New Representation of Plant Hydraulics Improves the Estimates of Transpiration in Land Surface Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Model Description
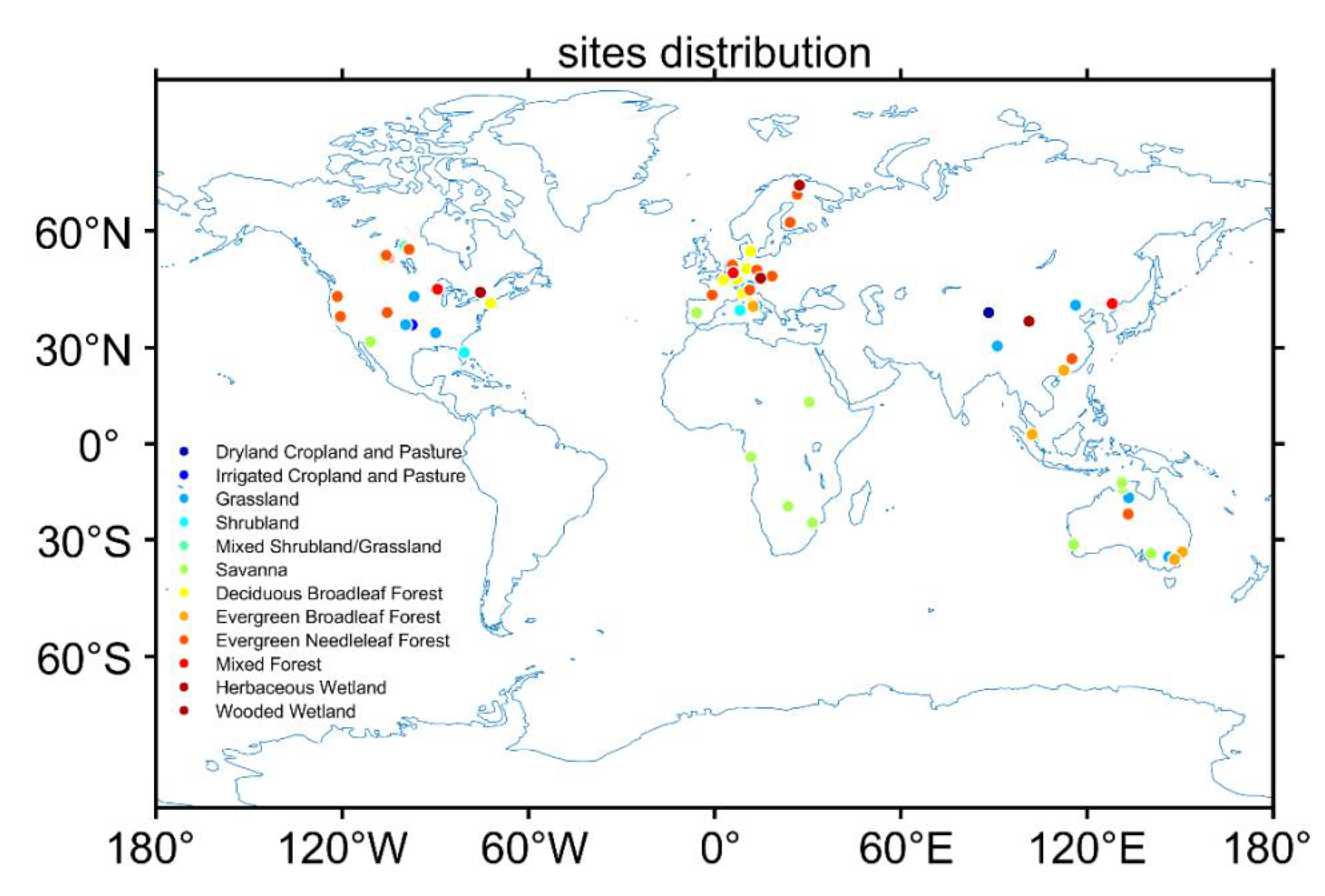
2.2. Forcing Data from FLUXNET Observations
2.3. Validation Datasets
2.4. Model Benchmarching
2.4.1. Bias Score
2.4.2. Root Mean Square Error (RMSE) Score
3. Results
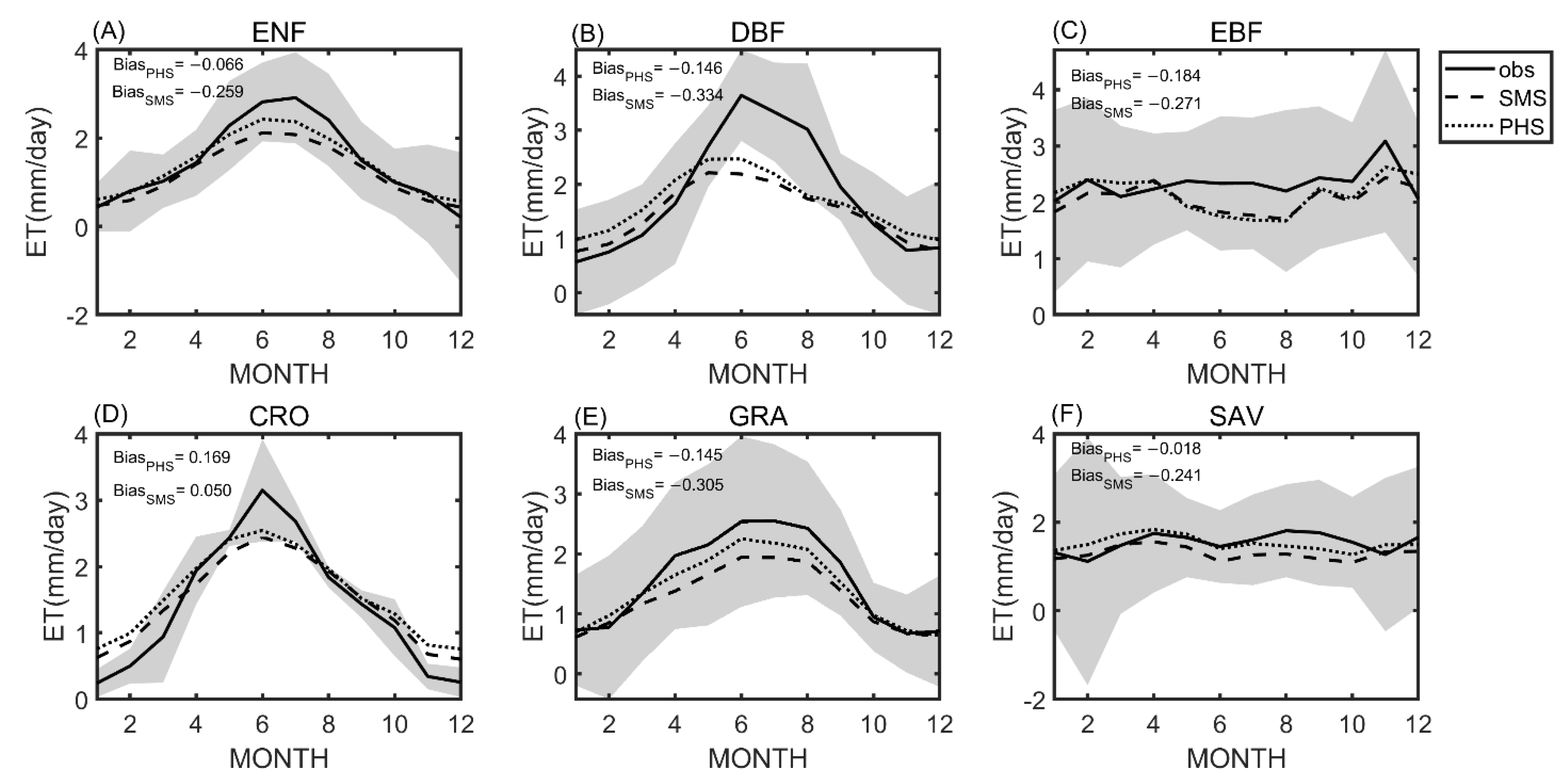
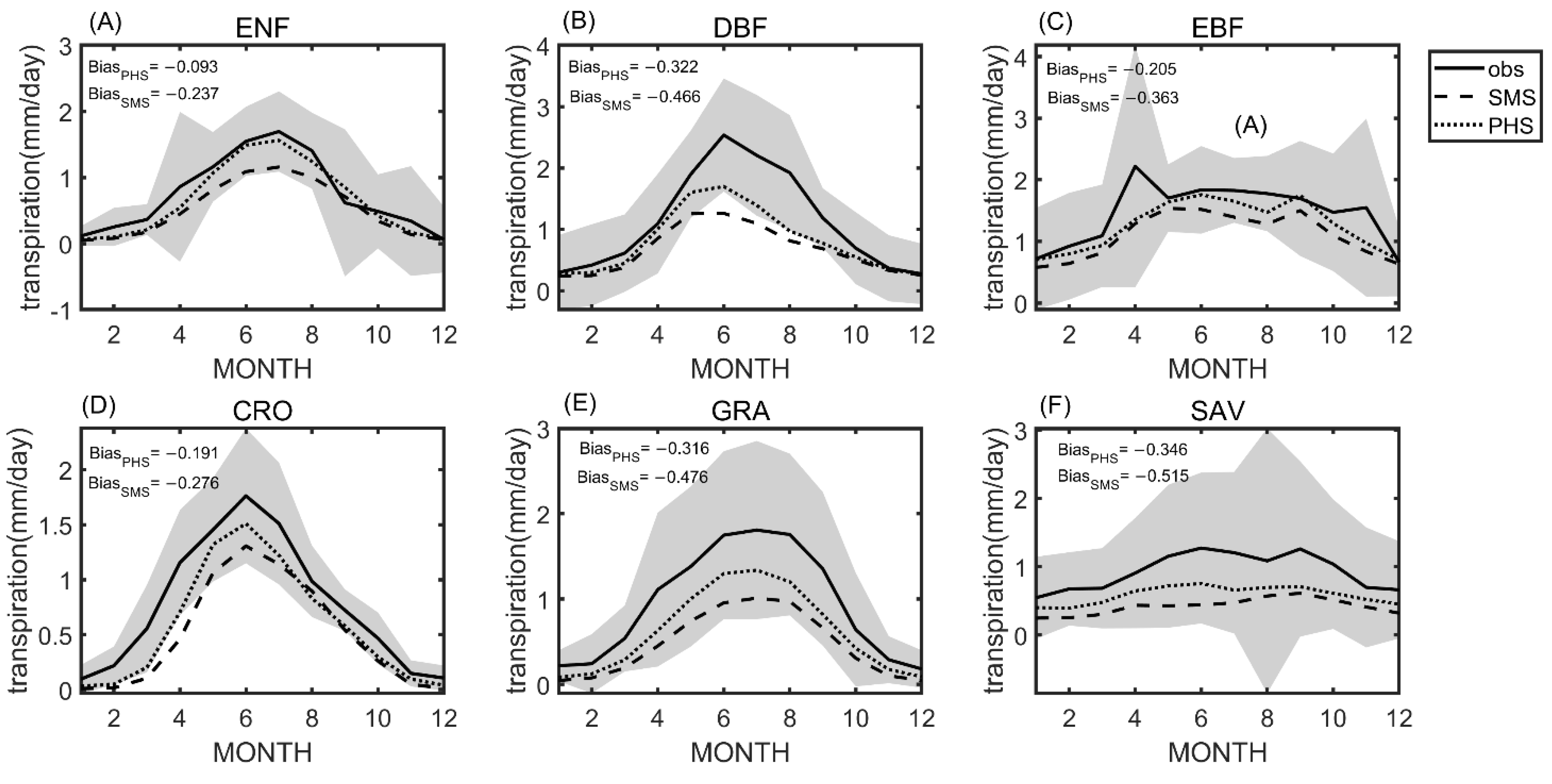
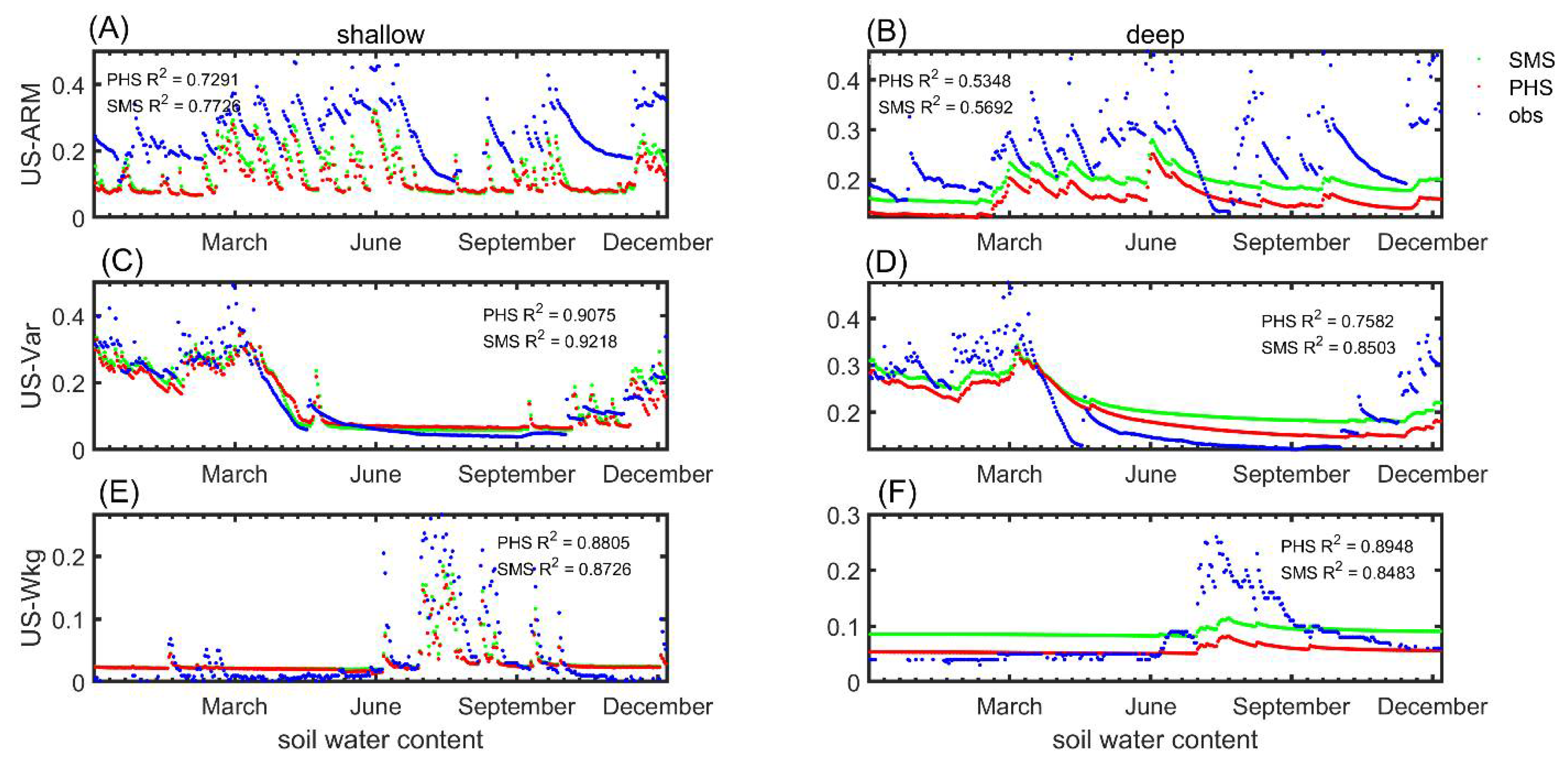
3.1. Seasonal Variation
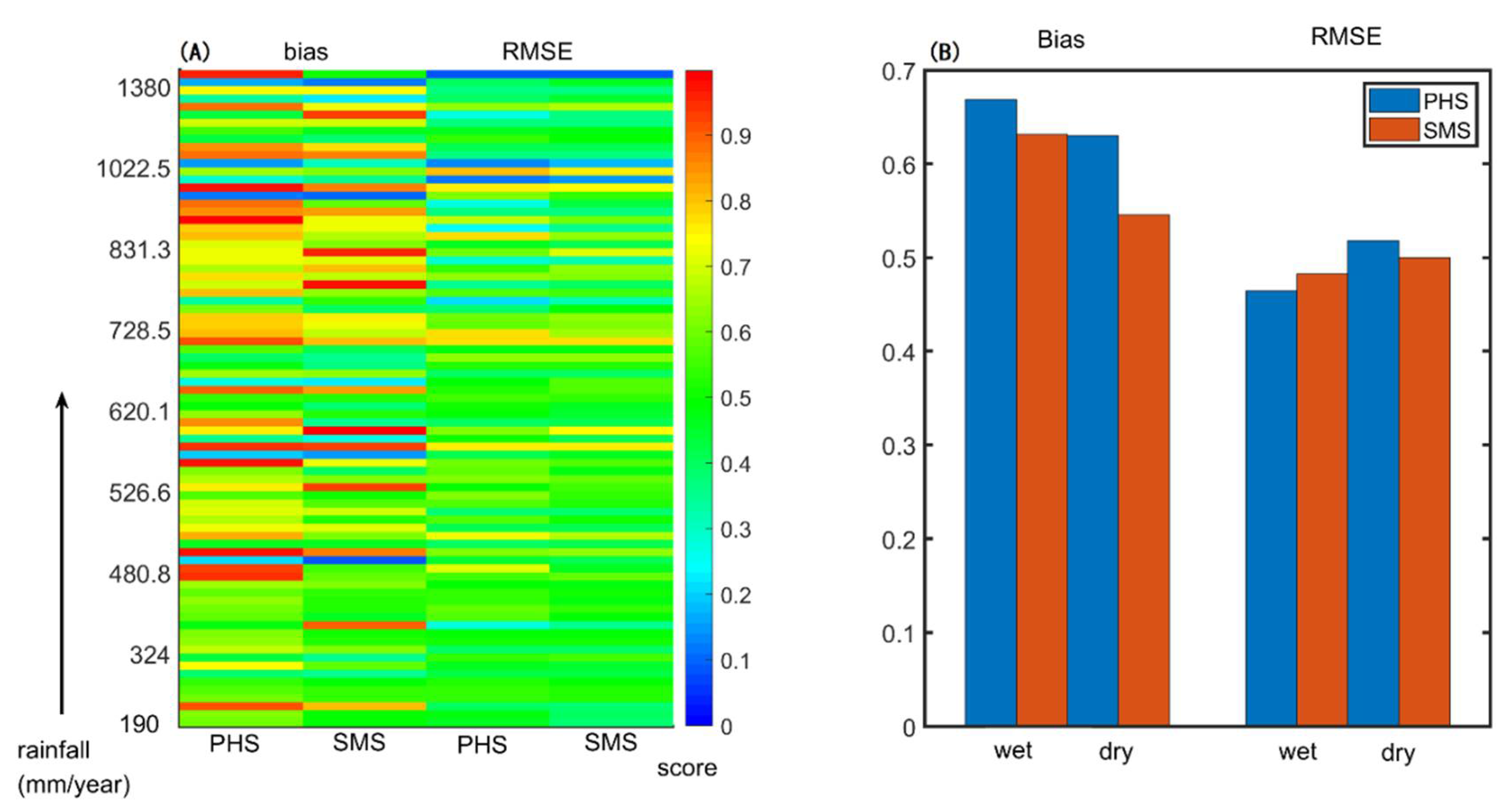
3.2. Benchmarking Analysis
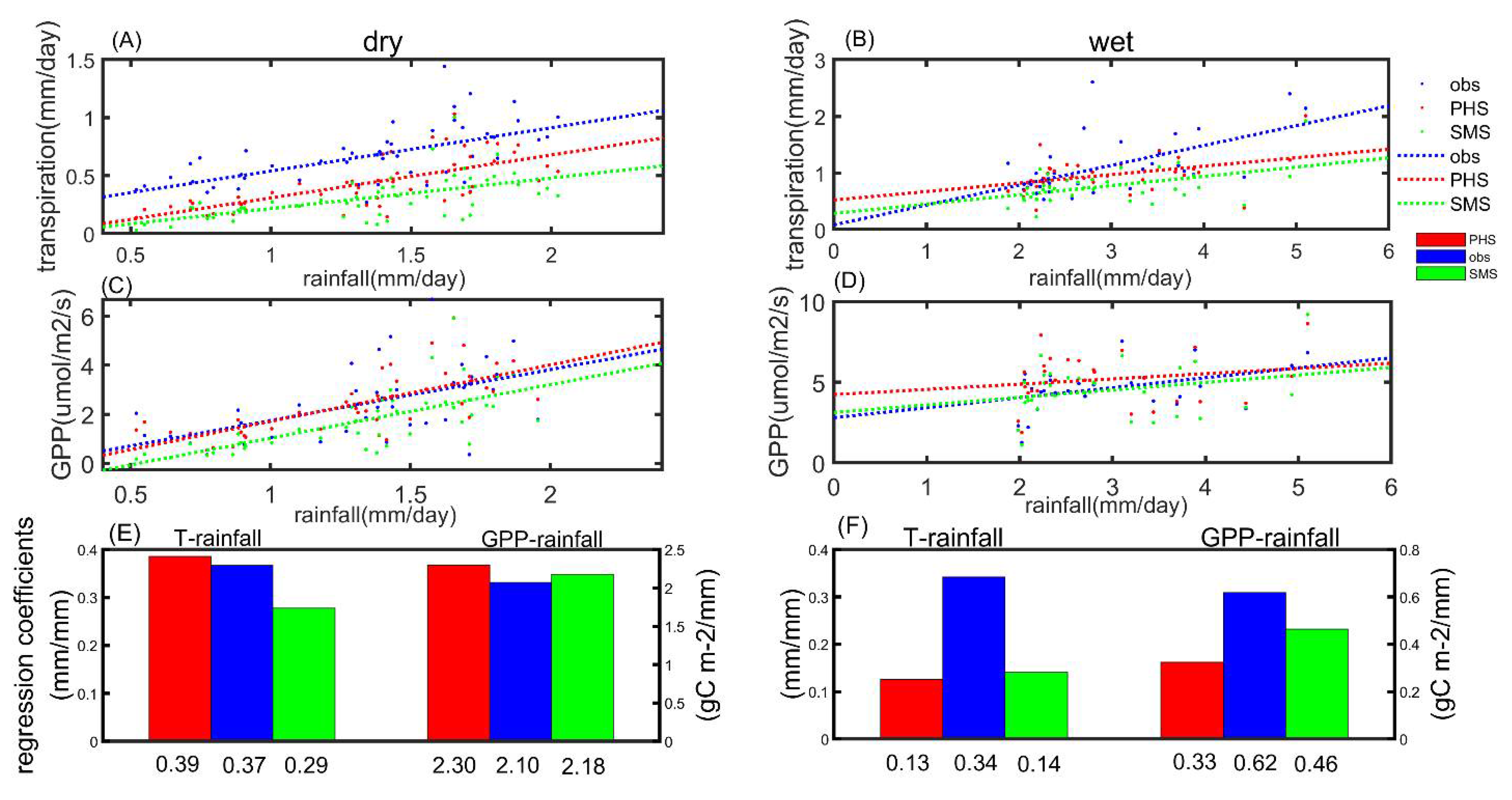
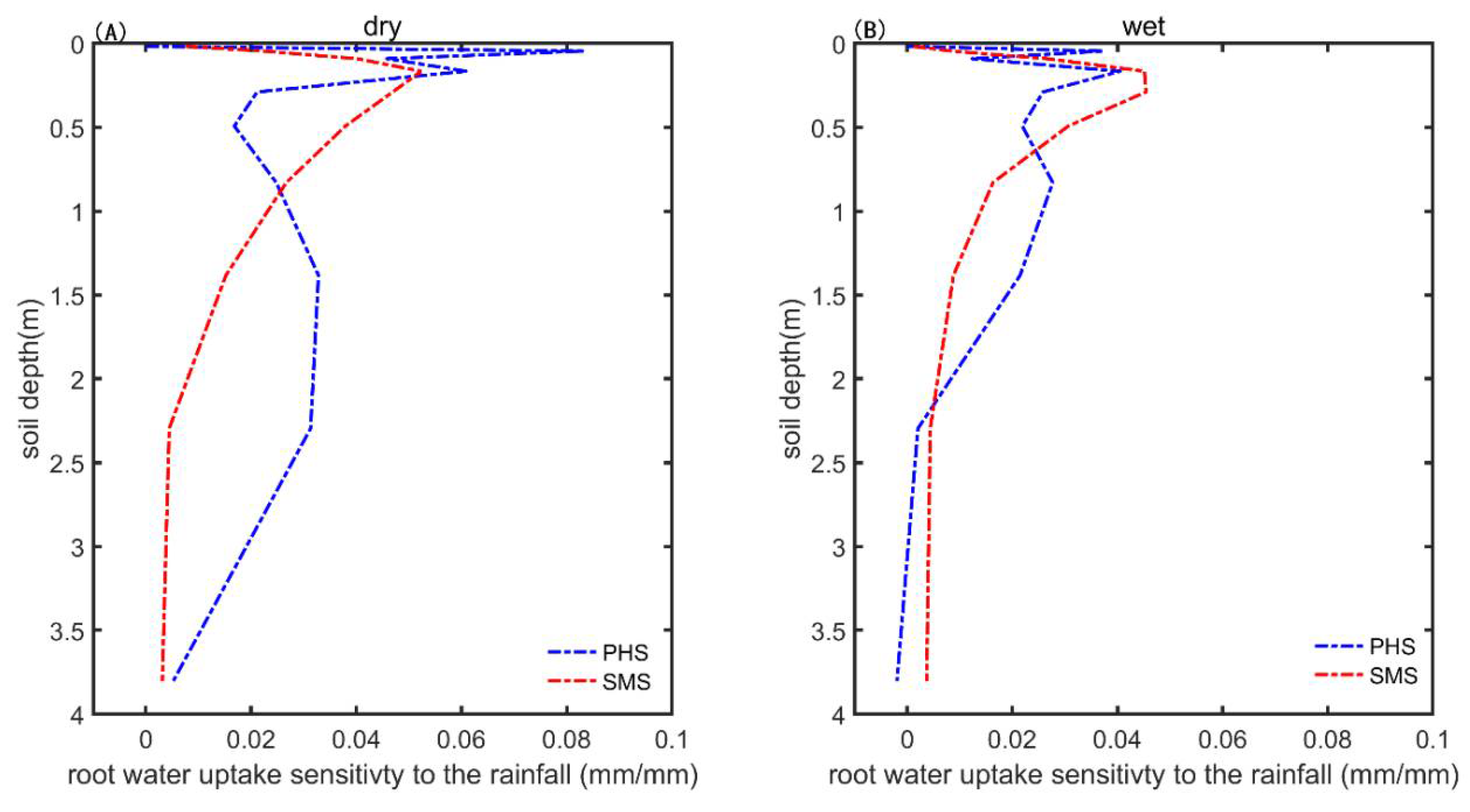
3.3. The Drought Sensitivity
4. Discussion
4.1. Benchmarking Analysis on Transpiration
4.2. Plant Hydraulic Schemes (PHS) Improved the Evapotranspiration Partitioning
4.3. Hydraulic Redistribution (HR) Implementation and the Implication
4.4. PHS Improved the Transpiration Modeling in Arid or Semi-Arid Regions
4.5. Uncertainties Sources
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
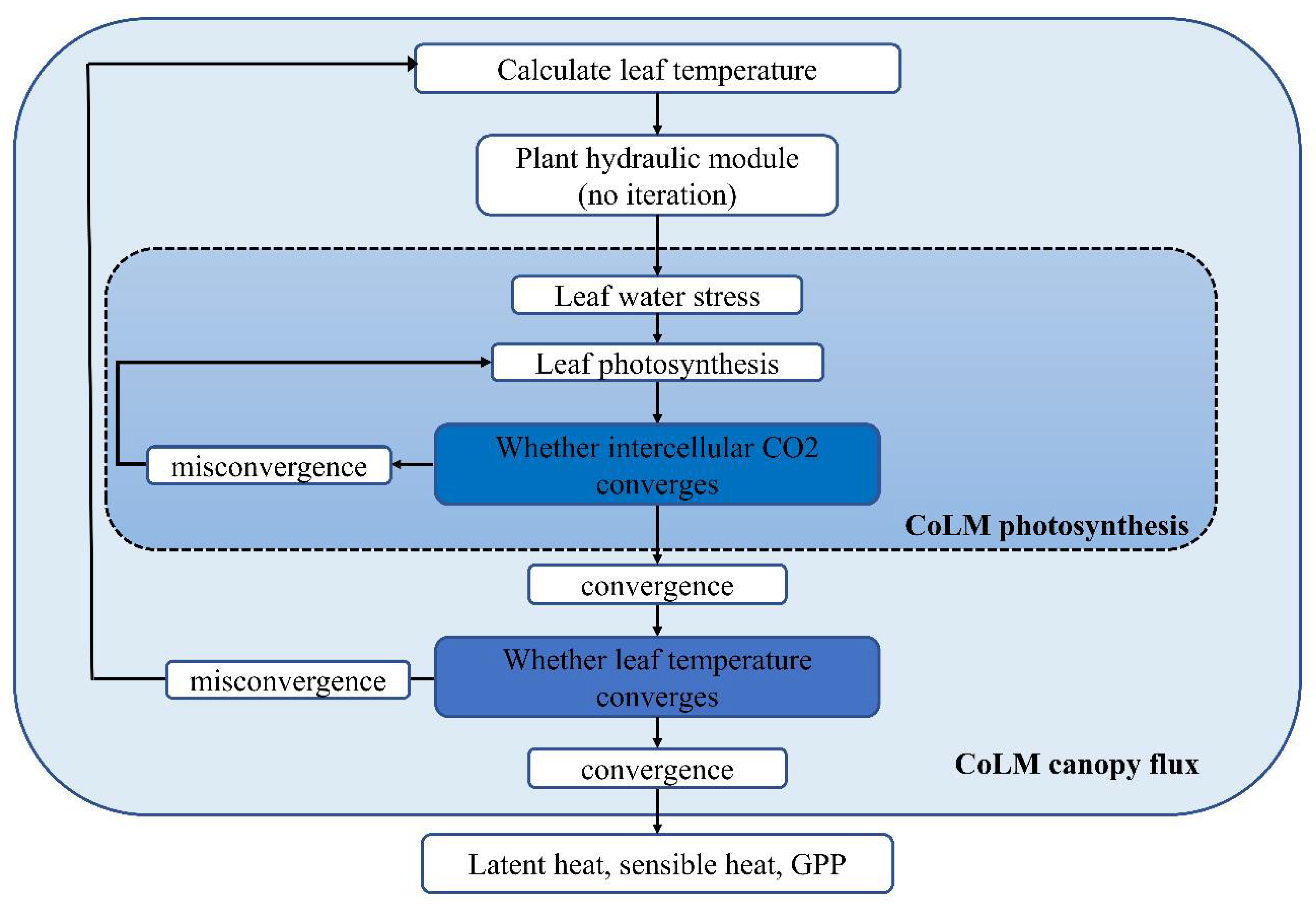
Appendix A. Processes and Parameterizations in the Plant Hydraulic Module
Appendix B. PHS Solution
- (1)
- calculate the leaf temperature based on canopy model (, ).
- (2)
- calculate the maximum stomatal conductance based on photosynthetic stomata model ( and ).
- (3)
- calculate the maximum leaf transpiration rate according to the maximum stomatal conductance ( and ).
- (4)
- calculate the water stress ().
- (5)
- update the water stress in the photosynthetic stomata model, and check whether the intercellular carbon dioxide concentration is converged. If it converges, go to step (6); Otherwise, repeat step (5).
- (6)
- update the stomata conductance, and check whether the water stress converges. If it converges, go to step (7); otherwise, go back to step (2);
- (7)
- update the plant water potential and leaf transpiration, and check whether the leaf temperatemperature based on canopy model n the plant hydraulic model are solved. Otherwise, go back to step (1).
| Plant Hydraulic Parameter | Parameter Name | Description | Land Surface Cover Type | Values | Units |
|---|---|---|---|---|---|
| P50 | Water potential at 50% loss of leaf tissue conductance | Evergreen Needleleaf Forest | −465,000.0 | mmH2O | |
| Deciduous Broadleaf Forest | −270,000.0 | ||||
| Evergreen Broadleaf Forest | −260,000.0 | ||||
| Cropland | −340,000.0 | ||||
| Grassland | −340,000.0 | ||||
| Savanna | −340,000.0 | ||||
| h | Canopy top | Evergreen Needleleaf Forest | 17.0 | m | |
| Deciduous Broadleaf Forest | 19.3 | ||||
| Evergreen Broadleaf Forest | 35.0 | ||||
| Cropland | 0.5 | ||||
| Grassland | 0.5 | ||||
| Savanna | 0.5 | ||||
| Kmax | Maximum hydraulic conductance | ALL | 2.010−8 | s−1 | |
| Ck | Shape-fitting parameter for vulnerability curve | ALL | 3.95 | ||
| Stomatal Conductance Parameter | Parameter name | Description | Plant type | Values | Units |
| G0 | Conductance-photosynthesis interception | C3 | 9.0 | ||
| C4 | 4.0 | ||||
| α1 | Conductance-photosynthesis slope parameter | C3 | 0.01 | mol CO2·m−2·s−1 | |
| C4 | 0.04 |
References
- Good, S.P.; Noone, D.; Bowen, G. Hydrologic connectivity constrains partitioning of global terrestrial water fluxes. Science 2015, 349, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yoshimura, K.; Wang, L.; Miralles, D.G.; Jasechko, S.; Lee, X. Revisiting the contribution of transpiration to global terrestrial evapotranspiration. Geophys. Res. Lett. 2017, 44, 2792–2801. [Google Scholar] [CrossRef]
- Sutanto, S.J.; Wenninger, J.; Coenders-Gerrits, A.M.J.; Uhlenbrook, S. Partitioning of evaporation into transpiration, soil evaporation and interception: A comparison between isotope measurements and a HYDRUS-1D model. Hydrol. Earth Syst. Sci. 2012, 16, 2605–2616. [Google Scholar] [CrossRef]
- Coenders-Gerrits, A.M.J.; Van Der Ent, R.J.; Bogaard, T.A.; Wang-Erlandsson, L.; Hrachowitz, M.; Savenije, H.H.G. Uncertainties in transpiration estimates. Nature 2014, 506, E1–E2. [Google Scholar] [CrossRef] [PubMed]
- Jasechko, S.; Sharp, Z.D.; Gibson, J.J.; Birks, S.J.; Yi, Y.; Fawcett, P.J. Terrestrial water fluxes dominated by transpiration. Nature 2013, 496, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, R.M.; Condon, L.E. Connections between groundwater flow and transpiration partitioning. Science 2016, 353, 377–380. [Google Scholar] [CrossRef]
- Miralles, D.G.; Jiménez, C.; Jung, M.; Michel, D.; Ershadi, A.; McCabe, M.F.; Hirschi, M.; Martens, B.; Dolman, A.J.; Fisher, J.B.; et al. The WACMOS-ET project–Part 2: Evaluation of global terrestrial evaporation data sets. Hydrol. Earth Syst. Sci. 2016, 20, 823–842. [Google Scholar] [CrossRef]
- Dirmeyer, P.A.; Gao, X.; Zhao, M.; Guo, Z.; Oki, T.; Hanasaki, N. GSWP-2: Multimodel analysis and implications for our perception of the land surface. Bull. Am. Meteorol. Soc. 2006, 87, 1381–1397. [Google Scholar] [CrossRef]
- Lawrence, D.M.; Oleson, K.W.; Flanner, M.G.; Thornton, P.E.; Swenson, S.C.; Lawrence, P.J.; Zeng, X.; Yang, Z.-L.; Levis, S.; Sakaguchi, K.; et al. Parameterization improvements and functional and structural advances in Version 4 of the Community Land Model. J. Adv. Model. Earth Syst. 2011, 3, 1–27. [Google Scholar]
- Lawrence, D.M.; Thornton, P.E.; Oleson, K.W.; Bonan, G.B. The Partitioning of Evapotranspiration into Transpiration, Soil Evaporation, and Canopy Evaporation in a GCM: Impacts on Land?Atmosphere Interaction. J. Hydrometeorol. 2007, 8, 862–880. [Google Scholar] [CrossRef]
- Yoshimura, K.; Miyazaki, S.; Kanae, S.; Oki, T. Iso-MATSIRO, a land surface model that incorporates stable water isotopes. Glob. Planet. Chang. 2006, 51, 90–107. [Google Scholar] [CrossRef]
- Wang-Erlandsson, L.; Van Der Ent, R.J.; Gordon, L.J.; Savenije, H.H.G. Contrasting roles of interception and transpiration in the hydrological cycle-Part 1: Temporal characteristics over land. Earth Syst. Dyn. 2014, 5, 441–469. [Google Scholar] [CrossRef]
- Oleson, K.W.; Lawrence, D.M.; Bonan, G.B.; Drewniak, B.; Huang, M.; Charles, D.; Levis, S.; Li, F.; Riley, W.J.; Zachary, M.; et al. Technical Description of Version 4.5 of the Community Land Model (CLM); NCAR Technical Note NCAR: Boulder, CO, USA, 2013. [Google Scholar]
- Kennedy, D.; Swenson, S.; Oleson, K.W.; Lawrence, D.M.; Fisher, R.; Lola da Costa, A.C.; Gentine, P. Implementing Plant Hydraulics in the Community Land Model, Version 5. J. Adv. Model. Earth Syst. 2019, 11, 485–513. [Google Scholar] [CrossRef]
- Klein, T.; Rotenberg, E.; Cohen-Hilaleh, E.; Raz-Yaseef, N.; Tatarinov, F.; Preisler, Y.; Ogée, J.; Cohen, S.; Yakir, D. Quantifying transpirable soil water and its relations to tree water use dynamics in a water-limited pine forest. Ecohydrology 2014, 7, 409–419. [Google Scholar] [CrossRef]
- Nadezhdina, N.; David, T.S.; David, J.S.; Ferreira, M.I.; Dohnal, M.; Tesař, M.; Gartner, K.; Leitgeb, E.; Nadezhdin, V.; Cermak, J.; et al. Trees never rest: The multiple facets of hydraulic redistribution. Ecohydrology 2010, 3, 431–444. [Google Scholar] [CrossRef]
- Schulze, E.D.; Caldwell, M.M.; Canadell, J.; Mooney, H.A.; Jackson, R.B.; Parson, D.; Scholes, R.; Sala, O.E.; Trimborn, P. Downward flux of water through roots (i.e., inverse hydraulic lift) in dry Kalahari sands. Oecologia 1998, 115, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Ryel, R.J.; Caldwell, M.M.; Yoder, C.K.; Or, D.; Leffler, A.J. Hydraulic redistribution in a stand of Artemisia tridentata: Evaluation of benefits to transpiration assessed with a simulation model. Oecologia 2002, 130, 173–184. [Google Scholar] [CrossRef]
- Amenu, G.G.; Kumar, P. A model for hydraulic redistribution incorporating coupled soil-root moisture transport. Hydrol. Earth Syst. Sci. 2008, 12, 55–74. [Google Scholar] [CrossRef]
- Poyatos, R.; Granda, V.; Flo, V.; Adams, M.A.; Oliveira, R.S. Global transpiration data from sap flow measurements: The SAPFLUXNET database. Earth Syst. Sci. Data Discuss. 2020, 1–57. [Google Scholar] [CrossRef]
- Pastorello, G.; Trotta, C.; Canfora, E.; Chu, H.; Christianson, D.; Cheah, Y.-W.; Poindexter, C.; Chen, J.; Elbashandy, A.; Humphrey, M.; et al. The FLUXNET2015 dataset and the ONEFlux processing pipeline for eddy covariance data. Sci. Data 2020, 7, 225. [Google Scholar] [CrossRef]
- Bonan, G.B. Land Surface Model (LSM Version 1.0) for Ecological, Hydrological, and Atmospheric Studies: Technical Description and User’s Guide; Technical note; Climate and Global Dynamics Division; National Center for Atmospheric Research: Boulder, CO, USA, 1996. [Google Scholar]
- Dai, Y.; Dickinson, R.E.; Wang, Y.-P. A Two-Big-Leaf Model for Canopy Temperature, Photosynthesis, and Stomatal Conductance. J. Clim. 2004, 17, 2281–2299. [Google Scholar] [CrossRef]
- Dai, Y.; Zeng, X.; Dickinson, R.E.; Baker, I.; Bonan, G.B.; Bosilovich, M.G.; Denning, A.S.; Dirmeyer, P.A.; Houser, P.R.; Niu, G.; et al. The common land model. Bull. Am. Meteorol. Soc. 2003, 84, 1013–1023. [Google Scholar] [CrossRef]
- Klein, T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- Wilson, K.; Goldstein, A.; Falge, E.; Aubinet, M.; Verma, S. Energy balance closure at FLUXNET sites. Agric. Meteorol. 2002, 113, 223–243. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, Y.; Cui, H.; Dickinson, R.E.; Zhu, S.; Wei, N.; Yan, B.; Yuan, H.; Shangguan, W.; Wang, L.; et al. Evaluating common land model energy fluxes using FLUXNET data. Adv. Atmos. Sci. 2017, 34, 1035–1046. [Google Scholar] [CrossRef]
- Nelson, J.A.; Pérez-Priego, O.; Zhou, S.; Poyatos, R.; Zhang, Y.; Blanken, P.D.; Gimeno, T.E.; Wohlfahrt, G.; Desai, A.R.; Gioli, B. Ecosystem transpiration and evaporation: Insights from three water flux partitioning methods across FLUXNET sites. Glob. Chang. Biol. 2020, 26, 6916–6930. [Google Scholar] [CrossRef]
- Yuan, H.; Dai, Y.; Xiao, Z.; Ji, D.; Shangguan, W. Reprocessing the MODIS Leaf Area Index products for land surface and climate modelling. Remote Sens. Environ. 2011, 115, 1171–1187. [Google Scholar] [CrossRef]
- Collier, N.; Hoffman, F.M.; Lawrence, D.M.; Keppel-Aleks, G.; Koven, C.D.; Riley, W.J.; Mu, M.; Randerson, J.T. The International Land Model Benchmarking (ILAMB) System: Design, Theory, and Implementation. J. Adv. Model. Earth Syst. 2018, 10, 2731–2754. [Google Scholar] [CrossRef]
- Luo, Y.Q.; Randerson, J.T.; Abramowitz, G.; Bacour, C.; Blyth, E.; Carvalhais, N.; Ciais, P.; Dalmonech, D.; Fisher, J.B.; Fisher, R.; et al. A framework for benchmarking land models. In Biogeosciences; Copernicus Publications: Göttingen, Germany, 2012; Volumn 9, pp. 3857–3874. [Google Scholar]
- Caldwell, M.M.; Dawson, T.E.; Richards, J.H. Hydraulic lift: Consequences of water efflux from the roots of plants. Oecologia 1998, 113, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.B.; Sperry, J.S.; Dawson, T.E. Root water uptake and transport: Using physiological processes in global predictions. Trends Plant Sci. 2000, 5, 482–488. [Google Scholar] [CrossRef]
- Fu, C.; Wang, G.; Bible, K.; Goulden, M.L.; Saleska, S.R.; Scott, R.L.; Cardon, Z.G. Hydraulic redistribution affects modeled carbon cycling via soil microbial activity and suppressed fire. Glob. Chang. Biol. 2018, 24, 3472–3485. [Google Scholar] [CrossRef]
- Fu, C.; Lee, X.; Griffis, T.J.; Wang, G.; Wei, Z. Influences of Root Hydraulic Redistribution on N2O Emissions at AmeriFlux Sites. Geophys. Res. Lett. 2018, 45, 5135–5143. [Google Scholar] [CrossRef]
- Neumann, R.B.; Cardon, Z.G. The magnitude of hydraulic redistribution by plant roots: A review and synthesis of empirical and modeling studies. New Phytol. 2012, 194, 337–352. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, H.; Zhang, X.; Wei, N.; Shangguan, W.; Yuan, H.; Zhang, S.; Wang, L.; Zhou, L.; Dai, Y. Incorporating root hydraulic redistribution and compensatory water uptake in the common land model: Effects on site level and global land modeling. J. Geophys. Res. 2017, 122, 7308–7322. [Google Scholar] [CrossRef]
- Lawrence, D.; Fisher, R.; Koven, C.; Oleson, K.; Swenson, S.; Vertenstein, M. CLM5 Documentation; NCAR Technical Note NCAR: Boulder, CO, USA, 2018. [Google Scholar]
- Bisht, G.; Riley, W.J.; Hammond, G.E.; Lorenzetti, D.M. Development and evaluation of a variably saturated flow model in the global E3SM Land Model (ELM) version 1.0. Geosci. Model. Dev. 2018, 11, 4085–4102. [Google Scholar] [CrossRef]
- Dai, Y.; Wei, N.; Yuan, H.; Zhang, S.; Shangguan, W.; Liu, S.; Lu, X.; Xin, Y. Evaluation of soil thermal conductivity schemes for use in land surface modeling. J. Adv. Modeling Earth Syst. 2019, 11, 3454–3473. [Google Scholar] [CrossRef]
- Baroni, G.; Facchi, A.; Gandolfi, C.; Ortuani, B.; Horeschi, D.; Van Dam, J.C. Uncertainty in the determination of soil hydraulic parameters and its influence on the performance of two hydrological models of different complexity. Hydrol. Earth Syst. Sci. Discuss. 2009, 6, 4065–4105. [Google Scholar]
- Christiaens, K.; Feyen, J. Analysis of uncertainties associated with different methods to determine soil hydraulic properties and their propagation in the distributed hydrological MIKE SHE model. J. Hydrol. 2001, 246, 63–81. [Google Scholar] [CrossRef]
- Freer, J.; Beven, K.; Ambroise, B. Bayesian Estimation of Uncertainty in Runoff Prediction and the Value of Data: An Application of the GLUE Approach. Water Resour. Res. 1996, 32, 2161–2173. [Google Scholar] [CrossRef]
- Walker, J.P.; Willgoose, G.R.; Kalma, J.D. In situ measurement of soil moisture: A comparison of techniques. J. Hydrol. 2004, 293, 85–99. [Google Scholar] [CrossRef]
- Dai, Y.; Xin, Q.; Wei, N.; Zhang, Y.; Shangguan, W.; Yuan, H.; Zhang, S.; Liu, S.; Lu, X. A global high-resolution data set of soil hydraulic and thermal properties for land surface modeling. J. Adv. Modeling Earth Syst. 2019, 11, 2996–3023. [Google Scholar] [CrossRef]
- Cai, W.; Wang, G.; Santoso, A.; Mcphaden, M.J.; Wu, L.; Jin, F.F.; Timmermann, A.; Collins, M.; Vecchi, G.; Lengaigne, M.; et al. Increased frequency of extreme La Niña events under greenhouse warming. Nat. Clim. Chang. 2015, 5, 132–137. [Google Scholar] [CrossRef]
- Jiménez-Muñoz, J.C.; Mattar, C.; Barichivich, J.; Santamaría-Artigas, A.; Takahashi, K.; Malhi, Y.; Sobrino, J.A.; Schrier, G. van der Record-breaking warming and extreme drought in the Amazon rainforest during the course of El Niño 2015–2016. Sci. Rep. 2016, 6, 33130. [Google Scholar] [CrossRef]
- Spinoni, J.; Vogt, J.V.; Naumann, G.; Barbosa, P.; Dosio, A. Will drought events become more frequent and severe in Europe? Int. J. Clim. 2018, 38, 1718–1736. [Google Scholar] [CrossRef]
- Zhang, M.; He, J.; Wang, B.; Wang, S.; Li, S.; Liu, W.; Ma, X. Extreme drought changes in Southwest China from 1960 to 2009. J. Geogr. Sci. 2013, 23, 3–16. [Google Scholar] [CrossRef]
- Cox, P.M.; Betts, R.A.; Jones, C.D.; Spall, S.A.; Totterdell, I.J. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 2000, 408, 184–187. [Google Scholar] [CrossRef]
- Cox, P.M.; Betts, R.A.; Collins, M.; Harris, P.P.; Huntingford, C.; Jones, C.D. Amazonian forest dieback under climate-carbon cycle projections for the 21st century. Appl. Clim. 2004, 78, 137–156. [Google Scholar] [CrossRef]
- Feeley, K.J.; Rehm, E.M. Amazon’s vulnerability to climate change heightened by deforestation and man-made dispersal barriers. Glob. Chang. Biol. 2012, 18, 3606–3614. [Google Scholar] [CrossRef]
- Wilson, K.B.; Hanson, P.J.; Baldocchi, D.D. Factors controlling evaporation and energy partitioning beneath a deciduous forest over an annual cycle. Agric. For. Meteorol. 2000, 102, 83–103. [Google Scholar]
- Kelliher, F.M.; Hollinger, D.Y.; Schulze, E.; Vygodskaya, N.N.; Byers, J.N.; Hunt, J.E.; Mcseveny, T.M.; Milukova, I.; Sogatchev, A.; Varlargin, A. Evaporation from an eastern Siberian larch forest. Agric. For. Meteorol. 1997, 85, 135–147. [Google Scholar]
- Gentine, P.; Guérin, M.; Uriarte, M.; McDowell, N.G.; Pockman, W.T. An allometry-based model of the survival strategies of hydraulic failure and carbon starvation. Ecohydrology 2016, 9, 529–546. [Google Scholar] [CrossRef]
- Neufeld, H.S.; Grantz, D.A.; Meinzer, F.C.; Goldstein, G.; Crisosto, G.M.; Crisosto, C. Genotypic Variability in Vulnerability of Leaf Xylem to Cavitation in Water-Stressed and Well-Irrigated Sugarcane. Plant Physiol. 1992, 100, 1020–1028. [Google Scholar] [CrossRef]
- Pammenter, N.W.; Van der Willigen, C. A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiol. 1998, 18, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Plaut, J.A.; Yepez, E.A.; Hill, J.; Pangle, R.; Sperry, J.S.; Pockman, W.T.; Mcdowell, N.G. Hydraulic limits preceding mortality in a piñon–juniper woodland under experimental drought. Plant Cell Environ. 2012, 35, 1601–1617. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.S.; Tyree, M.T. Mechanism of Water Stress-Induced Xylem Embolism. Plant Physiol. 1988, 88, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.M.; Fisher, R.A.; Koven, C.D.; Oleson, K.W.; Swenson, S.C.; Bonan, G.; Collier, N.; Ghimire, B.; van Kampenhout, L.; Kennedy, D.; et al. The Community Land Model Version 5: Description of New Features, Benchmarking, and Impact of Forcing Uncertainty. J. Adv. Model. Earth Syst. 2019, 11, 4245–4287. [Google Scholar] [CrossRef]
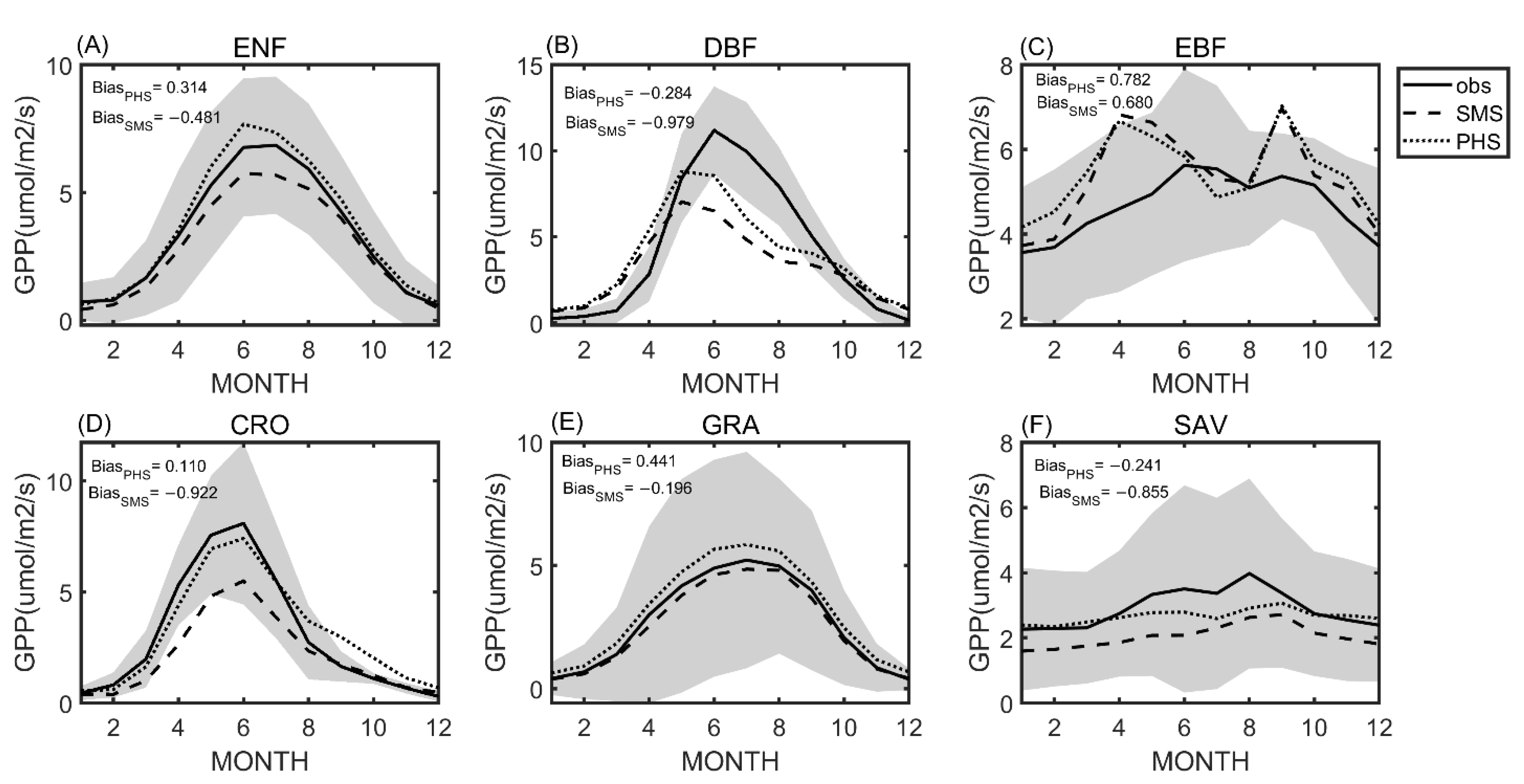
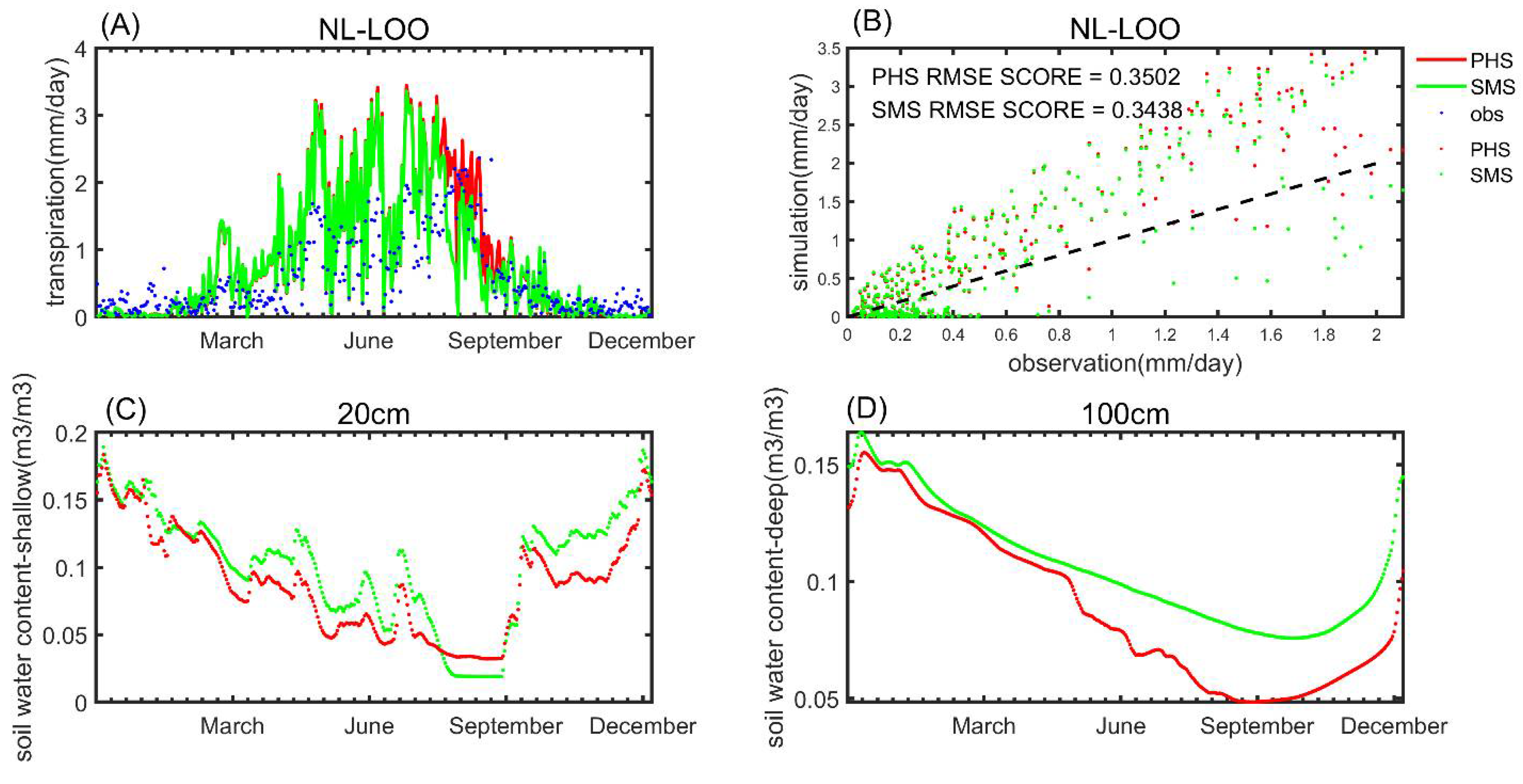
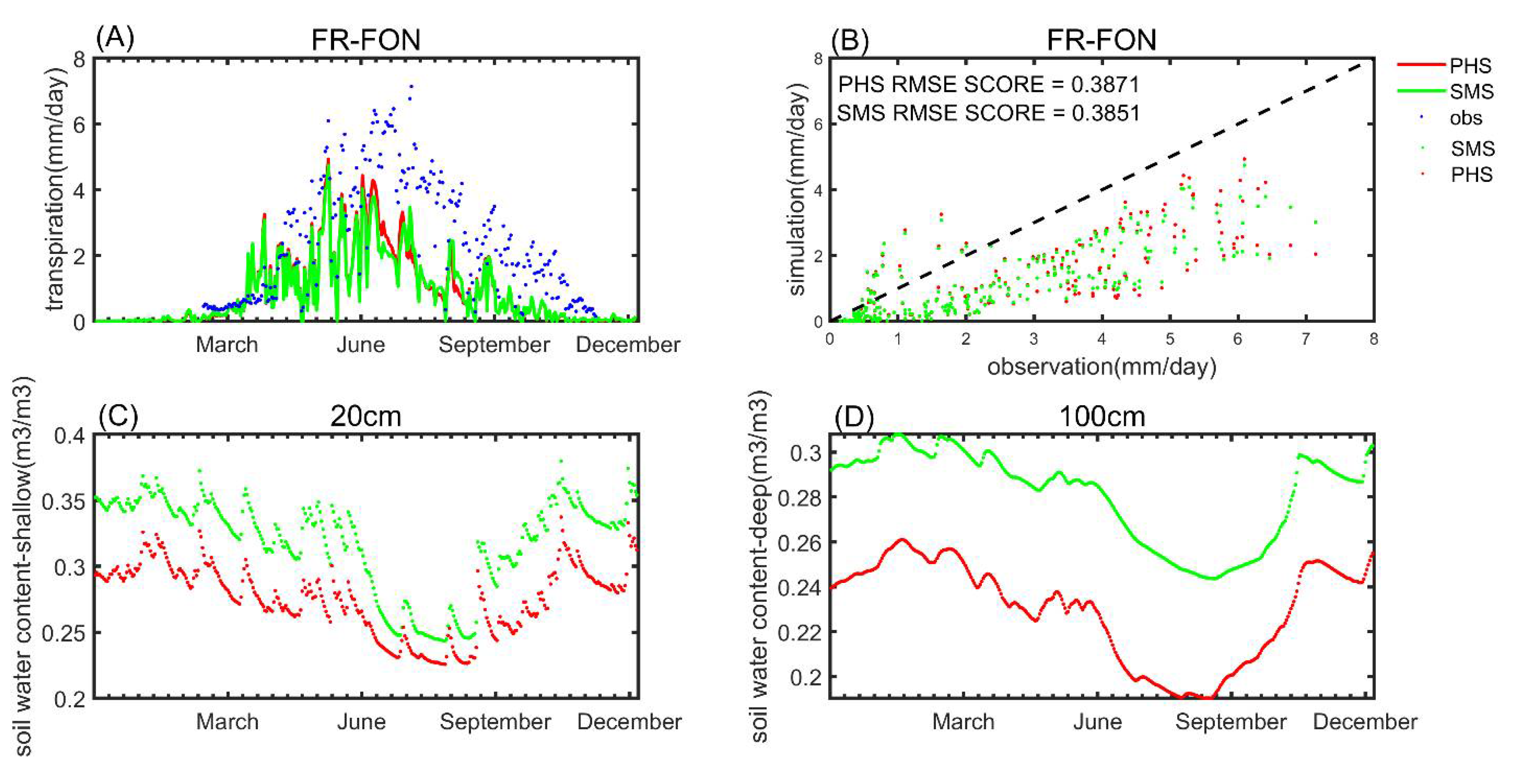
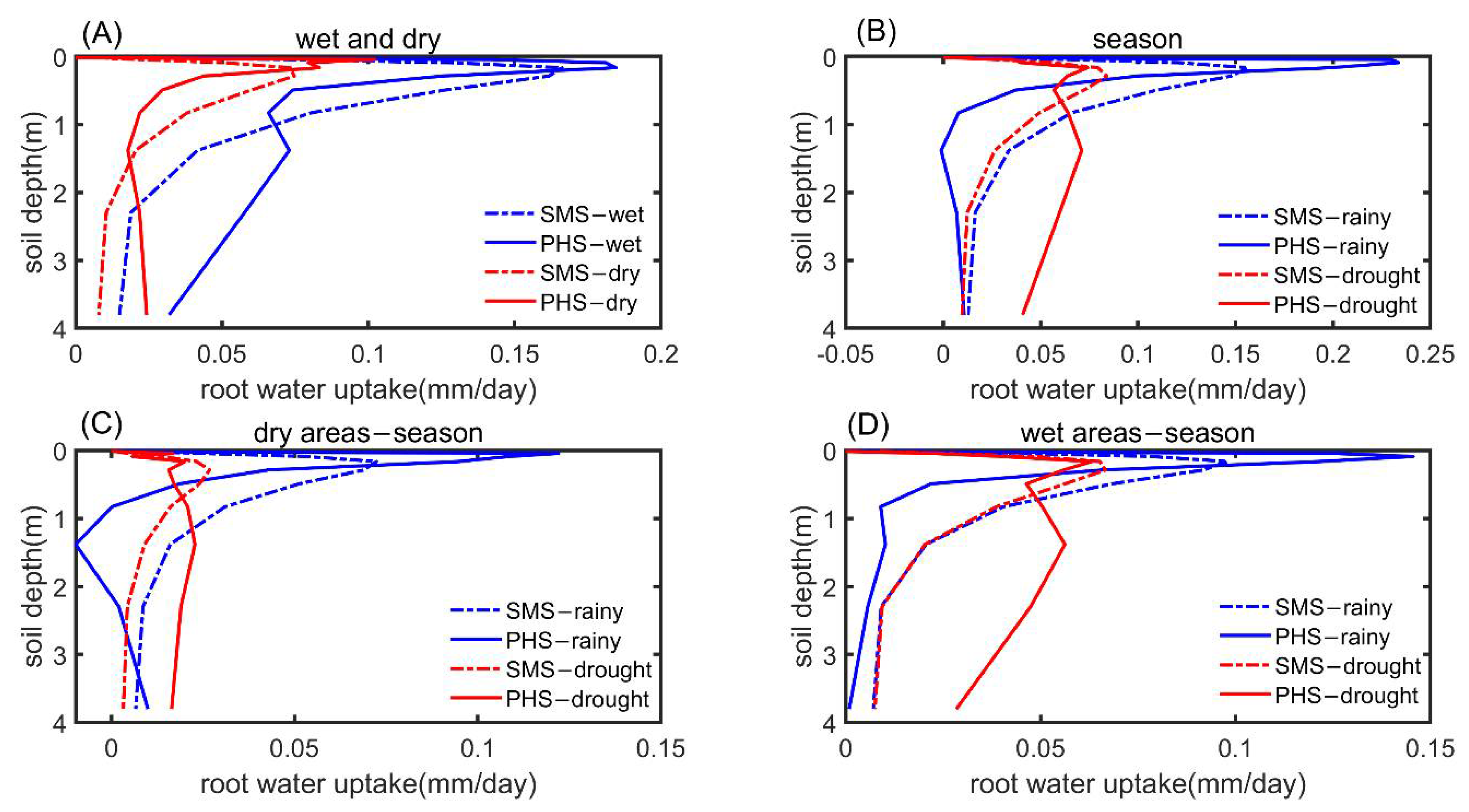
| Plant Cover Types | a | b |
|---|---|---|
| Needle-leaf forests | 0.48 | 0.32 |
| Broad-leaf forests | 0.64 | 0.15 |
| Mixed and forests | 0.52 | 0.26 |
| Shrubs and grasses | 0.69 | 0.28 |
| Crops | 0.66 | 0.18 |
| Wetlands | 0.65 | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Lu, X.; Wei, Z.; Zhu, S.; Wei, N.; Zhang, S.; Yuan, H.; Shangguan, W.; Liu, S.; Zhang, S.; et al. New Representation of Plant Hydraulics Improves the Estimates of Transpiration in Land Surface Model. Forests 2021, 12, 722. https://doi.org/10.3390/f12060722
Li H, Lu X, Wei Z, Zhu S, Wei N, Zhang S, Yuan H, Shangguan W, Liu S, Zhang S, et al. New Representation of Plant Hydraulics Improves the Estimates of Transpiration in Land Surface Model. Forests. 2021; 12(6):722. https://doi.org/10.3390/f12060722
Chicago/Turabian StyleLi, Hongmei, Xingjie Lu, Zhongwang Wei, Siguang Zhu, Nan Wei, Shupeng Zhang, Hua Yuan, Wei Shangguan, Shaofeng Liu, Shulei Zhang, and et al. 2021. "New Representation of Plant Hydraulics Improves the Estimates of Transpiration in Land Surface Model" Forests 12, no. 6: 722. https://doi.org/10.3390/f12060722
APA StyleLi, H., Lu, X., Wei, Z., Zhu, S., Wei, N., Zhang, S., Yuan, H., Shangguan, W., Liu, S., Zhang, S., Huang, J., & Dai, Y. (2021). New Representation of Plant Hydraulics Improves the Estimates of Transpiration in Land Surface Model. Forests, 12(6), 722. https://doi.org/10.3390/f12060722








