Physical and Mechanical Properties of Poplar Wood Modified by Glucose-Urea-Melamine Resin/Sodium Silicate Compound
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MUG Resin/Sodium Silicate Compound Modifier
2.3. Wood Impregnation
2.4. Physical and Mechanical Properties
2.5. Fourier Transform Infrared Spectroscopy (FTIR) Analysis of MUG
2.6. Micromorphology Characteristic
3. Results and Discussion
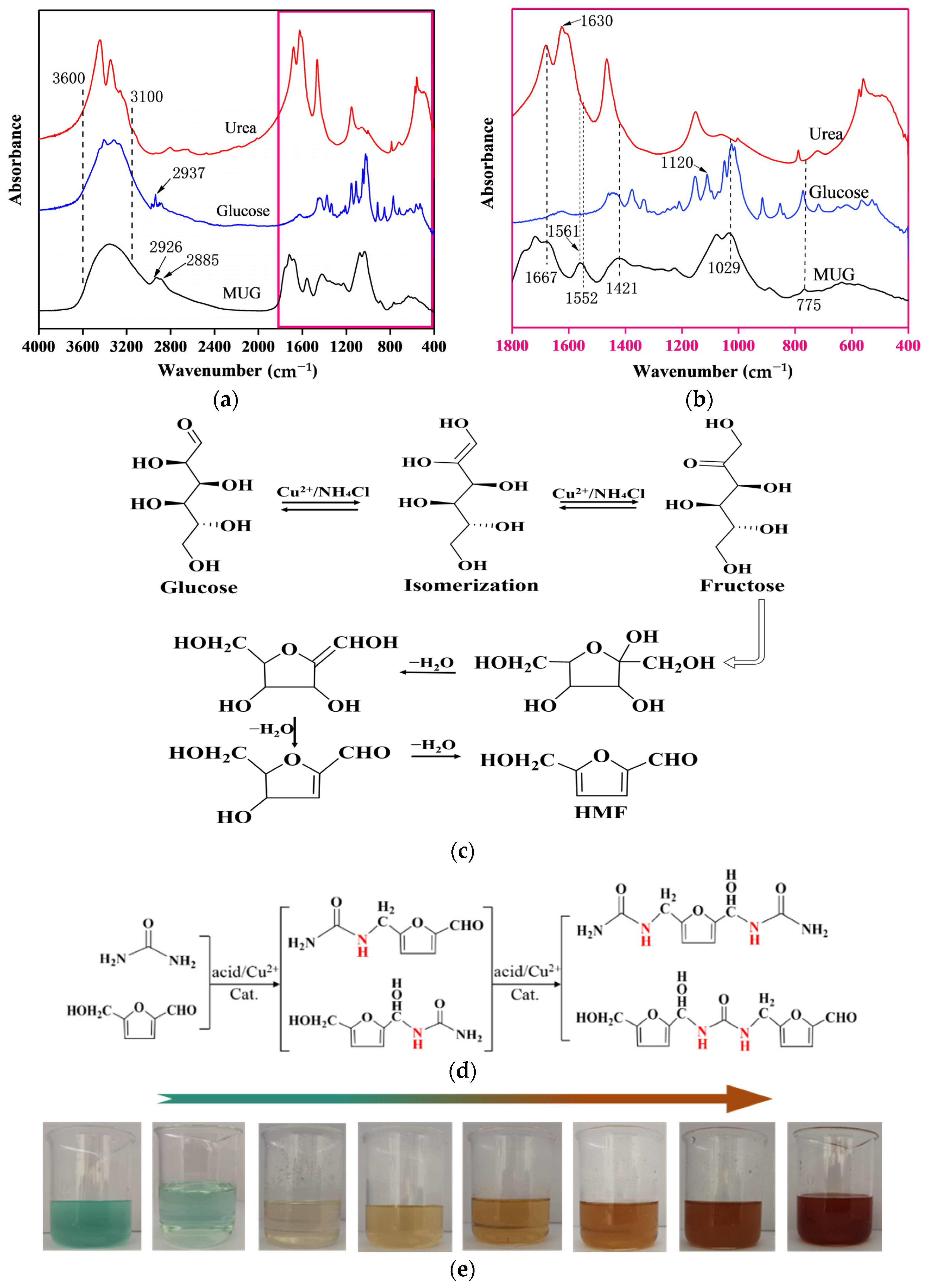
3.1. Infrared Spectroscopy (FTIR) Analysis of Resin
3.2. Physical Properties of Wood
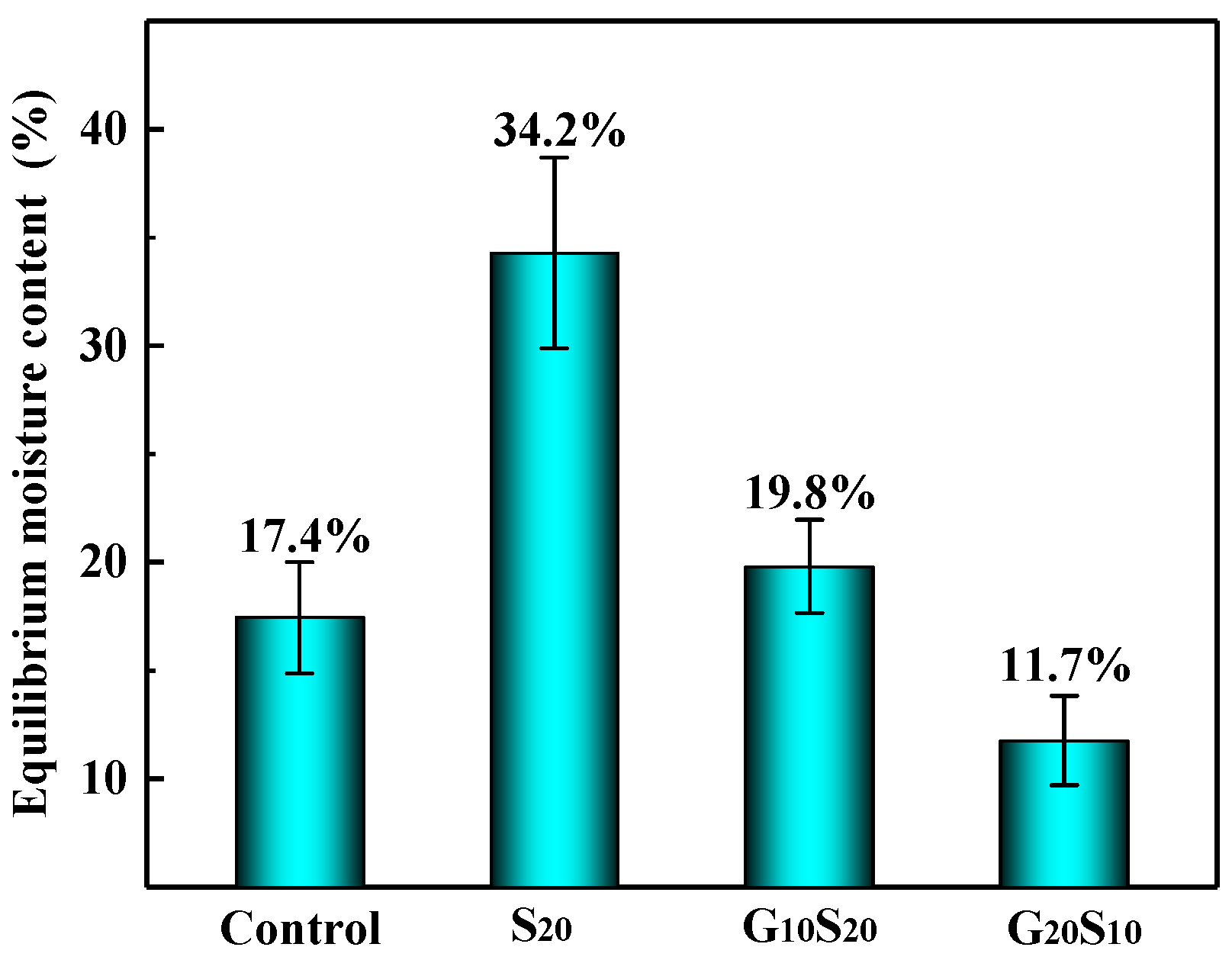
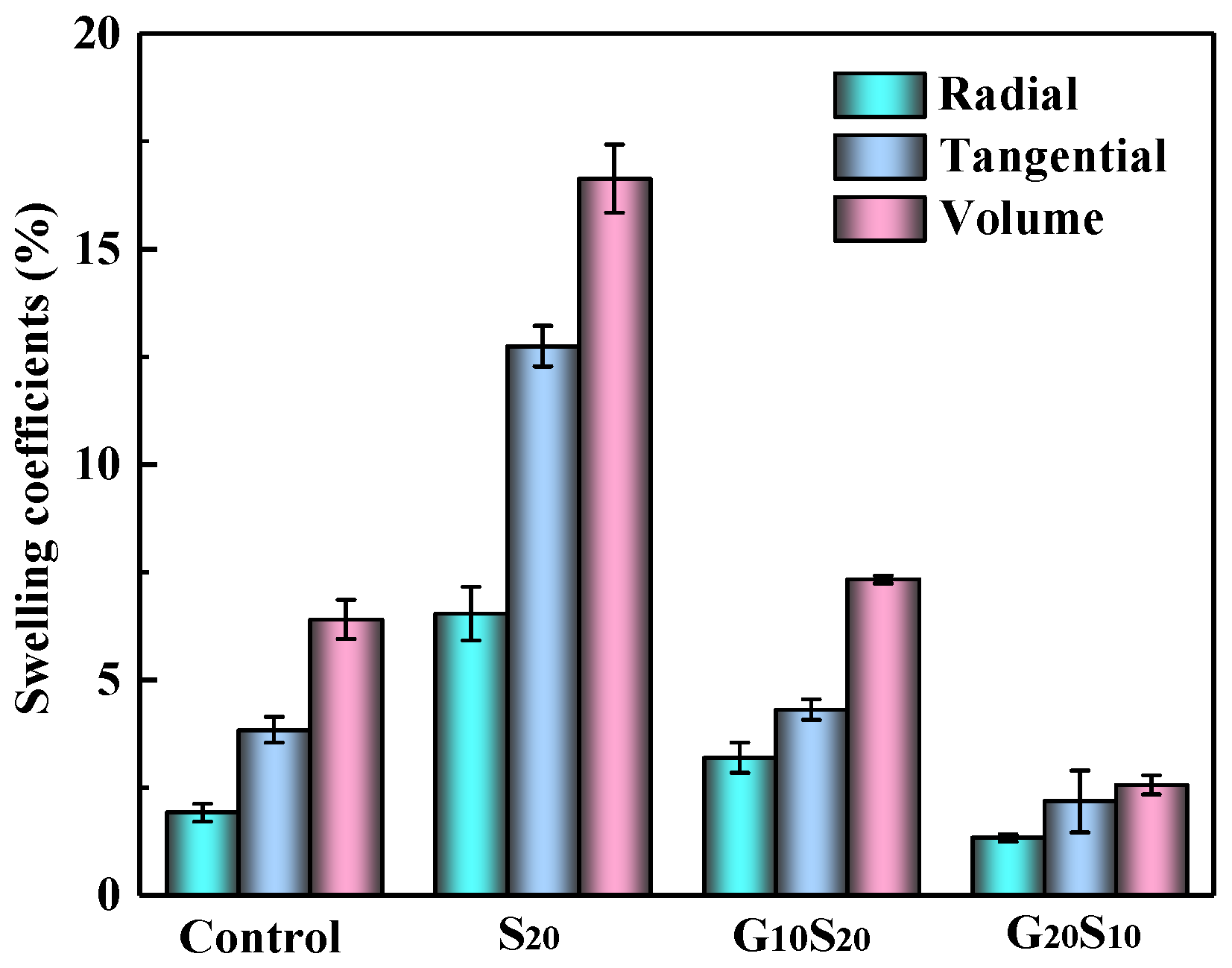
3.3. Hygroscopicity and Dimensional Stability of Wood
3.4. Mechanical Properties of Wood
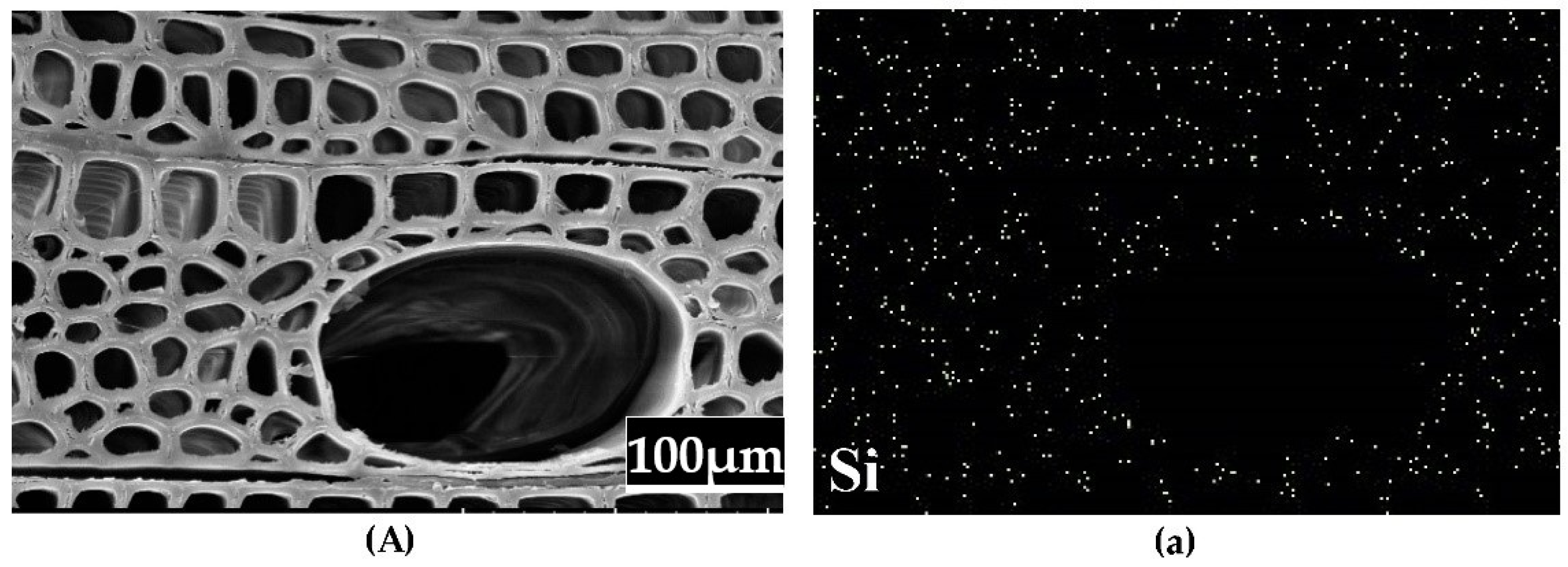
3.5. Microstructure Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, F.; Liu, J.L.; Lv, W.H. Thermal degradation and fire performance of wood treated with PMUF resin and boron compounds. Fire Mater. 2017, 41, 1051–1057. [Google Scholar] [CrossRef]
- Yue, K.; Liu, W.Q.; Chen, Z.J.; Lu, X.N.; Lu, W.D. Investigation of the Creep Property of Fast–growing Poplar Wood Modified with Low Molecular Weight Resins. BioResources 2016, 11, 1620–1633. [Google Scholar] [CrossRef]
- Gérardin, P. New alternatives for wood preservation based on thermal and chemical modification of wood—A review. Ann. For. Sci. 2016, 73, 559–570. [Google Scholar] [CrossRef]
- Qin, Y.L.; Long, D.J.; Li, J.Z. Research Progress in the Chemical Modification of Eucalyptus. Iop Conf. Ser. Mater. Sci. Eng 2019, 677, 022114. [Google Scholar] [CrossRef]
- Despres, A.; Pizzi, A.; Vu, C. Formaldehyde-free aminoresin wood adhesives based on dimethoxyethanal. J. Appl. Polym. Sci. 2008, 110, 3908–3916. [Google Scholar] [CrossRef]
- Mamiński, M.L.; Borysiuk, P.; Parzuchowski, P.G. Improved water resistance of particleboards bonded with glutaraldehyde-blended UF resin. Holzals Roh-Und Werkst. 2008, 66, 381–383. [Google Scholar] [CrossRef]
- He, X.; Xiao, Z.; Feng, X.; Sui, S.; Wang, Q.; Xie, Y. Modification of poplar wood with glucose crosslinked with citric acid and 1,3-dimethylol-4,5-dihydroxy ethyleneurea. Holzforschung 2016, 70, 47–53. [Google Scholar] [CrossRef]
- Kunaver, M.; Medved, S.; Cuk, N. Application of liquefied wood as a new particle board adhesive system. Bioresour. Technol. 2010, 101, 1361–1368. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Luo, H.; He, F. Mechanical, moisture absorption, and biodegradation behaviours of bacterial cellulose fibre-reinforced starch biocomposites. Compos. Ence Technol. 2009, 69, 1212–1217. [Google Scholar] [CrossRef]
- Viswanathan, T.; Richardson, T. Thermosetting adhesives resins from whey and whey by-products. Ind. Eng. Chem. Prod. Res. Dev. 1984, 23, 644–647. [Google Scholar] [CrossRef]
- Wei, X.G.; Hai, B.Y.; Yi, F.Z.; Sheldon, Q.S. Technology Development of Glycosyl Resins for Wood Composites. Mod. Appl. Bioequiv. Availab. 2017, 2, 555577. [Google Scholar]
- Huang, H.; Denard, C.A.; Alamillo, R. Tandem catalytic conversion of glucose to 5-hydroxymethylfurfural with an immobilized enzyme and a solid acid. ACS Catal. 2014, 4, 2165–2168. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, Z.; Cheng, S.; Leitch, M.; Xu, C.C. Synthesis of Novolac-Type Phenolic Resins Using Glucose as the Substitute for Formaldehyde. J. Appl. Polym. Sci. 2010, 118, 1191–1197. [Google Scholar] [CrossRef]
- Liu, S.L.; Zhao, K. Synthesis and response surface optimization of the phenol-glucose resin in non-aqueous phase. Thermosetting Resin 2015, 30, 29–32. (In Chinese) [Google Scholar]
- Li, D.; Wang, N.N. Preparation of resorcinol-glucose resin adhesive in near critical water. Adhes. China 2010, 31, 33–36. (In Chinese) [Google Scholar]
- Long, Y.F.; Yang, K.D. Synthesis of melamine-glucose resin adhesive. J. Guangdong Non-Ferr. Met. 2008, 2, 409–412. (In Chinese) [Google Scholar]
- Long, Y.F.; Yang, K.D. Study on synthesis of urea-glucose resin adhesive. J. Guangdong Non-Ferr. Met. 2008, 2, 400–404. (In Chinese) [Google Scholar]
- Nguyen, T.T.; Xiao, Z. Effects of modification with a combination of styrene-acrylic copolymer dispersion and sodium silicate on the mechanical properties of wood. J. Wood Sci. 2019, 65, 2–11. [Google Scholar] [CrossRef]
- Pfeffer, A.; Mai, C. Weathering characteristics of wood treated with water glass, siloxane or DMDHEU. Holzals Roh-Und Werkst. 2012, 70, 165–176. [Google Scholar] [CrossRef]
- Gong, M.X.; Cheng, R.X. Methods of Inorganic Modification of Wood. For. Eng. 2013, 29, 65–68. [Google Scholar]
- Yan, Y.; Dong, Y.; Li, J.; Zhang, S.; Xia, C.; Shi, S.Q.; Cai, L. Enhancement of mechanical and thermal properties of Poplar through the treatment of glyoxal-urea/nano-SiO2. RSC Adv. 2015, 5, 54148–54155. [Google Scholar] [CrossRef]
- Jiang, T.; Feng, X.; Wang, Q.; Xiao, Z.; Wang, F.; Xie, Y. Fire performance of oak wood modifed with N-methylol resin and methylolated guanylurea phosphate/boric acid-based fre retardant. Constr Build. Mater. 2014, 72, 1–6. [Google Scholar] [CrossRef]
- Testung Methods for Wood Adhesives and Their Resins; GB/T 14074-2006; State Administration for Market Regulation of China and Standardization Administration of China: Beijing, China, 2006.
- Method for Determination of the Swelling of Wood. ISO 4859:1982, Wood-Determination of Radial and Tangential Swelling; ISO 4860:1982, Wood-Determination of Volimetric Swelling; GB/T 1934.2-2009; State Administration for Market Regulation of China and Standardization Administration of China: Beijing, China, 2009.
- Method for Determination of the Density of Wood. ISO 3131:1975, Wood-Determination of Density for Physical and Mechanical Test; GB/T 1933-2009; State Administration for Market Regulation of China and Standardization Administration of China: Beijing, China, 2009.
- Method of in Bending Strength of Wood. ISO 3133:1975, Wood-Determination of Ultimate Strength in Static Bending; GB/T 1936.1-2009; State Administration for Market Regulation of China and Standardization Administration of China: Beijing, China, 2009.
- Method for Determination of the Modulus of Elasticity in Static Bending of Wood. ISO 3349: 1975, Wood-Determination of Modulus of Elasticity in Static Bending; GB/T 1936.2-2009; State Administration for Market Regulation of China and Standardization Administration of China: Beijing, China, 2009.
- Method of Testing in Compressive Strenth Parallel to Grain of Wood. ISO 3787:1976, Wood-Test Methods-Determination of Ultimate Stress in Compression Parallel to Grain; GB/T 1935-2009; State Administration for Market Regulation of China and Standardization Administration of China: Beijing, China, 2009.
- Li, M.M.; Hang, Z.S. Construction and Characterization of Semi-Interpenetrating Polymer Network of Polyethylene Glycol-Melamine-Formaldehyde and Polyvinyl Alcohol. Polym. Mater. Sci. Eng. 2015, 31, 158–163. [Google Scholar]
- Wang, K.L.; Gan, W.X. Thermal properties of glucose-melamine-formaldehyde resin. J. Guilin Univ. Technol. 2018, 38, 513–518. (In Chinese) [Google Scholar]
- Wang, K.L.; Liu, X.C. Synthesis and characterization of APF resin based on liquified amylum. J. Guilin Univ. Technol. 2019, 39, 446–452. (In Chinese) [Google Scholar]
- Zhang, Z.; Xiao, H.P. Relationship between Structure and Synthesis Process of Amino Resin. Plastics 2016, 45, 61–64. (In Chinese) [Google Scholar]
- Liu, M.; Thirumalai, R.V.K.G.; Wu, Y. Characterization of the crystalline regions of cured urea formaldehyde resin. RSC Adv. 2017, 7, 49536–49541. [Google Scholar] [CrossRef]
- Kong, L.; Guan, H.; Wang, X. In Situ Polymerization of Furfuryl Alcohol with Ammonium Dihydrogen Phosphate in Poplar Wood for Improved Dimensional Stability and Flame Retardancy. ACS Sustain. Chem. Eng. 2018, 6, 3349–3357. [Google Scholar] [CrossRef]
- Oishi, S.S.; Rezende, M.C.; Origo, F.D.; Damiao, A.J.; Botelho, E.C. Viscosity, pH, and moisture effect in the porosity of poly (furfuryl alcohol). J. Appl. Polym. Sci. 2013, 128, 1680–1686. [Google Scholar] [CrossRef]
- Huang, F.; Su, Y.; Tao, Y.; Sun, W.; Wang, W. Preparation of 5-hydroxymethylfurfural from glucose catalyzed by silica-supported phosphotungstic acid heterogeneous catalyst. Fuel 2018, 226, 417–422. [Google Scholar] [CrossRef]
- Feng, X.; Xiao, Z.; Sui, S.; Wang, Q.; Xie, Y. Esterification of wood with citric acid: The catalytic effects of sodium hypophosphite (SHP). Holzforschung 2014, 68, 427–433. [Google Scholar] [CrossRef]
- Shi, Z.; Yang, Q. Effects of lignin and hemicellulose contents on dissolution of wood pulp in aqueous NaOH/urea solution. Cellulose 2014, 21, 1205–1215. [Google Scholar] [CrossRef]
- Qin, Y.; Dong, Y.; Li, J. Effect of Modification with Melamine-Urea-Formaldehyde Resin on the Properties of Eucalyptus and Poplar. J. Wood Chem. Technol. 2019, 39, 360–371. [Google Scholar] [CrossRef]
- Suat, A.; Veysel, T. Modification with Melamine Formaldehyde and Melamine-Urea Formaldehyde Resin to Improve the Physical and Mechanical Properties of Wood. Bioresources 2017, 12, 586–596. [Google Scholar]
- Xie, Y.; Krause, A. Effect of treatments with 1, 3-dimethylol-4, 5-dihydroxy-ethyleneurea (DMDHEU) on the tensile properties of wood. Holzforschung 2007, 59, 484–500. [Google Scholar] [CrossRef]

| Groups | WPG (%) | BE (%) | Oven-Dry Density (g·cm−3) |
|---|---|---|---|
| Control | -- | -- | 0.385 ± 0.01 |
| S20 | 25.63 ± 0.29 | −14.90 ± 0.65 | 0.529 ± 0.01 |
| G10S20 | 43.23 ± 1.22 | −0.55 ± 2.37 | 0.522 ± 0.10 |
| G20S10 | 55.79 ± 2.89 | 7.84 ± 0.69 | 0.515 ± 0.10 |
| Group | Radial | Tangential | Volume |
|---|---|---|---|
| S20 | −241.11 ± 32.26 | −232.05 ± 12.14 | −221.71 ± 12.33 |
| G10S20 | −66.40 ± 18.07 | −12.33 ± 6.29 | −14.45 ± 1.37 |
| G20S10 | 30.85 ± 4.10 | 43.30 ± 18.86 | 60.01 ± 3.37 |
| Group | MOE (GPa) | MOR (MPa) | CS (MPa) |
|---|---|---|---|
| Control | 10.57 ± 0.61 | 92.17 ± 4.12 | 58.02 ± 4.79 |
| S20 | 16.20 ± 0.55 | 131.87 ± 2.98 | 119.17 ± 8.98 |
| G10S20 | 18.21 ± 0.91 | 139.33 ± 2.51 | 123.94 ± 2.18 |
| G20S10 | 18.02 ± 0.22 | 121.52 ± 2.05 | 122.20 ± 4.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Du, H.; Lyu, W. Physical and Mechanical Properties of Poplar Wood Modified by Glucose-Urea-Melamine Resin/Sodium Silicate Compound. Forests 2021, 12, 127. https://doi.org/10.3390/f12020127
Liu Q, Du H, Lyu W. Physical and Mechanical Properties of Poplar Wood Modified by Glucose-Urea-Melamine Resin/Sodium Silicate Compound. Forests. 2021; 12(2):127. https://doi.org/10.3390/f12020127
Chicago/Turabian StyleLiu, Qiangqiang, Haojia Du, and Wenhua Lyu. 2021. "Physical and Mechanical Properties of Poplar Wood Modified by Glucose-Urea-Melamine Resin/Sodium Silicate Compound" Forests 12, no. 2: 127. https://doi.org/10.3390/f12020127
APA StyleLiu, Q., Du, H., & Lyu, W. (2021). Physical and Mechanical Properties of Poplar Wood Modified by Glucose-Urea-Melamine Resin/Sodium Silicate Compound. Forests, 12(2), 127. https://doi.org/10.3390/f12020127





