Abstract
Determining adhesive bond performance for chemically modified wood is important not only for its commercial utility but also for understanding wood bond durability. Bulking modifications occupy space inside the cell wall, limiting the space available for water. We used two bulking modifications on yellow poplar (Liriodendron tulipifera L.): acetylation (Ac), which bulks and converts a wood hydroxyl group to an ester, while butylene oxide (BO) also bulks the wood but preserves a hydroxyl group. Both result in lower water uptake; however, the loss of the hydroxyl group with Ac reduces the wood’s ability to form hydrogen and other polar bonds with the adhesives. On the other hand, the BO reaction replaces a hydroxyl group with another one along a hydrocarbon chain; thus, this product may not be harder to bond than the unmodified wood. We investigated how these chemical modifications of wood affect bond performance with four adhesives: resorcinol-formaldehyde (RF), melamine-formaldehyde (MF), emulsion polymer isocyanate (EPI), and epoxy. The ASTM D 905 bond shear strength for both dry and wet samples showed that the BO results were quite similar to the unmodified wood, but the MF and EPI performed poorly on Ac-modified wood, in contrast to the results with RF and epoxy.
1. Introduction
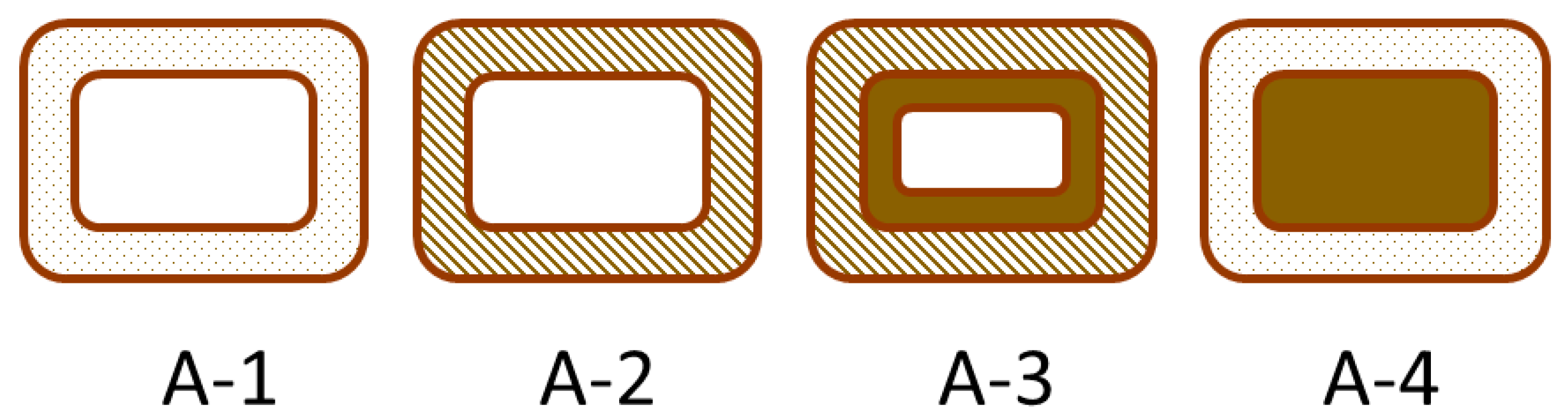
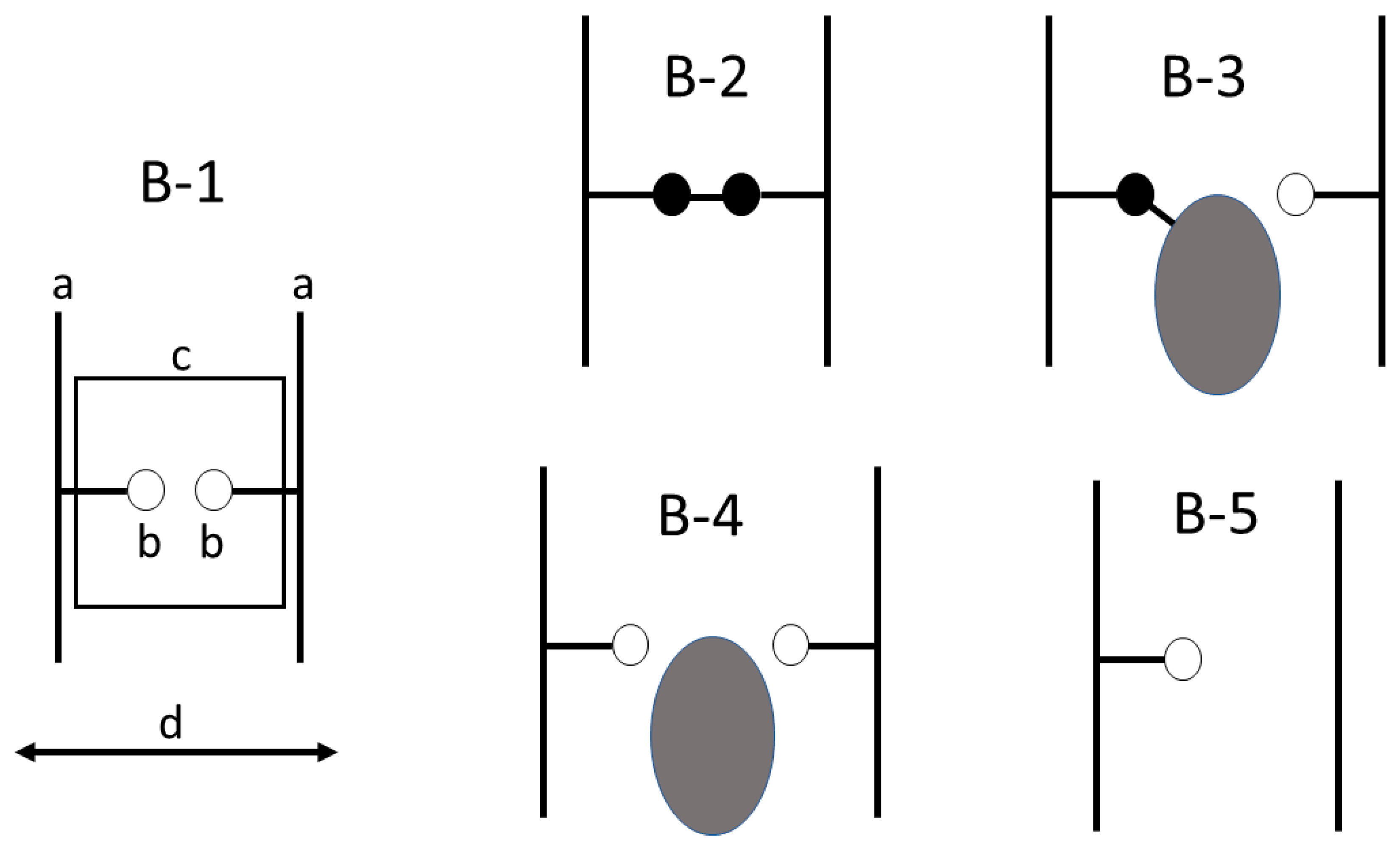
Wood is widely used because of its visual appealing characteristics, high strength for its weight and ready availability as a biomaterial in most countries. One factor that limits the utility of wood is its ability to readily absorb and desorb water resulting in undesired dimensional changes. Water absorption can lead to decay of wood, which is good for the natural recycling of wood, but is bad when used for buildings. Thus, wood protection has been an important research area for a long time. Traditionally, wood was preserved by impregnation (e.g., creosote treatment) and surface alteration (e.g., CCA or copper salt treatments), but these methods rely on hazardous chemicals. A less hazardous alternative is to chemically modify the wood, which involves adding chemicals directly to the wood to its chemical structure [1]. The most widely studied and used wood modification is acetylation, with the second most being thermal treatment of wood. To distinguish the different modes of wood modification and how they affect the wood structure, Norimoto et al. developed two simplistic models for classifying modifications [2,3], and these also work well for explaining adhesive bonding mechanisms [4]. The first model looks at the processes from a cellular point of view as depicted in Figure 1, while Figure 2 shows the processes from a cell wall polymer point of view. Thermal treatment Figure 1(A-2) and Figure 2(B-5), impregnation Figure 1(A-4) and surface treatment were not part of this study, so they will not be further considered.
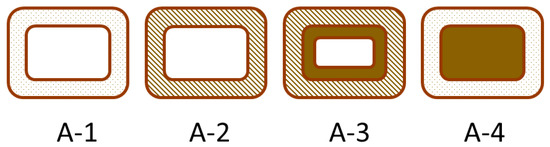
Figure 1.
Model for chemical modification of wood at the cellular level: (A-1), untreated; (A-2), treated cell wall with no chemical deposit in lumen; (A-3), treated cell wall with deposit on the lumen surface; (A-4), unaltered cell wall with filling of lumen [2].
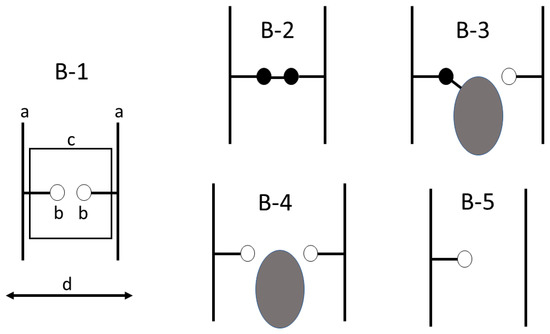
Figure 2.
Model for chemical modification of wood at the cell wall polymer level (B-1) with (a) being cell wall polymer, (b) being hydroxyl on the polymer, (c) enclosing the area of interest, (d) representing radial and tangential swelling and shrinking The following are different cases with (B-1), untreated wood: small open circle represents OH group available for hydrogen bonding; (B-2) small filled circle, substitution of OH group with cross-link; (B-3) large filled circle being a bulking group bonded to the hydroxyl; (B-4) large filled circle representing unbonded bulking agent, and (B-5), showing a dehydrated polymer [2].
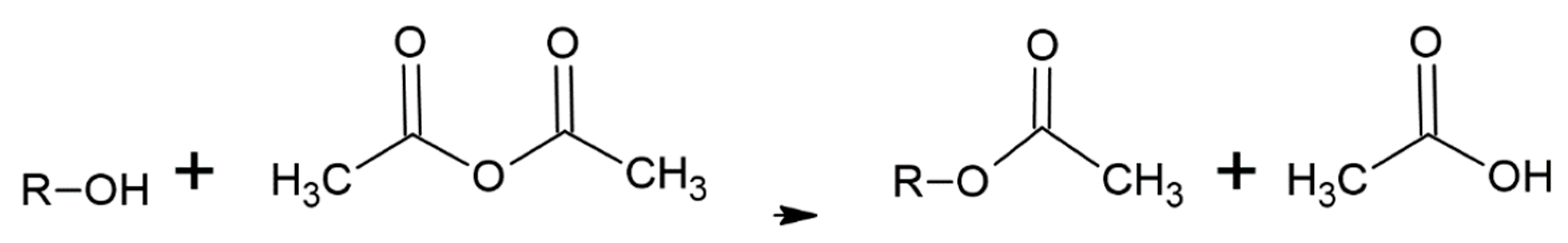
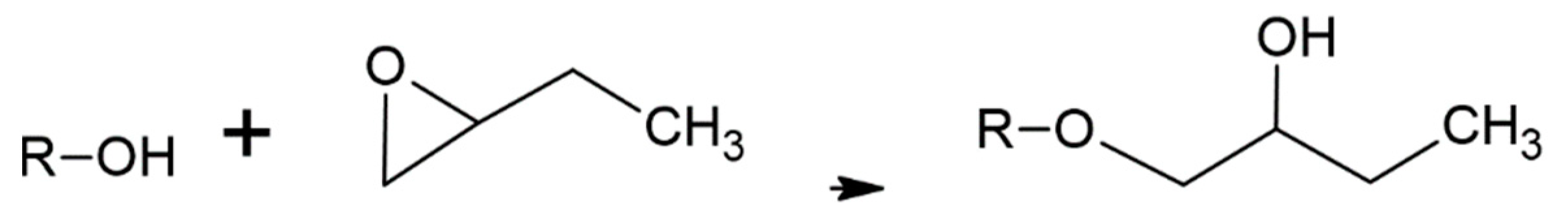
The literature on acetylation of wood is vast with many methods having been evaluated. The current commercial process treats dry wood with acetic anhydride under heat and pressure [1,2,5,6,7,8,9,10,11,12,13,14,15,16,17]. Although this process works quite well and the acetylated wood has some unique, beneficial properties, a significant problem is that only half the acetic anhydride reagent weight is attached to the wood with the other half becoming acetic acid (Figure 1(A-2), Figure 2(B-3) and Figure 3). This low use of the reagent with high acetic acid by-product volume requires separate steps to remove the acetic acid from the wood and reconvert it to acetic anhydride. Among the other ways to modify wood is to have their hydroxyls react with alkylene oxides to make hydroxyethers (Figure 1(A-2), Figure 2(B-3) and Figure 4. Both treatments modify the hydroxyl groups in the cell wall; in acetylation the hydroxyl group is converted to an ester and in the oxide case the hydroxyl group is converted to a 2-hydroxybutane-1-oxy. Both modifications bulk the cell wall Figure 2(B-4) and modify the polymer hydroxyl group Figure 2(B-3). The consequences are reduced wood polarity and that there is less room in the cell wall for water molecules and wood swelling. Compared to BO treatment, conversion of hydroxyl groups to esters in the Ac treatment lowers polarity further and limits the number and strength of hydrogen bonds with adhesives, because esters are only hydrogen bond acceptors while hydroxyls are both acceptors and donors.
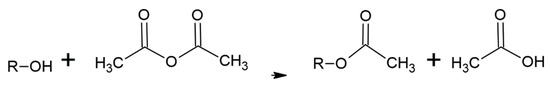
Figure 3.
Acetylation of wood polymer with acetic anhydride where R = wood polymers.
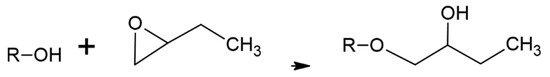
Figure 4.
Butylene oxide modification of wood polymer where R = wood polymers.
Wood modification is a well-studied area [1,11,18] because of the value in reducing wood swelling, microbial decay and insect attack. Available wood adhesives have very different functional groups, bonding mechanisms, morphologies, molecular weights and other properties; therefore, they are useful as probes of wood properties as well as to learn how they bond modified woods. Research in this area is greatly aided by a paper that studied the bonding of 18 different adhesives to yellow poplar acetylated at different modification levels [19]. Additional studies have been done on adhesive bonding of acetylated wood [20,21,22]. We are aware of only of two citations regarding bonding to alkylene oxide-modified wood, which are preliminary discussions of our results [23,24].
Further study is important because some obvious pre-conceived conclusions clash with the data on bond strengths. Due to its low polarity and few available hydroxyl groups, acetylated wood should not bond well with the aqueous and very polar phenolic adhesives, but these adhesives form strong bonds with the acetylated wood [19,20]. The analysis of this first study on the effect of acetylation showed that the low polarity of the acetylated wood affects adhesives in wet tests differently, with the resorcinol formaldehyde being unaffected, emulsion polymer isocyanate having poorer bonds, and epoxy having a better bond [20]. The question in this study was whether a small reduction in polarity but with retention of the hydroxyl groups (alkylene oxide modification) has a different effect on the adhesive bonding compared to a high reduction in polarity with loss of the hydroxyl groups (acetylation). The original intent was to compare unmodified and modified wood with Ac-, propylene oxide (PO)- and butylene oxide (BO)-modified wood. However, it was difficult to control the reaction of the PO to have sufficient but not excess modification of the wood. In our work, we found that the PO-modified wood could not give good wet strength values because of the over-treated wood fragmented during the water soaking test. Thus, we dropped the PO set of data from the evaluation in the results and discussion section because this is a very abnormal bondline failure mechanism.
Based on their performance, four adhesives (MF, RF, EPI, and epoxy) from the Vick et al. 1990 study [19] were selected for evaluating the bonding Ac- and BO-modified yellow popular wood. One group of durable adhesives, the reinforcing adhesives, create a swelling gradient by reinforcing the wood adjacent to the glueline [25,26,27]. These stiff, in-situ polymerized adhesives do not expand with the wood but avoid a zone of extreme shear concentration by diffusing into and reinforcing the cells adjacent to the glueline Figure 1(A-3) and Figure 2(B-3). This reinforced zone allows for a gradual transition from the bulk swelling of the wood to the low-swelling adhesive layer. Typical reinforcing adhesives are melamine formaldehyde (MF) and resorcinol formaldehyde (RF) [25]. The second group, the flexible, pre-polymerized adhesives, avoid producing a strain gradient by changing dimensionally with the wood; emulsion polymerized isocyanate (EPI) is an example of this class. Vick et al.’s 1990 study [19] showed that even though RF and MF are similar in-situ polymerized adhesives, they showed very different results. RF was insensitive to wood acetylation, while MF lost most of its wet but not dry strength upon wood acetylation. The EPI did not bond well to the acetylated compared to unmodified wood in either wet or dry conditions, and the epoxy was less sensitive to wet conditions for the acetylated compared to the unmodified wood. The current experiments adds the butylene-modified wood to the prior work [20], and in addition, we were able to study the MF adhesive by adding filler to eliminate the overpenetration issue encountered in the previous study [20].
2. Materials and Methods
2.1. Bond Strength Study
2.1.1. Experimental Design
The experiments were designed to survey four thermosetting adhesives for their ability to bond to acetic anhydride- and alkylene oxide-modified wood. The effectiveness of bonds was determined according to ASTM D905 [28] by measuring shear strength and wood failure in an ambient (dry) condition and in a wet condition after water soaking under vacuum and then pressure (VPS), keeping the samples soaking until being tested according to ASTM D2559 [29]. The design was a full factorial arrangement with four adhesives (RF, MF, EPI and epoxy) and three modifications (acetylated, propylene oxide-modified, butylene oxide-modified and an untreated control) yielding 16 treatment combinations. Each treatment combination (wood modification and adhesive used) was replicated nine times, and four samples were taken from each replicate since we had determined that variation between bonded specimens was larger than within specimen. After randomly assigning the specimens for dry or wet tests, the 576 samples (four adhesives, four wood modifications, four samples from each of the nine specimens) were tested for their shear strength and wood failure in dry and wet test conditions.
2.1.2. Acetylation of Wood
Yellow-poplar (poplar (Liriodendron tulipifera L.), also known as tulip tree and American whitewood sapwood lumber, from a Mississippi forest, free of defects, was sawn into strips 31.8 mm wide, 229 mm long, and 6.4 mm thick. After cutting, the strips were placed in an oven and dried at 105 °C for 24 h. The strips were removed from the oven, cooled in a desiccator for 1 h, and weighed. The strips were acetylated according to the following procedure. Strips were placed in a 2 L glass reactor fitted with a reflux condenser. The glass reactor was filled with enough acetic anhydride to cover the strips even after absorption of the chemicals. The acetic anhydride and wood were heated to boiling (139.8 °C) for 4 h and then cooled to room temperature (21 °C). Strips were removed, washed for 4 h in reversed osmosis water to remove acetic acid and excess acetic anhydride, air dried overnight, and then oven-dried for 24 h at 105 °C. The 21% weight gain due to acetylation was determined after oven-drying by calculation as a percentage of the original oven-dried weight.
2.1.3. Alkylene Oxide-Modification of Wood
Strips (31.8 mm wide, 229 mm long, and 6.4 mm thick) were placed in a stainless steel reactor with a mixture of propylene oxide or BO and triethylamine (95:5 (vol:vol)) at 120 °C and 635 mm of mercury, for 60 min for propylene oxide, and 4 h for butylene oxide. Strips were taken out of the reactor and air-dried under a fume hood overnight, water soaked for 4 h, air dried, and then oven dried for 24 h, to yield 23% weight gain.
2.1.4. Bonding
All strips, including the unmodified controls, were conditioned at 27 °C and 65% relative humidity until bonded. Specimens were prepared by laminating two strips of wood, 6.4 mm thick, 31.8 mm wide, and 229 mm long. Adhesive was placed on one piece of wood on a balance and rolled out for even coverage and desired adhesive weight.
Cold-setting adhesives, RF: Casophen resorcinol–formaldehyde RS-216 (five parts) with FM-60 M catalyst (one part) (Borden (Hexion), Bellevue, WA, USA) with 15 min open and 25 min closed time; EPI: Isoset emulsion polymer isocyanate WDC-154 (100 parts) with CX-11 catalyst (17 parts) (Ashland, Dublin, OH, USA) with 15 min open and closed assembly time, and Lord 305-1 epoxy (two parts) with 305-2 hardener (one part) (Lord Corporation, Cary, NC, USA) with one h closed assembly time, were spread at an approximate rate of 320–340 g/m2 and cured at room temperature. Adhesive was spread on both surfaces with a rubber-roll hand spreader. Adhesive spread rate was accurately controlled by automatically tare-weighing the adhesive on the laminates as they were spread. Pressure for the epoxy and emulsion polymer isocyanate was determined by increasing pressure until the beginning of squeeze out. After a 15-min open assembly time and 25-min closed time, pressure for the resorcinol–formaldehyde was maintained at 690 ± 35 kPa for 18 h. All nine replicates (joint assemblies) of a single treatment combination were pressed within the same press closure. Closed assembly varied between 15 and 60 min, depending on individual curing characteristics.
The hot setting melamine-formaldehyde (MF), Cascomel MF-600 (Hexion) plus 0.5 wt.% of walnut shell filler was spread at a rate of 200 kg/m2 with 35 min open time and 25 min closed assembly time and was cured in an electrically heated laboratory hot-press maintained at 138 ± 5 °C. Pressure was maintained at 862 ± 35 kPa for 10 min.
After removing material from both sides and ends, four block-shear samples with a shear area of 2.54 by 2.54 cm were cut from each specimen to form shear blocks as described in ASTM D905 and randomly assigned to either the dry or wet shear tests.
2.1.5. Adhesive Testing
Eighteen samples representative of each wood modification and adhesive combination (216 total) were subjected to a single vacuum pressure soak (VPS) and then tested for shear strength and wood failure while in the water-saturated condition. The saturation process consisted of the following events:
- Submerged specimens in tap water at room temperature in a pressure vessel.
- Maintained a vacuum of 635 ± 85 kPa for 30 min.
- Maintained a pressure of 448 ± 35 kPa for 30 min.
- Remained submerged in water until tested.
Dry and wet samples were tested in a compression-loading shearing tool as described in ASTM Method D905 [28] using an MTS 810 Material Test Machine (MTS Systems Corporation, Eden Prairie, MN, USA). Load was applied at a constant rate of 2.54 mm per minute until failure. The maximum load at failure was recorded, and then shear strength was calculated for each specimen based on the shear area. Wood failure was estimated to the nearest 5% on the sheared area, according to ASTM D5266-99 [30]. The wet-tested samples were air-dried before estimating wood failure. Estimating is easier after drying because of greater color and light reflection contrast between the dry wood fiber and the adhesive. Error bars represent one standard deviation.
2.2. Melamine Analysis
UV-absorbance spectra were acquired using the method of Gindl et al. [31] with a MPM800™ photometer microscope (Carl Zeiss). Briefly, UV absorbance was measured at 1 nm intervals with a 5 nm bandpass, of 0.5 µm wide spots on thin cross sections. The absorbance of S2 layers of tracheids were measured in cells near the glueline with resin-filled tracheids. These values were compared to spectra of control cells, positioned about 0.5 mm away from the glue line, and pure MF resin cured in tracheid lumina. Displayed spectra represent the average of 5–8 spots.
3. Results
Lap shear tests are common for measuring the strength of bonded wood products and then used to compare these results to shear strength parallel to grain for the wood, which is used in designing structures. Although other tests are needed for commercial adhesive approval, the ASTM D905 is usually the starting point. Wood structures are normally in the dry state (ambient temperature and humidity), to assure sufficiently good bond formation and durability, exposure to water is used in most wood approval processes. Therefore, the D905 test includes the requirement to fully saturate the wood and test the bond strength when wet.
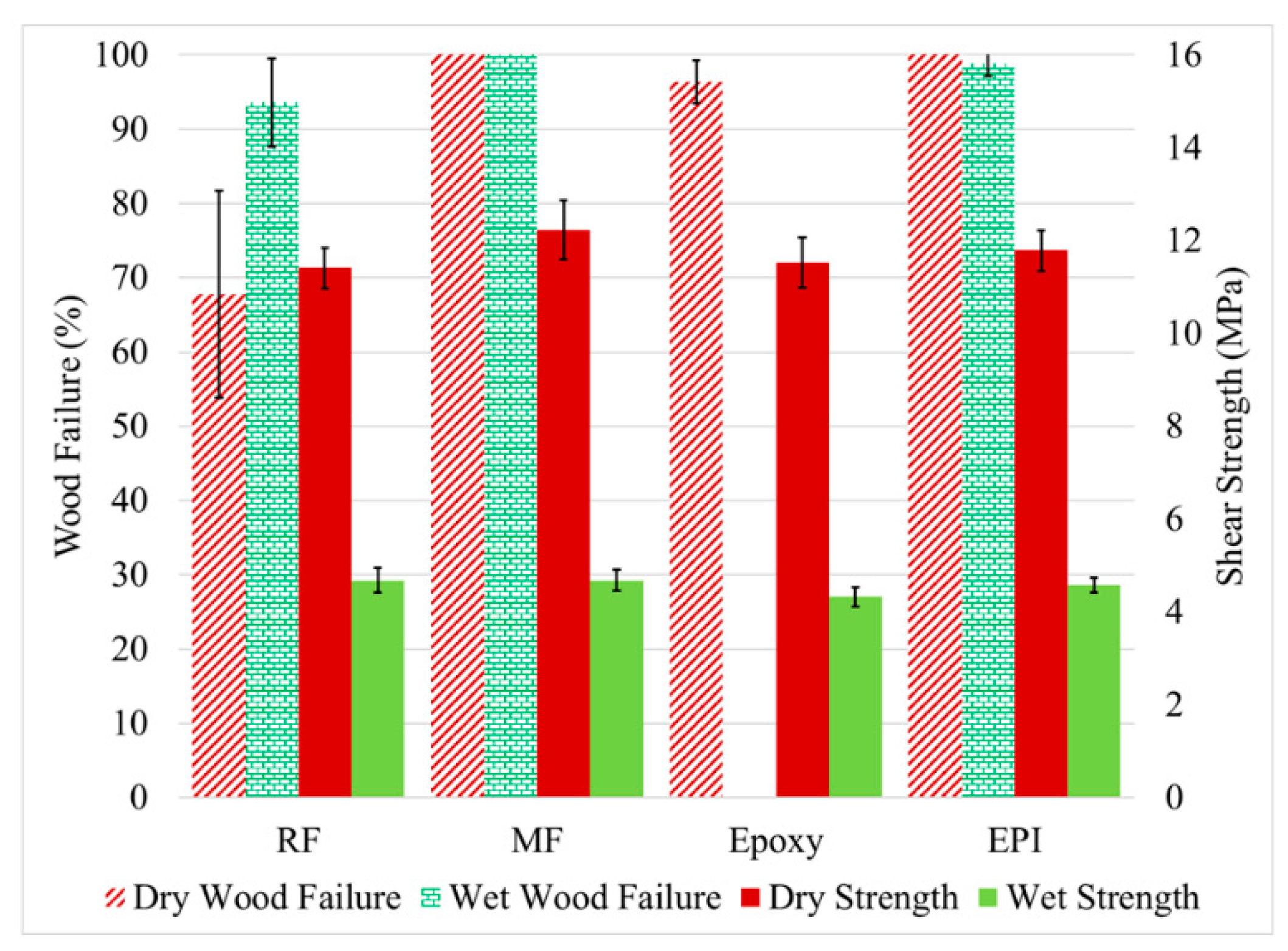
The shear test and wood failure results for the unmodified yellow poplar are presented in Figure 5. The desired case is for the adhesive to be stronger than the wood giving a 100% wood failure with the strength measuring the longitudinal strength of the wood. Less than 100% wood failure can be caused by insufficient ultimate adhesive strength, poor cure of the adhesive, insufficient adhesive added to the glue line (adhesive between the wood surfaces), overpenetration of the adhesive into the wood giving a starved glue line, or a weak layer between the adhesive and the wood. Just as the models in Figure 1 and Figure 2 were developed to consider how the modification alters the wood, the same models can be used to described the adhesive interaction with wood [25]. Of the adhesives tested, the RF, MF, and epoxy belong to the in situ-polymerized group that can develop strength through mechanisms in Figure 1(A-2,A-3) and Figure 2(B-3,B-4), and the EPI belongs to the pre-polymerized group that only develops strength through Figure 1(A-4) mechanism. It was expected that the in situ-polymerized group adhesives would be the most sensitive to the wood modification and therefore involved the greatest number of adhesives tested in this study. In addition, the selected adhesives also showed different responses in the examination of 18 adhesives with different degrees of wood acetylation [19].
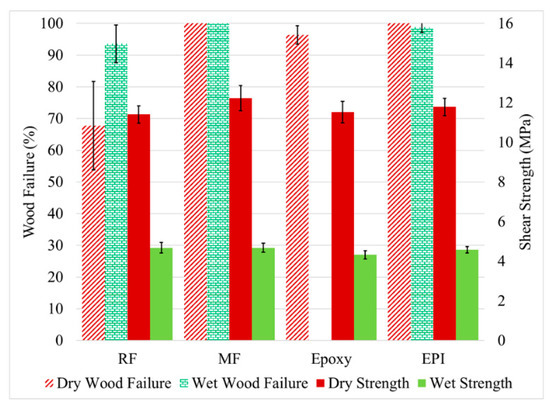
Figure 5.
The measured shear strength (left axis) and wood failure (right axis) for the untreated yellow poplar bonded with RF (resorcinol formaldehyde), MF (melamine formaldehyde), epoxy, and EPI (emulsion polymer isocyanate adhesives. The samples were tested dry and after water soaking (wet). Error bars = 1 standard deviation.
In Figure 5, all four of the adhesives had high dry wood failure and shear strength showing that all adhesives used suitable conditions. The lower than expected dry wood failure with RF was due to insufficient adhesive in the glueline, probably from over penetration. All but the epoxy did well after the water exposure in wood failure. Prior work has shown that the epoxy failed not due to lack of adhesion to the wood surface, but in the adhesive strength near the wood interface [32]. On the other hand, the shear strength of all the wood bonds decreased dramatically to a similar level under the wet test. Given the high wood failure (even higher than when tested dry), this drop is likely due to the plasticization of the wood by the water, a well-known phenomenon [33]. Even though the adhesive might be plasticized, the effect is even greater with the wood due its greater water uptake [34].
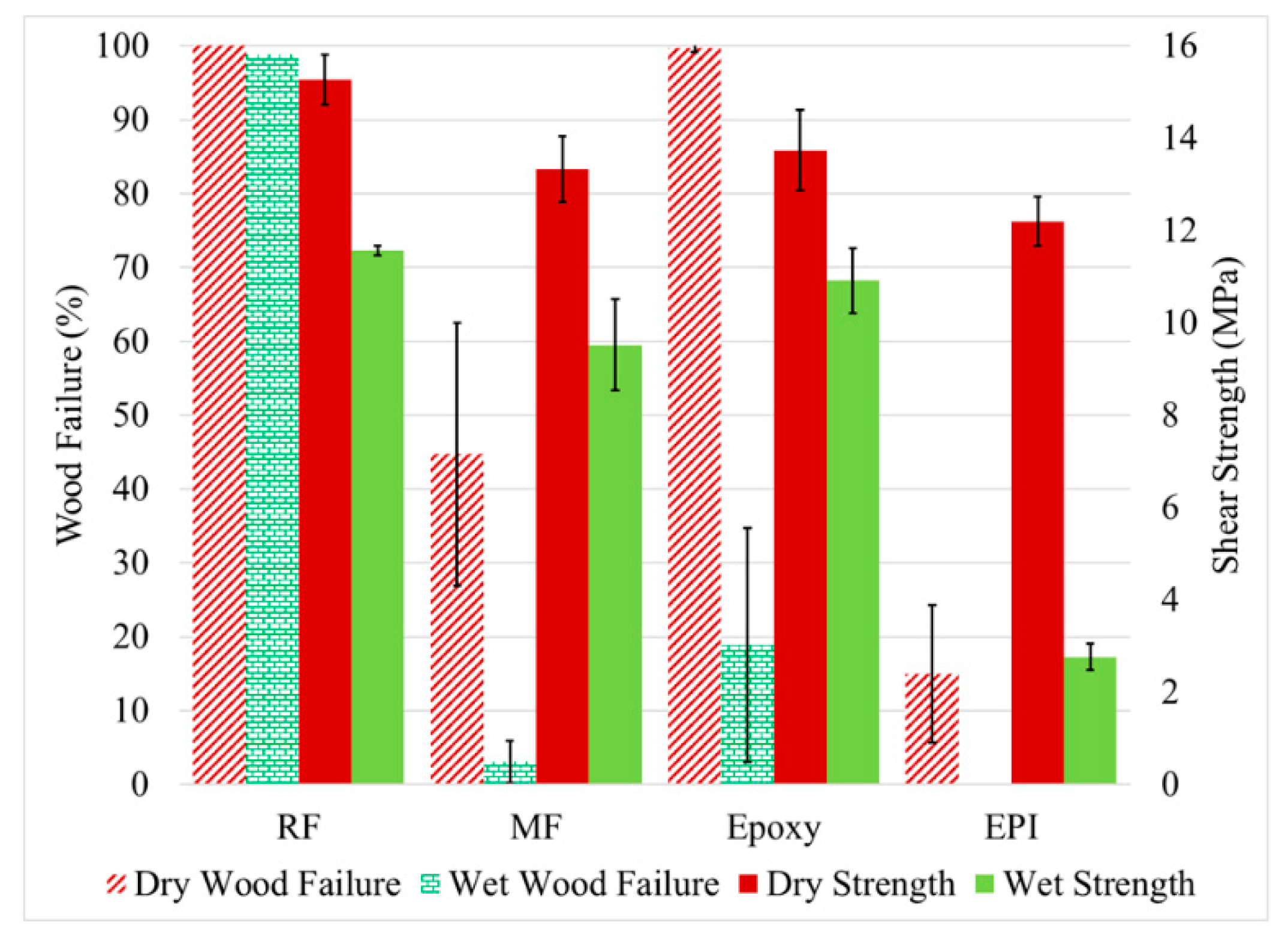
The performance of the adhesive bonds would normally be expected to change dramatically when the wood is acetylated, but the literature shows that simplistic assumption of poor adhesion with the water-borne adhesives (RF, MF, and EPI) was not found with all adhesives (Figure 6). Wetting of the wood surface and subsequent void penetration by the water-borne adhesives is expected to be poorer due to lower surface tension of the acetylated wood. Infiltration of the wood cell walls by these aqueous adhesives should be poorer due to bulking of the cell walls. Infiltration by highly polar molecules such as MF is expected to be less in Ac and BO wood because of the lower polarity of these substrates. Finally, water plasticizes the adhesive independent of the wood type, but the acetylated wood is much less plasticized than the unmodified wood [1,11,35]. The limited plasticization of acetylated wood results in higher forces on the adhesive under the wet conditions, which is shown by the higher wet strength (11 MPa) for the RF bonded Ac wood (Figure 6) compared to that (4 MPA) for the wet untreated wood (Figure 5). There is high wood failure in both cases. On the other hand, the MF had good bond strength, but poor wood failure with the acetylated wood: some in the dry state and greatly in the wet state. Although MF and RF are often considered similar in performance, this is certainly not the case with the acetylated wood. This pattern has been seen before [36], but the differences were not fully investigated. An explanation for this is suggested by a study using UV microscopy discussed later in this paper.
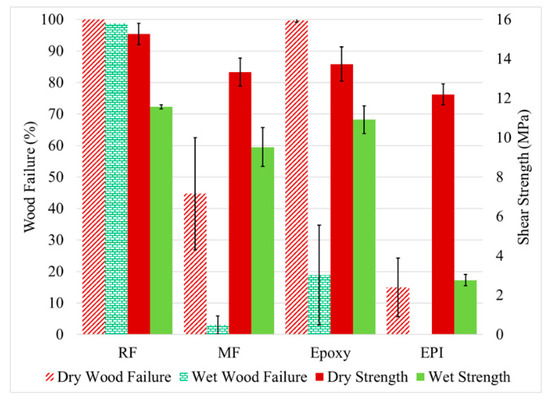
Figure 6.
The measured shear strength (left axis) and wood failure (right axis) for the acetylated yellow poplar bonded with RF (resorcinol formaldehyde), MF (melamine formaldehyde), epoxy, and EPI (emulsion polymer isocyanate adhesives. The samples were tested dry and after water soaking (wet).
Differences with the other two adhesives were also observed between the acetylated and non-acetylated wood. When bonding acetylated wood, the EPI not only had lower wood failure in the wet samples like the MF, but also had lower wood failure with the dry samples, which is not true for the MF. A rationalization is that the EPI is the only pre-polymerized adhesive tested and can be most sensitive to the lower surface energy of the acetylated wood. In contrast to the other adhesives, the epoxy adhesive showed higher wood failure and shear strength under wet conditions for acetylated wood compared to non-acetylated wood. This is not surprising since the epoxy is the least polar of the tested adhesives and would best wet the acetylated wood and is not subjected to the high strain experienced with unmodified wood [32].
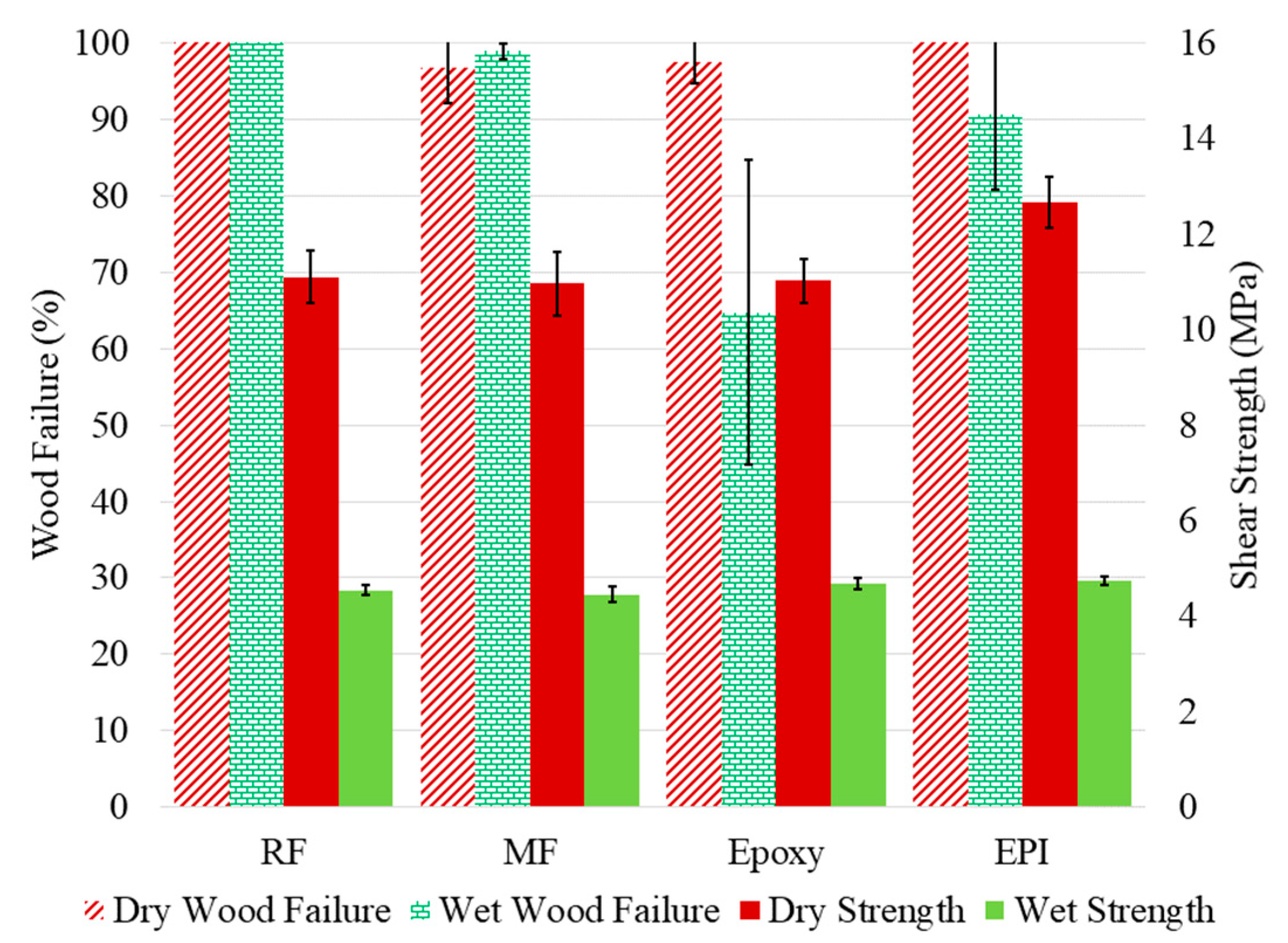
In contrast with the Ac modification, BO adds aliphatic character to the wood, but leaves a free hydroxyl group. The ASTM D905 compressive shear data (Figure 7) resemble those for the unmodified wood (Figure 5), but not the acetylated wood. The fact that the wet strength failure is similar for all four adhesives supports the conclusion that wood strength controls the bonded sample strength. This indicates that despite the BO modification reducing the water uptake [37,38], the water still plasticizes the wood more than with the Ac wood. Although there was not a difference in wet bond strength with the epoxy, the wood failure was much higher compared to the unmodified wood.
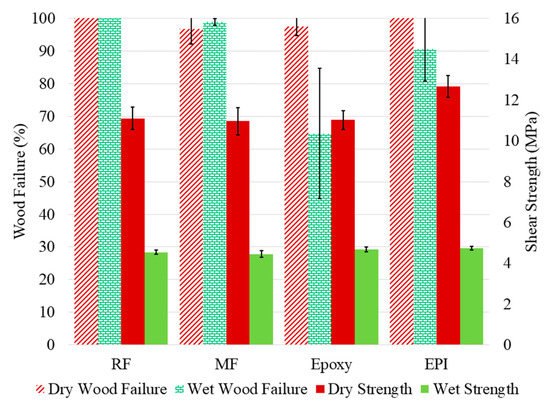
Figure 7.
The measured shear strength (left axis) and wood failure (right axis) for the butylene oxide-modified yellow poplar bonded with RF (resorcinol formaldehyde), MF (melamine formaldehyde), epoxy, and EPI (emulsion polymer isocyanate) adhesives. The samples were tested dry and after water soaking (wet).
The above discussion covers our observations in bonding modified woods with different adhesives, but the question remains: What does this mean as far as adhesive wood interactions and predictability of bond formation? With the BO-modified (Figure 7) being mainly similar to the untreated wood (Figure 5) while the Ac-wood being completely different (Figure 6), the bonding of modified wood depends more on the type of modification than whether it has been modified. The literature data [5,19,22,39] demonstrate that the adhesive formulation, wood species, and wood modification conditions all influence the outcome.
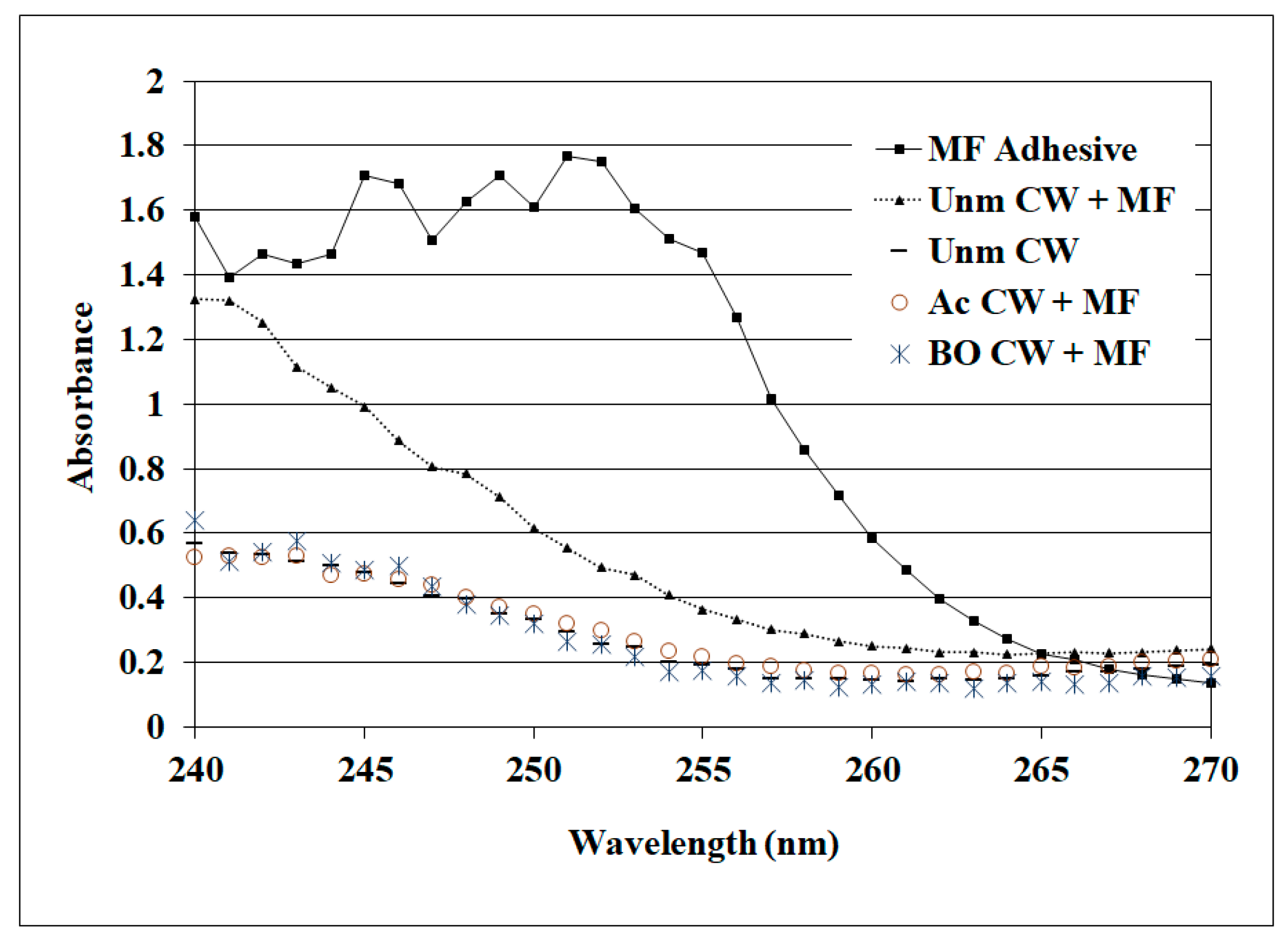
A startling prior observation was that the MF but not the RF was so poor in wood failure and bond strength with acetylated wood under wet conditions [19], which is supported by our observations. RF and MF both perform well with unmodified wood under both dry and wet conditions and are known to infiltrate the cell walls (i.e., cell wall penetration). Although we are not aware of a good method for measuring RF concentration in cell walls, a method has been developed to measure MF in cell walls using UV microscopy [31,40]. The analysis of these samples shows that MF has infiltrated the untreated wood cell walls near the glueline, but not the BO- or Ac-treated cell walls (Figure 8). While it is easy to jump to the conclusion that the poor wet shear strength and wood failure for the MF are a result of the failure to infiltrate the acetylated cell walls, it is contradicted by the good performance by the BO-modified wood results. Since the phenolics, including RF, are often highly basic, it could be postulated that this plays a role since MFs are acidic. However, this is not supported because the Casophen resorcinol–formaldehyde RS-216 had a pH 7.2. It may be that the phenolics are particularly effective at wetting acetylated wood, but insufficient data are available for drawing a firmer conclusion.
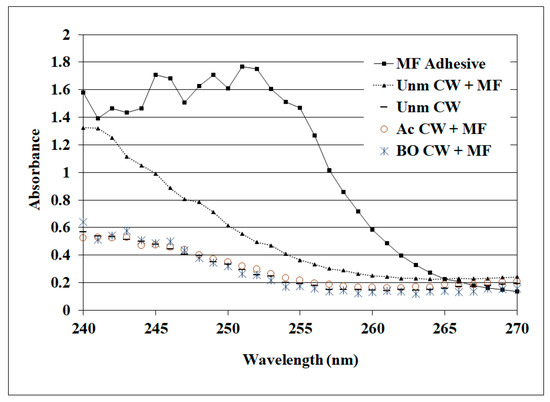
Figure 8.
UV microscopy to determine the MF (melamine formaldehyde) in wood cell walls where MF is the adhesive alone, and the cell walls (CW + MF) of unmodified, BO-modified and Ac-modified near the glueline, while the unmodified CW is the bonded specimen, away from the bondline.
4. Conclusions
Given the increased utilization of wood modification, it is important to know how well these modified woods can provide moisture-durable wood bonds, because most wood products are bonded. Two types of chemical wood modifications were used on yellow poplar; acetylation (Ac) which converts hydroxyl groups into ester groups, and butylene oxide (BO) modification, which does not reduce the number of hydroxyl groups but inserts a butylene group. A main issue is how these chemical modifications of wood alter the bond performance with four types of adhesives (resorcinol-formaldehyde (RF), melamine-formaldehyde (MF), emulsion polymer isocyanate (EPI), and epoxy). The main observation is that the BO-modified wood gave similar both shear strength and wood failure values to the untreated wood. On the other hand, only the RF gave good wet bonds to the acetylated wood. Modification to make the wood less polar improved the wet performance of the epoxy adhesive. These data were examined using the various models for wood modification and adhesive performance.
Author Contributions
Conceptualization, C.R.F.; methodology, C.R.F.; experimental, R.E.I., R.B. and W.G.-A.; formal analysis, C.R.F., C.G.H., and W.G.-A.; writing—original draft preparation, C.R.F.; writing—review and editing, C.R.F., R.E.I., and C.G.H.; visualization, C.G.H. and W.G.-A.; project administration, C.R.F.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not available except in non-digital notebooks.
Acknowledgments
We thank Roger Rowell (retired from FPL) for initial discussions and Boem-Goo Lee for modifying the wood.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hill, C.A.S. Wood Modification: Chemical, Thermal and Other Processes; John Wiley & Sons: Hong Kong, 2006; pp. 1–239. [Google Scholar]
- Norimoto, M. Chemical Modification of Wood. In Wood and Cellulose Chemistry; Hon, D.N.S., Shiraishi, N., Eds.; Marcel Dekker: New York, NY, USA, 2001; pp. 573–598. [Google Scholar]
- Norimoto, M.; Gril, J.; Rowell, R.M. Rheological properties of chemically modified wood Relationship between dimensional stability and creep stability. Wood Fiber Sci. 1992, 24, 25–35. [Google Scholar]
- Frihart, C.R.; Yelle, D.J.; Jakes, J.E.; Ralph, J.; Stone, D.S.; Beecher, J.F. Use of a new methodology to elucidate isocyanate adhesion to wood. In Proceedings of the 33rd Annual Meeting of The Adhesion Society, Daytona Beach, FL, USA, 21–24 February 2010. [Google Scholar]
- Bongers, F.; Meijerink, T.; Lütkemeier, B.; Lankveld, C.; Alexander, J.; Militz, H.; Lehringer, C. Bonding of acetylated wood. Int. Wood Prod. J. 2016, 7, 102–106. [Google Scholar] [CrossRef]
- Boonstra, M.G.; Pizzi, A.; Tekely, P.; Pendlebury, J. A study of the influence of solvents on the modification of wood with organic anhydrides. Holzforschung 1997, 51, 62–66. [Google Scholar]
- Brelid, P.L.; Simonson, R. Acetylation of solid wood using microwave heating: Part 2. Experiments in laboratory scale. Holz als Roh-und Werkst. 1999, 57, 383–389. [Google Scholar] [CrossRef]
- Çetin, N.S.; Özmen, N.; Birinci, E. Acetylation of Wood with Various Catalysts. J. Wood Chem. Technol. 2011, 31, 142–153. [Google Scholar] [CrossRef]
- Dunningham, E.A. Kinetic studies of the acetylation reaction of small Pinus radiata blocks. Holz als Roh-und Werkst. 2012, 70, 857–863. [Google Scholar] [CrossRef]
- Gu, X.; Sun, L.; Liu, G.; You, C.; Cheng, C.K.K.; Yao, J.; Gu, X. Chemical modification of poplar wood in gas-and liquid-phase acetylation. Wood Res. 2015, 60, 247–254. [Google Scholar]
- Hill, C.A.S. Wood modification: An update. BioResources 2011, 6, 918–919. [Google Scholar]
- Hunt, C.G.; Zelinka, S.L.; Frihart, C.R.; Lorenz, L.; Yelle, D.; Gleber, S.-C.; Vogt, S.; Jakes, J.E. Acetylation increases relative humidity threshold for ion transport in wood cell walls—A means to understanding decay resistance. Int. Biodeterior. Biodegrad. 2018, 133, 230–237. [Google Scholar] [CrossRef]
- Li, J.-Z.; Furuno, T.; Zhou, W.-R.; Ren, Q.; Han, X.-Z.; Zhao, J.-P. Properties of Acetylated Wood Prepared at Low Temperature in the Presence of Catalysts. J. Wood Chem. Technol. 2009, 29, 241–250. [Google Scholar] [CrossRef]
- Marcroft, J.; Bongers, F.; Perez, F.P.; Alexander, J.; Harrison, I. Structural Performance of Accoya® Wood under Service Class 3 Conditions. In Materials and Joints in Timber Structures; Aicher, S.R.H., Garrecht, H., Eds.; RILEM Bookseries; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Obataya, E.; Minato, K. Potassium acetate-catalyzed acetylation of wood at low temperatures I: Simplified method using a mixed reagent. J. Wood Sci. 2009, 55, 18–22. [Google Scholar] [CrossRef]
- Rowell, R.M. Acetylated Wood: A Stable and Durable Structural Building Material. In Proceedings of the World Conference on Timber Engineering (WCTE 2016), Vienna, Austria, 22–25 August 2016. [Google Scholar]
- Rowell, R.M.; Dickerson, J.P. Acetylation of wood. In Deterioration and Protection of Sustainable Biomaterials; ACS Symposium Series; ACS Publications: Washington, DC, USA, 2014; pp. 301–327. [Google Scholar]
- Rowell, R.M. Chemical Modification of Wood. In Handbook of Wood Chemistry and Wood Composites; Rowell, R.M., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 537–597. [Google Scholar]
- Vick, C.; Rowell, R. Adhesive bonding of acetylated wood. Int. J. Adhes. Adhes. 1990, 10, 263–272. [Google Scholar] [CrossRef]
- Frihart, C.R.; Brandon, R.; Beecher, J.F.; Ibach, R.E. Adhesives for Achieving Durable Bonds with Acetylated Wood. Polymers 2017, 9, 731. [Google Scholar] [CrossRef]
- Frihart, C.R.; Brandon, R.; Ibach, R.E. Selectivity of bonding for modified wood. In Proceedings of the 27th Annual Meeting of the Adhesion Society, Wilmington, NC, USA, 15–18 February2004. [Google Scholar]
- Vick, C.; Larsson, P.; Mahlberg, R.; Simonson, R.; Rowell, R. Structural bonding of acetylated Scandinavian softwoods for exterior lumber laminates. Int. J. Adhes. Adhes. 1993, 13, 139–149. [Google Scholar] [CrossRef]
- Brandon, R.; Ibach, R.E.; Frihart, C.R. Effects of chemically modified wood on bond durability. In Proceedings of the International Conference on Wood Adhesives 2005; Forest Products Society Publishing: Madison, WI, USA, 2006. [Google Scholar]
- Hunt, C.G.; Brandon, R.; Ibach, R.E.; Frihart, C.R. What does bonding to modified wood tell us about adhesion? In Bonding of Modified Wood: Proceedings of the 5th COST E34 International Workshop; Biotechnical Faculty, Department of Wood Science and Technology, University of Ljubljana: Bled, Slovenia, 2007. [Google Scholar]
- Frihart, C.R. Adhesive Groups and How They Relate to the Durability of Bonded Wood. J. Adhes. Sci. Technol. 2009, 23, 601–617. [Google Scholar] [CrossRef]
- Frihart, C.R. Adhesive Bonding and Performance Testing of Bonded Wood Products. J. ASTM Int. 2005, 2, 455–466. [Google Scholar] [CrossRef]
- Frihart, C.R.; Beecher, J.F. Factors that lead to failure with wood adhesive bonds. In World Conference on Timber Engineering 2016; World Conference on Timber Engineering: Vienna, Asutria, 2016. [Google Scholar]
- ASTM International. D 905-08 Standard Test Method for Strength Properties of Adhesive Bonds in Shear by Compression Loading; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- ASTM International. D 2559-12AE1 Standard Specification for Adhesives for Structural Laminated Wood Products for Use Under Exterior Exposure Conditions; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- ASTM International. D 5266-99 Standard Practice for Estimating the Percent Wood Failure in Adhesive Joints; ASTM International: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Gindl, W.; Dessipri, E.; Wimmer, R. Using UV-Microscopy to Study Diffusion of Melamine-Urea-Formaldehyde Resin in Cell Walls of Spruce Wood. Holzforschung 2002, 56, 103–107. [Google Scholar] [CrossRef]
- Frihart, C.R. Are epoxy-wood bonds durable enough? In Wood Adhesives 2005 Proceeding; Forest Product Society: San Diego, CA, USA, 2006. [Google Scholar]
- Wilson, T.R.C. Strength-Moisture Relations for Wood; Forest Products Laboratory: Madison, WI, USA, 1932. [Google Scholar]
- Green, D.W.; Winandy, J.E.; Kretschmann, D.E. Wood as an Engineering Material. In Wood Handbook; USDA Forest Service, Forest Products Laboratory: Madison, WI, USA, 1999; pp. 4.1–4.45. [Google Scholar]
- Rowell, R.M. Chemical Modification of Wood. In Handbook of Wood Chemistry and Wood Composites; Rowell, R.M., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 381–420. [Google Scholar]
- Vick, C.B.; Krzysik, A.; Wood, J.E. Acetylated, isocyanate-bonded flakeboards after accelerated aging. Holz als Roh-und Werkst. 1991, 49, 221–228. [Google Scholar] [CrossRef]
- Ibach, R.E.; Rowell, R.M. Improvements in Decay Resistance Based on Moisture Exclusion. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A. Mol. Cryst. Liq. Cryst. 2000, 353, 23–33. [Google Scholar] [CrossRef]
- Ibach, R.E.; Rowell, R.M.; Lee, B.-G. Decay protection based on moisture exclusion resulting from chemical modification of wood. In Proceedings of the 5th Pacific Rim Bio-Based Composites Symposium, Canberra, Australia, 10–13 December 2000; pp. 197–204. [Google Scholar]
- Treu, A.; Bredesen, R.; Bongers, F. Enhanced bonding of acetylated wood with an MUF-based adhesive and a resorcinol-formaldehyde-based primer. Holzforschung 2020, 74, 382–390. [Google Scholar] [CrossRef]
- Gierlinger, N.; Hansmann, C.; Röder, T.; Sixta, H.; Gindl, W.; Wimmer, R.; Gindl-Altmutter, W. Comparison of UV and confocal Raman microscopy to measure the melamine–formaldehyde resin content within cell walls of impregnated spruce wood. Holzforschung 2005, 59, 210–213. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).