The Impacts of Drought Stress and Phytophthora cinnamomi Infection on Short-Term Water Relations in Two Year-Old Eucalyptus obliqua
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design
2.3. Inoculation with Phytophthora and Re-Isolation
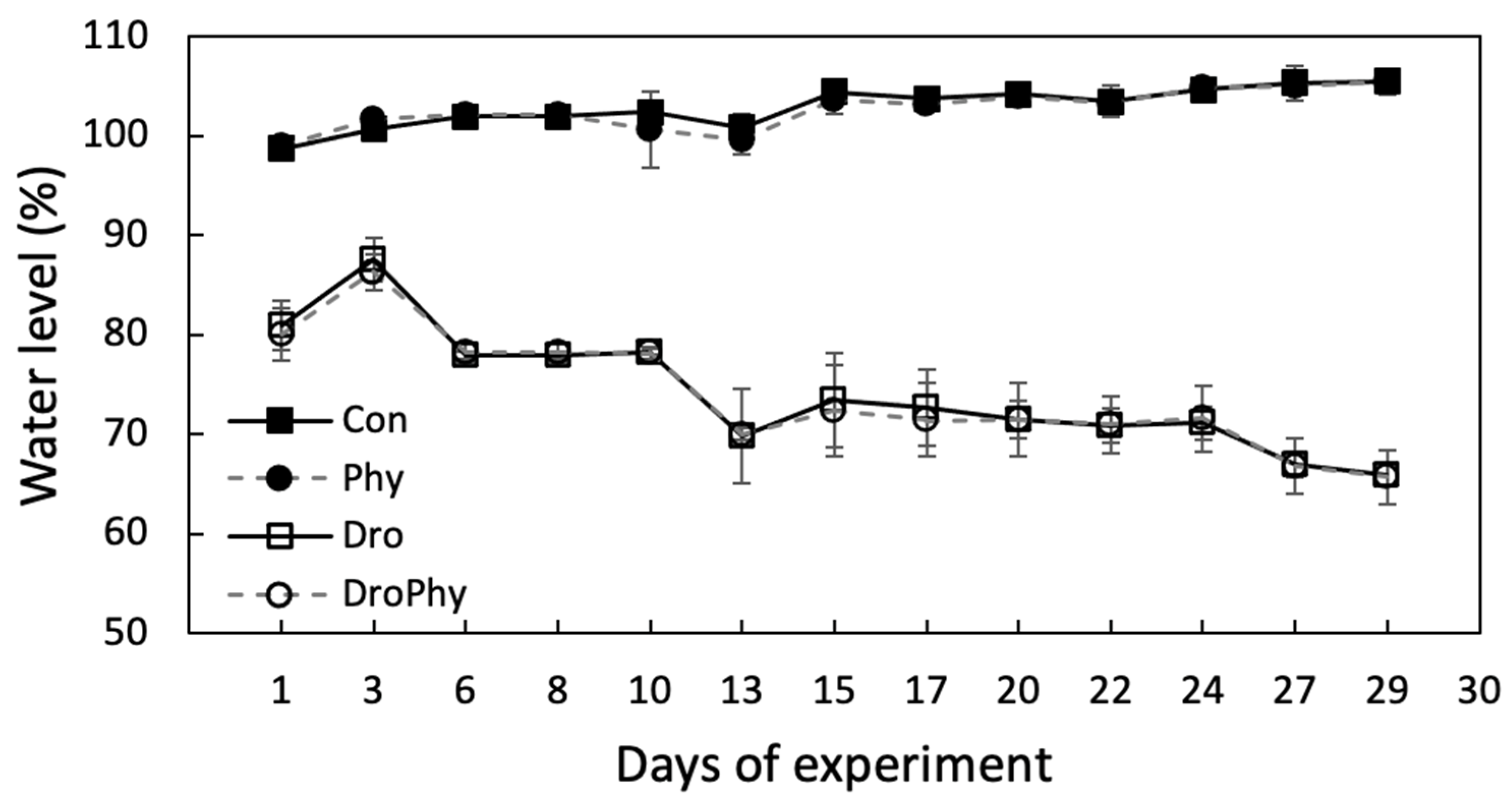
2.4. Watering
2.5. Data Collection
2.5.1. Water Relations
2.5.2. Gas Exchange
2.5.3. Pressure-Volume (PV) Curves
2.5.4. Final Harvest Biomass Assessment
2.6. Statistical Analysis
3. Results
3.1. Phenotypic Symptoms of Drought and Pathogen
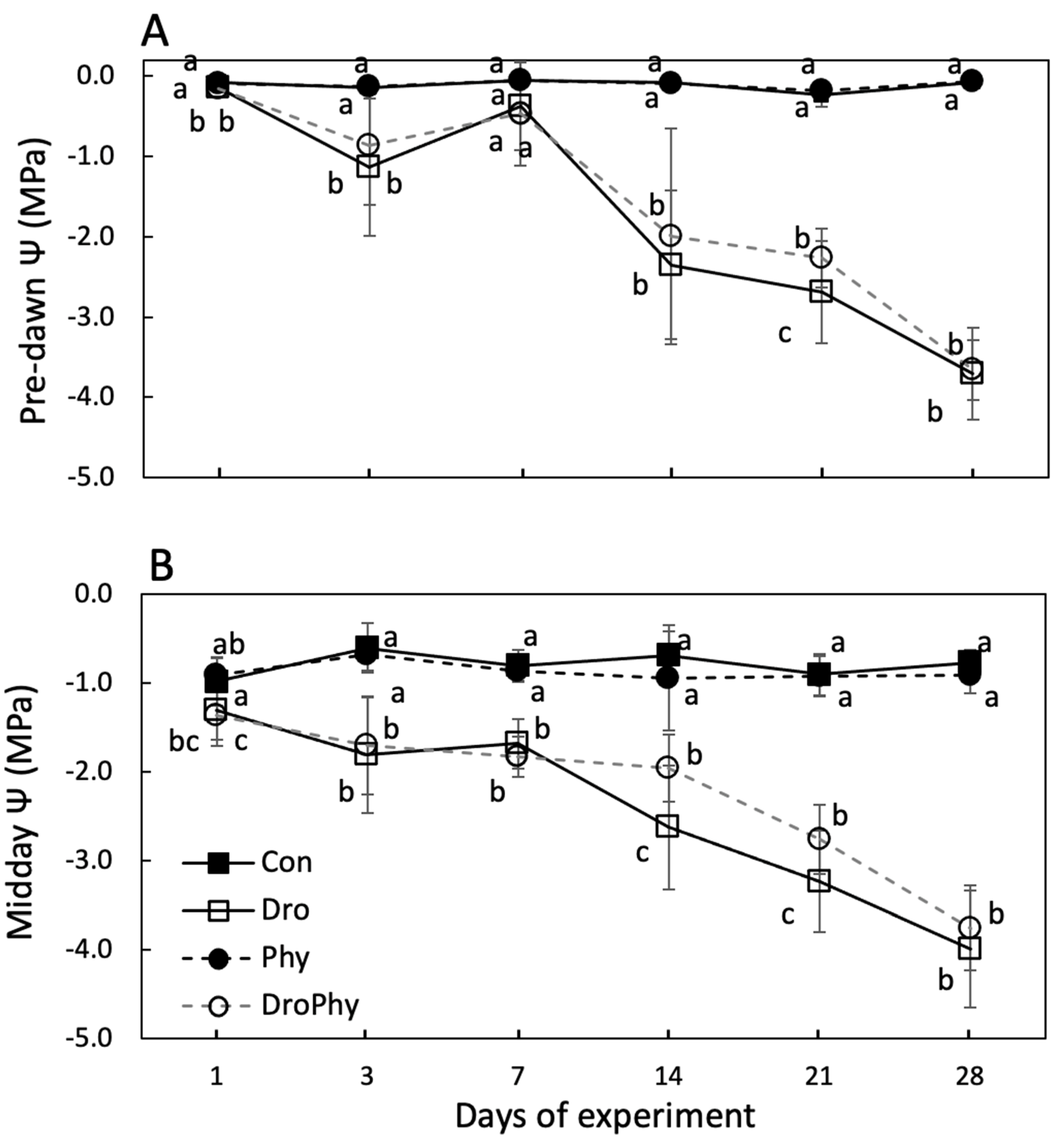
3.2. Water Relations
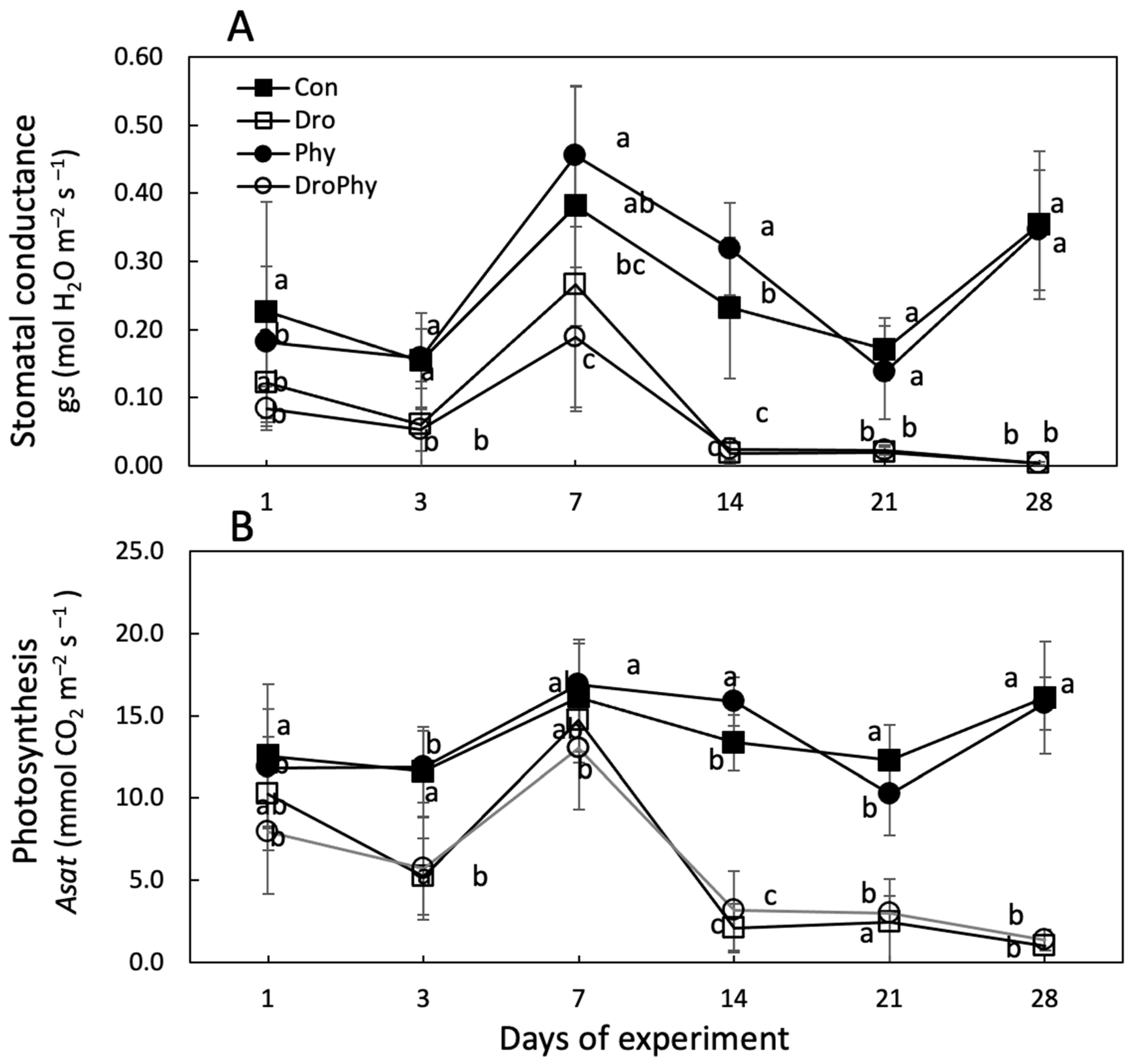
3.3. Gas Exchange
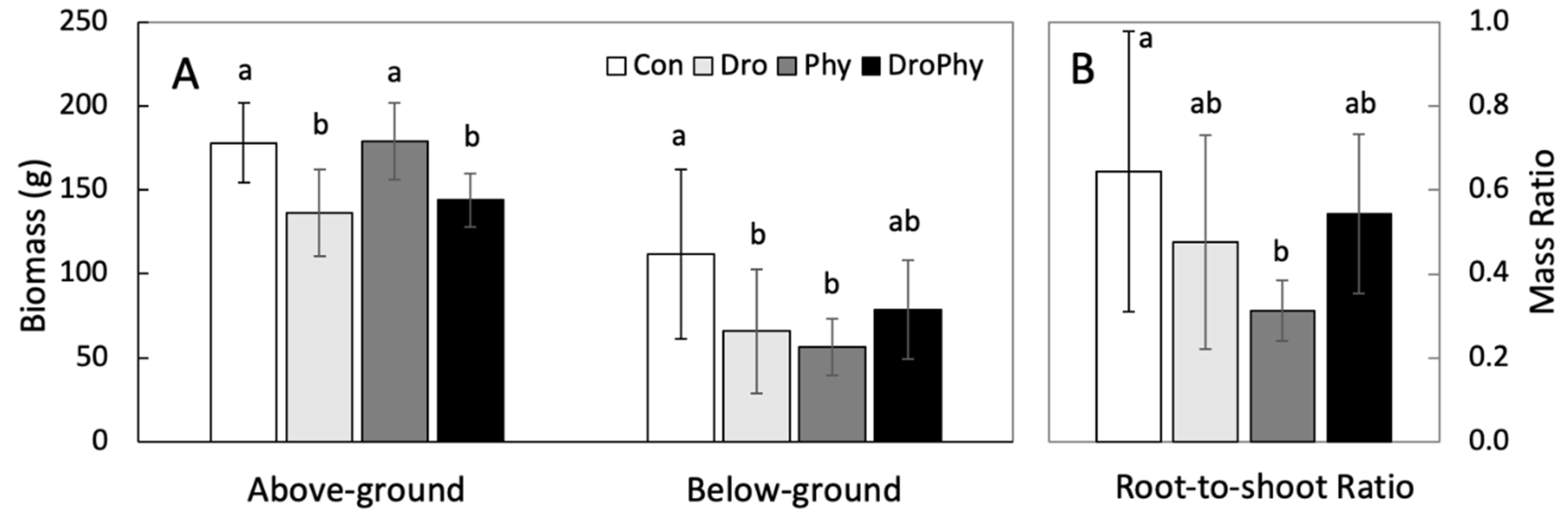
3.4. Biomass
3.5. Pressure-Volume (PV) Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adams, H.D.; Guardiola-Claramonte, M.; Barron-Gafford, G.A.; Villegas, J.C.; Breshears, D.D.; Zou, C.B.; Troch, P.A.; Huxman, T.E.; Mooney, H.A. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proc. Natl. Acad. Sci. USA 2009, 106, 7063–7066. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Anderegg, W.R.; Kane, J.M.; Anderegg, L.D. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Chang. 2013, 3, 30. [Google Scholar] [CrossRef]
- Matusick, G.; Ruthrof, K.X.; Brouwers, N.C.; Dell, B.; Hardy, G.S.J. Sudden forest canopy collapse corresponding with extreme drought and heat in a mediterranean-type eucalypt forest in southwestern Australia. Eur. J. For. Res. 2013, 132, 497–510. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Oliva, J.; Vicente-Serrano, S.M. To die or not to die: Early warnings of tree dieback in response to a severe drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef]
- Waller, M. Drought, disease, defoliation and death: Forest pathogens as agents of past vegetation change. J. Quat. Sci. 2013, 28, 336–342. [Google Scholar] [CrossRef]
- Desprez-Loustau, M.L.; Marçais, B.; Nageleisen, L.M.; Piou, D.; Vannini, A. Interactive effects of drought and pathogens in forest trees. Ann. For. Sci. 2006, 63, 597–612. [Google Scholar] [CrossRef]
- Garrett, K.A.; Dendy, S.P.; Frank, E.E.; Rouse, M.N.; Travers, S.E. Climate change effects on plant disease: Genomes to ecosystems. Annu. Rev. Phytopathol. 2006, 44, 489–509. [Google Scholar] [CrossRef]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; American Phytopathological Society (APS Press): St. Paul, MN, USA, 1996. [Google Scholar]
- Hansen, E.M. Phytophthora Species Emerging as Pathogens of Forest Trees. Curr. For. Rep. 2015, 1, 16–24. [Google Scholar] [CrossRef]
- Dunstan, W.A.; Howard, K.; Hardy, G.S.J.; Burgess, T.I. An overview of Australia’s Phytophthora species assemblage in natural ecosystems recovered from a survey in Victoria. IMA Fungus 2016, 7, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Sena, K.; Crocker, E.; Vincelli, P.; Barton, C. Phytophthora cinnamomi as a driver of forest change: Implications for conservation and management. For. Ecol. Manag. 2018, 409, 799–807. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Morel, O.; Perlerou, C.; Robin, C.; Diamandis, S.; Vannini, A. Occurrence and distribution of Phytophthora species in European chestnut stands, and their association with Ink Disease and crown decline. Eur. J. Plant Pathol. 2005, 111, 169. [Google Scholar] [CrossRef]
- Corcobado, T.; Cubera, E.; Moreno, G.; Solla, A. Quercus ilex forests are influenced by annual variations in water table, soil water deficit and fine root loss caused by Phytophthora cinnamomi. Agric. For. Meteorol. 2013, 169, 92–99. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Brockerhoff, E.G.; Garrett, L.J.; Parke, J.L.; Britton, K.O. Live plant imports: The major pathway for forest insect and pathogen invasions of the US. Front. Ecol. Environ. 2012, 10, 135–143. [Google Scholar] [CrossRef]
- Arentz, F.; Simpson, J.A. Distribution of Phytophthora cinnamomi in Papua New Guinea and notes on its origin. Trans. Br. Mycol. Soc. 1986, 87, 289–295. [Google Scholar] [CrossRef]
- Von Broembsen, S.; Kruger, F. Phytophthora cinnamomi associated with mortality of native vegetation in South Africa. Plant Dis. 1985, 69, 715–717. [Google Scholar] [CrossRef]
- Weste, G.M.; Taylor, P. The invasion of native forest by Phytophthora cinnamomi. I. Brisbane Ranges, Victoria. Aust. J. Bot. 1971, 19, 281. [Google Scholar] [CrossRef]
- Podger, F. Phytophthora cinnamomi a cause of lethal disease of indigenous plant communities. Phytopathology 1978, 62, 972–981. [Google Scholar] [CrossRef]
- Pratt, B.H.; Heather, W.A. The origin and distribution of Phytophthora cinnamomi Rands in Australian native plant communities and the significance of its association with particular plant species. Aust. J. Biol. Sci. 1973, 26, 559. [Google Scholar] [CrossRef][Green Version]
- Davison, E.M. Relative importance of site, weather and Phytophthora cinnamomi in the decline and death of Eucalyptus marginata–jarrah dieback investigations in the 1970s to 1990s. Australas. Plant Pathol. 2018, 47, 245–257. [Google Scholar] [CrossRef]
- Weste, G. The changing status of disease caused by Phytophthora cinnamomi in Victorian open forests, woodlands and heathlands. Australas. Plant Pathol. 1997, 26, 1–9. [Google Scholar] [CrossRef]
- Weste, G.; Marks, G.C. The biology of Phytophthora cinnamomi in Australasian Forests. Annu. Rev. Phytopathol. 1987, 25, 207–229. [Google Scholar] [CrossRef]
- Shearer, B.L.; Crane, C.E.; Barrett, S.; Cochrane, A. Phytophthora cinnamomi invasion, a major threatening process to conservation of flora diversity in the South-west Botanical Province of Western Australia. Aust. J. Bot. 2007, 55, 225–238. [Google Scholar] [CrossRef]
- Anderson, P.; Brundrett, M.; Grierson, P.; Robinson, R. Impact of severe forest dieback caused by Phytophthora cinnamomi on macrofungal diversity in the northern jarrah forest of Western Australia. Forest Ecol. Manag. 2010, 259, 1033–1040. [Google Scholar] [CrossRef]
- Hardham, A.R.; Blackman, L.M. Phytophthora cinnamomi. Mol. Plant Pathol. 2018, 19, 260–285. [Google Scholar] [CrossRef] [PubMed]
- Cahill, D.M.; Rookes, J.E.; Wilson, B.A.; Gibson, L.; McDougall, K.L. Phytophthora cinnamomi and Australias biodiversity: Impacts, predictions and progress towards control. Aust. J. Bot. 2008, 56, 279–310. [Google Scholar] [CrossRef]
- Kueh, K.H.; McKay, S.F.; Facelli, E.; Facelli, J.M.; Velzeboer, R.M.A.; Able, A.J.; Scott, E.S. Response of selected South Australian native plant species to Phytophthora cinnamomi. Plant Pathol. 2012, 61, 1165–1178. [Google Scholar] [CrossRef]
- Oßwald, W.; Fleischmann, F.; Rigling, D.; Coelho, A.C.; Cravador, A.; Diez, J.; Dalio, R.J.; Horta Jung, M.; Pfanz, H.; Robin, C.; et al. Strategies of attack and defence in woody plant–Phytophthora interactions. For. Pathol. 2014, 44, 169–190. [Google Scholar] [CrossRef]
- Hardham, A.R. Phytophthora cinnamomi. Mol. Plant Pathol. 2005, 6, 589–604. [Google Scholar] [CrossRef]
- Corcobado, T.; Cubera, E.; Juárez, E.; Moreno, G.; Solla, A. Drought events determine performance of Quercus ilex seedlings and increase their susceptibility to Phytophthora cinnamomi. Agric. For. Meteorol. 2014, 192–193, 1–8. [Google Scholar] [CrossRef]
- Sghaier-Hammami, B.; Valero-Galvàn, J.; Romero-Rodríguez, M.C.; Navarro-Cerrillo, R.M.; Abdelly, C.; Jorrín-Novo, J. Physiological and proteomics analyses of Holm oak (Quercus ilex subsp. ballota [Desf.] Samp.) responses to Phytophthora cinnamomi. Plant Physiol. Biochem. 2013, 71, 191–202. [Google Scholar] [CrossRef] [PubMed]
- León, I.; García, J.J.; Fernández, M.; Vázquez-Piqué, J.; Tapias, R. Differences in root growth of Quercus ilex and Quercus suber seedlings infected with Phytophthora cinnamomi. Silva Fenn. 2017, 51. [Google Scholar] [CrossRef]
- Maurel, M.; Robin, C.; Capron, G.; Desprez-Loustau, M.-L. Effects of root damage associated with Phytophthora cinnamomi on water relations, biomass accumulation, mineral nutrition and vulnerability to water deficit of five oak and chestnut species. For. Pathol. 2001, 31, 353–369. [Google Scholar] [CrossRef]
- Turco, E.; Close, T.J.; Fenton, R.D.; Ragazzi, A. Synthesis of dehydrin-like proteins in Quercus ilex L. and Quercus cerris L. seedlings subjected to water stress and infection with Phytophthora cinnamomi. Physiol. Mol. Plant Pathol. 2004, 65, 137–144. [Google Scholar] [CrossRef]
- Brasier, C.M.; Scott, J.K. European oak declines and global warming: A theoretical assessment with special reference to the activity of Phytophthora cinnamomi. EPPO Bull. 1994, 24, 221–232. [Google Scholar] [CrossRef]
- Burgess, T.I.; Scott, J.K.; McDougall, K.L.; Stukely, M.J.C.; Crane, C.; Dunstan, W.A.; Brigg, F.; Andjic, V.; White, D.; Rudman, T.; et al. Current and projected global distribution of Phytophthora cinnamomi, one of the world’s worst plant pathogens. Glob. Chang. Biol. 2017, 23, 1661–1674. [Google Scholar] [CrossRef]
- Homet, P.; Gonzalez, M.; Matias, L.; Godoy, O.; Perez-Ramos, I.M.; Garcia, L.V.; Gomez-Aparicio, L. Exploring interactive effects of climate change and exotic pathogens on Quercus suber performance: Damage caused by Phytophthora cinnamomi varies across contrasting scenarios of soil moisture. Agric. For. Meteorol. 2019, 276–277, 107605. [Google Scholar] [CrossRef]
- Marks, G.C.; Kassaby, F.Y. Detection of Phytophthora cinnamomi in Soils. Aust. For. 1972, 36, 198–203. [Google Scholar] [CrossRef]
- Masago, H.; Yoshikawa, M.; Fukada, M.; Nakanishi, N. Selective inhibition of Phythium spp. on a medium for direct isolation of Phytophthora spp. from soils and plants. Phytopathology 1977, 67, 425–428. [Google Scholar] [CrossRef]
- Zentmyer, G.A.; Guillemet, F.B. Evidence for strains of Phytophthora cinnamomi [Avocadoes, Camellia japonica, California]. Plant Dis. 1981, 65, 475–477. [Google Scholar] [CrossRef]
- Drenth, A.; Sendall, B. Practical Guide to Detection and Identification of Phytophthora; CRC for Tropical Plant Protection: Brisbane, Australia, 2001; pp. 1–41. [Google Scholar]
- Gallegly, M.E. Phytophthora: Identifying Species by Morphology and DNA Fingerprints; American Phytopathological Society (APS Press): St. Paul, MN, USA, 2008. [Google Scholar]
- Vettraino, A.M.; Natili, G.; Anselmi, N.; Vannini, A. Recovery and pathogenicity of Phytophthora species associated with a resurgence of ink disease in Castanea sativa in Italy. Plant Pathol. 2001, 50, 90–96. [Google Scholar] [CrossRef]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemmingsen, E.A. Sap pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.K.; Clifford, S.C.; Wanek, W.; Jones, H.G.; Popp, M. Physiological and morphological adaptations of the fruit tree Ziziphus rotundifolia in response to progressive drought stress. Tree Physiol. 2001, 21, 705–715. [Google Scholar] [CrossRef]
- Callister, A.N.; Arndt, S.K.; Adams, M.A. Comparison of four methods for measuring osmotic potential of tree leaves. Physiol. Plant. 2006, 127, 383–392. [Google Scholar] [CrossRef]
- Schulte, P.J.; Hinckley, T.M. A comparison of pressure—volume curve data analysis techniques. J. Exp. Bot. 1985, 36, 1590–1602. [Google Scholar] [CrossRef]
- Davidson, N.J.; Reid, J.B. Response of eucalypt species to drought. Aust. J. Ecol. 1989, 14, 139–156. [Google Scholar] [CrossRef]
- Rice, K.J.; Matzner, S.L.; Byer, W.; Brown, J.R. Patterns of tree dieback in Queensland, Australia: The importance of drought stress and the role of resistance to cavitation. Oecologia 2004, 139, 190–198. [Google Scholar] [CrossRef]
- Sinclair, R. Water potential and stomatal conductance of three Eucalyptus species in the Mount Lofty Ranges, South Australia: Responses to summer drought. Aust. J. Bot. 1980, 28, 499. [Google Scholar] [CrossRef]
- Merchant, A.; Callister, A.; Arndt, S.; Tausz, M.; Adams, M. Contrasting physiological responses of six Eucalyptus species to water deficit. Ann. Bot. 2007, 100, 1507–1515. [Google Scholar] [CrossRef]
- Farrell, C.; Szota, C.; Arndt, S.K. Does the turgor loss point characterize drought response in dryland plants? Plant Cell Environ. 2017, 40, 1500–1511. [Google Scholar] [CrossRef] [PubMed]
- Guarnaschelli, A.B.; Lemcoff, J.H.; Prystupa, P.; Basci, S.O. Responses to drought preconditioning in Eucalyptus globulus Labill. provenances. Trees 2003, 17, 501–509. [Google Scholar] [CrossRef]
- Sanders, G.; Arndt, S.K. Osmotic adjustment under drought conditions. In Plant Responses to Drought Stress; Aroca Alvarez, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 199–229. [Google Scholar]
- Roberts, S.W.; Strain, B.R.; Kenneth, R.K. Seasonal patterns of leaf water relations in four co-occurring forest tree species: Parameters from pressure-volume curves. Oecologia 1980, 46, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Terashima, I. Reversible decreases in the bulk elastic modulus of mature leaves of deciduous Quercus species subjected to two drought treatments. Plant Cell Environ. 2004, 27, 863–875. [Google Scholar] [CrossRef]
- Crombie, D.S.; Tippett, J.T. A comparison of water relations, visual symptoms, and changes in stem girth for evaluating impact of Phytophthora cinnamomi dieback on Eucalyptus marginata. Can. J. For. Res. 1990, 20, 233–240. [Google Scholar] [CrossRef]
- Sterne, R.E. Effect of Phytophthora root rot on water relations of avocado: Interpretation with a water transport model. Phytopathology 1978, 68, 595. [Google Scholar] [CrossRef]
- Maurel, M.; Robin, C.; Capdevielle, X.; Loustau, D.; Desprez-Loustau, M.L. Effects of variable root damage caused by Phytophthora cinnamomi on water relations of chestnut saplings. Ann. For. Sci. 2001, 58, 639–651. [Google Scholar] [CrossRef]
- Robin, C.; Capron, G.; Desprez-Loustau, M.L. Root infection by Phytophthora cinnamomi in seedlings of three oak species. Plant Pathol. 2001, 50, 708–716. [Google Scholar] [CrossRef]
- Ruiz Gomez, F.J.; Perez de Luque, R.; Sanchez-Cuesta, R.; Quero, J.L.; Navarro Cerillo, R.M. Differences in the response to acute drought and Phytophthora cinnamomi Rands infection in Quercus ilex L. seedlings. Forests 2018, 9, 634. [Google Scholar] [CrossRef]
- Tippett, J.; Mcgrath, J.; Hill, T. Site and seasonal effects on susceptibility of Eucalyptus marginata to Phytophthora cinnamomi. Aust. J. Bot. 1989, 37, 481–490. [Google Scholar] [CrossRef]
- David, M.C.; Gretna, M.W.; Bruce, R.G. Changes in cytokinin concentrations in xylem extrudate following Infection of Eucalyptus marginata Donn ex Sm with Phytophthora cinnamomi Rands. Plant Physiol. 1986, 81, 1103–1109. [Google Scholar]
- Crombie, D.; Tippett, J.; Gorddard, D. Water relations of root-pruned jarrah Eucalyptus marginata (Donn ex Smith) saplings. Aust. J. Bot. 1987, 35, 653–663. [Google Scholar] [CrossRef]
- Cahill, D.; Grant, B.; Weste, G. How does Phytophthora cinnamomi kill a susceptible eucalypt? Australas. Plant Pathol. 1985, 14, 59–60. [Google Scholar] [CrossRef]
- Tippett, J.; Hill, T.; Shearer, B. Resistance of Eucalyptus spp. to Invasion by Phytophthora cinnamomi. Aust. J. Bot. 1985, 33, 409–418. [Google Scholar] [CrossRef]
- Naidoo, S.; Külheim, C.; Zwart, L.; Mangwanda, R.; Oates, C.N.; Visser, E.A.; Wilken, F.E.; Mamni, T.B.; Myburg, A.A. Uncovering the defence responses of Eucalyptus to pests and pathogens in the genomics age. Tree Physiol. 2014, 34, 931–943. [Google Scholar] [CrossRef]
- Weste, G.; Ruppin, P. Factors affecting the population density of Phytophthora cinnamomi in native forests of the Brisbane Ranges, Victoria. Aust. J. Bot. 1975, 23, 77–85. [Google Scholar] [CrossRef]
- Lucas, A. Water Stress and Disease Development in Eucalyptus marginata (jarrah) Infected with Phytophthora cinnamomi. Ph.D. Thesis, Murdoch University, Perth, Australia, 2003. [Google Scholar]
- Brasier, C.M. Phytophthora cinnamomi and oak decline in southern Europe. Environmental constraints including climate change. Ann. Sci. For. 1996, 53, 347–358. [Google Scholar] [CrossRef]
- Shearer, B.L. Jarrah Dieback: The Dynamics and Management of Phytophthora Cinnamomi in the Jarrah (Eucalyptus Marginata) Forest of South-Western Australia; Deptartment of Conservation and Land Management: Como, WA, USA, 1989.
- Robin, C.; Desprez-Loustau, M.-L. Testing variability in pathogenicity of Phytophthora cinnamomi. Eur. J. Plant Pathol. 1998, 104, 465–475. [Google Scholar] [CrossRef]
- Stukely, M.; Crane, C. Genetically based resistance of Eucalyptus marginata to Phytophthora cinnamomi. Phytopathology 1994, 84, 650–656. [Google Scholar] [CrossRef]
| Treatment | π100 | ΨTLP | RWCTLP | ε |
|---|---|---|---|---|
| Con | −1.48 ± 0.05 | −1.63 ± 0.05 a | 0.91 ± 0.01 | 16.60 ± 2.25 ab |
| Phy | −1.52 ± 0.13 | −1.67 ± 0.09 a | 0.91 ± 0.01 | 20.47 ± 7.65 a |
| Dro | −1.75 ± 0.26 | −2.18 ± 0.60 b | 0.87 ± 0.07 | 12.14 ±4.77 b |
| DroPhy | −1.65 ± 0.13 | −1.93 ± 0.35 b | 0.87 ± 0.03 | 12.38 ± 4.96 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umami, M.; Parker, L.M.; Arndt, S.K. The Impacts of Drought Stress and Phytophthora cinnamomi Infection on Short-Term Water Relations in Two Year-Old Eucalyptus obliqua. Forests 2021, 12, 109. https://doi.org/10.3390/f12020109
Umami M, Parker LM, Arndt SK. The Impacts of Drought Stress and Phytophthora cinnamomi Infection on Short-Term Water Relations in Two Year-Old Eucalyptus obliqua. Forests. 2021; 12(2):109. https://doi.org/10.3390/f12020109
Chicago/Turabian StyleUmami, Mashlahatul, Linda M. Parker, and Stefan K. Arndt. 2021. "The Impacts of Drought Stress and Phytophthora cinnamomi Infection on Short-Term Water Relations in Two Year-Old Eucalyptus obliqua" Forests 12, no. 2: 109. https://doi.org/10.3390/f12020109
APA StyleUmami, M., Parker, L. M., & Arndt, S. K. (2021). The Impacts of Drought Stress and Phytophthora cinnamomi Infection on Short-Term Water Relations in Two Year-Old Eucalyptus obliqua. Forests, 12(2), 109. https://doi.org/10.3390/f12020109






