Abstract
Background and Objectives: The existence of common ash (Fraxinus excelsior) in Europe is severely endangered by ash dieback. To support its future sustainability, it is essential to improve the natural ash regeneration. The main aim of this study was to investigate the influence of light conditions, conceivably influenced by stand structure/ash dieback, on ash regeneration and the competition between ash seedlings and species growing in the understory. Materials and Methods: We selected 40 plots in a riparian forest located in Bavaria, Germany. Light-related variables (Leaf Area Index, gap fraction) were gathered with fish-eye photography, whereas other environmental factors were derived from vegetation surveys (Ellenberg indicator values). We assessed vegetation parameters such as species’ richness and coverage of the herb layer to account for competition with ash seedlings. Results: Our results indicate that ash regeneration is favoured under shady conditions. The majority of other abiotic factors were not statistically associated with the analysed ash metrics. In contrast, the coverage of grass was negatively related to LAI and positively to gap fraction. Higher herb and grass coverages were linked to a suppression of ash regeneration. A higher litter coverage was associated with a higher frequency of ash seedlings. Nonparametric partial correlation analyses demonstrated the influence of light and stressed that litter coverage is of particular importance. Conclusions: We conclude that gaps, inter alia induced by ash dieback, favour grass invasion. In turn, this invasion might suppress regeneration of ash. In this regard, rapid silvicultural management such as reforestation of gaps after dieback of mature trees is recommended. The influence of litter on interspecific competition during growth should be also considered. The pace of dieback might additionally influence the timing and quantity of litter accumulation; thus, further research should also focus on these interrelations.
1. Introduction
The broad-leaved common ash (Fraxinus excelsior L.) is a widespread tree species in Europe. Due to its flood tolerance, it is one of the main tree species in floodplain forest ecosystems [1,2]. Forestry faces major challenges in the management of riparian forests as some species are economically unattractive (e.g., poplar, willows), while others are exposed to major diseases (e.g., elm) [3]. For a long period, many ashes have been planted in order to revalue mixed forest ecosystems economically and ecologically [4]. The importance of ash—besides its ecological relevance—results from valuable wood characteristics such as stability and elasticity, monopodial but also rapid vertical growth [5,6]. Their roots dominate in the upper mineral soil horizon, forcing roots of other species such as beech downwards and allowing ashes to be the first acquiring infiltrated water [7,8]. Thus, ash is characterized by a high drought tolerance and is able to withstand high temperatures, making it an optimal tree species under climate change conditions [9].
The abundance of ash is currently declining due to the fungus Hymenoscyphus fraxineus, an invasive pathogen from East Asia [10,11]. First symptoms were already detected in the 1990s in Poland, but the causal agent could not been identified [12]. Only in 2006, the pathogen associated with ash dieback was identified as a new anamorphic form (Chalara fraxinea) of the fungus [13]. Now the disease is spreading over almost all of Europe [14,15]. The ascomycete infests the tree through its leaves or shoots [16], which results in dieback of branches and twigs and dwarf growth, and it may even lead to a gradual death of the tree within a few years [14]. Ash dieback mostly affects trees with an average or below-average size, which suggests that individual tree resistance is related to growth potential or tree vigour [17]. It is also known that a strong genetic variation in the susceptibility to ash dieback exists [18].
In the past, the high regeneration rate of ashes was discussed as a problem for mixed stands [19]. The well-appointed root system of ashes in combination with the high germination potential even under dense canopies are an obvious advantage over other tree species such as beech (not in riparian forests), oaks or poplars. In consideration of the current threat of ash dieback and in order to support the future viability and sustainability of ash, it is essential to improve its natural regeneration. It is the product of natural selection, which could offer opportunities to fight the disease [4,20]. Those seedlings from the natural regeneration, which are able to withstand interspecific or intraspecific competition, might present resistant phenotypes, which are also able to cope with the disease. Considering a high mortality of ash trees in affected areas along with a genetic variation in susceptibility may eventually result in more resistant juvenile ash trees [21]. A lower mortality and damage of seedlings of populations in Lithuania was already attributed to such a selection process [22]. Thus, breeding of resistant trees in future might be a powerful opportunity for the endangered ash [9,21].
Ash is usually characterized by a high seed production and regeneration rate. In Belgium, this rate was quantified with ca. 150,000 individuals per hectare under particularly good site conditions [23,24]. Ash juveniles have a high shade tolerance, whereas older trees are characterized by a higher light demand [6,8,25]. Therefore, ash is associated with a high juvenile survival rate in case of low incidence of light and a pronounced increase in height under amplified light availability.
Grass species in forests compete with tree seedlings or herbaceous plants for light, water and nutrients, which may result in allelopathy, mechanical obstruction of the roots or aboveground overgrowing [26,27]. Disturbances such as the dieback or the death of a large canopy tree can result in suddenly changed light conditions and subsequently in intense competition for above- and below-ground resources [28]. In the light of ash dieback, the crown transparency of old ash stocks represents a challenge for forest management, because grass invasion may additionally suppress ash regeneration [29,30]. Ash dieback might not only lead to an abundant growth of grass but also to an increasing growth of other light-demanding tree seedlings. In Lithuania’s forests, the disease already induced comprehensive changes in forest structure and species composition. Secondary succession in former ash-dominated locations resulted in a different tree composition consisting of birch and grey alder [31]. However, the effects on the composition of the herb layer due to changes in abiotic factors (e.g., light conditions) and biotic factors (e.g., competition) are largely unexplored.
In this study, we examine the influence of light-related variables, conceivably also modified by ash dieback, on the abundance of ash seedlings in a riparian forest in Southern Germany. In addition, we consider statistical relationships with other environmental factors, i.e., soil moisture, nutrient availability and soil reaction.
We compared the density and proportion of ash seedling to other seedlings growing in the herb layer to account for competition. Thus, the main aim was to test whether light availability is a key parameter for the development of natural ash regeneration and to identify especially the influence of structural pattern of understorey vegetation on their occurrence.
2. Study Area
Data were collected in a floodplain forest along the Danube between Neuburg and Ingolstadt (Bavaria, Germany; Figure 1). The study area is rather flat and situated at about 375 m a.s.l. According to Köppen-Geiger’s climate classification, the climate is oceanic (Cfb) [32]. The average annual precipitation is 715 mm and mean annual temperature is 7.8 °C (1961–1990) [33,34].
Figure 1.
Localization of the study site (black rectangle) in Bavaria, Germany. (Basis data: Bundesamt für Kartographie und Geodäsie, Germany—DEM: 200 m, GK3; DLM: 250 m, GK3, 2019).
Due to the proximity to the Danube, young riparian sediments of the last Danube terrace predominate [35]. Soils are influenced by former flooding and consist of overlaying calcareous, nutrient-rich substrate of mainly fine-grained alluvial loam [36]. The location allows for favourable growth conditions related to all types of floodplain forest trees [37].
Ca. 25% of the prevailing canopy trees belong to the species Acer pseudoplatanus. Other tree species with high abundances (ca. 15%) are Fraxinus excelsior and Carpinus betulus, and with lower abundances (<15%) Quercus robur, Tilia cordata, Ulmus minor and Acer campestre. Symptoms of ash dieback were frequently observed: 48.5% of the adult ash trees near the plots were characterized as (severely) damaged with a leaf loss greater than 30%, according to the scoring system of Lenz et al. (2012) [38]. In contrast, 33.3% of the adult ash trees were only slightly affected and 18.2% did not show any symptoms of ash dieback. In contrast, less than 10% of the ash seedlings growing within the selected plots were associated with symptoms related to ash dieback.
3. Materials and Methods
Data were collected within an area known for the occurrence of ash regeneration in 40 round plots, which were sized with a diameter of three meters (7.1 m2) in order to cover and characterize a large area of the forest floor. To obtain results for the whole range of the floodplain, forest areas on both sides of the river were investigated. The 20 plots in the southern part were located in the actual floodplain but were rarely flooded due to its elevation, whereas the 20 plots in the northern part were located in the former floodplain where possible flooding of the Danube is retained by a dam. Plots at these two sites were selected randomly, each in a forest sector of 9 ha (southern part) and of 4 ha (northern part) where ash frequently occurs (abundance of 15%). The health status of adult ash trees next to these plots was not used as criterion for plot selection. Adult tree density and structure of the forest differ within the area, among others due to ash dieback of individual trees. Thus, the canopy openness of our plots varies greatly.
We performed vegetation surveys in June and July 2019 determining the abundance of each species in the herb layer according to the scale of Londo (1975) [39]. In addition, we counted the number of individuals of the herb layer and we estimated the coverage of soil and litter as well as the grass coverage of the vegetation cover (plot area minus soil and litter coverage) in %. The distance between the plot centre and the nearest adult ash tree was recorded.
Data on ash regeneration were of particular interest: We counted the number of ash seedlings within each plot to calculate the density of ash regeneration (abbreviated “ash seedling density”, (m−2)), assessed the proportion of ash individuals among all prevailing individuals in the herb layer (abbreviated “proportion of ash”, (%)) and the percentage of ash seedlings among all tree seedlings (abbreviated “ash % among seedlings”, (%)). These variables were used as dependent variables in statistical analyses.
The natural abiotic conditions of a site determine species occurrence, especially of the herb layer. Therefore, species composition was analysed using Ellenberg indicator values [40]. In our study, we used the indicators for soil reaction (R) and nutrients (N) on a scale from 1 (low) to 9 (high), and for the moisture indicator (M) from 1 to 12. For each plot, the cover-weighted average of these indicator values of herb-layer species was calculated. Other indicators were ignored, since they are more suitable for larger scales (temperature and continentality) or were measured directly (light). The Leaf Area Index (LAI) of each plot was evaluated by digital hemispherical photography (Canon EOS 60 D (18 MP), 4.5 mm lens (Sigma model EX CD)). This dimensionless index describes the one-sided area of photosynthetically active material per ground surface area [41]. The photographs were taken during overcast sky conditions between 25 June and 12 July 2019 and were analysed using the software HemiView 2.1 [42]. In addition to LAI, we included gap fraction (G), which is defined as the fraction of visible sky [43] and can vary between 0% (completely obscured sky) and 100% (completely visible sky).
Statistical analyses were performed using IBM SPSS Statistics 25. If data did not meet the statistical requirements, i.e., normal distribution assessed using the Kolmogorow Smirnow test, nonparametric tests were applied, which included Spearman’s rank order correlation. In a second step, we investigated the effect of LAI when controlling for the most important biotic variable (i.e., coverage of litter) by nonparametric partial correlation analysis. We applied the Mann–Whitney U test to compare mean values for plots, which were categorized in classes according to the proportion of ash in the herb layer.
4. Results
4.1. Ash Metrics and Abiotic and Biotic Variables
We observed a mean density of 2.2 ash seedlings m−2 (range 0.1–8.5) at the selected plots (Table 1). On average, 13.2% (range 0.5%–41.6%) of the recorded individuals in the herb layer were ash seedlings and 38.3% (range 0%–100%) of all occurring seedlings belonged to ash. The mean distance to the nearest adult ash tree was 13.5 m (range 0.8–50 m).

Table 1.
Collected variables and their mean, minimum, maximum values including standard deviation. LAI: Leaf Area Index, G: gap fraction (%), M, R, N: Ellenberg indicator values for soil moisture, reaction, and nutrients, N: number, dist. ash (m): distance to the nearest adult ash tree.
The descriptive statistics related to LAI and gap fraction confirmed a wide range of different light conditions: LAI varied between 1.0 and 3.4 (mean 2.1) and gap fraction between 6.4% and 34.8% (mean 15.2%).
The Ellenberg indicator for soil moisture (M) varied between 3.7 and 6.0 (mean 5.4). The means of the other indicator values of the herbal layer were 7.1 (range 6.5–7.7) for soil reaction (R) and 6.7 (range 4.4–7.9) for nutrients (N) (Table 1).
An average of 10 different herb species (range 4–17) was found in the herb layer, which covers a mean surface of 73.1% (range 18%–98%) of the plot area (mean litter coverage 16.7%, range 0%–75% and mean soil coverage 3.4%, range 0%–40%). The coverage of grass species varied greatly among plots with values between 0% and 88.4% (mean 36.2%).
Table 2 lists all species observed in the understory and their respective coverage at the 40 plots. In total, 57 species were recorded but only three of them were associated with a coverage greater than 6%: Aegopodium podagraria (11.9%), and the two tree seedlings Acer pseudoplatanus (6.5%, N = 655) and Fraxinus excelsior (6.4%, N = 634).

Table 2.
Observed species and their coverage in the understorey at the 40 studied plots, t = tree, s = shrub, h = herb, l = liana.
4.2. Relationship between Ash Regeneration, Grass Cover and Abiotic and Biotic Variables
Ash regeneration is favoured under lower light incidence. This was documented by a positive correlation between LAI and the ash seedling density or the proportion of ashes (rs = 0.400, p = 0.011 and rs = 0.300, p = 0.037), and the negative correlation between ash seedling density and gap fraction (rs = −0.385, p = 0.014).
Soil reaction showed a statistically significant correlation with ash density (rs = −0.330, p = 0.038) and proportion of ash (rs = −0.355, p = 0.025). All other abiotic factors were not statistically associated with the analysed ash metrics.
The coverage of grass was negatively related to LAI (rs = −0.346, p = 0.029) and positively to gap fraction (rs = 0.319, p = 0.045) and thus showed the opposite pattern of ash regeneration. Figure 2a,b visualizes the relationships between gap fraction and the proportion of ash and coverage of grass. Steep regression slopes point to great changes in the proportion of ash and grass coverage under small variations of gap fraction. However, r2 remains quite low (<0.2) and statistical significance is only given for the relationship between gap fraction and grass coverage.
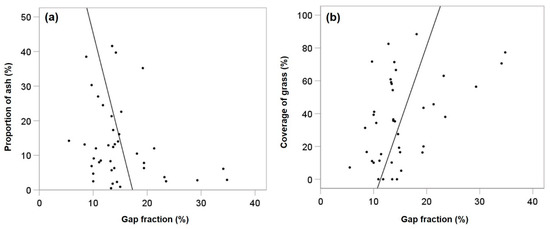
Figure 2.
Scatterplot visualisation of the relationships between gap fraction and proportion of ash (a) and coverage of grass (b).
Higher herb and grass coverages were related to a suppression of ash regeneration, regardless of the ash metric used but with highest correlation coefficients for ash seedling density (cf. coverage of the herb layer: rs = −0.523, p = 0.001; cf. coverage of grass: rs = −0.546, p < 0.001). The coverage of soil was not significantly related to the ash regeneration metrics. A higher litter coverage, however, was linked to increases in ash regeneration with the highest correlation coefficient of rs = 0.674 (p < 0.001, proportion of ash). Nonparametric partial correlation analyses that controlled for the effect of litter coverage revealed that LAI is still significantly related to ash density (rs = 0.396, p = 0.014), marginally significantly related to the proportion of ash among all individuals in the herb layer (rs = 0.314, p = 0.055), but not significantly related to the percentage of ash among seedlings (rs = 0.202, p = 0.224).
The coverage of grass was higher at plots with a higher coverage of the herb layer (rs = 0.571, p < 0.001) and litter (rs = 0.457, p = 0.003). In turn, the coverage of grass was lower at plots with higher soil coverage (rs = −0.347, p = 0.030).
There were no correlations between the distance to the nearest adult ash tree and ash-regeneration metrics or the number of species found in the herb layer.
The Mann–Whitney U test revealed that the mean grass coverage of selected classes categorised by the proportion of ash seedlings among all prevailing individuals in the herb layer (class 1: >15%, class 2: 7.5–15%, class 3: <7.5%) was significantly different for class 1 and 2 (p = 0.032), marginally significantly different for class 1 and 3 (p = 0.051), but not significantly different for class 2 and 3 (p = 0.621). The mean grass coverage was 20.9% for class 1, 40.0% for class 2 and 43.9% for class 3.
5. Discussion
In our 40 studied plots located in the floodplain forest and with a total size of 284 m2, we registered 634 ash seedlings which is equivalent to 22,324 individuals per ha. Existing literature documents an almost seven times higher regeneration rate of ash (150,000 individuals/ha) under favourable site conditions in a dense seminatural mixed hardwood forest in Belgium [23,24]. In our study, only seedlings of Acer pseudoplatanus (N = 655) were found more frequently than ash seedlings. More than 80% of the adult ash trees growing at our study site showed symptoms of ash dieback. Although we did not sample airborne Hymenoscyphus fraxineus spores, we suppose that their concentration is high enough to affect ashes from all age classes. However, only 10% of the ash regeneration showed symptoms of ash dieback.
5.1. Relationships between Variables Related to Light Conditions and Ash Regeneration
In the literature, it is well known that ash regeneration exhibits a remarkable shade tolerance [25,44], which is lower for oak or maple [5]. However, ash transforms into a light-demanding tree at an age of seven or eight [8]. A wide range of gap fraction (5.5% to 34.8%, see Table 1) characterized the studied plots. Plots associated with lower values of gap fraction and therefore lower light levels on the ground had higher ash seedling densities and a higher proportion of ash seedlings (Table 3). A coherent relationship was documented for LAI (c.f. N ash seedling).

Table 3.
Summary on Spearman’s rank correlation coefficients (rs) and p-values of all surveyed abiotic and biotic variables related to ash-regeneration metrics and coverage of grass and herb layer. LAI: Leaf Area Index, G: gap fraction, M, R, N: Ellenberg indicator values for soil moisture, reaction, and nutrients, dist. ash: distance to the next adult ash tree. Bold values indicate significant correlations (p < 0.050).
Related to the magnitude of the correlation coefficients, we conclude that especially LAI is the most appropriate measure for assessing the relation to ash regeneration. LAI accounts for photosynthetic and transpirational surface of plant canopies [45] and might therefore be suitable to account for the competition for light.
5.2. Relationships between Other Abiotic Factors and Ash Regeneration
Besides the great importance of light, there might be other environmental factors influencing regeneration such as soil nutrients [28]. Although soil characteristics are assumed to vary only slightly between our investigated plots, the Ellenberg indicator value for nutrients ranged between 4.4 and 7.9 (Table 1). However, this indicator was not statistically significantly associated with any of the selected ash regeneration metrics. The wide range of light availability seems to predominate the occurring variance in soil conditions such as moisture or nutrient availability—factors that were reported to be of importance in terms of the growth of seedlings [46]. In general, ash grows on a wide range of soils but preferably on nutrient- and base-rich soils with pH values greater than 4.2 [47]. We found a significant and negative correlation between ash density or ash proportion and soil reaction. However, the soil of our plots can be classified as base-rich (mean of the reaction indicator: 7.1; range: 6.5–7.7, Table 1). Optimal conditions related to soil moisture vary between very moist and fresh; however, slightly dry sites are also considered to be suitable for the growth of ash [48].
5.3. Seed Availability Inferred by Distance Measures
The distance to the nearest adult tree ranged between 0.8 m and 50 m (mean 13.5 m, Table 1), but was not significantly associated with ash-regeneration metrics. Thus, the abundance of ash seedlings is not primarily linked to the occurrence of adult trees. Mean distances for the dispersion of seeds from a 130-year-old mother tree (45 cm breast height diameter) was estimated with 43.5 m [49]. An experimental study investigating seed dispersal of a solitary ash tree documented that most of the seeds were found at 20 m distance to the tree [49]. In our study, for only seven plots the distance to the next adult tree was greater than the above-mentioned mean dispersion distance of 43.5 m. However, mean values for ash-regeneration metrics did not differ significantly between sites with a smaller (≤43.5 m) or greater distance (>43.5 m; data not shown). Thus, at our study site, which is characterized by a high proportion of ash trees in the canopy layer (ca. 15%), seed availability might not be a limiting factor.
5.4. Competition in the Herb Layer
Our results showed that high grass coverage is significantly accompanied by low ash regeneration. In general, the coverage of grass was remarkably high (mean 36.2%, Table 1) and exceeded up to 88.4%. Only four plots were associated with a complete absence of grass; three of them were linked to a high proportion of ash seedlings (over 20% and up to 40%). Fast-growing grass species that compete for above- and below-ground resources have a negative effect on the growth of young trees and therefore represent a major challenge for forest management [50]. Plots with a high proportion of ash (>15%) had significantly lower grass coverage than plots with a lower proportion (<7.5%). Grass coverage seems to be favoured by a high translucency of light indicated by both correlations with LAI and gap fraction (Table 3). Therefore, we assume that an ongoing damage of adult ash trees or other factors that increase the light perception on the herb layer will favour grass and reduce the amount of ash seedlings. Even if our results did not show clear correlations, herbaceous species are also able to occupy the herb layer below canopy gaps quickly [51,52]. Due to the generally higher species number of herb species compared to grasses and thus their higher variance in light demand, herbaceous species might suppress ash regeneration at sites with larger shaded areas as well.
In general, herbaceous species might have an advantage over tree seedlings in the competition for soil nutrients [53]. In our study, we found that the coverage of the herb layer was negatively correlated to the selected ash metrics (Table 3). Thus, an important biotic factor exposes the competition among species growing on the ground layer of the floodplain forest. The proportion of ash regeneration among other tree seedlings had a negative relationship to the coverage of the herb layer. However, whether herbaceous species suppress ash seedlings even more than other seedlings needs to be further studied.
5.5. Relationship between Litter and Ash Regeneration
The highest correlations were found between ash metrics and the coverage of litter (e.g., c.f. proportion of ash seedlings rs = 0.674, Table 3). Being aware that statistical correlations are not automatically causal relations, litter seems to suppress other species as documented by the negative correlations with the coverage of grass and herb layer (Table 3) and might favour the germination of ash seeds, also compared to other tree species (% of ash among seedlings). Thus, the influence of litter on the interspecific competition during growth is especially meaningful and associated with even higher correlation coefficients compared to light-related variables (Table 3). Partial correlations revealed that controlling for litter coverage reduces the effect of light (i.e., LAI) in magnitude. Light availability is still of importance when ash density or the proportion ash seedlings among all individuals in the herb layer are regarded. Considering the proportion of ash seedlings among all prevailing seedlings, a high litter coverage seems to support ash seedlings in contrast to others.
Strong and quickly intensifying symptoms of ash dieback might be rather associated with a high quantity of leaf litter during the vegetation period, which could prevent from grass invasion. A slower progress of the disease with a gradual leaf fall might not be linked to higher quantities of litter within a short time span and thus does not favour the growth of ash seedlings.
The tree species that dominate in our study area are characterized by a base- and nutrient-rich litter (Fraxinus excelsior and Carpinus betulus) or an intermediate quality of the litter (Acer pseudoplatanus) [54]. In general, base- and nutrient-rich litter increases nutrient availability and diversity of soil habitats in mixed forest stands [55]. Hence, the composition of canopy species influences the nutritional conditions of the soil (via leaf litter) and in turn affects the composition of the understorey. In our study, the Ellenberg indicator values for nutrients were not statistically significantly linked to ash regeneration. Since ash seedlings are extremely tolerant to shade [19,38] large litter quantities might also be beneficial for high survival rates compared to other tree seedlings.
6. Conclusions
The aim of this study was to investigate how light conditions and especially biotic factors influence the occurrence of the natural regeneration of ash. We conclude that crown gaps inter alia induced by mortality, leaf fall and damage of shoots caused by ash dieback favour grass invasion. In turn, this implies a feedback loop since the invasion of grass might suppress natural regeneration of ash, which might present resistant phenotypes that are more successful in coping with the disease. In this regard, rapid silvicultural management such as reforestation of gaps within the stand that occurred after dieback of mature trees is recommended. Since we found that the coverage of litter on the ground is an important factor influencing ash germination/growth, the speed of dieback might also be a so-far overlooked component. However, investigations on that might not be easy to trace in the natural environment and could be further addressed in controlled experiments. Silvicultural management might increase litter cover to reduce grass cover and to support ash regeneration. Further research on ash regeneration should especially focus on neighbouring canopy trees and their characteristics related to the timing of leaf fall, litter quantity/thickness and chemical composition of the leaf litter.
Author Contributions
Conceptualization, S.J.-O.; methodology, S.J.-O. and B.S.; field work, T.R.; data analyses, S.J.-O. and T.R.; writing—original draft preparation, T.R., S.J.-O. and B.S.; writing—review and editing, S.J.-O.; B.S., A.-K.E. and T.R.; visualization, S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
We gratefully acknowledge the Wittelsbacher Ausgleichsfonds for the permission to conduct scientific research in the riparian forest. We thank Rudolf Vierheilig and Christoph Gabler for their support in the field and Johanna Jetschni for proofreading of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bayerische Landesanstalt für Wald und Forstwirtschaft. Die Regionale Natürliche Waldzusammensetzung Bayerns; LWF-Wissen 32; Bayerische Landesanstalt für Wald und Forstwirtschaft: Freising, Germany, 2001. [Google Scholar]
- Mitchell, R.J.; Beaton, J.K.; Bellamy, P.E.; Broome, A.; Chetcuti, J.; Eaton, S.; Ellis, C.J.; Gimona, A.; Harmer, R.; Hester, A.J.; et al. Ash dieback in the UK: A review of the ecological and conservation implications and potential management options. Biol. Conserv. 2014, 175, 95–109. [Google Scholar] [CrossRef]
- Dichtl, T.; Stöger, W. Auenwald im Klimawandel: Forstliche Forschung an Donau und Rhein. LWF Aktuell 2020, 126, 28–29. [Google Scholar]
- Enderle, R.; Fussi, B.; Lenz, H.D.; Langer, G.; Nagel, R.; Metzler, B. Ash dieback in Germany: Research on disease development, resistance and management options. In Dieback of European Ash (Fraxinus spp.)—Consequences and Guidelines for Sustainable Management; Vasaitis, R., Enderle, R., Eds.; European Cooperation in Science & Technology (COST): Uppsala, Sewden, 2017; pp. 89–105. ISBN 978-91-576-8696-1. [Google Scholar]
- Tabari, K.M.; Lust, N.; Neirynk, J. Effect of light and humus on survival and height growth of ash (Fraxinus excelsior L.) seedlings. Silva Gandav. 1998, 63, 36–49. [Google Scholar] [CrossRef]
- Nüsslein, S. Waldbauliche Behandlung der Esche. In Beiträge zur Esche Fachtagung zum Baum des Jahres 2001; LWF-Wissen 34; Bayerische Landesanstalt für Wald und Forstwirtschaft: Freising, Germany, 2002; pp. 41–43. [Google Scholar]
- Gulder, H.-J. Standortansprüche und Wurzelwerk der Esche. In Beiträge zur Esche Fachtagung zum Baum des Jahres 2001; LWF-Wissen 34; Bayerische Landesanstalt für Wald und Forstwirtschaft: Freising, Germany, 2002; pp. 50–52. [Google Scholar]
- Schütz, J.P. Modellierung des Höhenwuchses der Esche in der Verjüngungsphase in Abhängigkeit von der Beschattung. In Proceedings of the Jahrestagung 2004 des Deutschen Verbandes forstlicher Forschungsanstalten, Stift Schlägl, Austria, 24–26 May 2004; Deutscher Verband Forstlicher Forschungsanstalten: Göttingen, Germany, 2004; p. 109. [Google Scholar]
- Enderle, R.; Nakou, A.; Thomas, K.; Metzler, B. Susceptibility of autochthonous German Fraxinus excelsior clones to Hymenoscyphus pseudoalbidus is genetically determined. Ann. For. Sci. 2015, 72, 183–193. [Google Scholar] [CrossRef]
- Zhao, Y.-J.; Hosoya, T.; Baral, H.-O.; Hosaka, K.; Kakishima, M. Hymenoscyphus pseudoalbidus, the correct name for Lambertella albida reported from Japan. Mycotaxon 2013, 122, 25–41. [Google Scholar] [CrossRef]
- Gross, A.; Holdenrieder, O.; Pautasso, M.; Queloz, V.; Sieber, T.N. Hymenoscyphus pseudoalbidus, the causal agent of European ash dieback. Mol. Plant Pathol. 2014, 15, 5–21. [Google Scholar] [CrossRef]
- Przybyl, K. Fungi associated with necrotic apical parts of Fraxinus excelsior shoots. For. Pathol. 2002, 32, 387–394. [Google Scholar] [CrossRef]
- Kowalski, T. Chalara fraxinea sp. nov. associated with dieback of ash (Fraxinus excelsior ) in Poland. For. Pathol. 2006, 36, 264–270. [Google Scholar] [CrossRef]
- Pautasso, M.; Aas, G.; Queloz, V.; Holdenrieder, O. European ash (Fraxinus excelsior) dieback—A conservation biology challenge. Biol. Conserv. 2013, 158, 37–49. [Google Scholar] [CrossRef]
- Giongo, S.; Oliveira Longa, C.M.; Dal Maso, E.; Montecchio, L.; Maresi, G. Evaluating the impact of Hymenoscyphus fraxineus in Trentino (Alps, Northern Italy): First investigations. iForest 2017, 10, 871–878. [Google Scholar] [CrossRef]
- Kowalski, T.; Holdenrieder, O. Pathogenicity of Chalara fraxinea. For. Pathol. 2009, 39, 1–7. [Google Scholar] [CrossRef]
- Skovsgaard, J.P.; Thomsen, I.M.; Skovgaard, I.M.; Martinussen, T. Associations among symptoms of dieback in even-aged stands of ash (Fraxinus excelsior L.). For. Pathol. 2010, 40, 7–18. [Google Scholar] [CrossRef]
- McKinney, L.V.; Nielsen, L.R.; Hansen, J.K.; Kjær, E.D. Presence of natural genetic resistance in Fraxinus excelsior (Oleraceae) to Chalara fraxinea (Ascomycota): An emerging infectious disease. Heredity 2011, 106, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Rysavy, T.; Roloff, A. Ursachen der Vereschung in Mischbeständen und Vorschläge zu ihrer Vermeidung. Forst Und Holz 1994, 49, 392–395. [Google Scholar]
- Metzler, B.; Enderle, R.; Karopka, M.; Toepfner, K.; Aldinger, E. Development of ash dieback in a provenance trial on different sites in southern Germany. In Allgmeine Forst und Jagdzeitung; JD Sauerlaender’s Verlag: Bad Orb, Germany, 2012; Volume 183, pp. 168–180. [Google Scholar]
- McKinney, L.V.; Nielsen, L.R.; Collinge, D.B.; Thomsen, I.M.; Hansen, J.K.; Kjaer, E.D. The ash dieback crisis: Genetic variation in resistance can prove a long-term solution. Plant Pathol. 2014, 63, 485–499. [Google Scholar] [CrossRef]
- Pliūra, A.; Lygis, V.; Suchockas, V. Performance of twenty four European Fraxinus excelsior populations in three Lithuanian progeny trials with a special emphasis on resistance to Chalara fraxinea. Balt. For. 2011, 17, 17–34. [Google Scholar]
- Tabari, K.M.; Lust, N. Monitoring of natural regeneration in a mixed deciduous forest. Silva Gandav. 1999, 64. [Google Scholar] [CrossRef]
- Dobrowolska, D.; Hein, S.; Oosterbaan, A.; Wagner, S.; Clark, J.; Skovsgaard, J.P. A review of European ash (Fraxinus excelsior L.): Implications for silviculture. Forestry 2011, 84, 133–148. [Google Scholar] [CrossRef]
- Petritan, A.M.; von Lupke, B.; Petritan, I.C. Effects of shade on growth and mortality of maple (Acer pseudoplatanus), ash (Fraxinus excelsior) and beech (Fagus sylvatica) saplings. Forestry 2007, 80, 397–412. [Google Scholar] [CrossRef]
- Coomes, D.A.; Grubb, P.J. Impacts of root competition in forests and woodlands: A theoretical framework and review of experiments. Ecol. Monogr. 2000, 70, 171–207. [Google Scholar] [CrossRef]
- Flory, S.L.; Clay, K. Non-native grass invasion suppresses forest succession. Oecologia 2010, 164, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, F.S. The Ecological Significance of the Herbaceous Layer in Temperate Forest Ecosystems. BioScience 2007, 57, 845–858. [Google Scholar] [CrossRef]
- ForstBW. Jahresbericht 2012 des Landesbetriebes ForstBW; ForstBW: Baden-Württemberg, Germany, 2012; p. 58. [Google Scholar]
- Langer, G.; Bressem, U. Eschentriebsterben—Praxisinformation Nr. 4; Bayerische Landesanstalt für Wald und Forstwirtschaft: Göttingen, Germany, 2016; Available online: https://www.lwf.bayern.de/mam/cms04/waldschutz/dateien/bonitur_von_alteschen.pdf (accessed on 4 December 2020).
- Lygis, V.; Vasiliauskas, R.; Larsson, K.-H.; Stenlid, J. Wood-inhabiting fungi in stems of Fraxinus excelsior in declining ash stands of northern Lithuania, with particular reference to Armillaria cepistipes. Scand. J. For. Res. 2005, 20, 337–346. [Google Scholar] [CrossRef]
- Geiger, R. Überarbeitete Neuausgabe von Geiger, P. Köppen-Geiger / Klima der Erde; Klett-Perthes: Gotha, Germany, 1961. [Google Scholar]
- Bayerische Landesanstalt für Landwirtschaft. Agrarmeteorologie by: Weather Data. (Open Document). Available online: https://dynamax.com/images/uploads/papers/HemiView_Manual.pdf (accessed on 4 December 2020).
- Schwab, A.; Stammel, B.; Kiehl, K. Seed dispersal via a new watercourse in a reconnected floodplain: Differences in species groups and seasonality. Restor. Ecol. 2018, 26, S103–S113. [Google Scholar] [CrossRef]
- Margraf, C. Die Vegetationsentwicklung der Donauauen Zwischen Ingolstadt und Neuburg: Vegetationskundlich-ökologische Studie über den Wandel Einer Auenlandschaft 30 Jahre Nach Staustufenbau; Hoppea, Denkschriften der Regensburgischen Botanischen Gesellschaft: Regensburg, Germany, 2004. [Google Scholar]
- Lang, P.; Ewald, J. Predictive modelling and monitoring of Ellenberg moisture value validates restoration success in floodplain forests. Appl. Veg. Sci. 2014, 17, 543–555. [Google Scholar] [CrossRef]
- Doben, K.; Doppler, G.; Freudenberger, W.; Jerz, H.; Meyer, R.K.F.; Mielke, H.; Ott, W.-D.; Rohrmüller, J.; Schmidt-Kaler, H.; Schwerd, K.; et al. Geologische Karte von Bayern; 4. Auflage; Bayerisches Geologisches Landesamt: Munich, Germany, 1996. [Google Scholar]
- Lenz, H.; Straßner, L.; Baumann, M.; Baier, U. Boniturschlüssel zur Einstufung der Vitalität von Alteschen. Afz-Der Wald 2012, 3, 18–129. [Google Scholar]
- Londo, G. The decimal scale for releves of permanent quadrats. Vegetatio 1976, 33, 61–64. [Google Scholar] [CrossRef]
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, V.; Werner, W.; Paulißen, D. (Eds.) Zeigerwerte von Pflanzen in Mitteleuropa; 2. Auflage; Verlag Erich Goltze GmbH & Co. KG: Göttingen, Germany, 1992. [Google Scholar]
- Monteith, J.L. Principles of Environmental Physics; Reprinted with corr.; Arnold: London, UK, 1973; ISBN 0713123753. [Google Scholar]
- Delta-T Devices. User Manual for HemiView, Version 2.1. 1999. Available online: https://dynamax.com/images/uploads/papers/HemiView_Manual.pdf (accessed on 4 December 2020).
- Welles, J.M.; Norman, J.M. Instrument for Indirect Measurement of Canopy Architecture. Agron. J. 1991, 83, 818–825. [Google Scholar] [CrossRef]
- Marigo, G.; Peltier, J.-P.; Girel, J.; Pautou, G. Success in the demographic expansion of Fraxinus excelsior L. Trees 2000, 15, 1–13. [Google Scholar] [CrossRef]
- Welles, J.M.; Cohen, S. Canopy structure measurement by gap fraction analysis using commercial instrumentation. J. Exp. Bot. 1996, 47, 1335–1342. [Google Scholar] [CrossRef]
- Mölder, A.; Bernhardt-Römermann, M.; Schmidt, W. Herb-layer diversity in deciduous forests: Raised by tree richness or beaten by beech? For. Ecol. Manag. 2008, 256, 272–281. [Google Scholar] [CrossRef]
- Thomas, P.A. Biological Flora of the British Isles: Fraxinus excelsior. J. Ecol. 2016, 104, 1158–1209. [Google Scholar] [CrossRef]
- Kerr, G.; Cahalan, C. A review of site factors affecting the early growth of ash (Fraxinus excelsior L.). For. Ecol. Manag. 2004, 188, 225–234. [Google Scholar] [CrossRef]
- Wagner, S. Ein Modell zur Fruchtausbreitung der Esche (Fraxinus excelsior L.) unter Berücksichtigung von Richtungseffekten. Allg. Forst Jagdztg. 1997, 168, 149–155. [Google Scholar]
- BMEL. The Forests in Germany—Selected Results of the Third National Forest Inventory; BMEL: Bonn, Germany, 2014. [Google Scholar]
- Gilliam, F.S. Response of the herbaceous layer of forest ecosystems to excess nitrogen deposition. J. Ecol. 2006, 94, 1176–1191. [Google Scholar] [CrossRef]
- Plue, J.; van Gils, B.; de Schrijver, A.; Peppler-Lisbach, C.; Verheyen, K.; Hermy, M. Forest herb layer response to long-term light deficit along a forest developmental series. Acta Oecol. 2013, 53, 63–72. [Google Scholar] [CrossRef]
- Lyon, J.; Sharpe, W.E. Impacts of Hay-Scented Fern on Nutrition of Northern Red Oak Seedlings. J. Plant Nutr. 2003, 26, 487–502. [Google Scholar] [CrossRef]
- Oijen, D.; Feijen, M.; Hommel, P.; Ouden, J.; Waal, R. Effects of tree species composition on within-forest distribution of understorey species. Appl. Veg. Sci. 2005, 8, 155–166. [Google Scholar] [CrossRef]
- Langenbruch, C.; Helfrich, M.; Flessa, H. Effects of beech (Fagus sylvatica), ash (Fraxinus excelsior) and lime (Tilia spec.) on soil chemical properties in a mixed deciduous forest. Plant Soil 2012, 352, 389–403. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).