ambient temperature prior to DNA extraction. Total genomic DNA was extracted and isolated using a DNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA) following the manufacturer’s instructions.
Seven microsatellite markers specific for
S. lanceolatum were sourced from [
17]. We screened an additional 22 primer sequences (
Table S1) designed for a variety of sandalwood congener species sourced from [
30,
31,
32]. The additional primers were assessed for the potential to cross-amplify with
S. lanceolatum with the aim of selecting a greater number of primers for analyses than had previously been utilised.
Twelve polymorphic microsatellite loci were selected that produced consistent Polymerase Chain Reaction (PCR) amplification, distinct allelic variation and clarity of fluorescent signal (electrophoretic signature) to evaluate population genetic variation. Forward primers for each locus were direct labelled with one of four fluorescent dyes (FAM, NED, VIC, PET). PCR was performed in a total reaction volume of 12.5 µL containing 7 µL ddH20, 1.25 µL PCR reaction buffer, 1 µL dNTP’s, 0.75 µL MgCl2, 0.25 µL of each forward and reverse primer, 0.25 µL Bovine Serum Albumin (BSA), 0.05 µL Taq F1 DNA polymerase and 1.5 µL (diluted to 1:10) genomic DNA. Amplification was performed with an Eppendorf Mastercycler (Hamburg, Germany) using the following cycling parameters: 95 °C for 3 min, followed by 35 cycles of 94 °C for 30 s, 56 °C for 30 s, 72 °C for 45 s, with a final extension at 72 °C for 10 min. PCR products were separated by capillary electrophoresis using an AB Genetic Analyser (Applied Biosystems). Fragment sizes were resolved corresponding to an internal lane standard (GS-600 LIZ; Applied Biosystems) using GENEMARKER v 2.4.0 software (SoftGenetics LLC, State College, PA, USA) followed by manual verification by a second person. Ambiguous and unresolved signals were assessed by repeat PCR and capillary electrophoresis.
To assess the extent of clonality, we used GenAlEx version 6.5 [
33]. We first applied the Find Clones function to produce a list of matching (i.e., identical) multilocus genotypes (MLGs). The list of matching MLGs was used to measure G/N, which determines the ratio between the matching MLGs (G) and total number of individuals in a population (N). The G/N ratio ranges between zero and one, with results close to zero suggesting strict clonality and a higher proportion of individuals sharing the same multilocus genotype. A G/N equal to one suggests sexual reproduction where individuals each have a unique multilocus genotype [
34]. The Find Clones function identifies clonality and clonal probability at the population level but does not handle missing data. From our dataset with identical individuals removed, we had 7.3% missing values. To overcome the missing values to run the Find Clones function, we interpolated the missing data for missing values. As most loci had at least one sample of missing data, we used the most common allele (mode allele) for each locus to replace all missing data for the clonal analyses. To determine whether the probability of the detection of MLGs was significant (
p ≤ 0.05), we calculated the Psex value where Psex is the probability of encountering a given genotype by chance, and PsexNgen is the probability of finding that MLG in the N sampling units collected and analysed. Whilst similar clonal analysis programs handle missing data by various methods such as using a ‘mean’ allele [
35] or treat missing values as ‘new’ alleles [
36], we acknowledge that using alternative methods or programs may produce different results.
2.1. Genetic Diversity
The polymorphic information content (PIC) was calculated using CERVUS Version 3.0.3 [
37]. Deviation from Hardy–Weinberg Equilibrium (HWE) at each locus and evidence for genotypic linkage disequilibrium (LD) among pairs of loci was calculated using FSTAT v2.9.3.2 [
38]. To estimate the possibility for large allele dropout, stuttering from genotyping errors and presence of null alleles, we used MICRO-CHECKER v2.2.3 [
39] and ML-NULLFREQ [
40] run with 1000 bootstraps at a 95% confidence interval. Three loci (SsA106, Lanc03 and Lanc08) displayed significant evidence of linkage disequilibrium and deviation from HWE (
Table S2). The low rate of natural recruitment and likelihood of the study populations being closely related or clonal may have contributed to homozygote excess, which influenced the HWE and LD test results. However, the locus Lanc03 was excluded from further analyses as it also showed evidence of potential null alleles (whereas SsA106 and Lanc08 did not). Thus, we obtained a final set of 12 loci suitable for the population genetics analysis of
S. lanceolatum. The average potential null allele frequencies for the 12 selected loci were estimated to be and 0.16 ± 0.07 by ML-NULLFREQ and 0.11 ± 0.03 by MICRO-CHECKER. Loci from natural populations are expected to present deviation from HWE as a result of a number of genetic factors such as genetic drift, level of relatedness, natural selection and founder effects, and null alleles are often prone to false positives in these situations [
41]. It is therefore unlikely that the estimated frequency of null alleles will significantly impact subsequent genetic analyses.
To provide a context to similar studies of Santalum species with reported clonal populations, all ramets were excluded from analyses, with one representative (genet) from each clonal group used for diversity and population structure analyses. The difference in diversity results was negligible when all individuals from each population including singletons, genets and ramets were included.
Allelic frequencies were assessed using GenAlEx version 6.5 [
33] to analyse population genetic diversity parameters including: mean number of alleles per locus (A), observed heterozygosity (H
O), expected heterozygosity (H
E) and the past inbreeding measure (
F) index [
42]. Allelic richness (
AR; mean number of alleles per locus) and private allelic richness (
PAR; mean number of private alleles per locus) were generated at a minimum sample size of 6 genes for age-class comparisons and 12 genes for populations without juveniles, using rarefaction from HP-Rare [
43].
Data from the southern populations (Rocky Creek and Price Creek) were unable to be resolved at the loci mSiCIR153 as it failed to amplify despite repeated PCR and capillary electrophoresis. Here, we report AR and PAR for these populations without loci mSiCIR153. However, it is worth noting that these missing data may have influenced the final results.
2.2. Population Structure and Progeny Dynamics
To analyse the population genetic structure and assess genetic change between generations (age class), we performed a number of analyses of molecular variance (AMOVAs) using GenAlEx version 6.5 [
33] with 999 permutations. The AMOVAs detected multilocus comparisons of the average pairwise levels of differentiation (
FST) within and between adult and juvenile populations. Further analysis of genetic differentiation within and between populations was performed using the R package Adegenet [
44] in the R environment (R studio, version 1.1.453) using a discriminant analysis of principal components (DAPC) to identify posterior probabilities of individual assignment to K means groups. The DAPC was based on 30 principal components retained from the cross-validation function. Groups were identified using the ‘snapclust’ function over a maximum of 20 groups to generate Bayesian Information Criterion (BIC) to identify the optimal K value. Snapclust evaluates clustering by modelling Hardy–Weinberg equilibrium combined with the maximum-likelihood estimation from an Expectation-Maximisation algorithm to determine the optimal number of genetic clusters (K) based on goodness-of-fit criteria [
45].
We also performed additional validation in STRUCTURE version 2.3.4 [
46] using the Evanno method [
47] to confirm assignments. Results of the DAPC K means clustering were visualised using the R package ggplot2 [
48]. To estimate genetic relationships between
S. lanceolatum populations and additional Santalum species we used the R package Ape, Version 5.1 [
49], to perform a Neighbour Joining (NJ) tree, hierarchal cluster analysis run at 999 bootstraps based on Nei’s genetic distance [
50]. Principal Coordinates of Analyses (PCoA) were produced for several axes from the R package Adegenet [
44] to assess genetic associations.
2.3. Detection of Bottlenecks
To detect the probability of a recent bottleneck event we used BOTTLENECK [
51]. Using a heterozygosity excess method, this program tests assumptions that a recent decline in effective population size leads to genetic bottlenecks as a result of deviations from mutation-drift equilibrium. We used the Wilcoxon’s signed-rank test as a minimum of 20 loci are needed for a standardized difference test. The two-phased mutation (TPM) model was used for its suitability with microsatellite markers [
51] with an 80% stepwise mutation model and 20% multistep mutations.
4. Discussion
There are several key findings from this study, which are highly relevant to the ecology and conservation of S. lanceolatum. Overall, we found a high proportion of clonality and low sexual reproduction, which in combination with low genetic variation may be exacerbating the decline of northern sandalwood populations in the NPA region. There is also evidence to suggest regional genetic differentiation between most northern Cape York and southern Cape York populations. Finally, our results indicated genetic similarity between one population of S. lanceolatum from the NPA and the two southern Cape York populations.
Clonality in the remnant populations of
S. lanceolatum in the NPA was more extensive than expected with over 43% of sampled trees shown to be ramets with identical multilocus genotypes to other trees. Somerset West and Injinoo had the highest clonal structure of
S. lanceolatum in the NPA, with each population containing over 46% of identical multilocus genotypes to other trees within their respective populations. Somerset West is also moderately genetically divergent from all other northern sandalwood populations probably as a result of long-term isolation from other breeding populations, possibly by distance and physical barriers. Nevertheless, outlying populations such as Somerset may be significant for ongoing preservation as populations for potential speciation events [
52]. The
Santalum genus is considered to have Australian origins, with some Pacific island sandalwoods thought to be directly divergent from
S. lanceolatum [
8], further highlighting the conservation significance of this study.
The humid regions of Australia are highly seasonal with over 90% of annual rainfall occurring in the summer period, followed by a distinct dry season where natural and human mediated fires can be frequent. Page et al. (2007) and Lee et al. (2019) reported that the majority of recruitment of
S. lanceolatum in Cape York was likely from vegetative reproduction following fire. We found root suckering to be contributing to recruitment in all NPA populations, including the nominal juveniles, which supports the reported lack of seedling regeneration for
S. lanceolatum in the NPA. Post-fire vegetative regeneration is a vital life-history strategy for many tropical savanna woody plants with the need to be highly resistant to fire events [
53]. Despite the benefits of clonal recruitment, seed production is a vital dispersal strategy to access more suitable environments and reduce competition [
54]. Seed production is significantly reduced in small populations of self-incompatible plant species due to limited amounts of outcrossing pollen, which is further restricted by a highly clonal structure [
55]. An ex situ pollination experiment showed
S. lanceolatum to be generally self-incompatible [
26], while a mixed accession, grafted clonal seed orchard of
S. lanceolatum trees sourced from across the NPA had abundant fruit production [
10]. This suggests the current genetic diversity within northern sandalwood from the NPA is capable of increased seed production when the proportions of outcrossing pollen are increased and disturbance is minimised.
Our data showed 68% of genetic variation was among the
S. lanceolatum populations. High levels of among-population variation are common among clonal plant species with extensive clonality, leading to populations that may have a single or few genets [
56]. While our results suggest populations have a proportion of unique multilocus genotypes to balance the levels of clonality, in severe cases this can cause sexual reproduction failure [
4]. Without sexual reproduction, clonality leads to a monoclonal population, which in the long term can increase the risk of extinction [
56]. Consequently, sexual reproduction is often balanced with vegetative propagation in clonal plant species [
57]. Our results indicate episodic disturbance from human activities including historical harvesting, frequent fires and grazing from livestock such as horses and cattle are likely compounding the capacity for natural regeneration and recovery of the species. These effects have influenced the level of clonality and may have reduced sexual reproduction potential of NPA
S. lanceolatum, which in turn has limited seed production.
Genetic diversity in NPA S. lanceolatum was relatively low, with heterozygosity (HO = 0.35–0.56, HE = 0.35–0.50), allelic richness (AR: 2.50–3.42), private allelic richness and frequency of private alleles consistently low across all populations. These diversity measures were similar to the closely related S. insulare (HO: 0.29–0.48, HE: 0.28–0.69, AR: 2.04–5.72), which reported extensive clonality across all populations, with some populations comprised entirely of multiple ramets from a single genet. Despite the low diversity in our study, we found that the two populations on the northern (Somerset West) and southern periphery (Muttee Heads) of the NPA were genetically distinct from the three geographically clustered populations of Bamaga, Seisia and Injinoo.
A wide-ranging review into the genetic diversity of Australian flora [
58] presented a number of considerations relevant to the
S. lanceolatum populations in the NPA. Importantly, plants in the Australian tropical biome were found to generally have low genetic diversity as a result of life-history strategies, biogeographical factors from climate oscillations and expansion of arid zones more than two million years ago during the Pleistocene [
58]. While these factors also influenced plant species in the western and eastern biomes, it was more pronounced in the northern tropical biome and resulted in extreme range contraction to the edges of the continent [
58]. This may support the results of higher diversity measures (
Na: 7.818, HE: 0.68), of the extensively harvested Western Australian sandalwood (
S. spicatum) [
32], compared to populations of
S. lanceolatum. Genetic diversity appears to vary considerably between sandalwood species. Studies of Indian sandalwood generally report variable diversity of
S. album depending on the study location [
3,
59], with some evidence of clonal structure found among natural populations [
22,
60]. Inconsistent reporting of diversity measures associated with differing molecular techniques makes direct comparisons difficult. However, the authors of [
61] reported low diversity (2–3 alleles per locus and
HE = 0.047) from Australian
S. album populations, while one population from India was reported to have high diversity based on the high frequency of amplified polymorphic DNA bands on UV visualized agarose gels. In contrast to other widely exploited sandalwoods, genetic diversity of
S. austrocaledonicum from islands in the New Caledonian archipelago had more variability in genetic diversity measures (
NA = 7.28–16,
HE = 0.12–0.79), depending on the island [
62].
Santalum insulare from French Polynesian islands (
NA = 8.75,
HE = 0.28–0.55, [
2]) and
S. yasi from Fijian and Tongan Islands (
NA = 2.88,
HE = 0.27–0.40, [
63]) had similar diversity features to
S. lanceolatum in the NPA. The only other genetic study to use microsatellites on
S. lanceolatum populations did not disclose diversity measures at the population level or sampling location but was informative in describing a high level of clonality in two out of ten populations investigated [
17]. Markers optimised by [
17] for northern sandalwood had H
E levels between 0.37 and 0.80 and was the first study to report marker diversity for
S. lanceolatum. Despite sampling locations not being reported, the higher levels of heterozygosity compared to our findings provide baseline data to support the fact that NPA populations may be in genetic decline.
The low diversity levels found in our study may be supported by several factors in common with the highly clonal populations of
S. leptocladum from Victoria, Australia, and
S. insulare from the Pacific region. These are: (1) the small and fragmented nature of populations has enhanced the rate of genetic erosion through loss of alleles from genetic drift; (2) overharvesting in the late 19th to the early 20th century caused a rapid loss of
S. lanceolatum resources in the NPA [
1], potentially exacerbating a genetic decline; (3) the evidence indicating a significant clonal structure is potentially resulting in limited sexual reproduction [
4]; and finally, (4) the overall population decline may be further increased by fire and browsing by free roaming livestock, which can reduce natural seed based regeneration [
1] and potential for improvement of genotypic diversity.
Within the adult
S. lanceolatum populations, the mean
FST indicated that the overall levels of population level genetic differentiation were relatively high (0.242) when compared to another economically important, threatened dry tropical plant species such as frankincense (
Boswellia papyrifera), which had a mean
FST value of 0.084 [
64]. Differentiation is variable among the commercially valuable sandalwood species. Dani et al. (2011) observed high mean
FST values (0.549) in three
S. album populations with some clonal structures, though clonal levels were not reported.
Santalum insulare, which was highly clonal (58%), had high differentiation (mean
FST = 0.50) between populations in French Polynesian islands, which is thought to be due to limited geneflow and overexploitation [
2].
Santalum spicatum has a long history of commercial extraction of natural populations with low differentiation at the species level (0.087) and strong regional differentiation [
11]. The relatively low and consistent differentiation between the NPA populations of Bamaga, Injinoo and Seisia (0.047–0.121) between the three geographically centralised suggest these populations once formed part of a larger, more continuous population prior to historical climate fluctuations and more anthropogenic disturbance. Conversely, our results indicated that populations on the northern (Somerset) and southern (Muttee Heads) periphery could be genetically distinct from the other NPA populations. There are a number of factors likely influencing limited observations of differentiation between the three proximate populations of Bamaga, Seisia and Injinoo compared to the more geographically isolated populations. As
S. lanceolatum is pollinated by small insects [
10], the potential for effective pollen dispersal from the central populations to the three peripheral populations would be limited due to the large distances and physical barriers such as wetlands, watercourses and small outcrops to the outlying populations. The Carnegie Range and associated rainforest vegetation lies between Somerset populations and the three central populations. Similarly, Cowel Creek and its associated mangrove system (300–400 m wide) may be isolating the Muttee Heads population. Further, the three central NPA populations are all proximate to local towns, which may explain the reports of fires over successive years [
10], including during the sampling period in this study. These frequent fires may influence pollinator populations, limiting the dispersal potential outside this main cluster of
S. lanceolatum populations. Fires in isolated populations can also have adverse genetic consequences, causing genetic bottlenecks and leading to increased genetic erosion [
28]. Finally, while low levels of
FST can infer low differentiation, this may be a historical feature, reflecting the structure of a larger, more connected metapopulation under minimal selection pressure before the current populations were fragmented and reduced in size through anthropogenic activities. There are numerous reports of species of Australian flora, with low levels of genetic diversity that are highly adapted to efficiently survive in narrow ecological niches [
65,
66]. Conclusions drawn from the levels of differentiation must therefore be tempered with the small population sizes and low genetic variation found in this study.
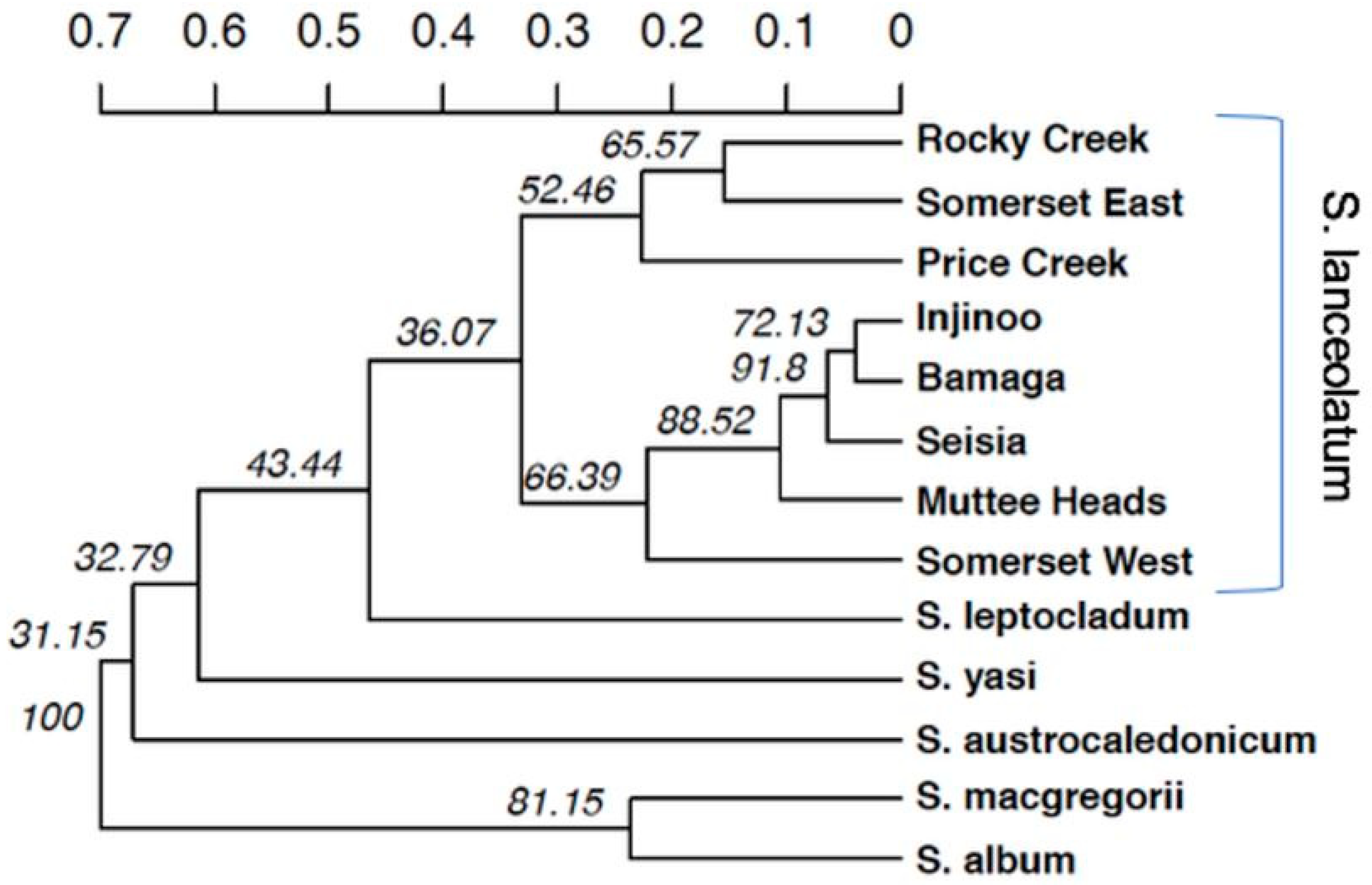
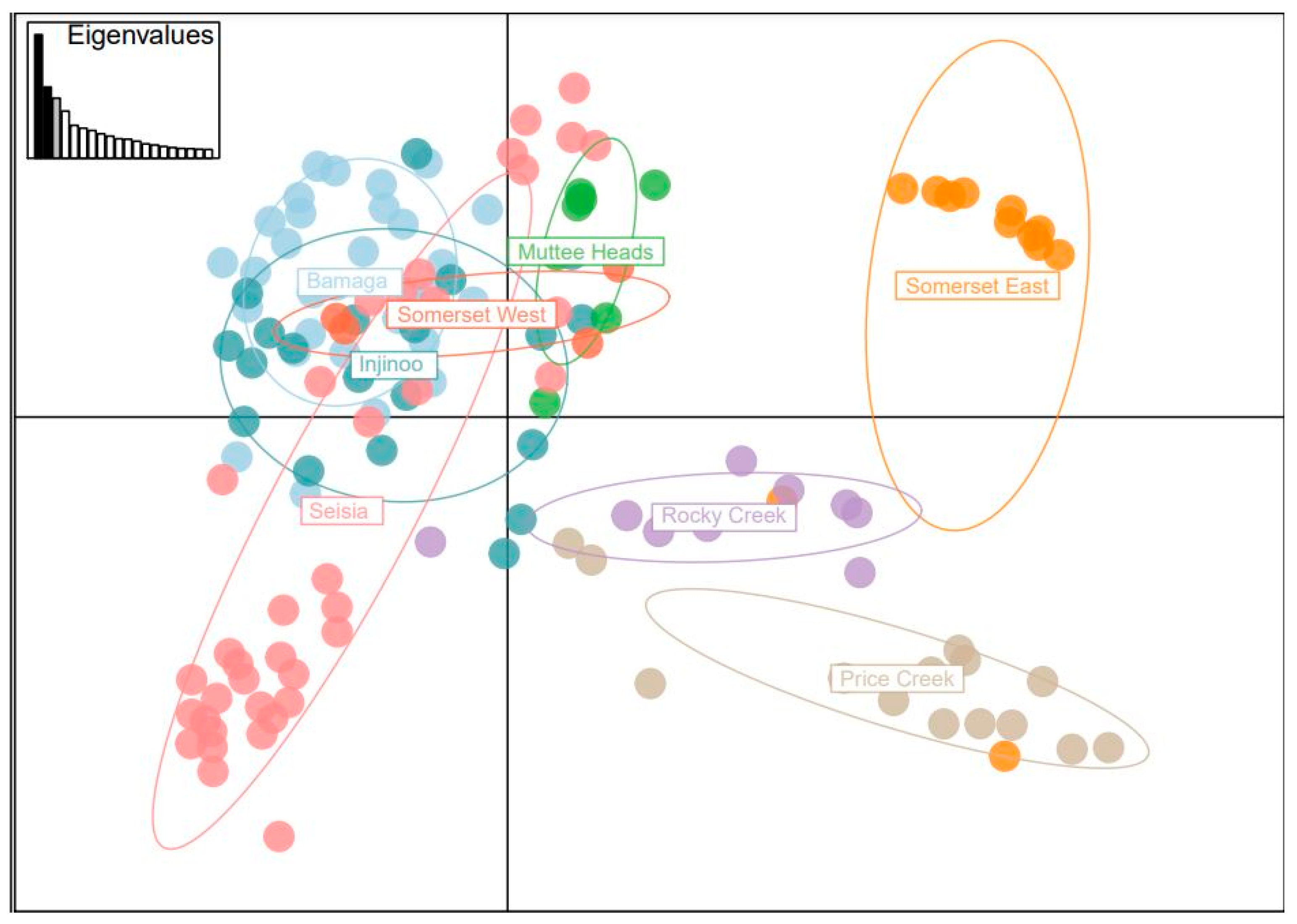
Our population genetic structure results highlighted the three central populations are genetically similar. Conversely, Somerset West and Muttee Heads appear to be genetically distinct from this central group but are still part of the broader NPA population group. Somerset East was an outlier of this group, with the PCoA and the NJ tree suggesting that Somerset East may be a genetic relative of
S. lanceolatum from southern Cape York. It is plausible that Somerset East is an artefact population that was grown from seed or plant material transported from outside the NPA. Alternatively, the genetics of the Somerset East population may suggest that there are two distinct
Santalum genotypes found in the greater Cape York area. In the adult age class, Seisia had a relatively low clonal structure (16%), indicating that this population may have historically contributed a disproportionate amount of geneflow between some populations. This could be another insight into what may have been a relatively similar structure before the current fragmentation and decline of populations. Muttee Heads is relatively genetically distinct with weak associations with Seisia and Injinoo. Seisia appears to have limited genetic relationships with Injinoo and Bamaga despite the population’s close proximity and lack of physical barriers limiting geneflow, suggesting pollen/seed dispersal has been restricted to the more coastal populations. Somerset East showed virtually no evidence of genetic association with the west coast populations. Interestingly, Somerset is mentioned in previous studies as an area where trees were commercially wild harvested for export [
20], suggesting a larger population existed before harvesting commenced and then depleted to a level where only a limited number of individuals (possibly non-commercial size trees and seedlings) remained. To investigate more precise relationships influencing geneflow, a thorough paternal analysis would be required by collecting wild seed. However, seed production across this species in the NPA is limited, with only nine seeds collected over a 3-year period [
10].
Generally, our results showed an overall negligible level of inbreeding despite the small number of trees found in the NPA and the isolation of populations. However, a number of studies have reported self-incompatible breeding mechanisms from
S. lanceolatum [
4,
26], which could explain our observations of limited inbreeding values. The limited homozygote excess observed in most populations may be a result of low natural recruitment as a result of frequent fires destroying seedlings, together with a clonal structure. However,
FST was relatively high between some age classes, for example, between Seisia adults and juveniles (
FST = 0.131), which indicates a recent increase in the extent of drift, causing greater genetic structure among juveniles than was operating on the adult cohort. Seisia is a site observed to be burnt frequently [
10] with repeated, intense fires, which may be interrupting flowering and fruiting cycles limiting effective geneflow between unrelated individuals [
51]. This is supported by the mean
PAR, which was significantly lower in the juvenile class. Therefore, while the overall evidence for inbreeding or loss of diversity is low between generations, the
PAR result (reflected in the
FST) might provide initial evidence of genetic erosion. Finally, in the context of age class, our results indicated a significant proportion of reproduction is vegetative with over 35% of juvenile trees from asexual reproduction. It may be that the clonal structure of northern sandalwood in the NPA is one feature of a complex life-history strategy to avoid mating between closely related individuals and persistence in an environment under frequent disturbance.
There are several conservation implications that have been identified in this study. One of the most significant findings is the extensive clonal structure of
S. lanceolatum in the NPA which may place the species at significant risk in this region. Fire is reported to have impacted adult northern sandalwood trees in the NPA with an overall 19% decline of the regional population of northern sandalwood over the last 8 years [
10]. If a severe event were to destroy more mature plants within these populations, the probability of pollen dispersal between unique genets would continue to decrease. The small population size and fragmented distribution of these populations would exacerbate this effect with increases in clonal propagation, decreases in sexual reproduction, further loss of genetic variation, and continued population decline expected. With this is mind, it is unlikely protecting these populations from further human mediated impacts alone will improve seed production and natural regeneration of the species. With the potential for five out of the six northern sandalwood populations in the NPA to be genetically distinct from other northern sandalwood populations, there is evidence to support conservation measures of what may be a genetically unique species.
Therefore, we believe a range of conservation strategies are necessary for northern sandalwood in the NPA. We recommend a genetic translocation program as per [
67] that captures 95% of the alleles from multiple source populations. This genetic material can be used to develop seedling stock to be replanted following genetic rescue guidelines [
67] into remnant source populations to increase genetic diversity and ultimately sexual reproduction of
S. lanceolatum in the NPA. As wild-open pollinated seed is scarce for this species, a clonal seed orchard is the suggested method of producing seedling stock for enrichment plantings into recipient populations.
Specifically, we recommend that the three central populations of Seisia, Bamaga and Injinoo, which are genetically closely related, which likely once formed a larger, continuous population of S. lanceolatum, should be the focus of the initial genetic translocation. Together, these three populations contain 56% (132 out of 234 alleles) of the alleles identified in this study, but only 42–47 alleles in each population. Target genotypes from these three populations should be selected to ensure that 95% of the genetic diversity found in these three combined populations is captured and subsequently distributed within each population to increase local population genetic diversity by 20%. We also recommend that Muttee Heads and Somerset West populations should be managed separately as a conservation priority as there is evidence to suggest they are differentiated from the three central populations. The genetic bank that would result via the initial genetic capture would not only help to protect NPA S. lanceolatum, but would also allow further research aiming to conserve the species. The urgency of these activities is highlighted by the fact that since our study was completed, the Somerset East population has been badly damaged by land clearing during a road-widening project in the area.
Several studies have identified important phylogenetic relationships of the
Santalum taxa [
16,
68]. However, there is evidence from our study to suggest the taxonomy of
S. lanceolatum may not yet be resolved and warrants further investigation. Our results indicate that Somerset East may not be a genetic representative of
S. lanceolatum from the NPA. All analyses highlight that Somerset East has a genetic profile closely related to southern Cape York populations. This is especially surprising given that Somerset West is only 5.5 km to the west of Somerset East. The PCoA, NJ tree and genetic group cluster analyses highlighted that the five other NPA populations (Bamaga, Seisia, Injinoo, Muttee Heads and Somerset West) may form yet another cryptic, distinct species within the
Santalum lanceolatum clade. However, acknowledging the limitations of inferring phylogeny from microsatellite markers, we suggest clearer conclusions of the genetic relationships of
S. lanceolatum both in the context of the species and the genus, additional analyses from multiple populations across a much broader range of northern Australia, using genome-wide markers such as SNPs is required.