Short-Rotation Willows as a Wastewater Treatment Plant: Biomass Production and the Fate of Macronutrients and Metals
Abstract
1. Introduction
2. Materials and Methods
2.1. Pilot Evapotranspirative Willow System
2.2. Wastewater and Soil Analyses
2.3. Estimation of Willow Growth and Biomass Production
2.4. Nutrient and Metal Content in the Woody Biomass
2.5. Statistical Analyses
3. Results
3.1. Wastewater Characteristics
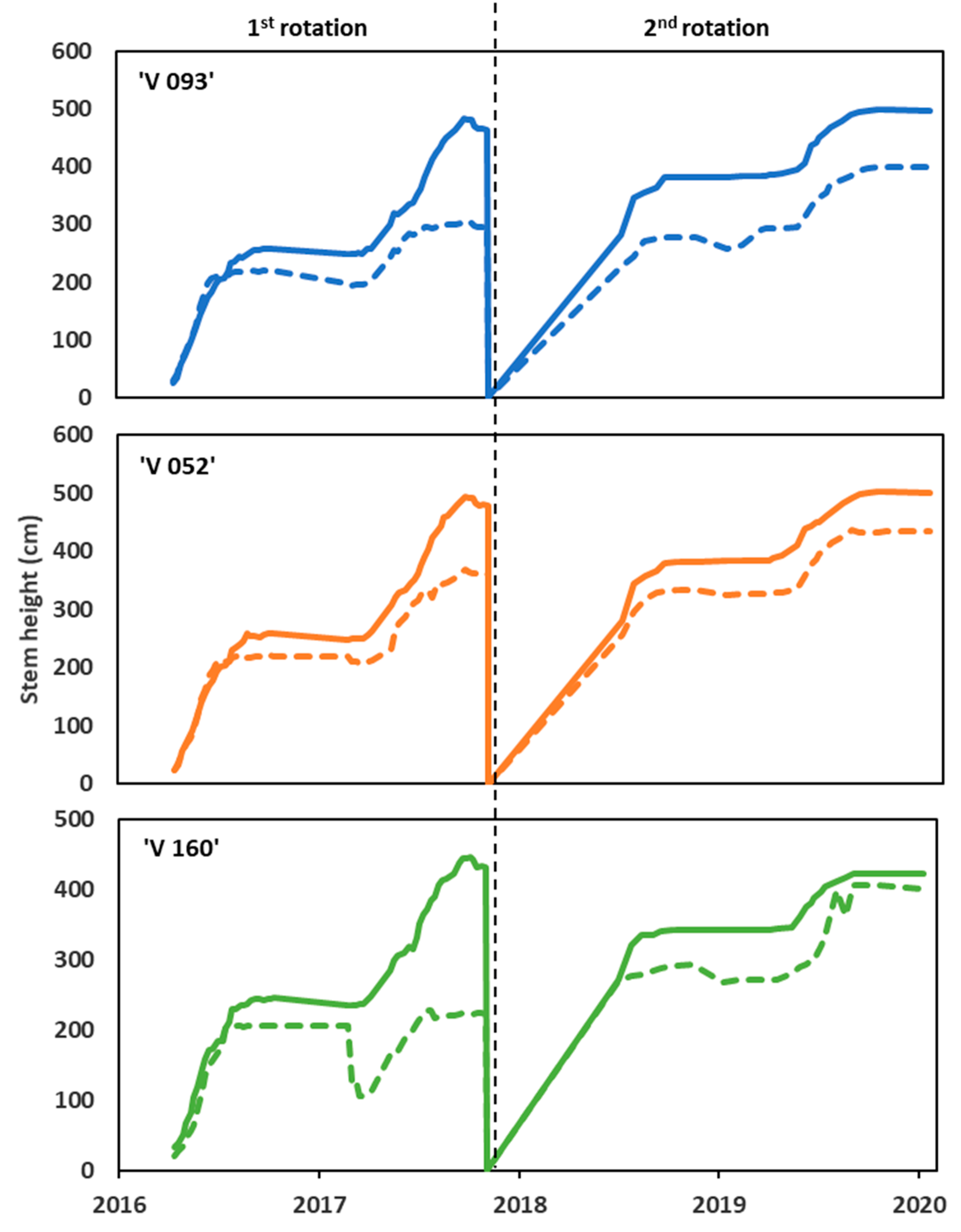
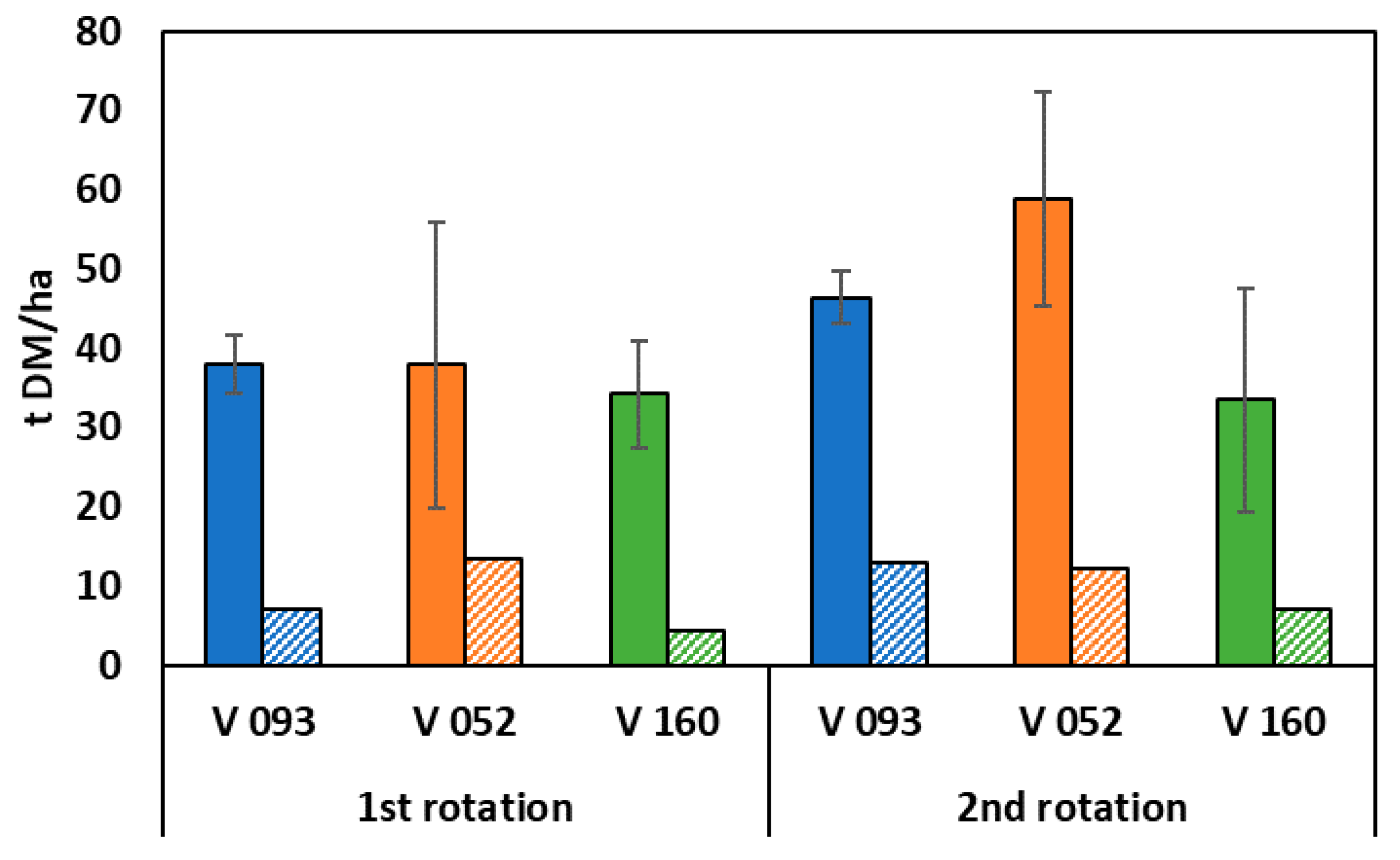
3.2. Willow Growth and Biomass Production
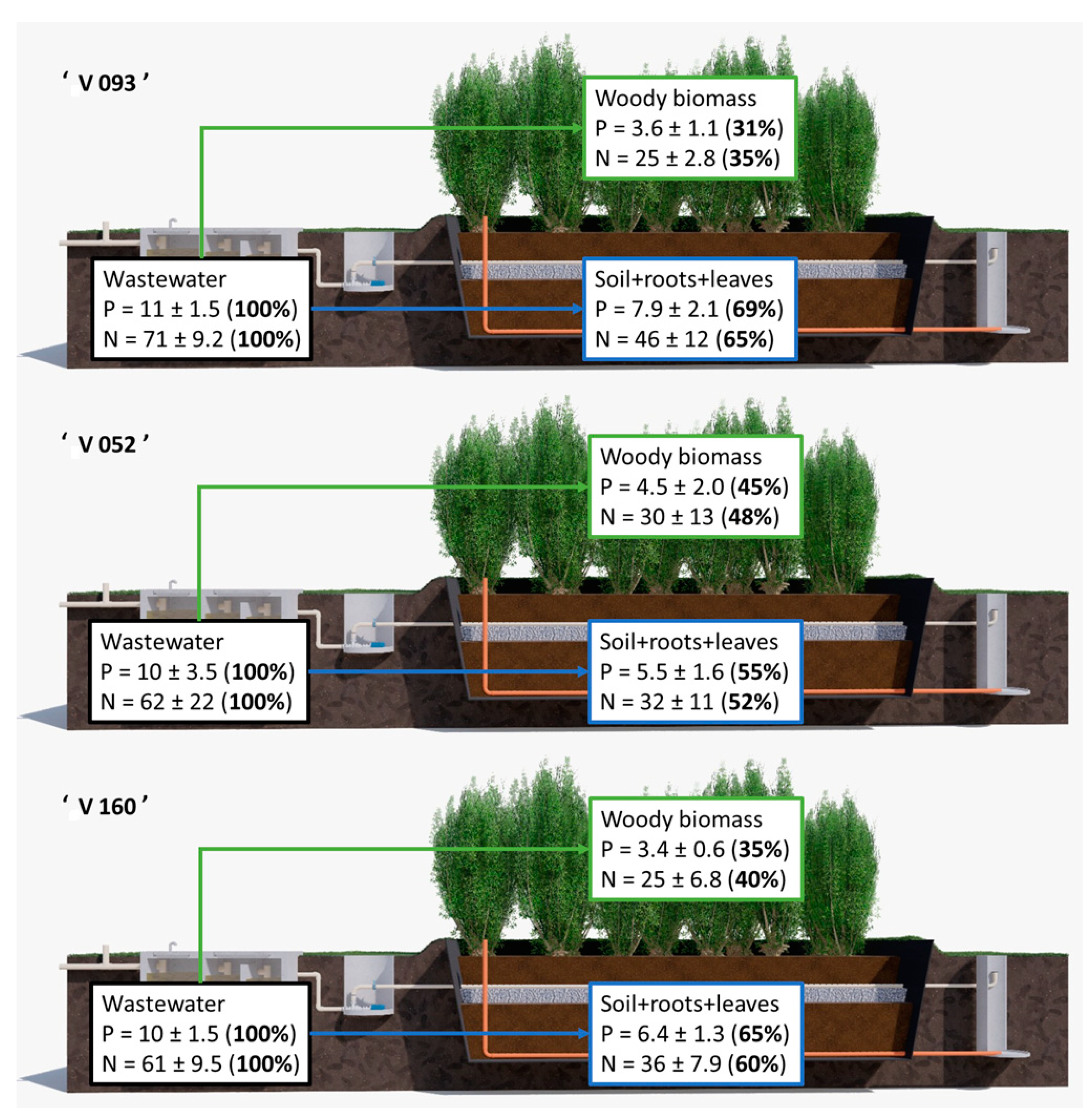
3.3. Fate of Macronutrients
3.4. Fate of Metals
4. Discussion
4.1. Wastewater
4.2. Willow Growth and Biomass Production
4.3. Fate of Macronutrients
4.3.1. Carbon and Organic Matter
4.3.2. Nitrogen
4.3.3. Phosphorus
4.3.4. Potassium
4.3.5. Sulfur
4.3.6. Calcium and Magnesium
4.3.7. Removal of Nutrients by Harvesting
4.4. Fate of Metals
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aronsson, P.; Perttu, K. A complete system for wastewater treatment using vegetation filters. In Proceedings of the Willow Vegetation Filters for Municipal Wastewaters and Sludges; Aronsson, P., Perttu, K., Eds.; Swedish University of Agricultural Sciences: Uppsala, Sweden, 1994; pp. 211–213. [Google Scholar]
- Dimitriou, I.; Aronsson, P. Willows for energy and phytoremediation in Sweden. Unasylva 2005, 56, 47–50. [Google Scholar]
- Aronsson, P.; Perttu, K. (Eds.) Willow Vegetation Filters for Municipal Wastewaters and Sludges: A Biological Purification System. Swedish University of Agriclutural Sciences: Uppsala, Sweden, 1994; ISBN 91-576-4916-2. [Google Scholar]
- Larsson, S.; Arronson, P.; Backlund, A.; Carlander, A. Short-rotation Willow Biomass Plantations Irrigated and Fertilised with Wastewaters. Dan. Environ. Prot. Agency 2003, 37, 1–58. [Google Scholar] [CrossRef]
- Holm, B.; Heinsoo, K. Municipal wastewater application to Short Rotation Coppice of willows—Treatment efficiency and clone response in Estonian case study. Biomass Bioenergy 2013, 57, 126–135. [Google Scholar] [CrossRef]
- Guidi Nissim, W.; Jerbi, A.; Lafleur, B.; Fluet, R.; Labrecque, M. Willows for the treatment of municipal wastewater: Performance under different irrigation rates. Ecol. Eng. 2015, 81, 395–404. [Google Scholar] [CrossRef]
- Jurse, A.; Vrhovsek, D.; Justin, M.Z.; Bozic, G.; Gaberscik, A. Effect of wastewater salinity on treatment performance of vertical constructed wetland and growth of poplars in sand filters. Sylwan 2013, 158, 1–15. [Google Scholar]
- Aronsson, P.; Dahlin, T.; Dimitriou, I. Treatment of landfill leachate by irrigation of willow coppice—Plant response and treatment efficiency. Environ. Pollut. 2010, 158, 795–804. [Google Scholar] [CrossRef]
- Zalesny, R.S.; Bauer, E.O. Evaluation of Populus and Salix continuously irrigated with landfill leachate II. Soils and early tree development. Int. J. Phytoremediation 2007, 9, 307–323. [Google Scholar] [CrossRef][Green Version]
- Dimitriou, I.; Mola-Yudego, B. Poplar and willow plantations on agricultural land in Sweden: Area, yield, groundwater quality and soil organic carbon. For. Ecol. Manag. 2017, 383, 99–107. [Google Scholar] [CrossRef]
- Dimitriou, I.; Aronsson, P. Wastewater and sewage sludge application to willows and poplars grown in lysimeters–Plant response and treatment efficiency. Biomass Bioenergy 2011, 35, 161–170. [Google Scholar] [CrossRef]
- Gregersen, P.; Brix, H. Zero-discharge of nutrients and water in a willow dominated constructed wetland. Water Sci. Technol. 2001, 44, 407–412. [Google Scholar] [CrossRef]
- Brix, H.; Arias, C.A. Use of willows in evapotranspirative systems for onsite wastewater management—Theory and experiences from Denmark. In Proceedings of the “STREPOW” International Workshop; Orlović, S., Ed.; Institute of Lowland Forestry and Environment: Novi Sad, Serbia, 2011; pp. 15–29. [Google Scholar]
- O’Hogain, S.; McCarton, L.; Reid, A.; Turner, J.; Fox, S. A review of zero discharge wastewater treatment systems using reed willow bed combinations in Ireland. Water Pract. Technol. 2011, 6. [Google Scholar] [CrossRef]
- Wu, Y.; Xia, L.; Hu, Z.; Liu, S.; Liu, H.; Nath, B.; Zhang, N.; Yang, L. The application of zero-water discharge system in treating diffuse village wastewater and its benefits in community afforestation. Environ. Pollut. 2011, 159, 2968–2973. [Google Scholar] [CrossRef]
- Istenič, D.; Božič, G.; Arias, C.A.; Bulc, T.G. Growth dynamic of three different white willow clones used in a zero-discharge wastewater treatment system in the sub-Mediterranean region—An early evaluation. Desalin. Water Treat. 2017, 91, 260–267. [Google Scholar] [CrossRef]
- Curneen, S.J.; Gill, L.W. A comparison of the suitability of different willow varieties to treat on-site wastewater effluent in an Irish climate. J. Environ. Manag. 2014, 133, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Curneen, S.; Gill, L.W. Willow-based evapotranspiration systems for on-site wastewater effluent in areas of low permeability subsoils. Ecol. Eng. 2016, 92, 199–209. [Google Scholar] [CrossRef]
- Frédette, C.; Grebenshchykova, Z.; Comeau, Y.; Brisson, J. Evapotranspiration of a willow cultivar (Salix miyabeana SX67) grown in a full-scale treatment wetland. Ecol. Eng. 2019, 127, 254–262. [Google Scholar] [CrossRef]
- Perttu, K.L. Biomass Production and Nutrient Removal from Municipal Wastes Using Willow Vegetation Filters. J. Sustain. For. 1994, 1, 57–70. [Google Scholar] [CrossRef]
- Börjesson, P.; Berndes, G. The prospects for willow plantations for wastewater treatment in Sweden. Biomass Bioenergy 2006, 30, 428–438. [Google Scholar] [CrossRef]
- Lachapelle-T, X.; Labrecque, M.; Comeau, Y. Treatment and valorization of a primary municipal wastewater by a short rotation willow coppice vegetation fi lter. Ecol. Eng. 2019, 130, 32–44. [Google Scholar] [CrossRef]
- Guidi, W.; Piccioni, E.; Bonari, E. Evapotranspiration and crop coefficient of poplar and willow short-rotation coppice used as vegetation filter. Bioresour. Technol. 2008, 99, 4832–4840. [Google Scholar] [CrossRef]
- Guidi, W.; Bonari, E.; Superiore, S.; Anna, S.; Bertolacci, M.; Nazionale, L. Water Consumption of Poplar and Willow Short Rotation Forestry Used as Vegetation Filter: Preliminary Results. In Proceedings of the ICID 21st European Regional Conference, Furt (Oder), Germany, 15–19 May 2005; pp. 19–20. [Google Scholar]
- Guidi, W.; Labrecque, M. Effects of high water supply on growth, water use, and nutrient allocation in willow and poplar grown in a 1-year pot trial. Water Air Soil Pollut. 2009, 207, 85–101. [Google Scholar] [CrossRef]
- Quaye, A.K.; Volk, T.A. Biomass production and soil nutrients in organic and inorganic fertilized willow biomass production systems. Biomass Bioenergy 2013, 57, 113–125. [Google Scholar] [CrossRef]
- Kuzovkina, Y.A.; Volk, T.A. The characterization of willow (Salix L.) varieties for use in ecological engineering applications: Co-ordination of structure, function and autecology. Ecol. Eng. 2009, 35, 1178–1189. [Google Scholar] [CrossRef]
- Licht, L.A.; Isebrands, J.G. Linking phytoremediated pollutant removal to biomass economic opportunities. In Proceedings of the Biomass d Bioenergy; Pergamon: Oxford, UK, 2005; Volume 28, pp. 203–218. [Google Scholar]
- Fillion, M.; Brisson, J.; Teodorescu, T.I.; Sauvé, S.; Labrecque, M. Performance of Salix viminalis and Populus nigra×Populus maximowiczii in short rotation intensive culture under high irrigation. Biomass Bioenergy 2009, 33, 1271–1277. [Google Scholar] [CrossRef]
- Francis, R.A.; Gurnell, A.M.; Petts, G.E.; Edwards, P.J. Survival and growth responses of Populus nigra, Salix elaeagnos and Alnus incana cuttings to varying levels of hydric stress. For. Ecol. Manag. 2005, 210, 291–301. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Responses of Woody Plants to Flooding and Salinity; Heron Publishing: Victoria, BC, Canada, 1997. [Google Scholar]
- Caslin, B.; Finnan, J.; Johnston, C.; McCracken, A.; Walsh, L. Short Rotation Coppice Willow Best Practice Guide; Teagasc Agriculture and Food Development Authority: Carlow, Ireland; AFBI Agri-Food and Bioscience Institute: Belfast, Northern Ireland, UK, 2015; ISBN 1841705683. [Google Scholar]
- Weih, M.; Nordh, N. Characterising willows for biomass and phytoremediation: Growth, nitrogen and water use of 14 willow clones under different irrigation and fertilisation regimes. Biomass Bioenergy 2002, 23, 397–413. [Google Scholar] [CrossRef]
- Rastas Amofah, L.; Mattsson, J.; Hedström, A. Willow bed fertigated with domestic wastewater to recover nutrients in subarctic climates. Ecol. Eng. 2012, 47, 174–181. [Google Scholar] [CrossRef]
- Justin, M.Z.; Zupančič, M. Combined purification and reuse of landfill leachate by constructed wetland and irrigation of grass and willows. Desalination 2009, 246, 157–168. [Google Scholar] [CrossRef]
- Białowiec, A.; Wojnowska-Baryła, I.; Agopsowicz, M. The efficiency of evapotranspiration of landfill leachate in the soil–plant system with willow Salix amygdalina L. Ecol. Eng. 2007, 30, 356–361. [Google Scholar] [CrossRef]
- Mleczek, M.; Rutkowski, P.; Rissmann, I.; Kaczmarek, Z.; Golinski, P.; Szentner, K.; Strażyńska, K.; Stachowiak, A. Biomass productivity and phytoremediation potential of Salix alba and Salix viminalis. Biomass Bioenergy 2010, 34, 1410–1418. [Google Scholar] [CrossRef]
- Kajba, D.; Andrić, I. Selection of Willows (Salix sp.) for biomass production. S. East Eur. For. 2014, 5, 145–151. [Google Scholar] [CrossRef][Green Version]
- Landberg, T.; Greger, M. Differences in uptake and tolerance to heavy metals in Salix from unpolluted and polluted areas. Appl. Geochem. 1996, 11, 175–180. [Google Scholar] [CrossRef]
- Istenič, D.; Arias, C.A.; Vollertsen, J.; Nielsen, A.H.; Wium-Andersen, T.; Hvitved-Jacobsen, T.; Brix, H. Improved urban stormwater treatment and pollutant removal pathways in amended wet detention ponds. J. Environ. Sci. Health A Toxic Hazard. Subst. Environ. Eng. 2012, 47, 1466–1477. [Google Scholar] [CrossRef]
- Henze, M.; Comeau, Y. Wastewater Characterization. In Biological Wastewater Treatment: Principles Modelling and Design; Henze, M., van Loosdrecht, M.C.M., Ekama, G.A., Brdjanovic, D., Eds.; IWA Publishing: London, UK, 2008; pp. 33–52. ISBN 9781843391883. [Google Scholar]
- Federation, W.E.; APH Association. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Burt, R. Soil Survey Laboratory Methods Manual (Soil Survey Investigations Report No. 4, Version 5.02); Burt, R., Ed.; U.S. Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Black, C.A. Methods of Soil Analysis: Part 1 Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling; American Society of Agronomy: Madison, WI, USA, 1965. [Google Scholar]
- Rytter, R.M. Biomass production and allocation, including fine-root turnover, and annual N uptake in lysimeter-grown basket willows. For. Ecol. Manag. 2001, 140, 177–192. [Google Scholar] [CrossRef]
- Amiot, S.; Jerbi, A.; Lachapelle-T, X.; Frédette, C.; Labrecque, M.; Comeau, Y. Optimization of the wastewater treatment capacity of a short rotation willow coppice vegetation filter. Ecol. Eng. 2020, 158, 106013. [Google Scholar] [CrossRef]
- Pistocchi, C.; Guidi, W.; Piccioni, E.; Bonari, E. Water requirements of poplar and willow vegetation filters grown in lysimeter under Mediterranean conditions: Results of the second rotation. Desalination 2009, 246, 137–146. [Google Scholar] [CrossRef]
- Keoleian, G.A.; Volk, T.A. Renewable energy from willow biomass crops: Life cycle energy, environmental and economic performance. Crc. Crit. Rev. Plant Sci. 2005, 24, 385–406. [Google Scholar] [CrossRef]
- Fabio, E.S.; Smart, L.B. Effects of nitrogen fertilization in shrub willow short rotation coppice production—A quantitative review. GCB Bioenergy 2018, 10, 548–564. [Google Scholar] [CrossRef]
- Leskošek, M.; Mihelič, R.; Grčman, H.; Pavlič, E. Oskrbljenost kmetijskih tal s fosforjem in kalijem v Sloveniji. In Novi izzivi v Poljedelstvu; Tajnšek, A., Šantavec, I., Eds.; Slovensko agronomsko društvo: Ljubljana, Slovenia, 1998; pp. 37–41. [Google Scholar]
- Leonel, L.P.; Tonetti, A.L. Wastewater reuse for crop irrigation: Crop yield, soil and human health implications based on giardiasis epidemiology. Sci. Total Environ. 2021, 775, 145833. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Krzyżaniak, M.; Warminski, K.; Zaluski, D.; Olba-Ziety, E. Willows biomass as enery feedstock: The effect of habitat, genotype and harvest rotation on thermophysical properties and elemental composition. Energies 2020, 13, 4130. [Google Scholar] [CrossRef]
- Matthews, G. The Carbon CONTENT of Trees; Forestry Commission: Edinburgh, UK, 1993; Volume 4.
- Lamlom, S.H.; Savidge, R.A. A reassessment of carbon content in wood: Variation within and between 41 North American species. Biomass Bioenergy 2003, 25, 381–388. [Google Scholar] [CrossRef]
- Arienzo, M.; Christen, E.W.; Quayle, W.; Kumar, A. A review of the fate of potassium in the soil-plant system after land application of wastewaters. J. Hazard. Mater. 2009, 164, 415–422. [Google Scholar] [CrossRef]
- Adegbidi, H.G.; Volk, T.A.; White, E.H.; Abrahamson, L.P.; Briggs, R.D.; Bickelhaupt, D.H. Biomass and nutrient removal by willow clones in experimental bioenergy plantations in New York State. Biomass Bioenergy 2001, 20, 399–411. [Google Scholar] [CrossRef]
- Stolarski, M. Content of carbon, hydrogen and sulphur in biomass of some shrub willow species. J. Elem. 2008, 13, 655–663. [Google Scholar]
- Hytönen, J.; Saarsalmi, A. Long-term biomass production and nutrient uptake of birch, alder and willow plantations on cut-away peatland. Biomass Bioenergy 2009, 33, 1197–1211. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S.D. Treatment Wetlands; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9781566705264. [Google Scholar]
- Pokharel, P.; Chang, S.X. Manure pellet, woodchip and their biochars differently affect wheat yield and carbon dioxide emission from bulk and rhizosphere soils. Sci. Total Environ. 2018, 659, 463–472. [Google Scholar] [CrossRef]
- Zalesny, J.A.; Zalesny, R.S.; Wiese, A.H.; Sexton, B.; Hall, R.B. Sodium and chloride accumulation in leaf, woody, and root tissue of Populus after irrigation with landfill leachate. Environ. Pollut. 2008, 155, 72–80. [Google Scholar] [CrossRef]
- Loncnar, M.; Zupancic, M.; Bukovec, P.; Justin, M.Z. Fate of saline ions in a planted landfill site with leachate recirculation. Waste Manag. 2010, 30, 110–118. [Google Scholar] [CrossRef]
- Nissim, W.G.; Palm, E.; Pandolfi, C.; Mancuso, S.; Azzarello, E. Willow and poplar for the phyto-treatment of landfill leachate in Mediterranean climate. J. Environ. Manag. 2021, 277, 111454. [Google Scholar] [CrossRef]
- Meers, E.; Vandecasteele, B.; Ruttens, A.; Vangronsveld, J.; Tack, F.M.G. Potential of five willow species (Salix spp.) for phytoextraction of heavy metals. Environ. Exp. Bot. 2007, 60, 57–68. [Google Scholar] [CrossRef]


| BOD5 | COD | TP | PO4-P | TN | NH4-N | NO3-N | NO2-N | TSS | SS | O2 | O2 | T | pH | EC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | mg/L | mL/L | mg/L | % | °C | μS/cm | |||||||||
| Average | 452 | 739 | 5.75 | 2.84 | 50.6 | 23.3 | 0.298 | 0.092 | 207 | 7.4 | 2.87 | 33.0 | 23.9 | 6.68 | 441 |
| SD | 182 | 294 | 2.45 | 2.13 | 14.4 | 10.5 | 0.792 | 0.307 | 161 | 10.3 | 2.47 | 27.0 | 4.8 | 0.58 | 482 |
| Nr | 40 | 40 | 40 | 40 | 36 | 36 | 36 | 36 | 20 | 14 | 19 | 19 | 22 | 39 | 39 |
| BOD5 | COD | TP | PO4-P | TN | NH4-N | NO3-N | NO2-N | |
|---|---|---|---|---|---|---|---|---|
| 1st rotation, (aged 1/2) | ||||||||
| ‘V 093’ | 181 ± 9 | 278 ± 14 | 2.84 ± 0.15 | 1.50 ± 0.08 | 17.2 ± 0.9 | 11.1 + 0.1 | 0.023 ± 0.001 | 0.015 + 0.001 |
| ‘V 052’ | 183 ± 64 | 281 ± 98 | 2.87 ± 1.0 | 1.51 ± 0.53 | 17.4 ± 6.1 | 11.2 ± 3.9 | 0.023 ± 0.008 | 0.015 ± 0.005 |
| ‘V 160’ | 170 ± 21 | 262 ± 32 | 2.67 ± 0.32 | 1.41 ± 0.17 | 16.2 ± 2.0 | 10.4 ± 1.3 | 0.022 ± 0.003 | 0.014 ± 0.002 |
| 1st rotation, (aged 2/3) | ||||||||
| ‘V 093’ | 392 ± 61 | 600 ± 93 | 8.62 ± 1.33 | 6.32 ± 0.98 | 53.6 ± 8.3 | 34.7 ± 5.4 | 0.278 ± 0.043 | 0.072 ± 0.111 |
| ‘V 052’ | 323 ± 114 | 496 ± 174 | 7.12 ± 2.51 | 5.21 ± 1.84 | 44.3 ± 15.6 | 28.6 ± 10.1 | 0.230 ± 0.081 | 0.060 ± 0.021 |
| ‘V 160’ | 326 ± 55 | 499 ± 84 | 7.17 ± 1.21 | 5.25 ± 0.89 | 44.6 ± 7.5 | 28.8 ± 4.9 | 0.231 ± 0.039 | 0.060 ± 0.010 |
| 2nd rotation, (aged 1/4) | ||||||||
| ‘V 093’ | 485 ± 7 | 793 ± 11 | 6.17 ± 0.08 | 3.05 ± 0.04 | 54.3 ± 0.7 | 25.0 ± 0.3 | 0.319 ± 0.004 | 0.099 ± 0.001 |
| ‘V 052’ | 469 ± 54 | 766 ± 88 | 5.97 ± 0.69 | 2.95 ± 0.34 | 52.4 ± 6.1 | 24.2 ± 2.8 | 0.309 ± 0.036 | 0.095 ± 0.011 |
| ‘V 160’ | 406 ± 103 | 665 ± 169 | 5.17 ± 1.31 | 2.56 ± 0.65 | 45.5 ± 11.6 | 21.0 ± 5.3 | 0.268 ± 0.068 | 0.083 ± 0.021 |
| 2nd rotation, (aged 2/5) | ||||||||
| ‘V 093’ | 592 ± 31 | 1019 ± 54 | 4.89 ± 0.26 | 1.30 ± 0.07 | 68.6 ± 3.6 | 18.8 ± 1.0 | 0.599 ± 0.032 | 0.182 ± 0.010 |
| ‘V 052’ | 619 ± 130 | 1064 ± 224 | 5.11 ± 1.08 | 1.35 ± 0.29 | 71.6 ± 15.1 | 19.7 ± 4.1 | 0.626 ± 0.132 | 0.190 ± 0.040 |
| ‘V 160’ | 471 ± 148 | 810 ± 254 | 3.89 ± 1.22 | 1.03 ± 0.32 | 54.5 ± 17.1 | 15.0 ± 4.7 | 0.476 ± 0.149 | 0.145 ± 0.045 |
| Parameter | Percentage/Classification |
|---|---|
| Sand | 26.1 |
| Silt—coarse | 18.1 |
| Silt—fine | 30.4 |
| Silt—total | 48.5 |
| Clay | 25.4 |
| Classification * | clay |
| Unit | Start * | 1st Year | 2nd Year | |
|---|---|---|---|---|
| pH in CaCl2 | 7.1 | 7.1 ± 0.06 | 7.3 ± 0.06 | |
| P2O5 | mg/100 g | 144 | 162 ± 46.6 | 177 ± 3.13 |
| K2O | mg/100 g | 13.0 | 13.7 ± 0.66 | 14.4 ± 0.32 |
| Organic matter | % | 7.0 | 7.5 ± 0.3 | 7.2 ± 0.5 |
| Organic carbon | % | 4.1 | 4.3 ± 0.15 | 4.2 ± 0.3 |
| TN | % | 0.43 | 0.48 ± 0.01 | 0.40 ± 0.04 |
| C/N ratio | 9.5 | 9.0 ± 0.32 | 10.5 ± 0.40 | |
| Ca | mmol/100 g | 35.2 | 30.0 ± 0.68 | 36.8 ± 1.57 |
| Mg | mmol/100 g | 2.74 | 3.11 ± 0.12 | 2.87 ± 0.09 |
| K | mmol/100 g | 0.30 | 0.20 ± 0.02 | 0.30 ± 0.02 |
| Na | mmol/100 g | 0.05 | 0.17 ± 0.02 | 0.43 ± 0.10 |
| Exchangable acidity | mmol/100 g | 3.55 | NA | 4.80 ± 0.22 |
| Sum of base cations | mmol/100 g | 38.3 | 33.4 ± 0.78 | 40.4 ± 1.74 |
| CEC | mmol/100 g | 41.8 | NA | 45.2 ± 1.54 |
| Base saturation | % | 91.6 | NA | 89.4 ± 0.84 |
| Control Trees | Test Trees | ||||||
|---|---|---|---|---|---|---|---|
| ‘V 093’ | ‘V 052’ | ‘V 160’ | ‘V 093’ | ‘V 052’ | ‘V 160’ | P | |
| C | 477 ± 1.8 | 473 ± 0.28 | 475 ± 0.0 | 477 ± 0.40 a | 474 ± 0.31 b | 474 ± 1.8 ab | 0.845 |
| N | 7.6 ± 0.07 | 10 ± 0.2 | 7.6 ± 0.21 | 6.5 ± 1.0 | 7.9 ± 0.76 | 7.1 ± 0.73 | 0.081 |
| P | 0.99 ± 0.16 | 1.1 ± 0.40 | 0.91 ± 0.07 | 0.92 ± 0.23 ab | 1.2 ± 0.04 a | 1.0 ± 0.08 b | 0.775 |
| K | 2.4 ± 0.07 | 2.9 ± 0.28 | 2.2 ± 0.14 | 1.8 ± 0.25 | 2.2 ± 0.21 | 2.0 ± 0.25 | 0.029 |
| Ca | 8.1 ± 2.8 | 8.2 ± 2.1 | 9.1 ± 0.07 | 5.0 ± 0.25 | 5.7 ± 0.78 | 5.8 ± 1.2 | 0.005 |
| Mg | 0.93 ± 0.18 | 1.1 ± 0.1 | 0.84 ± 0.16 | 0.75 ± 0.06 b | 0.75 ± 0.04 b | 0.87 ± 0.04 a | 0.060 |
| S | 1.6 ± 0.42 | 1.7 ± 0.07 | 1.1 ± 0.50 | 1.9 ± 0.27 | 1.8 ± 0.23 | 1.9 ± 0.15 | 0.044 |
| Control Trees | Test Trees | ||||||
|---|---|---|---|---|---|---|---|
| ‘V 093’ | ‘V 052’ | ‘V 160’ | ‘V 093’ | ‘V 052’ | ‘V 160’ | P | |
| Fe | 105 ± 7 | 95 ± 7 | 75 ± 7 | 57 ± 6 | 63 ± 6 | 67 ± 6 | 0.003 |
| Al | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | |
| Na | 20 ± 0 | 15 ± 7 | 25 ± 7 | 33 ± 6 | 33 ± 15 | 47 ± 12 | 0.003 |
| Mo | 0.04 ± 0.03 | 0.06 ± 0.00 | 0.05 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.00 | 0.03 ± 0.02 | 0.008 |
| Cu | 7.8 ± 1.7 | 11.1 ± 1.0 | 8.8 ± 2.2 | 6.2 ± 0.6 c | 10.0 ± 0.5 a | 7.9 ± 0.4 b | 0.271 |
| Pb | 1.22 ± 1.11 | 0.19 ± 0.04 | 0.16 ± 0.00 | 0.12 ± 0.01 | 1.55 ± 2.48 | 0.14 ± 0.03 | 0.885 |
| Zn | 46 ± 5 | 58 ± 24 | 40 ± 7 | 42 ± 2 | 42 ± 4 | 45 ± 5 | 0.436 |
| Ni | 0.70 ± 0.28 | 0.45 ± 0.07 | 0.30 ± 0.14 | 0.23 ± 0.12 | 0.30 ± 0.17 | 0.53 ± 0.40 | 0.343 |
| Co | 0.10 ± 0.01 | 0.11 ± 0.01 | 0.06 ± 0.00 | 0.35 ± 0.33 | 0.06 ± 0.01 | 0.08 ± 0.02 | 0.322 |
| Mn | 9.0 ± 2.8 | 10.0 ± 4.2 | 9.0 ± 1.4 | 9.3 ± 1.2 b | 8.7 ± 0.6 b | 15.3 ± 2.5 a | 0.265 |
| As | 0.25 ± 0.07 | 0.40 ± 0.00 | <LOD | 0.29 ± 0.36 | 0.20 ± 0.10 | 0.11 ± 0.07 | 0.678 |
| U | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 0.254 |
| Th | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 1.000 |
| Sr | 10.8 ± 4.6 | 10.9 ± 0.2 | 10.8 ± 1.8 | 6.6 ± 0.8 | 7.0 ± 1.0 | 7.6 ± 0.7 | 0.007 |
| Cd | 0.88 ± 0.56 | 0.72 ± 0.01 | 0.57 ± 0.04 | 0.43 ± 0.07 | 1.00 ± 0.96 | 0.49 ± 0.01 | 0.713 |
| Sb | 0.03 ± 0.01 | 0.02 ± 0.01 | <LOD | <LOD | 0.02 ± 0.00 | <LOD | 0.139 |
| Bi | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 0.254 |
| V | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | |
| La | 0.02 ± 0.02 | <LOD | <LOD | <LOD | <LOD | <LOD | 0.363 |
| Cr | 2.50 ± 0.85 | 2.05 ± 0.07 | 1.95 ± 0.21 | 2.17 ± 0.25 | 2.20 ± 0.20 | 2.50 ± 0.61 | 0.608 |
| Ba | 4.65 ± 2.05 | 4.85 ± 0.78 | 3.65 ± 0.07 | 2.20 ± 0.00 | 3.70 ± 3.03 | 2.40 ± 0.61 | 0.046 |
| Ti | 13.5 ± 0.7 | 27.0 ± 2.8 | 24.5 ± 9.2 | 4.0 ± 2.6 | 11.7 ± 7.8 | 18.7 ± 9.6 | 0.037 |
| B | 15.5 ± 4.9 | 14.5 ± 0.7 | 13.0 ± 0.0 | 9.3 ± 0.6 | 9.7 ± 1.5 | 9.7 ± 1.5 | 0.004 |
| W | <LOD | 0.20 ± 0.00 | 0.14 ± 0.09 | 2.29 ± 2.27 | 0.15 ± 0.13 | 0.15 ± 0.13 | 0.201 |
| Sc | 0.30 ± 0.14 | 0.25 ± 0.07 | 0.20 ± 0.00 | 0.23 ± 0.06 | 0.30 ± 0.00 | 0.27 ± 0.06 | 0.674 |
| Tl | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 0.254 |
| Se | 0.30 ± 0.14 | 0.20 ± 0.00 | 0.20 ± 0.14 | 0.20 ± 0.00 | 0.23 ± 0.06 | 0.17 ± 0.06 | 0.488 |
| Te | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 0.254 |
| Ga | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 1.000 |
| Ag | 3.21 ± 2.54 | 3.50 ± 0.71 | 3.00 ± 0.00 | 4.67 ± 1.53 | 6.67 ± 1.15 | 9.67 ± 1.53 | 0.002 |
| Au | <LOD | <LOD | <LOD | <LOD | 0.11 ± 0.17 | <LOD | 0.347 |
| Hg | 4.00 ± 1.41 | 2.50 ± 0.71 | 3.00 ± 1.41 | 1.90 ± 1.15 | 2.33 ± 1.53 | 3.33 ± 0.58 | 0.321 |
| Start | After 1st Rotation | |||
|---|---|---|---|---|
| ‘V 093’ | ‘V 052’ | ‘V 160’ | ||
| Fe | 28,700 | 29,200 | 29,300 | 31,000 |
| Cd | 0.7 | 0.6 | 0.7 | 0.6 |
| Cu | 66.2 | 67.2 | 66.5 | 72.8 |
| Ni | 80.9 | 78.1 | 81.3 | 86.6 |
| Pb | 36.4 | 34.1 | 35.5 | 42.2 |
| Zn | 153 | 161 | 172 | 166 |
| Cr | 63 | 60 | 59 | 67 |
| Hg | 0.56 | 0.79 | 0.76 | 0.65 |
| Co | 16.1 | 16.3 | 16.5 | 18.3 |
| Mo | 1.2 | 1.3 | 1.3 | 1.5 |
| As | 8.7 | 8 | 8.2 | 8.1 |
| Mn | 1012 | 1146 | 1110 | 1069 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Istenič, D.; Božič, G. Short-Rotation Willows as a Wastewater Treatment Plant: Biomass Production and the Fate of Macronutrients and Metals. Forests 2021, 12, 554. https://doi.org/10.3390/f12050554
Istenič D, Božič G. Short-Rotation Willows as a Wastewater Treatment Plant: Biomass Production and the Fate of Macronutrients and Metals. Forests. 2021; 12(5):554. https://doi.org/10.3390/f12050554
Chicago/Turabian StyleIstenič, Darja, and Gregor Božič. 2021. "Short-Rotation Willows as a Wastewater Treatment Plant: Biomass Production and the Fate of Macronutrients and Metals" Forests 12, no. 5: 554. https://doi.org/10.3390/f12050554
APA StyleIstenič, D., & Božič, G. (2021). Short-Rotation Willows as a Wastewater Treatment Plant: Biomass Production and the Fate of Macronutrients and Metals. Forests, 12(5), 554. https://doi.org/10.3390/f12050554






