Trees at a Moderately Arid Site Were More Sensitive to Long-Term Drought
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Sites
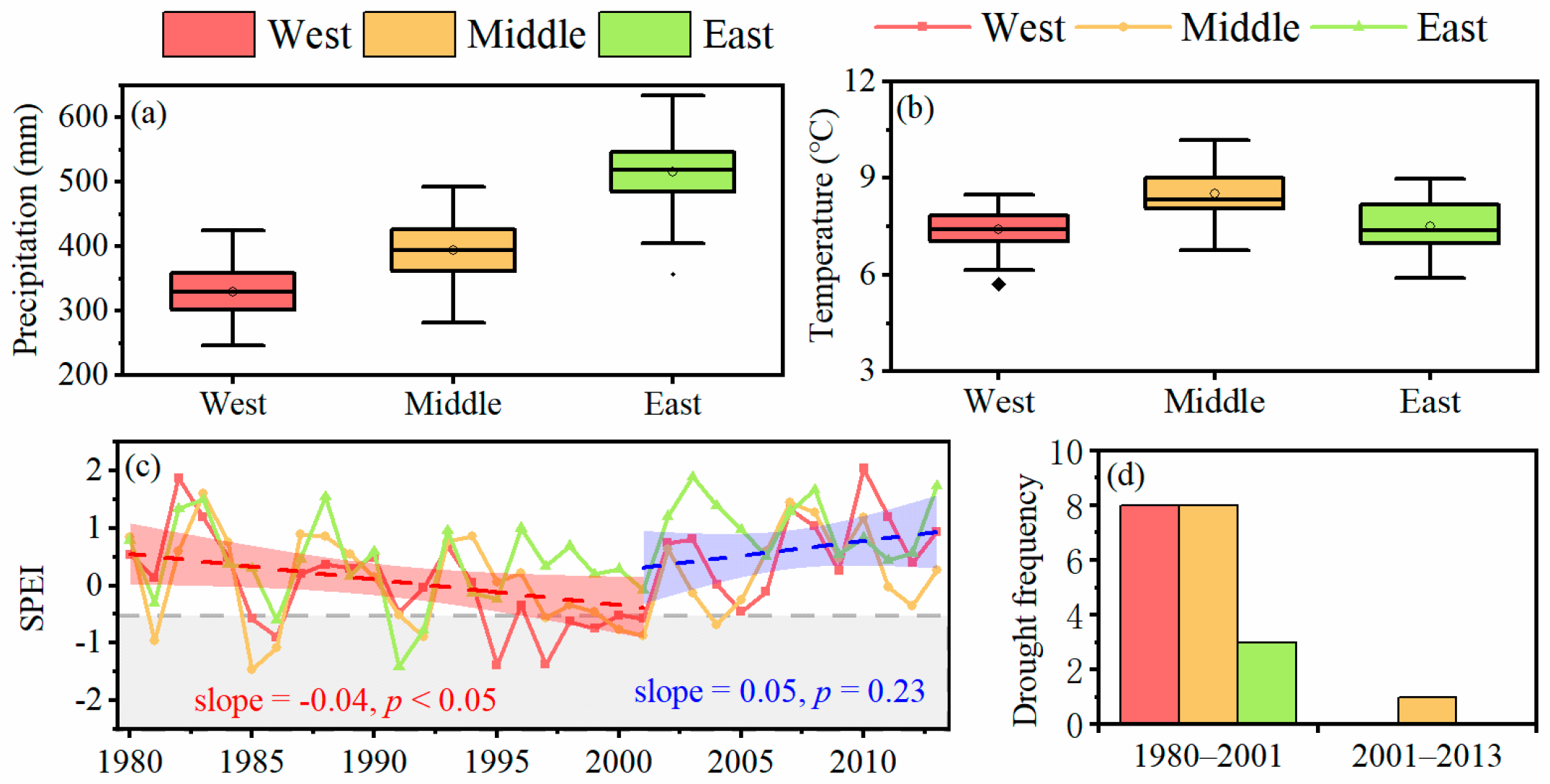
2.2. Climate Data and Drought Periods
2.3. Tree-Ring Sampling and Laboratory Treatment
2.4. Biomass Increment Data
2.5. Indices of Tree Resistance to and Recovery from Long-Term Drought
2.6. Statistical Analyses
3. Results
3.1. Tree Resistance to and Recovery from Long-Term Drought
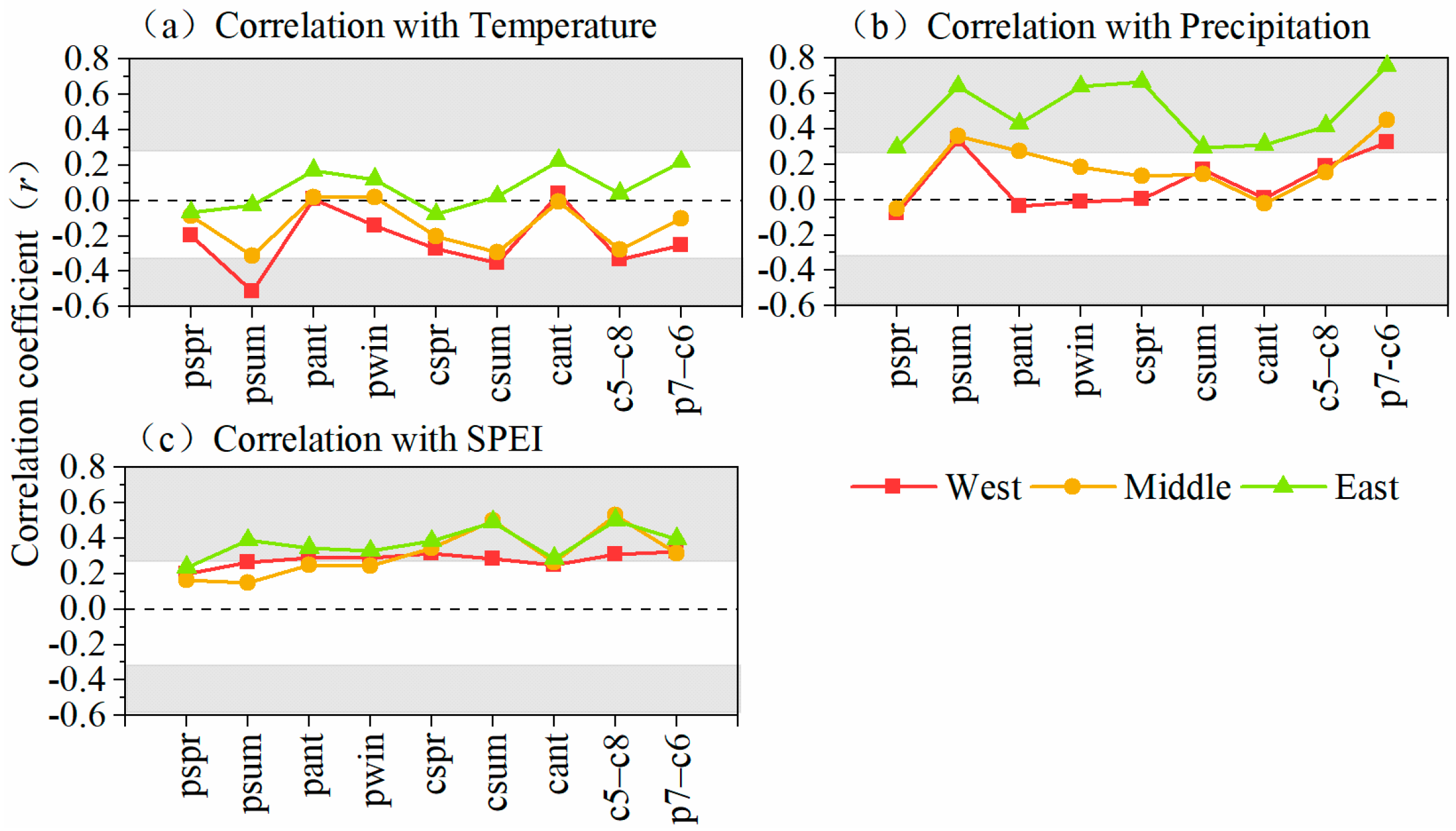
3.2. Growth–Climate Relationships
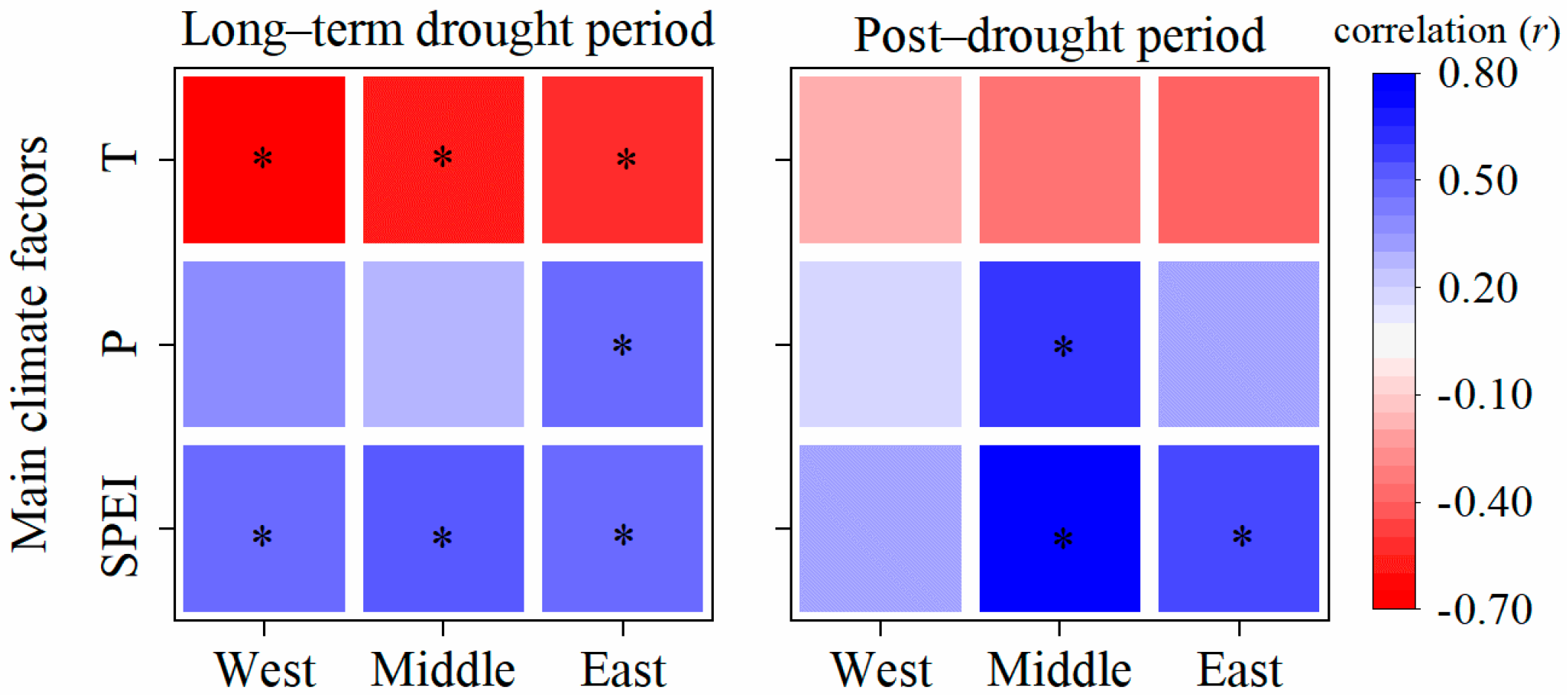
3.3. Impacts of Main Climate Factors on Tree Resistance to and Recovery from Drought
3.4. Climate Attribution in Different Sites
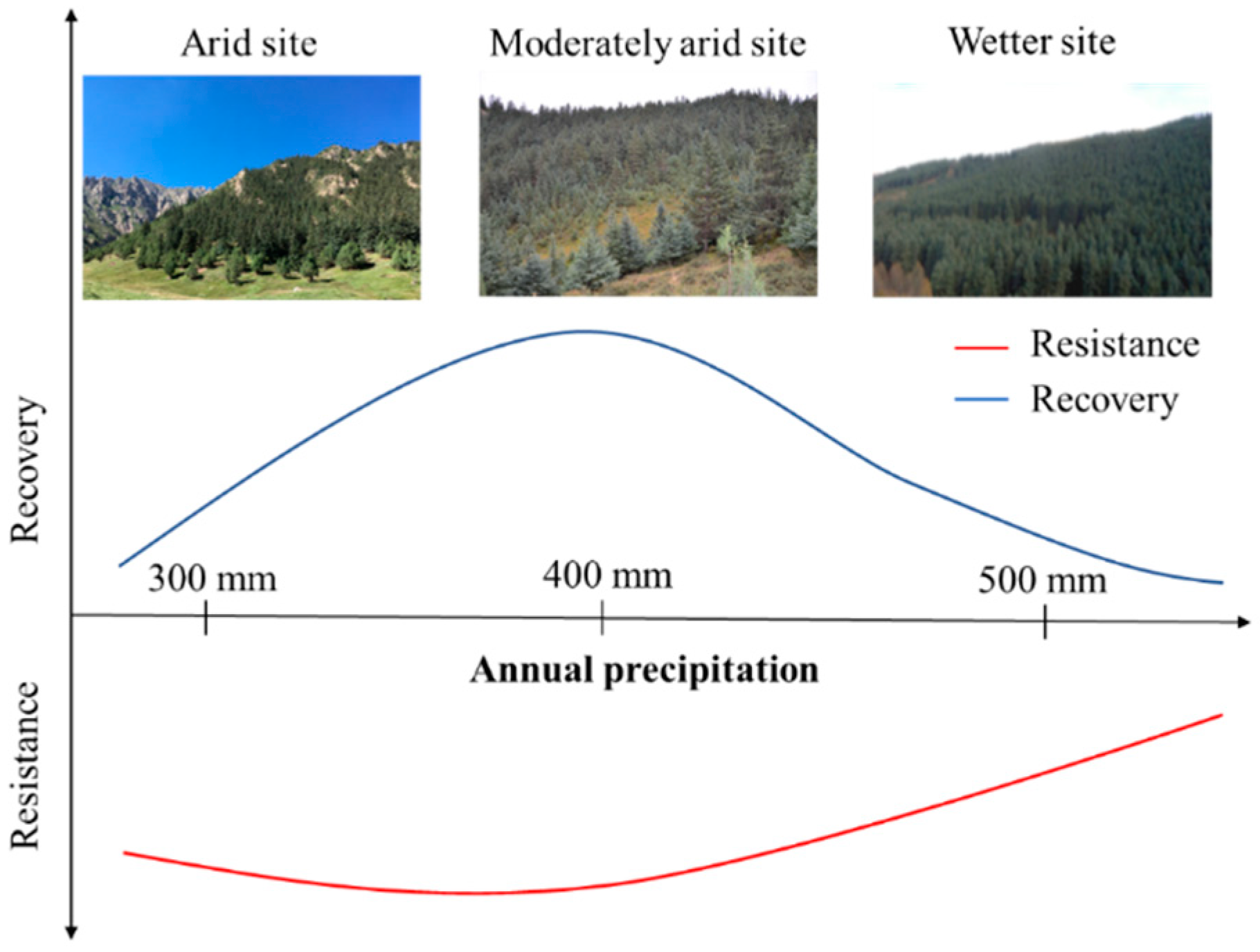
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, C.; Liu, H.; Anenkhonov, O.A.; Korolyuk, A.Y.; Sandanov, D.V.; Balsanova, L.D.; Naidanov, B.B.; Wu, X. Long-term forest resilience to climate change indicated by mortality, regeneration, and growth in semiarid southern Siberia. Glob. Chang. Biol. 2016, 23, 2370–2382. [Google Scholar] [CrossRef]
- Levine, N.M.; Zhang, K.; Longo, M.; Baccini, A.; Phillips, O.L.; Lewis, S.L.; Alvarez-Dávila, E.; De Andrade, A.C.S.; Brienen, R.J.W.; Erwin, T.L.; et al. Ecosystem heterogeneity determines the ecological resilience of the Amazon to climate change. Proc. Natl. Acad. Sci. USA 2016, 113, 793–797. [Google Scholar] [CrossRef]
- Humphrey, V.; Zscheischler, J.; Ciais, P.; Gudmundsson, L.; Sitch, S.; Seneviratne, S.I. Sensitivity of atmospheric CO2 growth rate to observed changes in terrestrial water storage. Nat. Cell Biol. 2018, 560, 628–631. [Google Scholar] [CrossRef]
- Jung, M.; Reichstein, M.; Schwalm, C.R.; Huntingford, C.; Sitch, S.; Ahlström, A.; Arneth, A.; Camps-Valls, G.; Ciais, P.; Friedlingstein, P.; et al. Compensatory water effects link yearly global land CO2 sink changes to temperature. Nat. Cell Biol. 2017, 541, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Berdugo, M.; Delgado-Baquerizo, M.; Soliveres, S.; Hernández-Clemente, R.; Zhao, Y.; Gaitán, J.J.; Gross, N.; Saiz, H.; Maire, V.; Lehmann, A.; et al. Global ecosystem thresholds driven by aridity. Science 2020, 367, 787–790. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, art129. [Google Scholar] [CrossRef]
- DeSoto, L.; Cailleret, M.; Sterck, F.; Jansen, S.; Kramer, K.; Robert, E.M.R.; Aakala, T.; Amoroso, M.M.; Bigler, C.; Camarero, J.J.; et al. Low growth resilience to drought is related to future mortality risk in trees. Nat. Commun. 2020, 11, 545. [Google Scholar] [CrossRef]
- Vacchiano, G.; Garbarino, M.; Mondino, E.B.; Motta, R. Evidences of drought stress as a predisposing factor to Scots pine decline in Valle d’Aosta (Italy). Eur. J. For. Res. 2011, 131, 989–1000. [Google Scholar] [CrossRef]
- Flores, B.M.; Holmgren, M.; Xu, C.; Van Nes, E.H.; Jakovac, C.C.; Mesquita, R.C.G.; Scheffer, M. Floodplains as an Achilles’ heel of Amazonian forest resilience. Proc. Natl. Acad. Sci. USA 2017, 114, 4442–4446. [Google Scholar] [CrossRef]
- Helman, D.; Lensky, I.M.; Yakir, D.; Osem, Y. Forests growing under dry conditions have higher hydrological resilience to drought than do more humid forests. Glob. Chang. Biol. 2017, 23, 2801–2817. [Google Scholar] [CrossRef]
- Huang, K.; Xia, J. High ecosystem stability of evergreen broadleaf forests under severe droughts. Glob. Chang. Biol. 2019, 25, 3494–3503. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Piao, S.; Wang, K.; Wang, X.; Wang, T.; Ciais, P.; Chen, A.; Lian, X.; Peng, S.; Peñuelas, J. Temporal trade-off between gymnosperm resistance and resilience increases forest sensitivity to extreme drought. Nat. Ecol. Evol. 2020, 4, 1075–1083. [Google Scholar] [CrossRef]
- Jump, A.S.; Hunt, J.M.; Peñuelas, J. Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Glob. Chang. Biol. 2006, 12, 2163–2174. [Google Scholar] [CrossRef]
- Peng, C.; Ma, Z.; Lei, X.; Zhu, Q.; Chen, H.; Wang, W.; Liu, S.; Li, W.; Fang, X.; Zhou, X. A drought-induced pervasive increase in tree mortality across Canada’s boreal forests. Nat. Clim. Chang. 2011, 1, 467–471. [Google Scholar] [CrossRef]
- Wang, B.; Yu, P.; Zhang, L.; Wang, Y.; Yu, Y.; Wang, S. Differential Trends of Qinghai Spruce Growth with Elevation in Northwestern China during the Recent Warming Hiatus. Forests 2019, 10, 712. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Schwalm, C.; Biondi, F.; Camarero, J.J.; Koch, G.; Litvak, M.; Ogle, K.; Shaw, J.D.; Shevliakova, E.; Williams, A.P.; et al. Pervasive drought legacies in forest ecosystems and their implications for carbon cycle models. Science 2015, 349, 528–532. [Google Scholar] [CrossRef]
- Huang, M.; Wang, X.; Keenan, T.F.; Piao, S. Drought timing influences the legacy of tree growth recovery. Glob. Chang. Biol. 2018, 24, 3546–3559. [Google Scholar] [CrossRef]
- Vitasse, Y.; Bottero, A.; Cailleret, M.; Bigler, C.; Fonti, P.; Gessler, A.; Lévesque, M.; Rohner, B.; Weber, P.; Rigling, A.; et al. Contrasting resistance and resilience to extreme drought and late spring frost in five major European tree species. Glob. Chang. Biol. 2019, 25, 3781–3792. [Google Scholar] [CrossRef]
- Liu, H.; Williams, A.P.; Allen, C.D.; Guo, D.; Wu, X.; Anenkhonov, O.A.; Liang, E.; Sandanov, D.V.; Yin, Y.; Qi, Z.; et al. Rapid warming accelerates tree growth decline in semi-arid forests of Inner Asia. Glob. Chang. Biol. 2013, 19, 2500–2510. [Google Scholar] [CrossRef]
- Gao, L.; Gou, X.; Deng, Y.; Wang, Z.; Gu, F.; Wang, F. Increased growth of Qinghai spruce in northwestern China during the recent warming hiatus. Agric. For. Meteorol. 2018, 260–261, 9–16. [Google Scholar] [CrossRef]
- Piovesan, G.; Biondi, F.; Di Filippo, A.; Alessandrini, A.; Maugeri, M. Drought-driven growth reduction in old beech (Fagus sylvatica L.) forests of the central Apennines, Italy. Glob. Chang. Biol. 2008, 14, 1265–1281. [Google Scholar] [CrossRef]
- Prior, L.D.; Bowman, D.M. Big eucalypts grow more slowly in a warm climate: Evidence of an interaction between tree size and temperature. Glob. Chang. Biol. 2014, 20, 2793–2799. [Google Scholar] [CrossRef] [PubMed]
- Cabon, A.; Mouillot, F.; Lempereur, M.; Ourcival, J.-M.; Simioni, G.; Limousin, J.-M. Thinning increases tree growth by delaying drought-induced growth cessation in a Mediterranean evergreen oak coppice. For. Ecol. Manag. 2018, 409, 333–342. [Google Scholar] [CrossRef]
- D’Amato, A.W.; Bradford, J.B.; Fraver, S.; Palik, B.J. Effects of thinning on drought vulnerability and climate response in north temperate forest ecosystems. Ecol. Appl. 2013, 23, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Fang, O.; Zhang, Q. Tree resilience to drought increases in the Tibetan Plateau. Glob. Chang. Biol. 2019, 25, 245–253. [Google Scholar] [CrossRef]
- Teets, A.; Fraver, S.; Weiskittel, A.R.; Hollinger, D.Y. Quantifying climate-growth relationships at the stand level in a mature mixed-species conifer forest. Glob. Chang. Biol. 2018, 24, 3587–3602. [Google Scholar] [CrossRef]
- Foster, J.R.; Finley, A.O.; D’Amato, A.W.; Bradford, J.B.; Banerjee, S. Predicting tree biomass growth in the temperate-boreal ecotone: Is tree size, age, competition, or climate response most important? Glob. Chang. Biol. 2016, 22, 2138–2151. [Google Scholar] [CrossRef]
- Li, D.L.; Liu, X.H. Climate in Gansu; China Meteorological Press: Beijing, China, 2000. [Google Scholar]
- Wang, J.; Wang, J.; Ye, H.; Liu, Y.; He, H. An interpolated temperature and precipitation dataset at 1-km grid resolution in China (2000–2012). China Sci. Data 2017, 2, 73–80. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull 1983, 43, 69–75. [Google Scholar]
- Monserud, R.A.; Sterba, H. A basal area increment model for individual trees growing in even- and uneven-aged forest stands in Austria. For. Ecol. Manag. 1996, 80, 57–80. [Google Scholar] [CrossRef]
- Liang, E.; Shao, X.; Eckstein, D.; Liu, X. Spatial variability of tree growth along a latitudinal transect in the Qilian Mountains, northeastern Tibetan Plateau. Can. J. For. Res. 2010, 40, 200–211. [Google Scholar] [CrossRef]
- De Keersmaecker, W.; Lhermitte, S.; Tits, L.; Honnay, O.; Somers, B.; Coppin, P. A model quantifying global vegetation resistance and resilience to short-term climate anomalies and their relationship with vegetation cover. Glob. Ecol. Biogeogr. 2015, 24, 539–548. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Zhao, S.; Jiao, L.; Wen, Y. Relationships between Tree Age and Climate Sensitivity of Radial Growth in Different Drought Conditions of Qilian Mountains, Northwestern China. Forests 2018, 9, 135. [Google Scholar] [CrossRef]
- Williams, A.P.; Allen, C.D.; Macalady, A.K.; Griffin, D.; Woodhouse, C.A.; Meko, D.M.; Swetnam, T.W.; Rauscher, S.A.; Seager, R.; Grissino-Mayer, H.D.; et al. Temperature as a potent driver of regional forest drought stress and tree mortality. Nat. Clim. Chang. 2013, 3, 292–297. [Google Scholar] [CrossRef]
- Ryan, M.G. Tree responses to drought. Tree Physiol. 2011, 31, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Zhao, Y.; Chen, X.; Li, J.; Da, G.; Zhu, Y. Soil water characteristics in Picea crassifolia forestry lands in Qilian Mountains. J. Appl. Ecol. 2004, 15, 1148–1152. [Google Scholar]
- Wang, B.; Yu, P.; Wang, S.; Wang, Y.; Liu, X.; Jin, M.; Zhang, X. Response of soil water storage to slope position of forestland in Qilian Mountains. Sci. Soil Water Conserv. 2016, 14, 101–108. [Google Scholar]
- Bai, X. Estimation of Soil Moisture and Its Temporal-Spatial Variability by Remote Sensing in Qilian Mountain, Northwestern China; Lanzhou University: Lanzhou, China, 2017. [Google Scholar]
- Giardina, F.; Konings, A.G.; Kennedy, D.; Alemohammad, S.H.; Oliveira, R.S.; Uriarte, M.; Gentine, P. Tall Amazonian forests are less sensitive to precipitation variability. Nat. Geosci. 2018, 11, 405–409. [Google Scholar] [CrossRef]
- Gou, X.; Chen, F.; Yang, M.; Li, J.; Peng, J.; Jin, L. Climatic response of thick leaf spruce (Picea crassifolia) tree-ring width at different elevations over Qilian Mountains, northwestern China. J. Arid. Environ. 2005, 61, 513–524. [Google Scholar] [CrossRef]



| Plots | Elevation (m a.s.l.) | Aspect (°) | Slope (°) | Stand Density (Trees/ha) | DBH (cm) | Tree Height (m) | Number of Trees in Plot |
|---|---|---|---|---|---|---|---|
| E1 | 2800 | NE59 | 21 | 1025 | 27.74 (9.5–43.4) | 18.8 (6.5–24.0) | 41 |
| E2 | 2900 | NE42 | 31 | 1300 | 18.59 (5.2–39) | 12.2 (3.0–23.5) | 52 |
| M1 | 2800 | NE7 | 23 | 3025 | 14.1 (6.6–29.5) | 10.3 (3.5–14.6) | 122 |
| M2 | 2900 | NE24 | 32 | 2725 | 12.7 (5.5–31.9) | 7.9 (2.6–14.2) | 107 |
| W1 | 2800 | NE7 | 36 | 950 | 14.78 (8.5–24) | 7.7 (3.5–12.4) | 38 |
| W2 | 2930 | NE16 | 37 | 1425 | 17.67 (7.4–39) | 9.4 (2.8–13.0) | 57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Yu, P.; Yu, Y.; Wang, Y.; Zhang, L.; Wan, Y.; Wang, S.; Liu, X. Trees at a Moderately Arid Site Were More Sensitive to Long-Term Drought. Forests 2021, 12, 579. https://doi.org/10.3390/f12050579
Wang B, Yu P, Yu Y, Wang Y, Zhang L, Wan Y, Wang S, Liu X. Trees at a Moderately Arid Site Were More Sensitive to Long-Term Drought. Forests. 2021; 12(5):579. https://doi.org/10.3390/f12050579
Chicago/Turabian StyleWang, Bin, Pengtao Yu, Yipeng Yu, Yanhui Wang, Lei Zhang, Yanfang Wan, Shunli Wang, and Xiande Liu. 2021. "Trees at a Moderately Arid Site Were More Sensitive to Long-Term Drought" Forests 12, no. 5: 579. https://doi.org/10.3390/f12050579
APA StyleWang, B., Yu, P., Yu, Y., Wang, Y., Zhang, L., Wan, Y., Wang, S., & Liu, X. (2021). Trees at a Moderately Arid Site Were More Sensitive to Long-Term Drought. Forests, 12(5), 579. https://doi.org/10.3390/f12050579







