In Planta Analysis of the Radial Movement of Minerals from Inside to Outside in the Trunks of Standing Japanese Cedar (Cryptomeria japonica D. Don) Trees at the Cellular Level
Abstract
1. Introduction
2. Materials and Methods
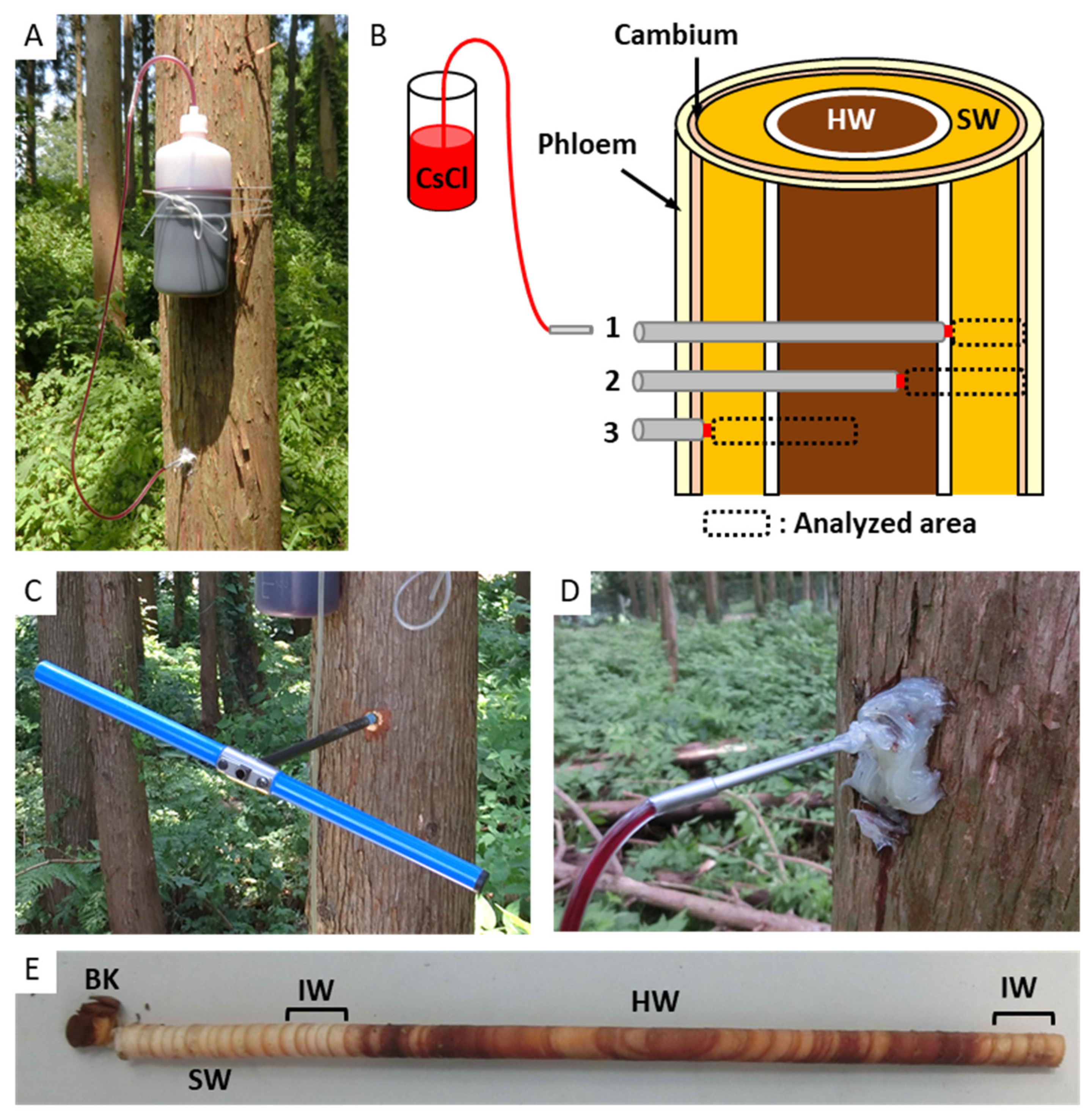
2.1. Plant Materials
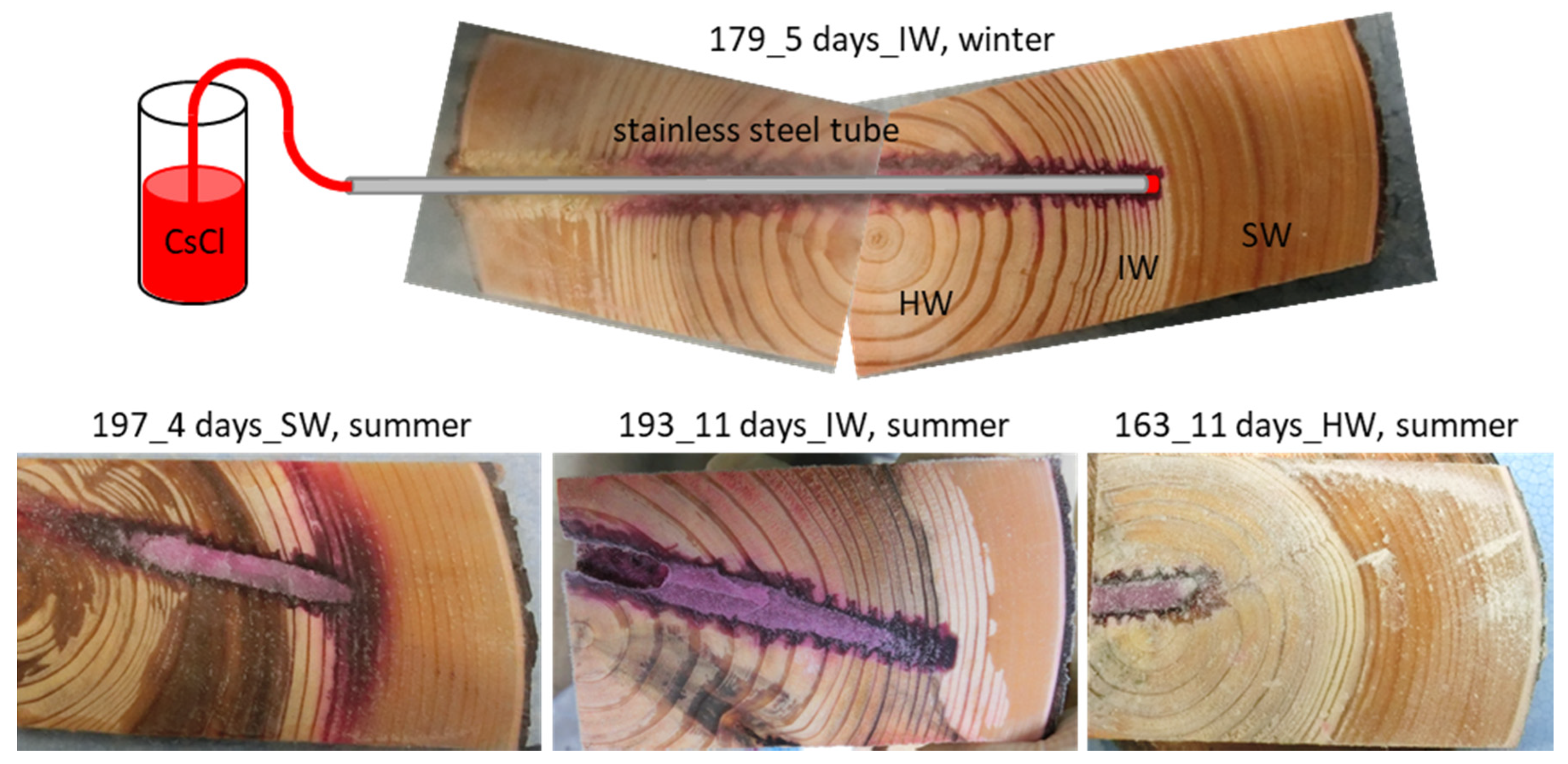
2.2. Cs Injection in Standing Tree Trunk and Sample Collection
2.3. Cryo-SEM/Energy-Dispersive X-ray Spectroscopy (EDX)
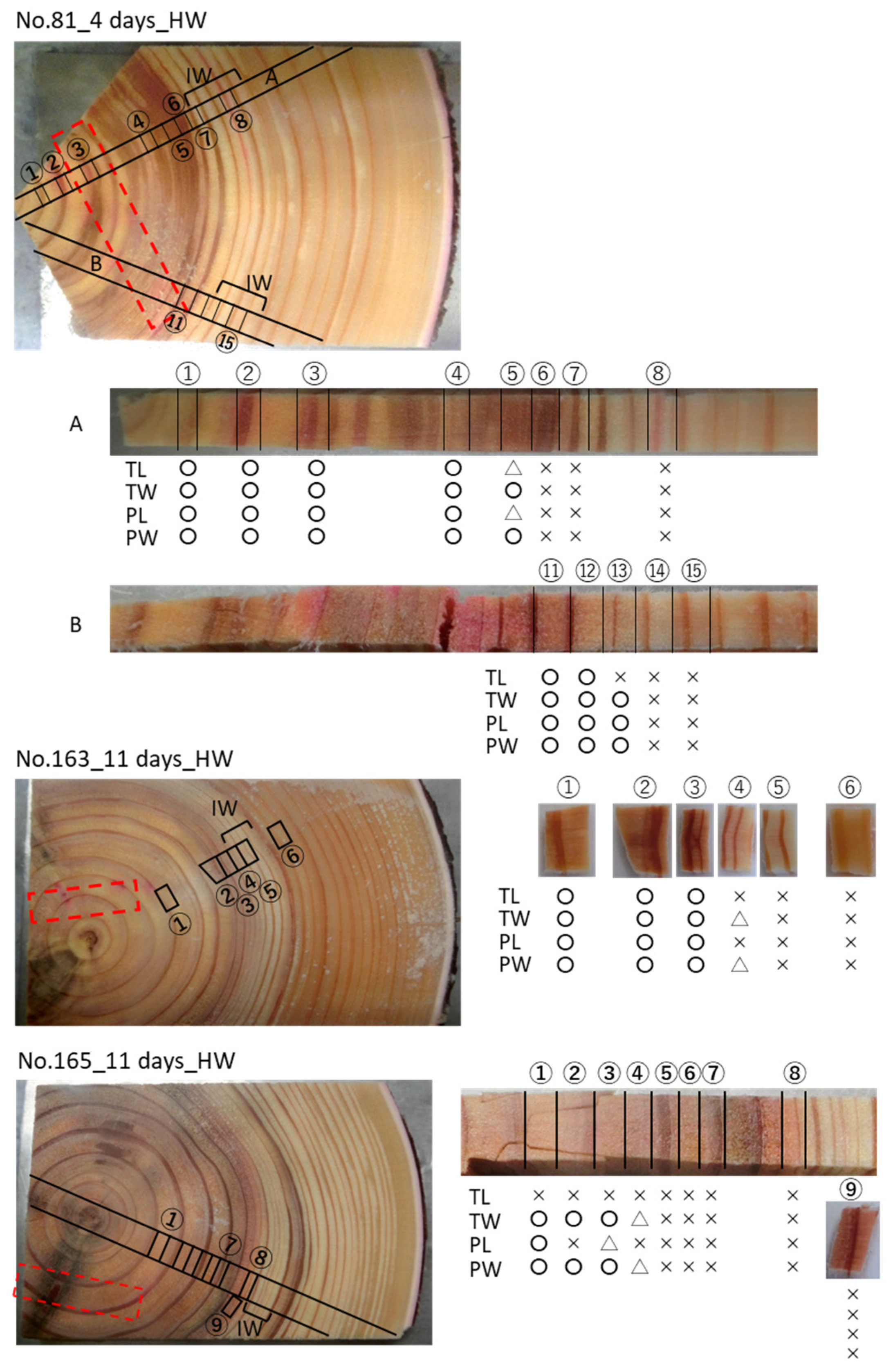
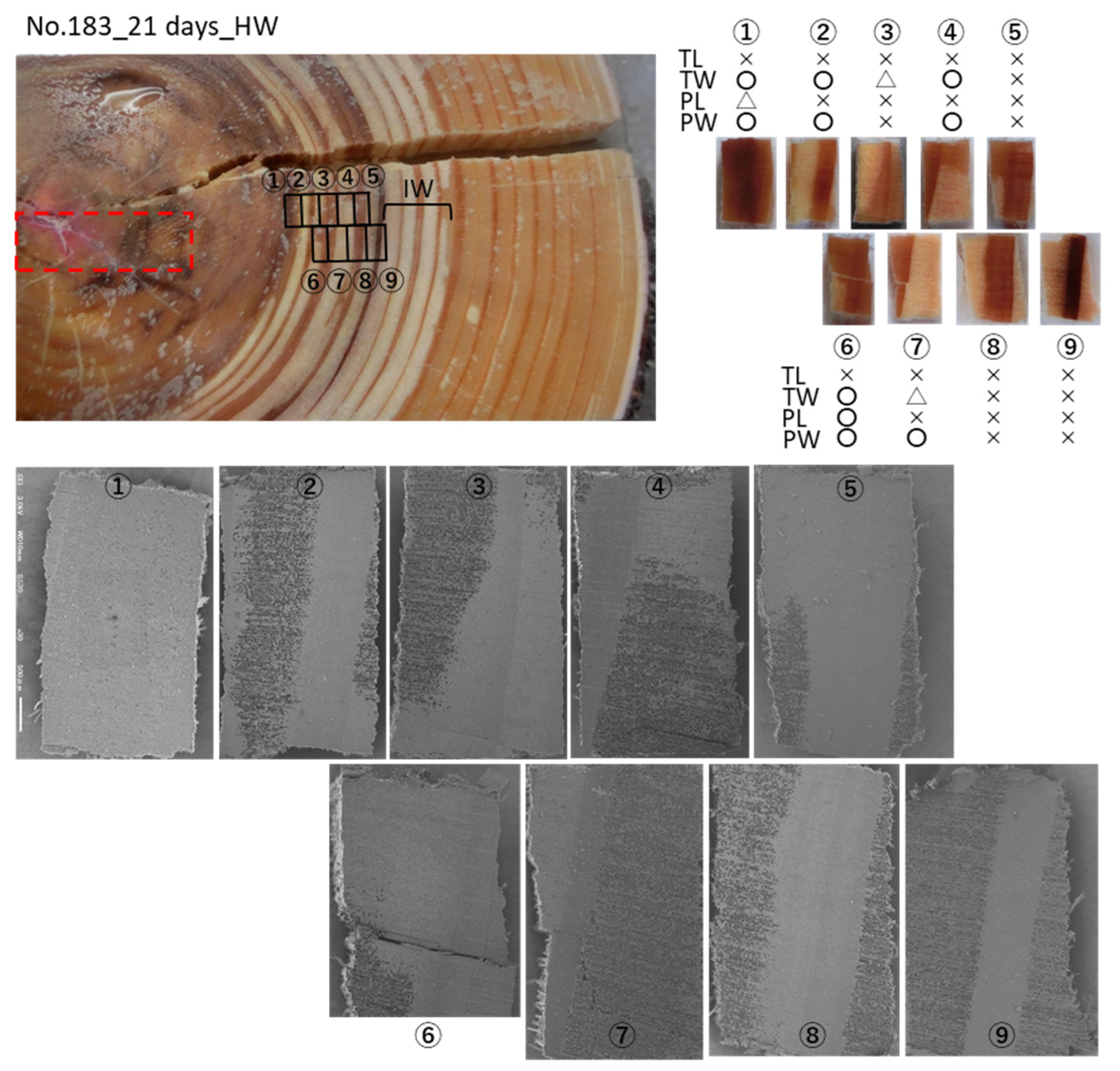
3. Results
3.1. Cs Distribution in the Xylem Following Cs Injection into the Sapwood and Intermediate Wood in Summer
3.2. Cs Distribution in the Xylem Following Cs Injection into the Heartwood in Summer
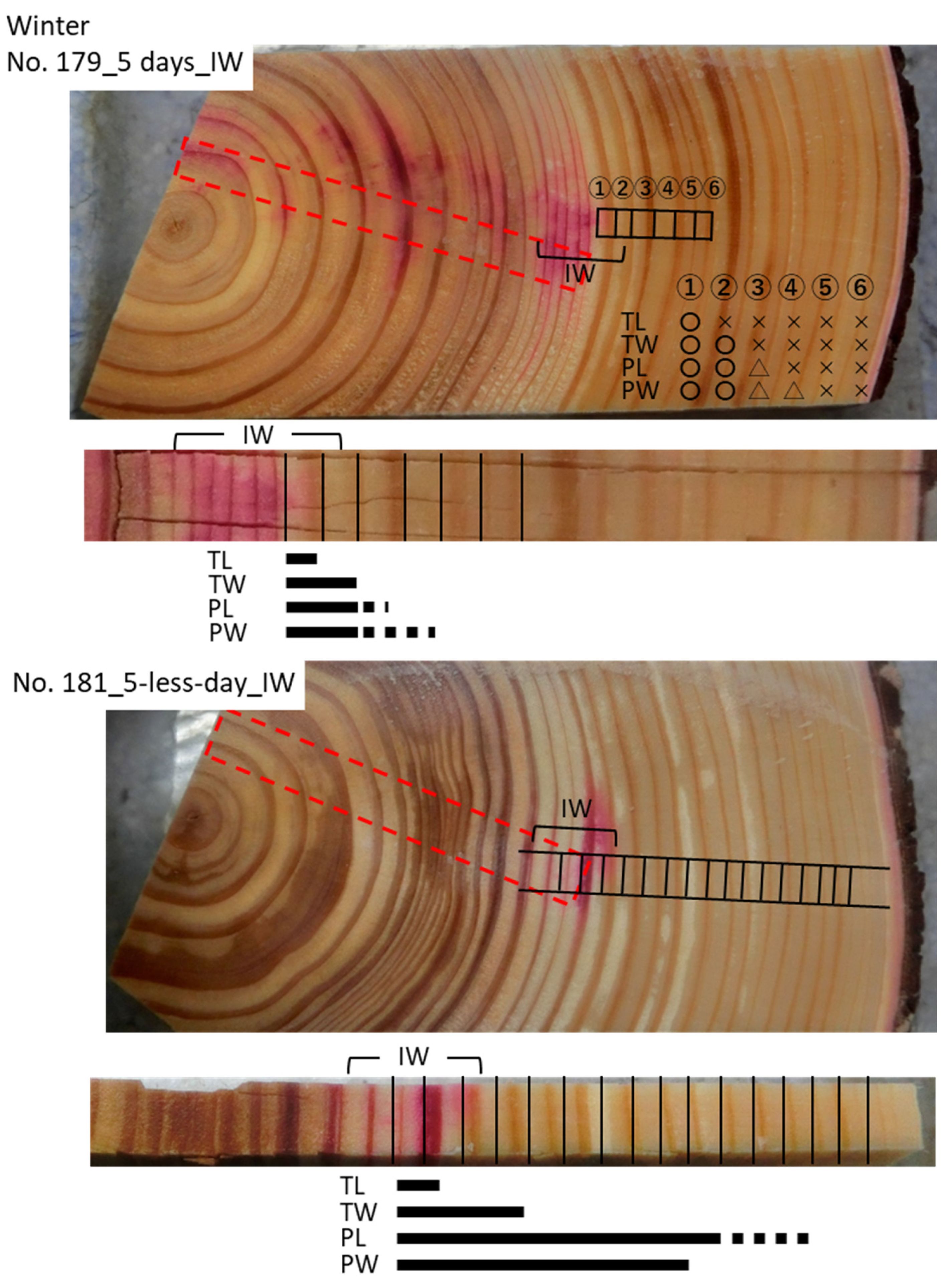
3.3. Cs Distribution in the Xylem Following Cs Injection into the Intermediate Wood in Winter
4. Discussion
4.1. Mineral Transport in Both Directions in the Sapwood by Ray Parenchyma Cells
4.2. Minerals Rarely Move from the Heartwood to the Sapwood
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pallardy, S. Physiology of Woody Plants, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Sauter, J.J.; Kloth, S. Plasmodesmatal frequency and radial translocation rates in ray cells of poplar (Populus × canadensis Moench ‘robusta’). Planta 1986, 168, 7–380. [Google Scholar] [CrossRef]
- Holbrook, N.M.; Zwieniecki, M.A. Integration of long distance transport systems in plants: Perspectives and prospects for future research. In Vascular Transport in Plants; Holbrook, N.M., Zwieniecki, M.A., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2005; pp. 537–545. [Google Scholar]
- Sokołowska, K. Symplasmic transport in wood: The importance of living xylem cells Symplasmic Transport in Vascular Plants. In Symplasmic Transport in Vascular Plants; Sokołowska, K., Sowiński, P., Eds.; Springer Science-Business Media: New York, NY, USA, 2013; pp. 101–132. [Google Scholar]
- Spicer, R. Symplasmic networks in secondary vascular tissues: Parenchyma distribution and activity supporting long-distance transport. J. Exp. Bot. 2014, 65, 1829–1848. [Google Scholar] [CrossRef]
- Baker, H.; James, W.O. The behaviour of dyes in the transpiration stream of sycamores (Acer pseudoplatanus L.). New Phytol. 1933, 32, 245–260. [Google Scholar] [CrossRef]
- Stout, P.R.; Hoagland, D.R. Upward and lateral movement of salt in certain plants as indicated by radioactive isotopes of potassium, sodium, and phosphorus absorbed by roots. Am. J. Bot. 1939, 26, 320–324. [Google Scholar] [CrossRef]
- Tyree, M.T.; Zimmermann, M.H. Xylem Structure and the Ascent of Sap; Springer: Berlin, Germany, 2002. [Google Scholar]
- Umebayashi, T.; Utsumi, Y.; Koga, S.; Inoue, S.; Shiiba, Y.; Arakawa, K.; Matsumura, J.; Oda, K. Optimal conditions for visualizing water-conducting pathways in a living tree by the dye injection method. Tree Physiol. 2007, 27, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Umebayashi, T.; Utsumi, Y.; Koga, S.; Inoue, S.; Fujikawa, S.; Arakawa, K.; Matsumura, J.; Oda, K. Conducting pathways in north temperate deciduous broadleaved trees. IAWA J. 2008, 29, 247–263. [Google Scholar] [CrossRef]
- van der Schoot, C.; van Bel, A.J.E. Mapping membrane potential differences and dye-coupling in internodal tissues of tomato (Solanum lycopersicum L.). Planta 1990, 182, 9–21. [Google Scholar] [CrossRef]
- Pfautsch, S.; Renard, J.; Tjoelker, M.G.; Salih, A. Phloem as capacitor: Radial transfer of water into xylem of tree stems occurs via symplastic transport in ray parenchyma. Plant Physiol. 2015, 167, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Sokołowska, K.; Zagórska-Marek, B. Seasonal changes in the degree of symplasmic continuity between the cells of cambial region of Acer pseudoplatanus and Ulmus minor. Acta Soc. Bot. Pol. 2007, 76, 277–286. [Google Scholar] [CrossRef]
- Sokołowska, K.; Zagórska-Marek, B. Symplasmic, long-distance transport in xylem and cambial regions in branches of Acer pseudoplatanus (Aceraceae) and Populus tremula × P. tremuloides (Salicaceae). Am. J. Bot. 2012, 99, 1745–1755. [Google Scholar]
- Okada, N.; Hirakawa, Y.; Katayama, Y. Application of activable tracers to investigate radial movement of minerals in the stem of Japanese cedar (Cryptomeria japonica). J. Wood Sci. 2011, 57, 421–428. [Google Scholar] [CrossRef]
- Okada, N.; Hirakawa, Y.; Katayama, Y. Radial movement of sapwood-injected rubidium into heartwood of Japanese cedar (Cryptomeria japonica) in the growing period. J. Wood Sci. 2012, 58, 1–8. [Google Scholar] [CrossRef]
- Chaffey, N.; Barlow, P. The cytoskeleton facilitates a three-dimensional symplasmic continuum in the long-lived ray and axial parenchyma cells of angiosperm trees. Planta 2001, 213, 811–823. [Google Scholar] [CrossRef]
- Kuroda, K.; Yamane, K.; Itoh, Y. Cellular level in planta analysis of radial movement of artificially injected caesium in Cryptomeria japonica xylem. Trees 2018, 32, 1505–1517. [Google Scholar] [CrossRef]
- Kuroda, K.; Yamane, K.; Itoh, Y. Radial movement of minerals in the trunks of standing Japanese cedar (Cryptomeria japonica D. Don) trees in summer by tracer analysis. Forests 2020, 11, 562. [Google Scholar] [CrossRef]
- Sauter, J.J.; van Cleve, B. Storage, mobilization and interrelations of starch, sugars, protein and fat in the ray storage tissue of poplar trees. Trees 1994, 8, 297–304. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Hiura, T.; Kato, E.; Funada, R. Allocation of Resources to Reproduction in Styrax obassia in a Masting Year. Ann. Bot. 2002, 89, 767–772. [Google Scholar] [CrossRef]
- van Bel, A.J.E. Xylem-phloem exchange via the rays: The undervalued route of transport. J. Exp. Bot. 1990, 41, 631–644. [Google Scholar] [CrossRef]
- Aubry, E.; Dinant, S.; Vilaine, F.; Bellini, C.; Le Hir, R. Lateral transport of organic and inorganic solutes. Plants 2019, 8, 20. [Google Scholar] [CrossRef]
- Stewart, C.M. Excretion and heartwood formation in living trees. Science 1966, 153, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Hillis, W.E. Heartwood and Tree Exudates; Springer: Berlin/Heidelberg, Germany, 1987. [Google Scholar]
- Taylor, A.M.; Gartner, B.L.; Morrell, J.J. Heartwood formation and natural durability-a review. Wood Fiber Sci. 2002, 34, 587–611. [Google Scholar]
- Okada, N.; Katayama, Y.; Nobuchi, T.; Ishimaru, Y.; Aoki, A. Trace elements in the stems of trees V. Comparisons of radial distributions among softwood stems. Mokuzai Gakkaishi 1993, 10, 1111–1118. [Google Scholar]
- Okada, N.; Katayama, Y.; Nobuchi, T.; Ishimaru, Y.; Aoki, A. Trace elements in the stems of trees VI. Comparisons of radial distributions among hardwood stems. Mokuzai Gakkaishi 1993, 10, 1119–1127. [Google Scholar]
- Penninckx, V.; Glineur, S.; Gruber, W.; Herbauts, J.; Meerts, P. Radial variations in wood mineral element concentrations: A comparison of beech and pedunculate oak from the Belgian Ardennes. Ann. For. Sci. 2001, 58, 253–260. [Google Scholar] [CrossRef]
- Meerts, P. Mineral nutrient concentrations in sapwood and heartwood: A literature review. Ann. For. Sci. 2002, 59, 713–722. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Shimogaki, H.; Abe, H.; Kagawa, A. Inorganic elements in typical trees for woody biomass fuel. J. Wood Sci. 2010, 56, 53–63. [Google Scholar] [CrossRef]
- Kuroda, K.; Yamashita, K.; Fujiwara, T. Cellular level observation of water loss and the refilling of tracheids in the xylem of Cryptomeria japonica during heartwood formation. Trees 2009, 23, 1163–1172. [Google Scholar] [CrossRef]
- Nobuchi, T.; Harada, H. Physiological features of the ‘white zone’ of sugi (Cryptomeria japonica D. Don) -cytological structure and moisture content. Mokuzai Gakkaishi 1983, 29, 824–832. [Google Scholar]
- Arakawa, I.; Funada, R.; Nakaba, S. Changes in the morphology and functions of vacuoles during the death of ray parenchyma cells in Cryptomeria japonica. J. Wood Sci. 2018, 64, 177–185. [Google Scholar] [CrossRef]
- Kean-Jim, L.; Paasela, T.; Harju, A.; Venäläinen, M.; Paulin, L.; Auvinen, P.; Kärkkäinen, K.; Teeriet, T. Developmental changes in Scots pine transcriptome during heartwood formation. Plant Physiol. 2016, 172, 1403–1417. [Google Scholar]
- Nakaba, S.; Morimoto, H.; Arakawa, I.; Yamagishi, Y.; Nakada, R.; Funada, R. Responses of ray parenchyma cells to wounding differ between earlywood and latewood in the sapwood of Cryptomeria japonica. Trees 2017, 31, 27–39. [Google Scholar] [CrossRef]
- Begum, S.; Kudo, K.; Rahman, M.H.; Nakaba, S.; Yamagishi, Y.; Nabeshima, E.; Nugroho, W.D.; Oribe, Y.; Kitin, P.; Jin, H.-O.; et al. Climate change and the regulation of wood formation in trees by temperature. Trees 2018, 32, 3–15. [Google Scholar] [CrossRef]
- Okada, N.; Katayama, Y.; Nobuchi, T.; Ishimaru, Y.; Yamashita, H.; Aoki, A. Trace elements in the stems of trees I. Radial distributions in Sugi (Cryptomeria japonica D. Don). Mokuzai Gakkaishi 1987, 12, 913–920. [Google Scholar]
- Kubo, T.; Ataka, S. Blackening of Sugi (Cryptomeria japonica D. Don) heartwood in relation to metal content and moisture content. J. Wood Sci. 1998, 44, 137–141. [Google Scholar] [CrossRef]
- Nakada, R.; Okada, N.; Nakai, T.; Kuroda, K.; Nagai, S. Water potential gradient between sapwood and heartwood as a driving force in water accumulation in wetwood in conifers. Wood Sci. Technol. 2019, 53, 407–424. [Google Scholar] [CrossRef]
- Yamamoto, K. Moisture distribution in stems of Acacia mangium, A. auriculiformis and hybrid Acacia trees. Jpn. Agric. Res. Q. 2003, 37, 207–212. [Google Scholar] [CrossRef][Green Version]
- Nakada, R. Within-stem water distribution in living trees of some conifers. IAWA J. 2006, 27, 313–327. [Google Scholar] [CrossRef]


| Injection | |||||||
|---|---|---|---|---|---|---|---|
| Tree Number | * Injection Hole Depth | Period of Injection | Starting Date (D/M/Y) | Harvesting Date (D/M/Y) | Age | Tree Height (m) | Girth at 1.2 m (cm) |
| Summer | |||||||
| 191 | SW | 4 days | 03/08/2020 | 07/08/2020 | 38 | 17.2 | 55 |
| 197 | SW | 4 days | 03/08/2020 | 07/08/2020 | 39 | 18.2 | 58 |
| 195 | SW | 11 days | 03/08/2020 | 14/08/2021 | 37 | 16.0 | 52 |
| 193 | IW | 11 days | 03/08/2020 | 14/08/2020 | 38 | 14.8 | 57 |
| 81 | HW | 4 days | 10/07/2017 | 14/07/2017 | 34 | 16.3 | 52 |
| 163 | HW | 11 days | 18/06/2018 | 29/06/2018 | 35 | 16.5 | 47 |
| 165 | HW | 11 days | 18/06/2018 | 29/06/2018 | 35 | 14.2 | 48 |
| 185 | HW | 16 days | 10/07/2019 | 26/07/2019 | 38 | 17.3 | 56 |
| 183 | HW | 21 days | 26/06/2019 | 17/07/2019 | 35 | 17.4 | 45 |
| Winter | |||||||
| 179 | IW | 5 days | 08/02/2019 | 13/02/2019 | 37 | 18.2 | 57.5 |
| 181 | IW | 5-less days | 08/02/2019 | 13/02/2019 | 38 | 16.4 | 59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuroda, K.; Yamane, K.; Itoh, Y. In Planta Analysis of the Radial Movement of Minerals from Inside to Outside in the Trunks of Standing Japanese Cedar (Cryptomeria japonica D. Don) Trees at the Cellular Level. Forests 2021, 12, 251. https://doi.org/10.3390/f12020251
Kuroda K, Yamane K, Itoh Y. In Planta Analysis of the Radial Movement of Minerals from Inside to Outside in the Trunks of Standing Japanese Cedar (Cryptomeria japonica D. Don) Trees at the Cellular Level. Forests. 2021; 12(2):251. https://doi.org/10.3390/f12020251
Chicago/Turabian StyleKuroda, Katsushi, Kenichi Yamane, and Yuko Itoh. 2021. "In Planta Analysis of the Radial Movement of Minerals from Inside to Outside in the Trunks of Standing Japanese Cedar (Cryptomeria japonica D. Don) Trees at the Cellular Level" Forests 12, no. 2: 251. https://doi.org/10.3390/f12020251
APA StyleKuroda, K., Yamane, K., & Itoh, Y. (2021). In Planta Analysis of the Radial Movement of Minerals from Inside to Outside in the Trunks of Standing Japanese Cedar (Cryptomeria japonica D. Don) Trees at the Cellular Level. Forests, 12(2), 251. https://doi.org/10.3390/f12020251






