Litterfall and Accumulated Nutrients in Pinus taeda Plantation and Native Forest in Southern Brazil
Abstract
1. Introduction
2. Materials and Methods
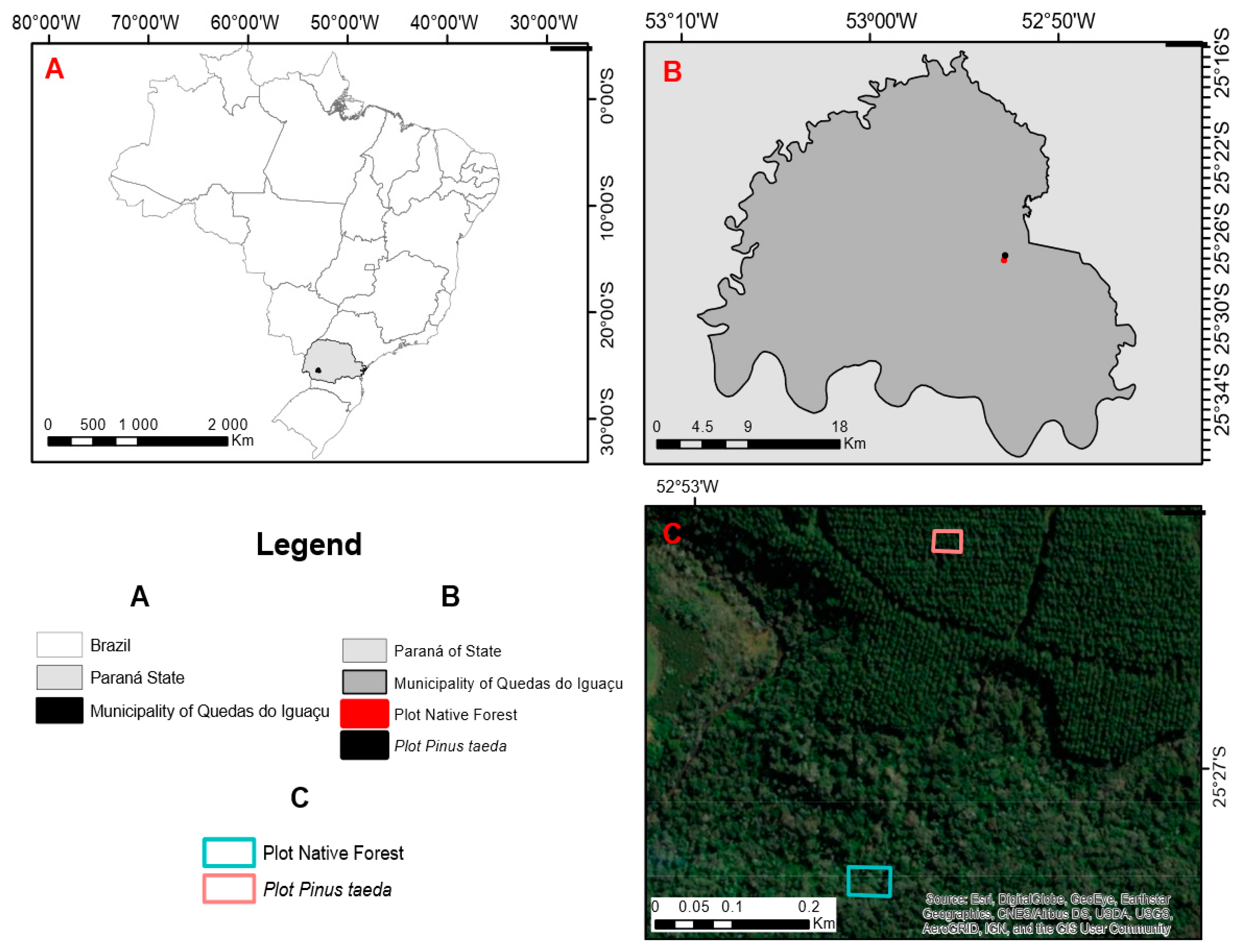
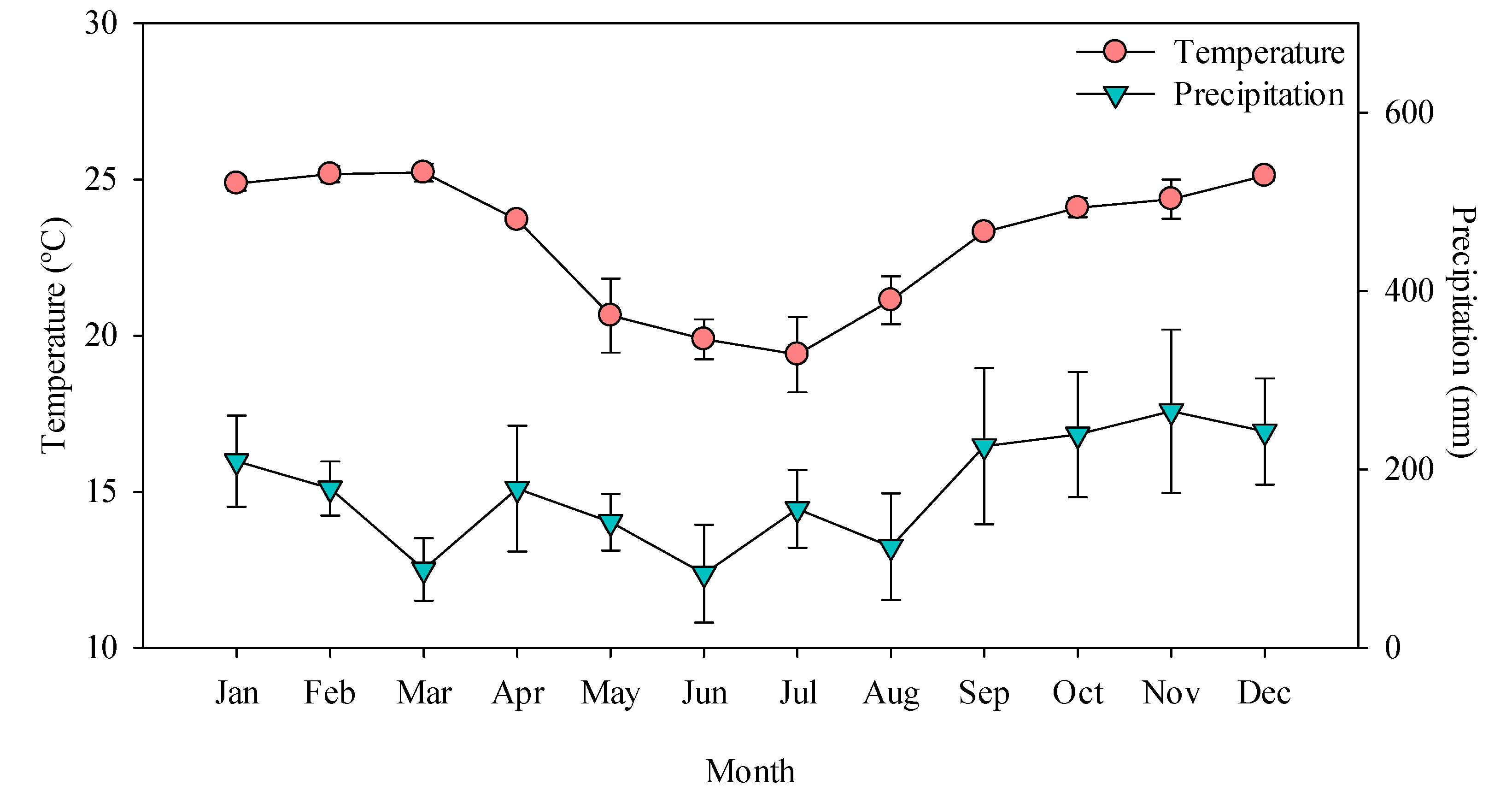
2.1. Site Description
2.2. Litterfall Collection
2.3. Accumulated and Nutrient Use Efficiency
2.4. Statistical Analysis
3. Results
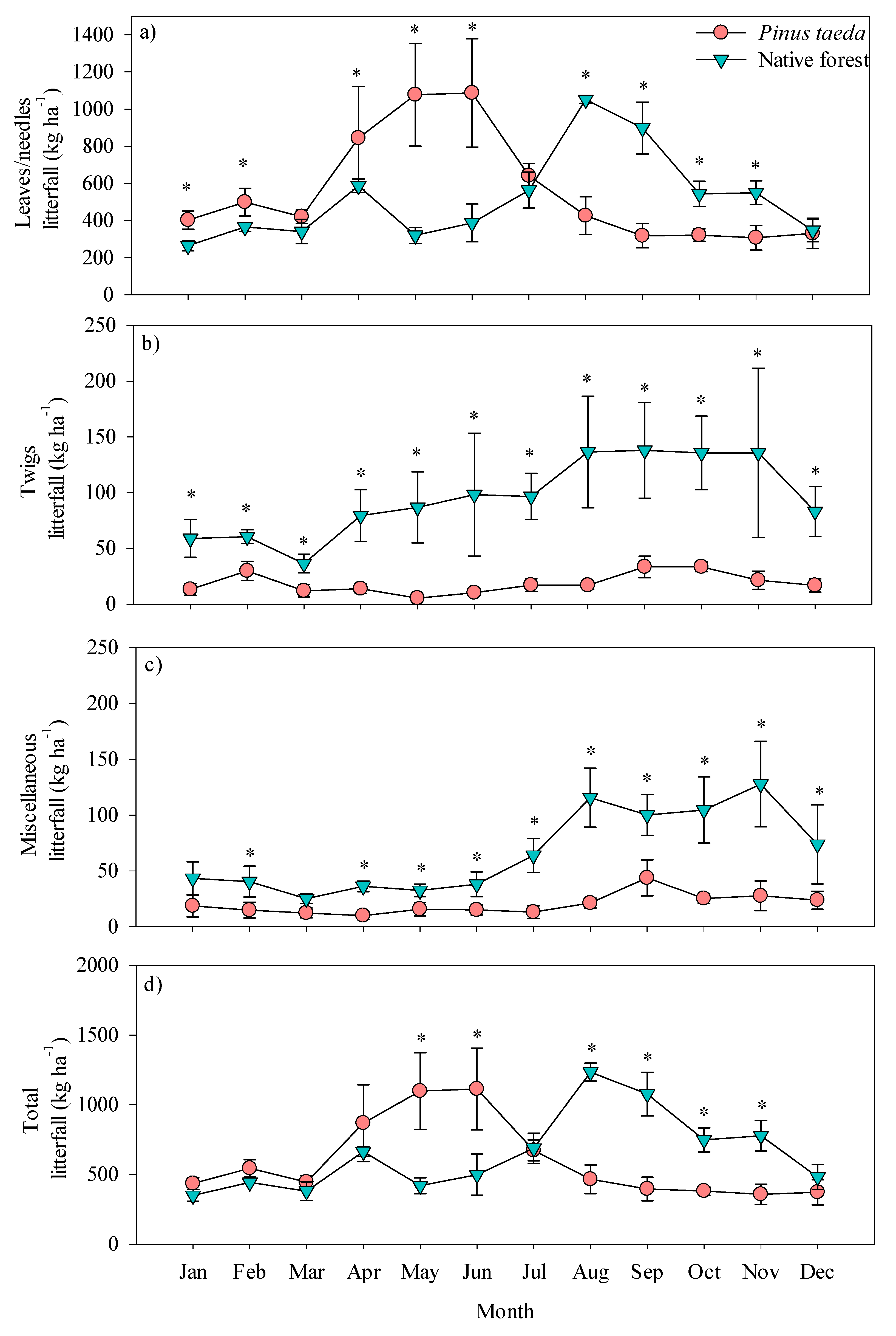
3.1. Monthly and Annual Dynamics of Litterfall Production
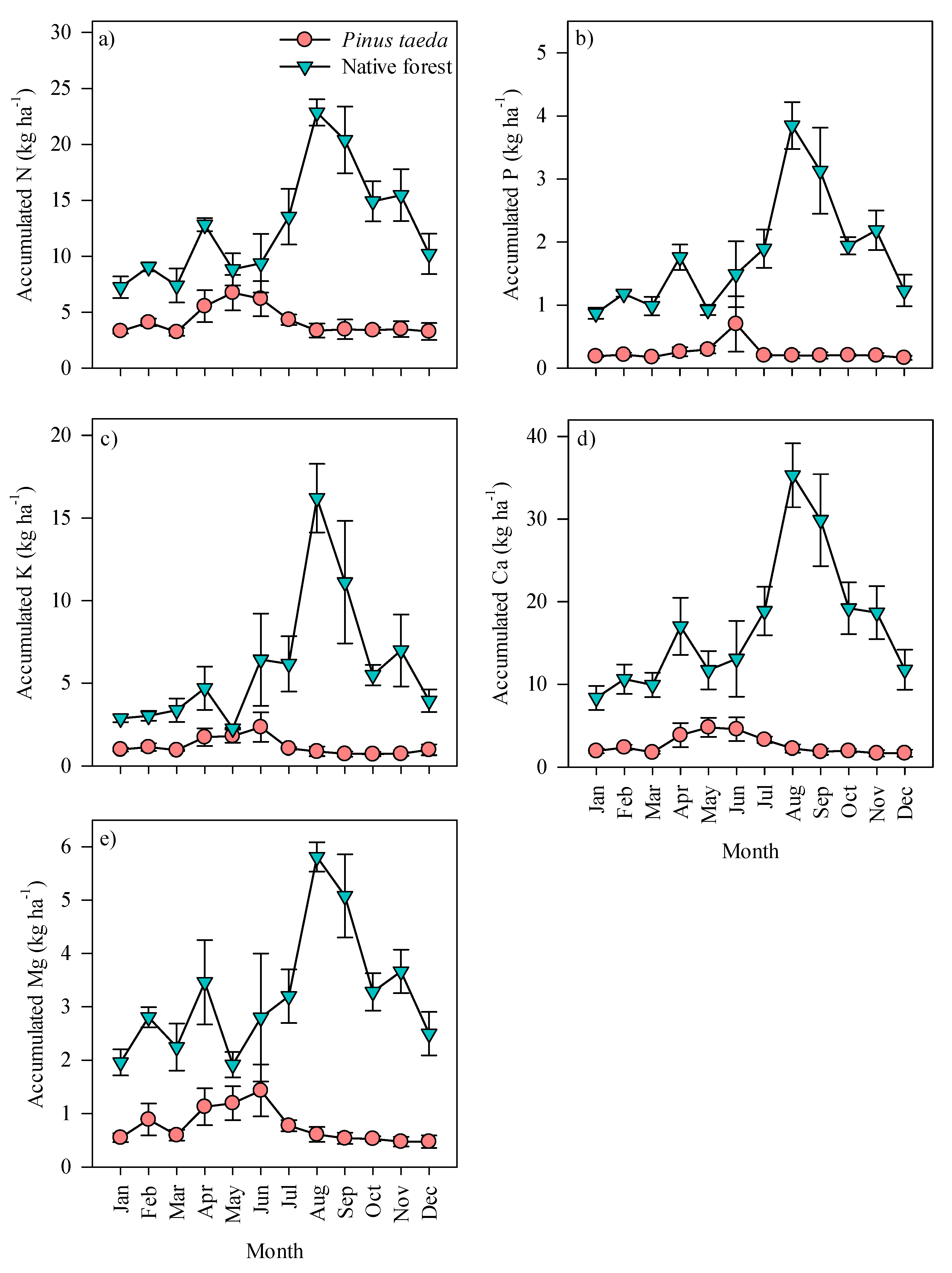
3.2. Monthly and Annual Dynamics of Accumulated Nutrient
3.3. Nutrient Use Efficiency of Litterfall
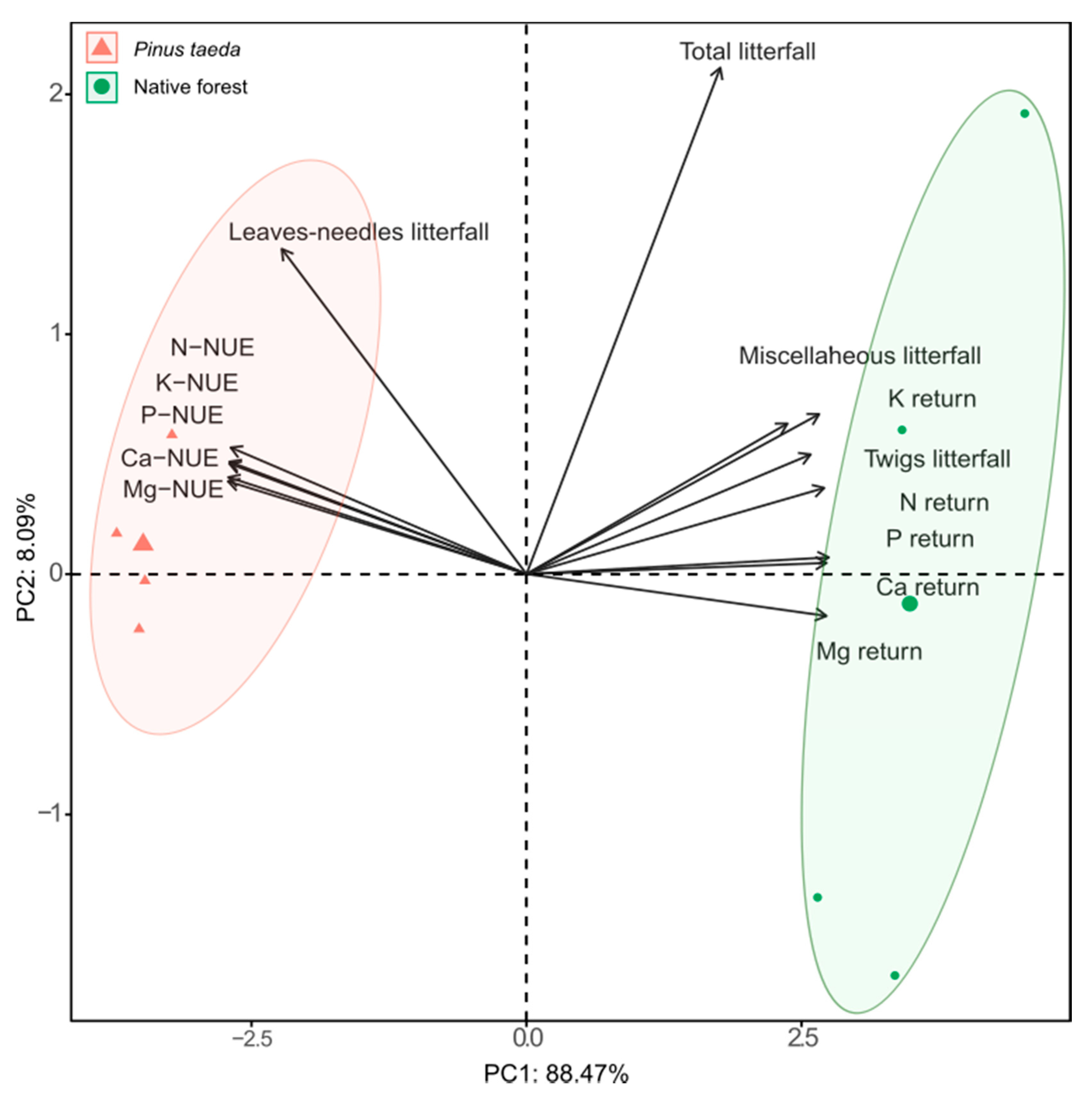
3.4. Principal Component Analysis
4. Discussion
4.1. Monthly and Annual Dynamics of Litterfall Production
4.2. Monthly and Annual Dynamics of Accumulated Nutrients in Litterfall
4.3. Nutrient Use Efficiency of Litterfall
4.4. Principal Component Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Global Forest Resources Assessment. In Forest Resources Assessment (2020) Working Paper; FAO: Rome, Italy, 2020. [Google Scholar]
- Zhou, L.; Shalom, A.-D.D.; Wu, P.; Li, S.; Jia, Y.; Ma, X. Litterfall production and nutrient return in different-aged Chinese fir (Cunninghamia lanceolata) plantations in South China. J. For. Res. 2015, 26, 79–89. [Google Scholar] [CrossRef]
- Xu, D.; Yang, Z.; Zhang, N. Effects of Site Management on tree Growth and Soil Properties of a Second-Rotation Plantation of Eucalyptus Urophylla in Guangdong Province, China. 2004. Available online: http://www.cifor.org/publications/pdf_files/Books/BKallio0801.pdf (accessed on 19 October 2021).
- Eufrade Junior, H.J.; de Melo, R.X.; Sartori, M.M.P.; Guerra, S.P.S.; Ballarin, A.W. Sustainable use of eucalypt biomass grown on short rotation coppice for bioenergy. Biomass Bioenergy 2016, 90, 15–21. [Google Scholar] [CrossRef]
- IBÁ Relatório Anual da Indústria Brasileira de Árvores-ano Base 2019. 2020 [Brazilian Tree Industry Annual Report-Base Year 2019]. Assoc. Brasleira Árvores, 160. Available online: https://iba.org/datafiles/publicacoes/relatorios/relatorio-iba-2020.pdf (accessed on 19 October 2021).
- Braga, R.C.; Paludeto, J.G.Z.; Souza, B.M.; Aguiar, A.V.; Pollnow, M.F.M.; Carvalho, A.G.M.; Tambarussi, E.V. Genetic parameters and genotype × environment interaction in Pinus taeda clonal tests. For. Ecol. Manag. 2020, 474, 118342. [Google Scholar] [CrossRef]
- Bi, J.B.; Blanco, J.A.B.A.; Seely, B.S.; Kimmins, J.P.K.P.; Ding, Y.D.; Welham, C.W. Yield decline in Chinese-fir plantations: A simulation investigation with implications for model complexity. Can. J. For. Res. 2007, 37, 1615–1630. [Google Scholar] [CrossRef]
- O’Hehir, J.F.; Nambiar, E.K.S. Productivity of three successive rotations of P. radiata plantations in South Australia over a century. For. Ecol. Manag. 2010, 259, 1857–1869. [Google Scholar] [CrossRef]
- Egnell, G. Is the productivity decline in Norway spruce following whole-tree harvesting in the final felling in boreal Sweden permanent or temporary? For. Ecol. Manag. 2011, 261, 148–153. [Google Scholar] [CrossRef]
- Nambiar, E.K.S.; Harwood, C.E. Productivity of acacia and eucalypt plantations in southeast Asia. 1. Bio-physical determinants of production: Opportunities and challenges. Int. For. Rev. 2014, 16, 225–248. [Google Scholar] [CrossRef]
- Subedi, P.; Jokela, E.J.; Vogel, J.G.; Martin, T.A. Sustained productivity of intensively managed loblolly pine plantations: Persistence of fertilization and weed control effects across rotations. For. Ecol. Manag. 2019, 446, 38–53. [Google Scholar] [CrossRef]
- An, J.Y.; Han, S.H.; Youn, W.B.; Lee, S.I.; Rahman, A.; Dao, H.T.T.; Seo, J.M.; Aung, A.; Choi, H.-S.; Hyun, H.J.; et al. Comparison of litterfall production in three forest types in Jeju Island, South Korea. J. For. Res. 2019, 31, 945–952. [Google Scholar] [CrossRef]
- Michopoulos, P.; Kaoukis, K.; Karetsos, G.; Grigoratos, T.; Samara, C. Nutrients in litterfall, forest floor and mineral soils in two adjacent forest ecosystems in Greece. J. For. Res. 2019, 31, 291–301. [Google Scholar] [CrossRef]
- Dick, G.; Schumacher, M.V. Litterfall in the Semideciduous Seasonal Forest in Southern Brazil. Floresta Ambiente 2020, 27, 1–7. [Google Scholar] [CrossRef]
- Han, L.; Tao, H.; Cheng-Zhen, W.U.; Hui, C.; Can, C.; Li, J.; Lin, Y.-M.; Fan, H.-L. Monthly variation in litterfall and the amount of nutrients in an Aleurites montana plantation. For. Stud. China 2012, 14, 30–35. [Google Scholar] [CrossRef]
- González-Rodríguez, H.; Ramírez-Lozano, R.G.; Cantú-Silva, I.; Gómez-Meza, M.V.; Estrada-Castillón, E.; Arévalo, J.R. Deposition of litter and nutrients in leaves and twigs in different plant communities of northeastern Mexico. J. For. Res. 2017, 29, 1307–1314. [Google Scholar] [CrossRef]
- An, J.Y.; Park, B.B.; Chun, J.H.; Osawa, A. Litterfall production and fine root dynamics in cool-temperate forests. PLoS ONE 2017, 12, e0180126. [Google Scholar] [CrossRef]
- Bray, J.R.; Gorham, E. Litter Production in Forests of the World. Adv. Ecol. Res. 1964, 2, 101–157. [Google Scholar] [CrossRef]
- Zhou, G.; Guan, L.; Wei, X.; Zhang, D.; Zhang, Q.; Yan, J.; Wen, D.; Liu, J.; Liu, S.; Huang, Z.; et al. Litterfall production along successional and altitudinal gradients of subtropical monsoon evergreen broadleaved forests in Guangdong, China. Plant Ecol. 2006, 188, 77–89. [Google Scholar] [CrossRef]
- Yusheng, Y.; Yinxiu, C.; Zongming, H.; Jianfen, G.; Chunhua, L. Comparatively study on litter properties between plantations of Fokienia hodginsii and Cunninghamia lanceolata. Sci. Silvae Sin. 2004, 40, 2–9. [Google Scholar]
- Forrester, D.I. Linking forest growth with stand structure: Tree size inequality, tree growth or resource partitioning and the asymmetry of competition. For. Ecol. Manag. 2019, 447, 139–157. [Google Scholar] [CrossRef]
- Binkley, D.; Stape, J.L.; Ryan, M.G. Thinking about efficiency of resource use in forests. For. Ecol. Manag. 2004, 193, 5–16. [Google Scholar] [CrossRef]
- Fundação de Pesquisas Florestais do Paraná-FUPEF. Conservação do Bioma Floresta com Araucária: Relatório Final: Diagnóstico dos Remanescentes Florestais, 1st ed.; Fundação de Pesquisas Florestais do Paraná-FUPEF: Curitiba, Brazil, 2001. [Google Scholar]
- Matzenauer, R.; Radin, B.; Almeida, I. Atlas Climático do Rio Grande do Sul; Secretaria da Agricultura Pecuária e Agronegócio: Porto Alegre, Brazil, 2011. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy; USDA -Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Santos, H.G. dos Sistema Brasileiro de Classificação de Solos. Embrapa Inf. Tecnol. 2013, 3, 353. [Google Scholar]
- Viani, R.A.G.; Costa, J.C.; Rozza, A.D.F.; Bufo, L.V.B.; Ferreira, M.A.P.; Oliveira, A.C.P.D. Caracterização florística e estrutural de remanescentes florestais de Quedas do Iguaçu, Sudoeste do Paraná. Biota Neotrop. 2011, 11, 115–128. [Google Scholar] [CrossRef]
- Lopes, M.; Domingos, M.; Struffaldide, Y. Ciclagem de nutrientes minerais. In Manual Metodológico para Estudos Botânicos na Mata Atlântica; Sylvestre, L.S., Orgs Rosa, M.M.T., Eds.; Infraestrutura e Meio Ambiente: Seropédica, Brazil, 2002. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; de Oliveira, S.A. Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações; POTAFOS: Piracicaba, Brazil, 1997. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing. 2019. Available online: https://www.r-project.org/ (accessed on 10 September 2021).
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. Available online: https://cran.r-project.org/web/packages/factoextra/index.html (accessed on 10 September 2021).
- De Souza Kulmann, M.S.; de Paula, B.V.; Sete, P.B.; Arruda, W.S.; Sans, G.A.; Tarouco, C.P.; Tabaldi, L.A.; Nicoloso, F.T.; Brunetto, G. Morphological and kinetic parameters of the absorption of nitrogen forms for selection of Eucalyptus clones. J. For. Res. 2020, 1, 3. [Google Scholar] [CrossRef]
- De Moraes Goncalves, J.L.; Alvares, C.A.; Higa, A.R.; Silva, L.D.; Alfenas, A.C.; Stahl, J.; Ferraz, S.F.d.B.; Lima, W.d.P.; Brancalion, P.H.S.; Hubner, A.; et al. Integrating genetic and silvicultural strategies to minimize abiotic and biotic constraints in Brazilian eucalypt plantations. For. Ecol. Manag. 2013, 301, 6–27. [Google Scholar] [CrossRef]
- Rocha, J.H.T.; du Toit, B.; de Moraes Goncalves, J.L. Ca and Mg nutrition and its application in Eucalyptus and Pinus plantations. For. Ecol. Manag. 2019, 442, 63–78. [Google Scholar] [CrossRef]
- Poggiani, F.; Zamberlan, E.; Monteiro, E.J.; Gava, I.C. Quantificação da deposição de folhedo em talhões experimentais de Pinus taeda, Eucalyptus viminalis e Mimosa scabrella plantados em uma área degradada pela mineração do xisto betuminoso. Sci. For. 1987, 37, 21–29. [Google Scholar]
- Schumacher, M.V.; Viera, M.; Witschoreck, R. Produção de serapilheira e transferência de nutrientes em área de segunda rotação com floresta de Pinus taeda L. no município de Cambará do sul, RS. Ciênc. Florest. 2008, 18, 471–480. [Google Scholar] [CrossRef][Green Version]
- Quichimbo, P.; Jiménez, L.; Veintimilla, D.; Potthast, K.; Tischer, A.; Günter, S.; Mosandl, R.; Hamer, U. Nutrient dynamics in an Andean forest region: A case study of exotic and native species plantations in southern Ecuador. New For. 2019, 51, 313–334. [Google Scholar] [CrossRef]
- Kulmann, M.S.S.; Stefanello, L.O.S.; Schwalbert, R.A.; Berghetti, Á.L.P.; Araujo, M.M.; Piccin, R.; Gatiboni, L.C.; Tiecher, T.; Ferreira, P.A.A.; Brunetto, G. Effects of phosphorus fertilizer application on phosphorus fractions in different organs of Cordia trichotoma. J. For. Res. 2021, 32, 725–732. [Google Scholar] [CrossRef]
- Cuevas, E.; Lugo, A.E. Dynamics of organic matter and nutrient return from litterfall in stands of ten tropical tree plantation species. For. Ecol. Manag. 1998, 112, 263–279. [Google Scholar] [CrossRef]
- Kalinitchenko, V.P.; Glinushkin, A.P.; Swidsinski, A.V.; Minkina, T.M.; Andreev, A.G.; Mandzhieva, S.S.; Sushkova, S.N.; Makarenkov, D.A.; Ilyina, L.P.; Chernenko, V.V.; et al. Thermodynamic mathematical model of the Kastanozem complex and new principles of sustainable semiarid protective silviculture management. Environ. Res. 2021, 194, 110605. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Shane, M.; Cramer, M.; Pearse, S.; Veneklaas, E. Root structure and functioning for efficient acquisition of phosphorus: Matching morphological and physiological traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef] [PubMed]
- Elanchezhian, R.; Krishnapriya, V.; Pandey, R.; Rao, A.S.; Abrol, Y.P. Physiological and Molecular Approaches for Improving Phosphorus Uptake Efficiency of Crops. Available online: https://www.jstor.org/stable/24905488 (accessed on 3 August 2021).
- Resquin, F.; Navarro-Cerrillo, R.M.; Carrasco-Letelier, L.; Casnati, C.R.; Bentancor, L. Evaluation of the nutrient content in biomass of Eucalyptus species from short rotation plantations in Uruguay. Biomass Bioenergy 2020, 134, 105502. [Google Scholar] [CrossRef]
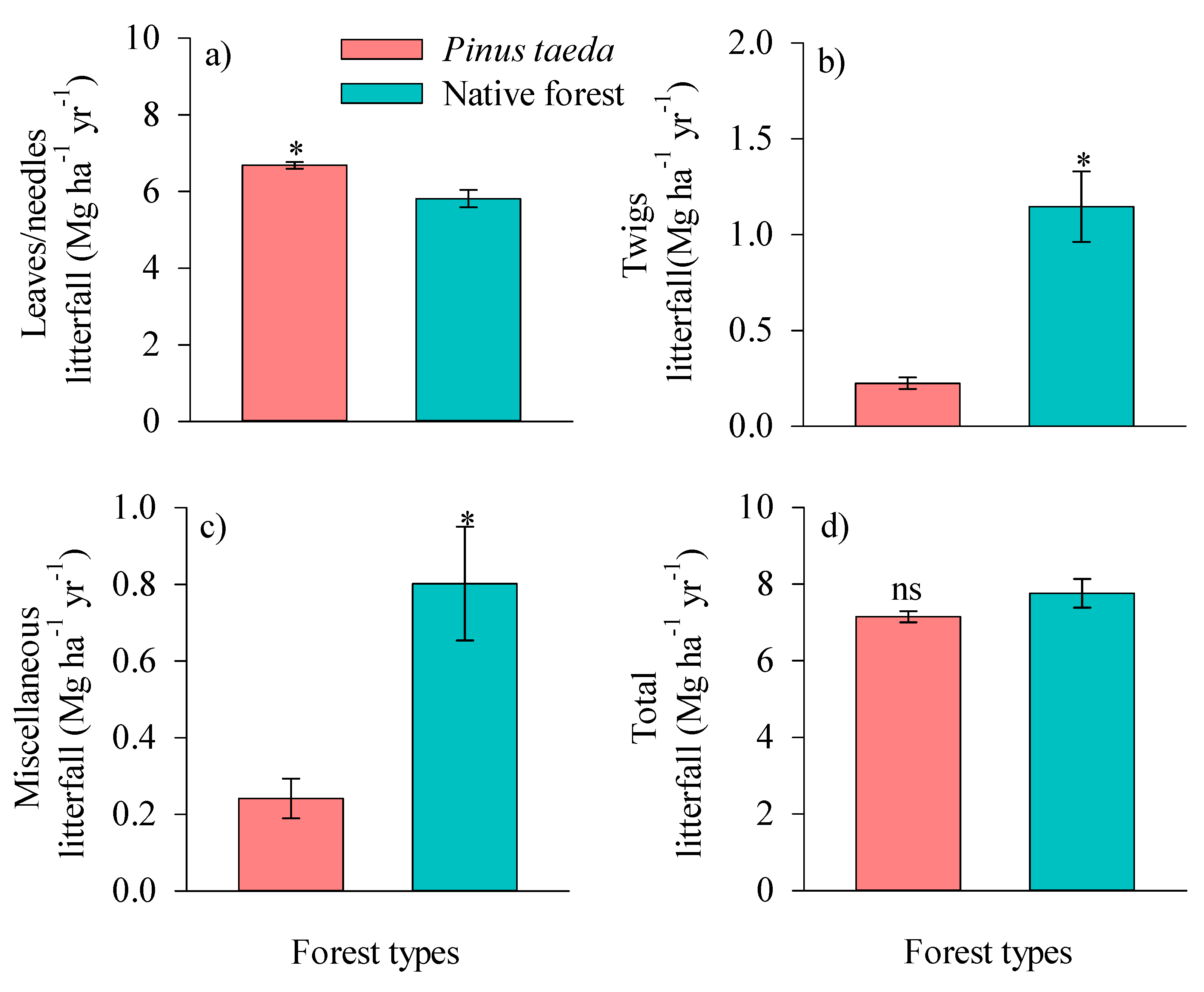
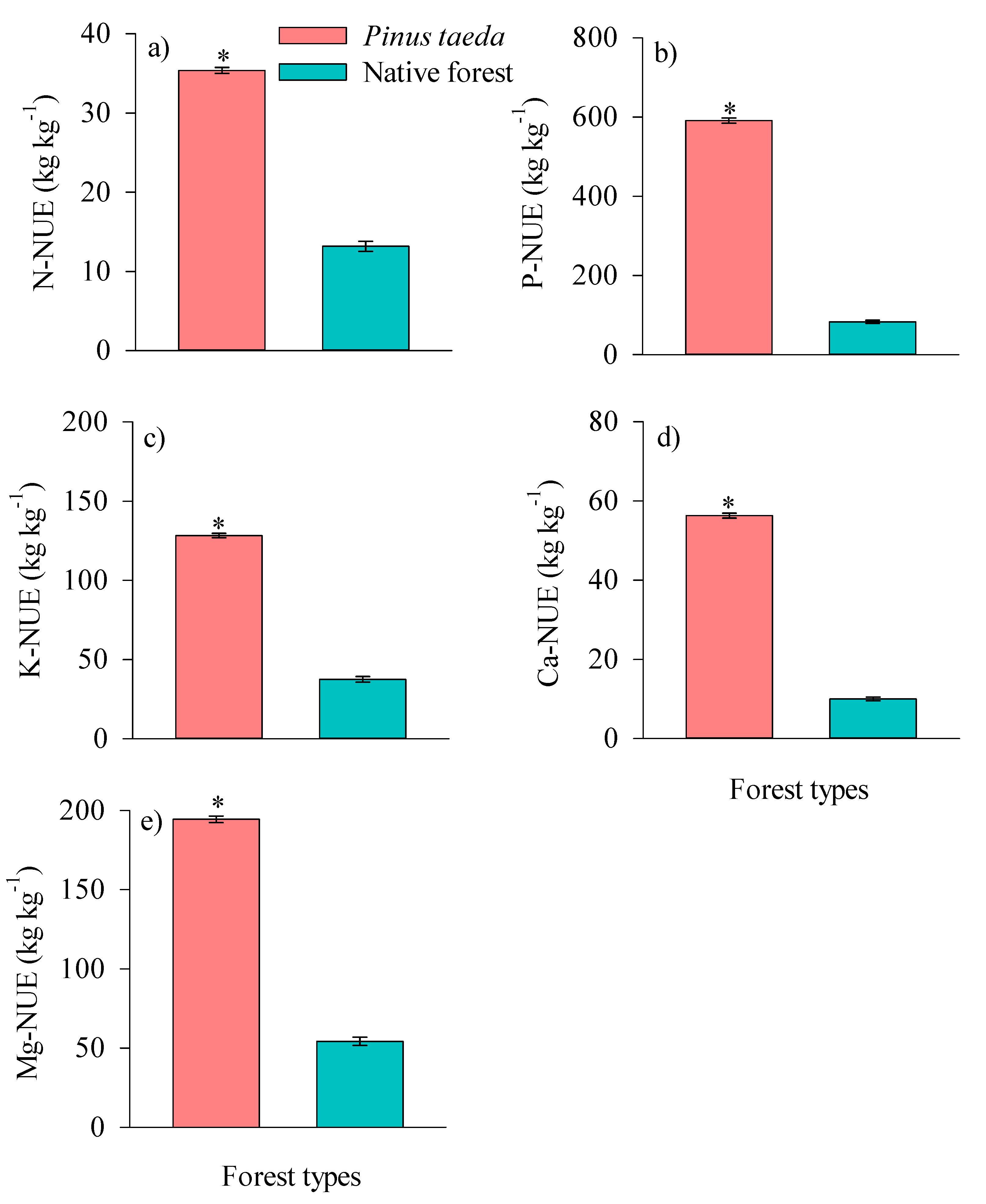
| Variables | Effect | ||
|---|---|---|---|
| Forest | Month | Interaction | |
| Leaves/needles litterfall (kg ha−1) | 0.1341 ns | <0.001 * | <0.001 * |
| Twigs litterfall (kg ha−1) | <0.001 * | 0.3838 ns | 0.7551 ns |
| Miscellaneous litterfall (kg ha−1) | <0.001 * | <0.001 * | 0.0735 ns |
| Total litterfall (kg ha−1) | 0.3273 ns | <0.0001 * | <0.0001 * |
| Accumulated N in litterfall (kg ha−1) | <0.001 * | <0.001 * | <0.001 * |
| Accumulated P in litterfall (kg ha−1) | <0.001 * | <0.001 * | <0.0001 * |
| Accumulated K in litterfall (kg ha−1) | <0.001 * | <0.001 * | <0.001 * |
| Accumulated Ca in litterfall (kg ha−1) | <0.001 * | <0.001 * | <0.001 * |
| Accumulated Mg in litterfall (kg ha−1) | <0.001 * | <0.001 * | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulmann, M.S.d.S.; Dick, G.; Valdir Schumacher, M. Litterfall and Accumulated Nutrients in Pinus taeda Plantation and Native Forest in Southern Brazil. Forests 2021, 12, 1791. https://doi.org/10.3390/f12121791
Kulmann MSdS, Dick G, Valdir Schumacher M. Litterfall and Accumulated Nutrients in Pinus taeda Plantation and Native Forest in Southern Brazil. Forests. 2021; 12(12):1791. https://doi.org/10.3390/f12121791
Chicago/Turabian StyleKulmann, Matheus Severo de Souza, Grasiele Dick, and Mauro Valdir Schumacher. 2021. "Litterfall and Accumulated Nutrients in Pinus taeda Plantation and Native Forest in Southern Brazil" Forests 12, no. 12: 1791. https://doi.org/10.3390/f12121791
APA StyleKulmann, M. S. d. S., Dick, G., & Valdir Schumacher, M. (2021). Litterfall and Accumulated Nutrients in Pinus taeda Plantation and Native Forest in Southern Brazil. Forests, 12(12), 1791. https://doi.org/10.3390/f12121791







