Budget of Plant Litter and Litter Carbon in the Subalpine Forest Streams
Abstract
:1. Introduction
2. Material and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Monitoring the Input and Output of Litter and Litter Carbon
2.4. Analytical Methods and Calculations
3. Results
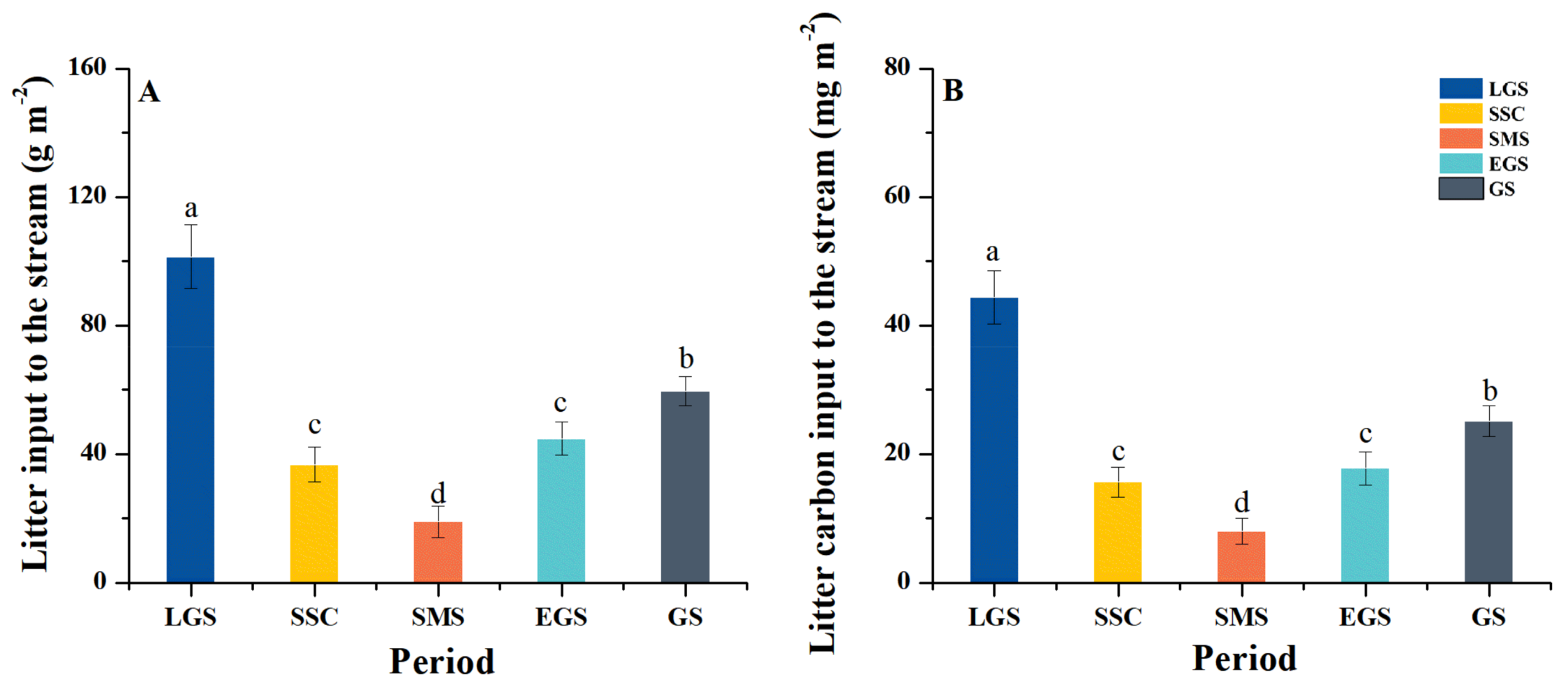
3.1. Litter and Litter Carbon Input to the Stream
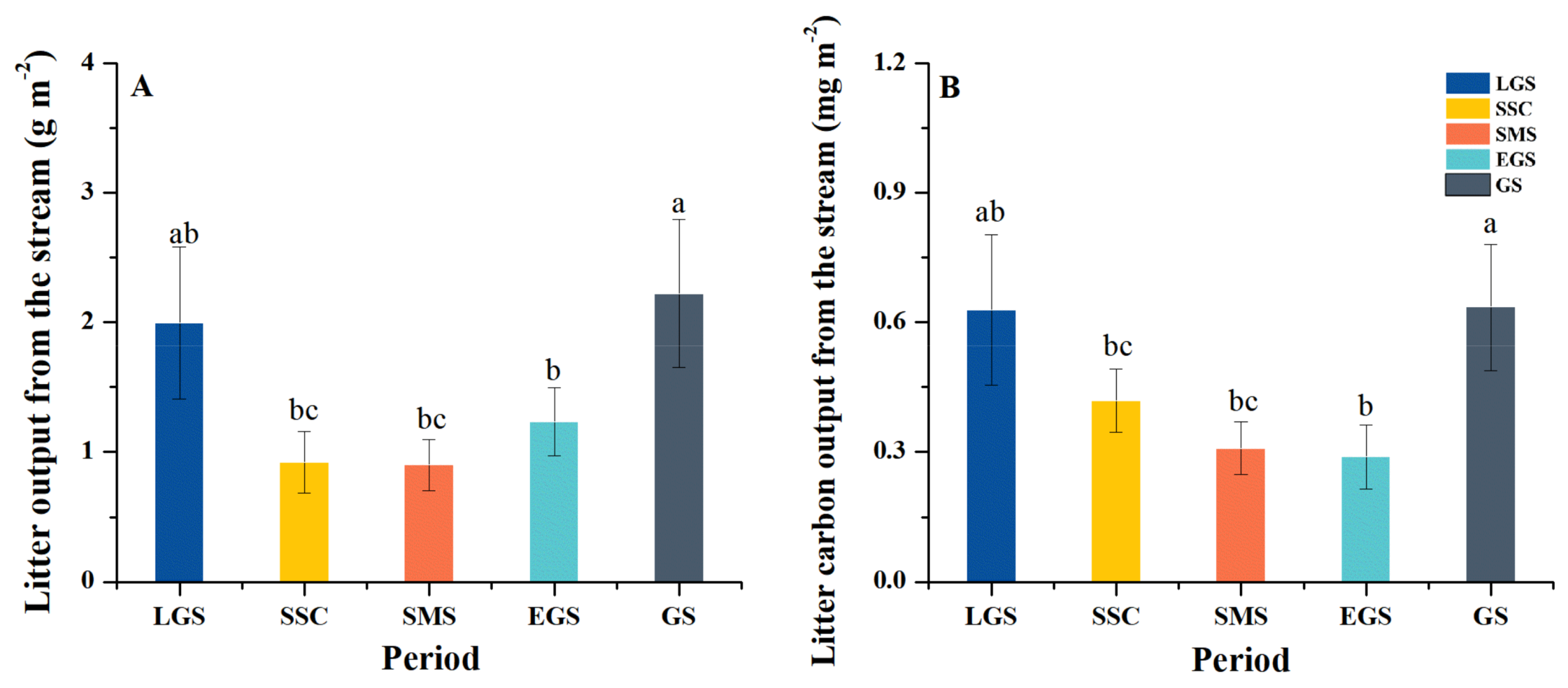
3.2. Litter and Litter Carbon Output from the Stream
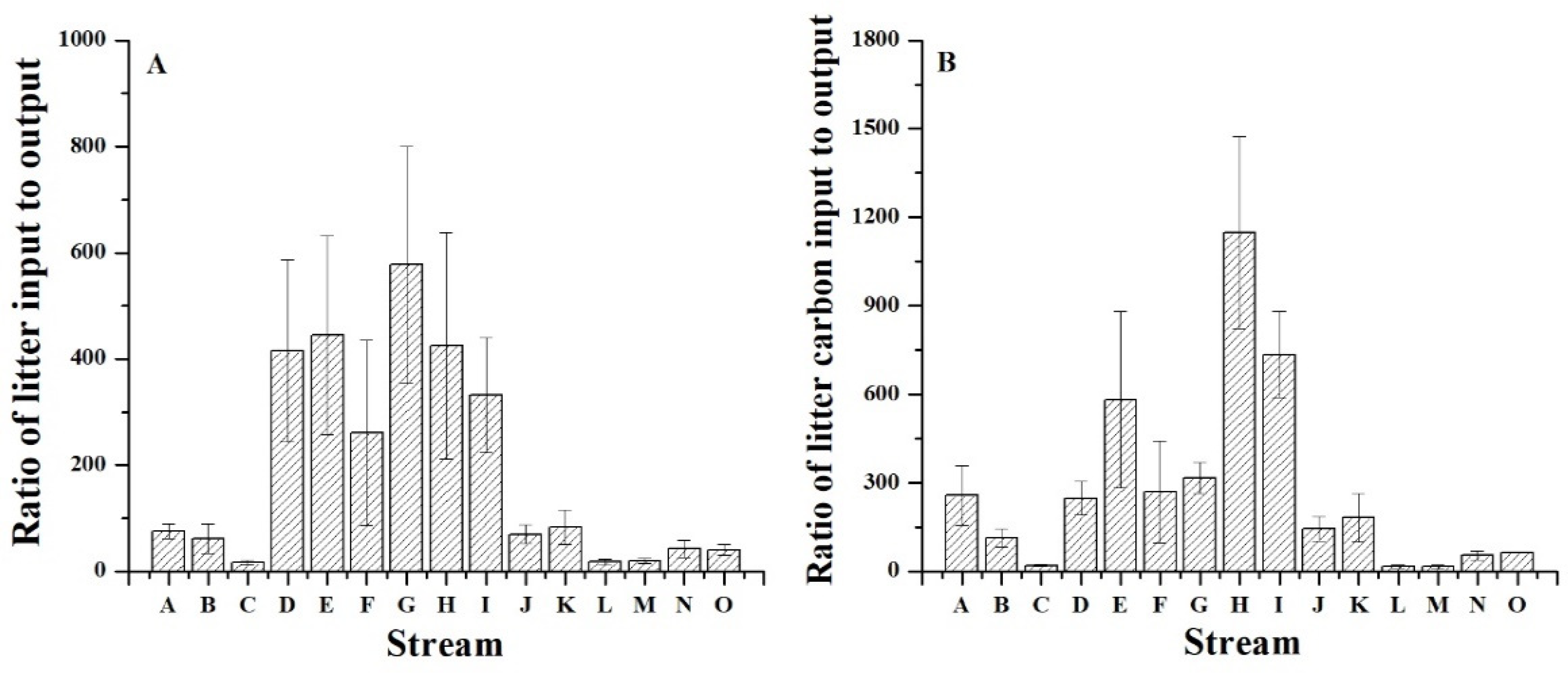
3.3. The Ratios of the Input to Output of Litter and Litter Carbon
3.4. Relationships of Litter and Litter Carbon Input/Output with Relative Variables
4. Discussion
4.1. Dynamics of Litter and Carbon Input in the Subalpine Forest Streams
4.2. Seasonal Dynamics of Litter and Carbon Output from Subalpine Forest Streams
4.3. Litter Carbon Budget in the Subalpine Forest Stream
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sutherland, A.B.; Meyer, J.L.; Gardiner, E.P. Effects of land cover on sediment regime and fish assemblage structure in four southern Appalachian streams. Freshw. Biol. 2002, 47, 1791–1805. [Google Scholar] [CrossRef]
- Gessner, M.O.; Swan, C.M.; Dang, C.K.; McKie, B.G.; Bardgett, R.D.; Wall, D.H.; Hattenschwiler, S. Diversity meets decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.B.; Eggert, S.L.; Meyer, J.L.; Webster, J.R. Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science 1997, 277, 102–104. [Google Scholar] [CrossRef] [Green Version]
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Ballatore, T.J.; Ballatore, T.J.; Dillon, P.; Finlay, K.; Fortino, K.; et al. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanograohy 2009, 54, 2298–2314. [Google Scholar] [CrossRef] [Green Version]
- Battin, T.J.; Kaplan, L.A.; Findlay, S.; Hopkinson, C.S.; Marti, E.; Packman, A.I.; Newbold, J.D.; Sabater, F. Biophysical controls on organic carbon fluxes in fluvial networks. Nat. Geosci. 2008, 1, 95–100. [Google Scholar] [CrossRef]
- Loreau, M.; Mouquet, N.; Holt, R.D. Meta-ecosystems: A theoretical framework for a spatial ecosystem ecology. Ecol. Lett. 2003, 6, 673–679. [Google Scholar] [CrossRef] [Green Version]
- Yue, K.; Yang, W.Q.; Peng, C.H.; Peng, Y.; Zhang, Z.; Huang, C.P.; Tan, Y.; Wu, F.Z. Foliar litter decomposition in an alpine forest meta-ecosystem on the eastern Tibetan Plateau. Sci. Total Environ. 2016, 566, 279–287. [Google Scholar] [CrossRef]
- Coûteaux, M.M.; Bottner, P.; Berg, B. Litter decomposition, climate, and liter quality. Trends Ecol. Evol. 1995, 10, 63–66. [Google Scholar] [CrossRef]
- Abril, M.; Muñoz, I.; Menéndez, M. Heterogeneity in leaf litter decomposition in a temporary Mediterranean stream during flow fragmentation. Sci. Total Environ. 2016, 553, 330–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, V.; Canhoto, C. Future increase in temperature may stimulate litter decomposition in temperate mountain streams: Evidence from a stream manipulation experiment. Freshw. Biol. 2015, 60, 881–892. [Google Scholar] [CrossRef]
- Berg, B.; Meentemeyer, V. Litterfall in some European coniferous forests as dependent on climate: A synthesis. Can. J. For. Res. 2001, 31, 292–301. [Google Scholar] [CrossRef]
- Louzada, J.N.C.; Schoereder, J.H.; Marco, J.P. Litter decomposition in semidecidous forest and Eucalyptus spp. crop in Brazil: A comparison. For. Ecol. Manag. 1997, 94, 31–36. [Google Scholar] [CrossRef]
- Bachega, L.R.; Bouillet, J.P.; Piccolo, M.C.; Saint-André, L.; Bouvet, J.M.; Nouvellon, Y.; Goncalves, J.L.M.; Robin, A.; Laclau, J.P. Decomposition of Eucalyptus grandis and Acacia mangium leaves and fine roots in tropical conditions did not meet the home field advantage hypothesis. For. Ecol. Manag. 2016, 359, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Cornwell, W.K.; Cornelissen, J.H.C.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Pérez-Harguindeguy, N.; et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yang, Y.; Shen, Y.; Chen, Z.; Dong, Y.; Wu, F.; Zhang, L. Litter production and its dynamic pattern in a dark coniferous forest in the alpine gorge region. Chin. J. Appl. Environ. Biol. 2017, 23, 745–752. [Google Scholar]
- Guo, J.; Yu, L.H.; Fang, X.; Xiang, W.H.; Deng, X.W.; Lu, X. Litter production and turnover in four types of subtropical forests in China. Acta Ecol. Sin. 2015, 35, 4668–4677. [Google Scholar]
- Rueda-Delgado, G.; Wantzen, K.M.; Tolosa, M.B. Leaf-litter decomposition in an Amazonian floodplain stream: Effects of seasonal hydrological changes. J. N. Am. Benthol. Soc. 2006, 25, 233–249. [Google Scholar] [CrossRef]
- Taylor, B.R.; Chauvet, E.E. Relative influence of shredders and fungi on leaf litter decomposition along a river altitudinal gradient. Hydrobiologia 2014, 721, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Tiegs, S.D.; Akinwole, P.O.; Gessner, M.O. Litter decomposition across multiple spatial scales in stream networks. Oecologia 2009, 161, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Benfield, E.F. Comparison of Litterfall Input to Streams. Freshw. Sci. 1997, 16, 104–108. [Google Scholar] [CrossRef]
- Webster, J.R. Stream organic matter budgets-introduction. J. N. Am. Benthol. Soc. 1997, 16, 2–13. [Google Scholar] [CrossRef]
- Triska, F.J.; Sedell, J.R.; Gregory, S.V. Coniferous forest streams. In Analysis of Coniferous Forest Ecosystems in the Western United States; Edmonds, R.L., Ed.; Hutchinson Ross: Stroudsburg, PA, USA, 1982; pp. 292–332. [Google Scholar]
- Soininen, J.; Bartels, P.; Heino, J.; Miska, L.; Hillebrand, H. Toward more integrated ecosystem research in aquatic and terrestrial environments. BioScience 2015, 65, 174–182. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Q.; Wang, Y.J. Evolution of study on the forest stream water quality. Res. Soil Water Conserv. 2004, 10, 242–246. [Google Scholar]
- Bünemann, E.; Bossio, D.; Smithson, P.; Frossard, E.; Oberson, A. Microbial community composition and substrate use in a highly weathered soil as affected by crop rotation and P fertilization. Soil Biol. Biochem. 2004, 36, 889–901. [Google Scholar] [CrossRef]
- Perakis, S.S.; Hedin, L.O. Nitrogen loss from unpolluted South American forests mainly via dissolved organic compounds. Nature 2002, 415, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.H.; Cheng, X.L.; Geng, Q.H.; Shi, Z.; Luo, Y.Q.; Xu, X. Leaf litter traits predominantly control litter decomposition in streams worldwide. Glob. Ecol. Biogeogr. 2019, 28, 1469–1486. [Google Scholar] [CrossRef]
- Baldy, V.; Gobert, V.; Guerold, F.; Chauvet, E.; Lambrigot, D.; Charcosset, J.Y. Leaf litter breakdown budgets in streams of various trophic status: Effects of dissolved inorganic nutrients on microorganisms and invertebrates. Freshw. Biol. 2007, 52, 1322–1335. [Google Scholar] [CrossRef] [Green Version]
- Leroy, C.J.; Whitham, T.G.; Keim, P.M.; Jane, C. Plant genes link forests and streams. Ecology 2006, 87, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, W.Q.; Tan, B.; Liang, Z.Y.; Wu, F.Z. Storage and content of organic carbon in alpine forest stream in the upper reaches of the Yangtze River. Fresenius Environ. Bull. 2017, 26, 3402–3408. [Google Scholar]
- Yuan, Z.Q.; Li, B.H.; Bai, X.J.; Lin, F.; Shi, S.; Ye, J.; Wang, X.G.; Hao, Z.Q. Composition and seasonal dynamics of litterfalls in a broad-leaved Korean pine (Pinus koraiensis) mixed forest in Changbai Mountains, Northeast China. Chin. J. Appl. Ecol. 2010, 21, 2171–2178. [Google Scholar]
- Skalny, A.V. Bioelementology as an interdisciplinary integrative approach in life sciences: Terminology, classification, perspectives. J. Trace Elem. Med. Biol. 2011, 25, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Finlay, J.C. Controls of stream water dissolved inorganic carbon dynamics in a forested watershed. Biogeochemistry 2003, 62, 231–252. [Google Scholar] [CrossRef]
- Easthouse, K.B.; Mulder, J.; Christophersen, N.; Seip, H.M. Dissolved organic carbon fractions in soil and stream water during variable hydrological conditions at Birkenes, Southern Norway. Water Resourc. Res. 1992, 28, 1585–1596. [Google Scholar] [CrossRef]
- Gomes, T.F.; Broek, M.V.; Govers, G.; Silva, R.W.C.; Moraes, J.M.; Camargo, P.B.; Mazzi, E.A.; Martinelli, L.A. Runoff, soil loss, and sources of particulate organic carbon delivered to streams by sugarcane and riparian areas: An isotopic approach. Catena 2019, 181, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yu, G.R.; Zheng, Z.M.; Wang, Q.F. Carbon Emission and Spatial Pattern of Soil Respiration of Terrestrial Ecosystems in China: Based on Geostatistic Estimation of Flux Measurement. Prog. Geogr. 2012, 1, 97–108. [Google Scholar]
- Duan, L.; Zheng, W.; Li, M. Geologic hazards on the western Sichuan plateau and their controls. Sediment. Geol. Tethyan Geol. 2005, 25, 95–99. [Google Scholar]
- Zhang, H.; Yang, W.Q.; Wang, M.; Liao, S. Carbon, nitrogen and phosphorus storage of woody debris in headwater streams in an alpine forest in upper reaches of the Mingjiang River. Acta Ecol. Sin. 2016, 36, 1967–1974. [Google Scholar]
- Alvarez, S.; Guerrero, M. Enzymatic activities associated with decomposition of particulate organic matter in two shallow ponds. Soil Biol. Biochem. 2000, 32, 1941–1951. [Google Scholar] [CrossRef]
- Yang, W.Q.; Wu, F.Z. Ecosystem Processes and Management of Subalpine Coniferous Forest in the Upper Reaches of the Yangtze River; Science Press: Beijing, China, 2021. [Google Scholar]
- Sun, H.L. Ecological and Environmental Problems in the Upper Reaches of the Yangtze River; China Environmental Press: Beijing, China, 2008. [Google Scholar]
- Wu, F.Z.; Peng, C.H.; Zhu, J.; Zhang, J.; Tan, B.; Yang, W.Q. Impact of freezing and thawing dynamics on foliar litter carbon release in alpine/subalpine forests along an altitudinal gradient in the eastern Tibetan Plateau. Biogeosciences 2014, 11, 6471–6481. [Google Scholar]
- Wu, F.Z.; Yang, W.Q.; Zhang, J.; Deng, R. Litter decomposition in two subalpine forests during the freeze–thaw season. Acta Oecol. 2010, 36, 135–140. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, W.Q.; Zhang, H.; Zhang, B.; Yue, K.; Peng, Y.; Wu, F.Z. Standing biomass and carbon-storage of non-woody debris and their distribution in the alpine forest streams of Western Sichuan in the upper reaches of Minjiang River. Ecol. Environ. Sci. 2014, 23, 1509–1514. [Google Scholar]
- Lu, R.K. Soil Agrochemical Analysis Methods; China Agricultural Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67. [Google Scholar] [CrossRef]
- Hart, J.J.; Welch, R.M.; Norvell, W.; Kochian, L.V. Characterization of cadmium uptake, translocation and storage in near-isogenic lines of durum wheat that differ in grain cadmium concentration. New Phytol. 2006, 172, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Muto, E.A. The Characteristics and Fate of Leaf Litter Inputs to Boreal Shield Streams in Relation to Riparian Stand Structure; The University of Guelph: Guelph, ON, Canada, 2008. [Google Scholar]
- Tonin, A.M.; Goncalves, J.F.; Bambi, P.; Couceiro, S.R.M.; Feitoza, L.A.M.; Fontana, L.E.; Hamada, N.; Hepp, L.U.; Lezan-Kowalczuk, V.G.; Leite, G.F.M.; et al. Plant litter dynamics in the forest-stream interface: Precipitation is a major control across tropical biomes. Sci. Rep. 2006, 7, 10799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Railoun, M.Z. Impacts of the Invasive Tree Acacia Mearnsii on Riparian and Instream Aquatic Environments in the Cape Floristic Region, South Africa; Stellenbosch University: Stellenbosch, South Africa, 2018. [Google Scholar]
- Wenger, S.A. A Review of the Scientific Literature in Riparian Buffer Width, Extent and Vegetation; University of Georgia Press: Athens, GA, USA, 1999; pp. 6–51. [Google Scholar]
- Yang, W.Q.; Wang, K.Y.; Kellomaki, S.; Gong, H.D. Litter dynamics of three subalpine forests in Western Sichuan. Pedosphere 2005, 15, 653–659. [Google Scholar]
- Wei, X.Y.; Yang, Y.L.; Shen, Y.; Chen, Z.H.; Dong, Y.L.; Wu, F.Z.; Zhang, L. Effects of litterfall on the accumulation of extracted soil humic substances in subalpine forests. Front. Plant Sci. 2020, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.L.; Jones, J.A. Stream temperature responses to forest harvest and debris flows in western Cascades, Oregon. Can. J. Fish. Aquat. Sci. 2000, 57, 30–39. [Google Scholar] [CrossRef]
- Graça, M.A.; Ferreira, V.; Canhoto, C.; Encalada, A.C.; Guerrero-Bolaño, F.; Wantzen, K.M.; Boyero, L. A conceptual model of litter breakdown in low order streams. Int. Rev. Hydrobiol. 2015, 100, 1–12. [Google Scholar] [CrossRef]
- Bilby, R.E. Role of organic debris dams in regulating the export of dissolved organic and particulate matter from a forested watershed. Ecology 1981, 62, 1234–1243. [Google Scholar] [CrossRef]
- Drewitt, G.B.; Black, T.A.; Nesic, Z.; Humpgreys, E.R.; Morgenstern, K. Measuring Forest floor CO2 fluxes in a Douglas-fir forest. Agric. For. Meteorol. 2002, 110, 299–317. [Google Scholar] [CrossRef]
- Rantakari, M.; Mattsson, T.; Kortelainen, P.; Mattsson, T.; Piirainen, S. Organic and inorganic carbon concentrations and fluxes from managed and unmanaged boreal first-order catchments. Sci. Total Environ. 2010, 408, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.J.; Prairie, Y.T.; Caraco, N.F.; McDowell, W.H.; Tranvik, L.J.; Striegl, R.G.; Duarte, C.M.; Kortelaien, P.; Downing, J.A.; Middelburg, J.J.; et al. Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems 2007, 10, 172–185. [Google Scholar] [CrossRef] [Green Version]
- Wallace, T.A.; Ganf, G.G.; Brookes, J.D. A comparison of phosphorus and DOC leachates from different types of leaf litter in an urban environment. Freshw. Biol. 2008, 53, 1902–1913. [Google Scholar] [CrossRef]

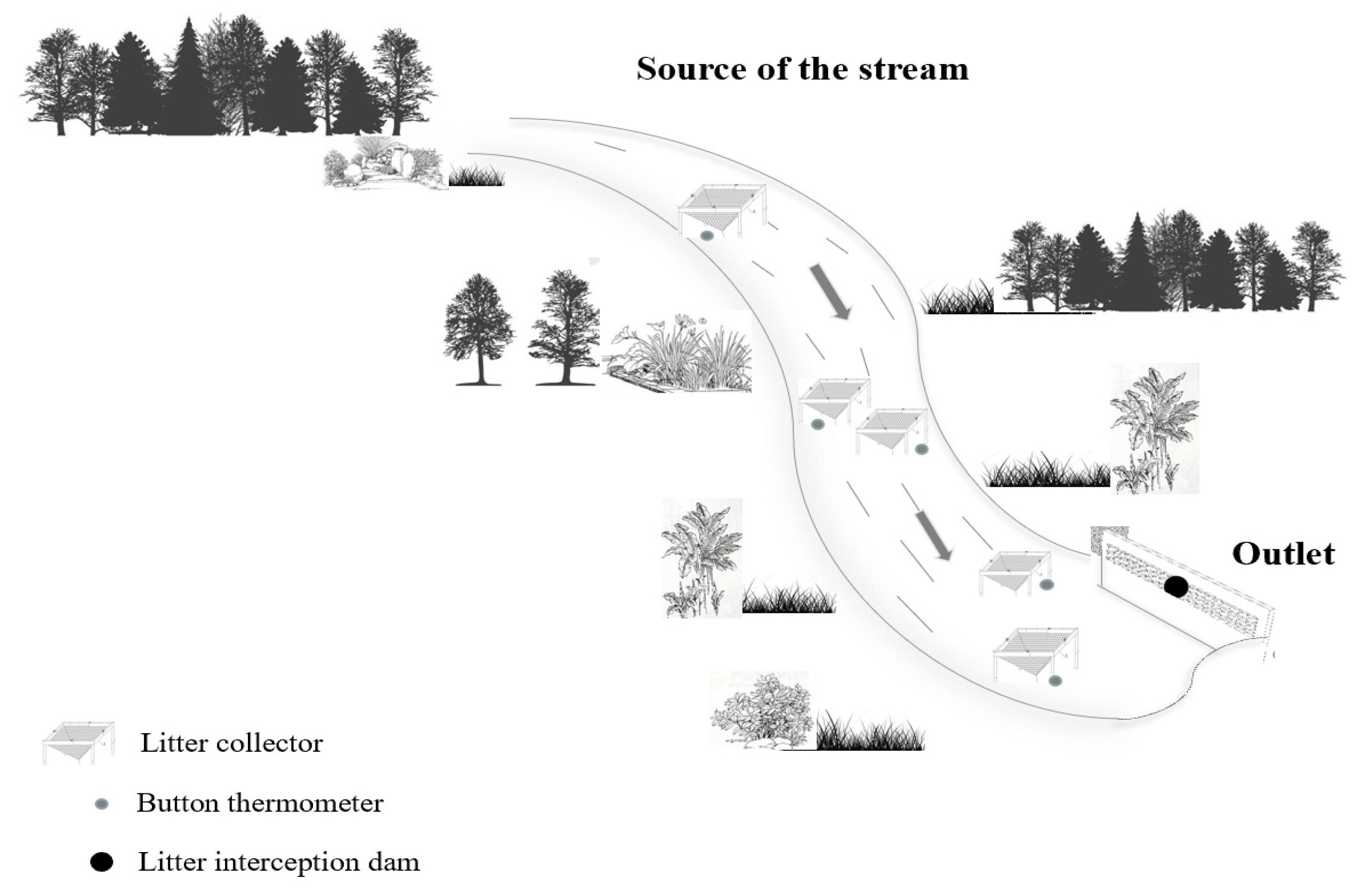

| Stream | Elevation (m) | Length (m) | Width (m) | Water Level (cm) | Flow Velocity (m3/s) | Main Plants |
|---|---|---|---|---|---|---|
| A | 3668 | 220 | 0.63 ± 0.16 | 8.57 ± 3.20 | 0.11 ± 0.11 | A. faxoniana, Cyperus spp., S saltuaria |
| B | 3667 | 66 | 0.69 ± 0.14 | 5.15 ± 1.60 | 0.07 ± 0.07 | A. faxoniana, Cyperus spp., S. saltuaria |
| C | 3658 | 13 | 0.63 ± 0.22 | 7.16 ± 3.83 | 0.01 ± 0.02 | A. faxoniana, Cyperus spp., S. saltuaria |
| D | 3658 | 92.4 | 0.86 ± 0.19 | 4.81 ± 1.08 | 0.06 ± 0.07 | A. faxoniana, Cyperus spp., S. saltuaria |
| E | 3657 | 47 | 0.34 ± 0.30 | 3.73 ± 3.43 | 0.01 ± 0.01 | A. faxoniana, Cyperus spp., S. saltuaria |
| F | 3640 | 65 | 1.11 ± 0.30 | 6.90 ± 1.44 | 0.11 ± 0.12 | S. saltuaria, R. lapponicum |
| G | 3640 | 186 | 1.02 ± 0.33 | 8.96 ± 1.91 | 0.06 ± 0.06 | S. saltuaria, Carex spp., R. weginzowii |
| H | 3634 | 108 | 0.82 ± 0.28 | 6.73 ± 4.38 | 0.04 ± 0.07 | S. saltuaria, Carex spp., S. rufopilosa |
| I | 3634 | 256 | 1.02 ± 0.22 | 7.19 ± 1.80 | 0.13 ± 0.12 | S. saltuaria, R. weginzowii, Carex spp. |
| J | 3634 | 18 | 1.29 ± 1.00 | 3.85 ± 3.05 | 0.04 ± 0.07 | S. saltuaria, R. weginzowii, Carex spp. |
| K | 3611 | 36 | 1.00 ± 0.24 | 7.93 ± 2.32 | 0.10 ± 0.08 | R. lapponicum, S. saltuaria |
| L | 3611 | 11 | 0.93 ± 0.58 | 3.70 ± 1.89 | 0.03 ± 0.05 | R. lapponicum, S. saltuaria |
| M | 3610 | 12 | 0.85 ± 0.23 | 6.26 ± 1.42 | 0.03 ± 0.08 | R. lapponicum, S. saltuaria |
| N | 3607 | 28 | 0.84 ± 0.32 | 11.72 ± 4.88 | 0.15 ± 0.14 | S. mastersiana, Cyperus spp., S. rufopilosa |
| O | 3607 | 17 | 0.65 ± 0.41 | 4.34 ± 3.84 | 0.02 ± 0.04 | S. mastersiana, S. rufopilosa, Cyperus spp. |
| Factors | d.f. | p | r2 | |
|---|---|---|---|---|
| Litter input | Precipitation | 73 | 0.97 | <0.01 |
| Temperature | 73 | <0.01 ** | 0.32 | |
| Sediment depth | 73 | 0.27 | <0.01 | |
| Water level | 73 | 0.50 | 0.04 | |
| Flow velocity | 73 | 0.27 | <0.01 | |
| Width | 73 | −0.29 | <0.01 | |
| Length | 73 | <0.01 ** | 0.11 | |
| Litter carbon input | Precipitation | 73 | 0.63 | 0.02 |
| Temperature | 73 | <0.01 ** | 0.30 | |
| Sediment depth | 73 | 0.21 | 0.04 | |
| Water level | 73 | 0.22 | 0.01 | |
| Flow velocity | 73 | 0.21 | 0.01 | |
| Width | 73 | −0.43 | <0.01 | |
| Length | 73 | 0.01 * | 0.06 |
| Factors | d.f. | p | r2 | |
|---|---|---|---|---|
| Litter output | Precipitation | 73 | 0.002 ** | 0.15 |
| Temperature | 73 | 0.02 * | 0.08 | |
| Sediment depth | 73 | −0.04 * | 0.05 | |
| Water level | 73 | 0.24 | 0.02 | |
| Flow velocity | 73 | 0.02 * | 0.07 | |
| Width | 73 | −0.68 | <0.01 | |
| Length | 73 | −0.07 | 0.04 | |
| Litter input | 73 | 0.002 ** | 0.18 | |
| Litter carbon output | Precipitation | 73 | 0.04 * | 0.01 |
| Temperature | 73 | 0.003 ** | 0.08 | |
| Sediment depth | 73 | −0.03 * | 0.04 | |
| Water level | 73 | 0.33 | <0.01 | |
| Flow velocity | 73 | 0.04 * | 0.06 | |
| Width | 73 | −0.41 | <0.01 | |
| Length | 73 | −0.26 | <0.01 | |
| Litter carbon input | 73 | <0.001 ** | 0.22 |
| Site | Tree Spices | Input (g m−2 y−1) | References |
|---|---|---|---|
| Upper Reaches of Yangtze River, China | Coniferous forest | 262 | This study |
| Ogeechee River, Georgia, USA | Coniferous forest | 537 | Triska et al. (1982) |
| Satellite Br, North Carolina, USA | Mixed deciduous forest | 629 | Benfield (1997) |
| Devil’s Club Cr, Oregon, USA | Coniferous forest | 736 | Benfield (1997) |
| Deep Cr, Idaho, USA | Shrub/grass cover | 3 | Benfield (1997) |
| Mack Cr, Oregon, USA | Coniferous forest | 730 | Benfield (1997) |
| Sycamore Cr, Arizona, USA | Shrub cover | 20 | Benfield (1997) |
| North Carolina, USA | Deciduous forest | 1000 | Webster et al. (1997) |
| Coast Range of Oregon, USA | Deciduous forest | 613 | Hart (2006) |
| Domtar Inc. White River Forest, Canada | Mixed wood forest | 167 | Muto (2008) |
| Brazil | Atlantic forest | 131 | Tonin et al. (2017) |
| Brazil | Amazon forest | 165 | Tonin et al. (2017) |
| Brazil | Cerrado savanna, | 213 | Tonin et al. (2017) |
| Wit River, Western Cape, South Africa | typical mountain fynbos | 69 | Railoun (2018) |
| Du Toit’s River, Western Cape, South Africa | typical mountain fynbos | 68 | Railoun (2018) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, J.; Li, F.; Wang, Z.; Li, X.; Yang, W. Budget of Plant Litter and Litter Carbon in the Subalpine Forest Streams. Forests 2021, 12, 1764. https://doi.org/10.3390/f12121764
Hou J, Li F, Wang Z, Li X, Yang W. Budget of Plant Litter and Litter Carbon in the Subalpine Forest Streams. Forests. 2021; 12(12):1764. https://doi.org/10.3390/f12121764
Chicago/Turabian StyleHou, Jianfeng, Fei Li, Zhihui Wang, Xuqing Li, and Wanqin Yang. 2021. "Budget of Plant Litter and Litter Carbon in the Subalpine Forest Streams" Forests 12, no. 12: 1764. https://doi.org/10.3390/f12121764
APA StyleHou, J., Li, F., Wang, Z., Li, X., & Yang, W. (2021). Budget of Plant Litter and Litter Carbon in the Subalpine Forest Streams. Forests, 12(12), 1764. https://doi.org/10.3390/f12121764







