Tree Rings Reveal the Impact of Soil Temperature on Larch Growth in the Forest-Steppe of Siberia
Abstract
:1. Introduction
2. Materials and Methods
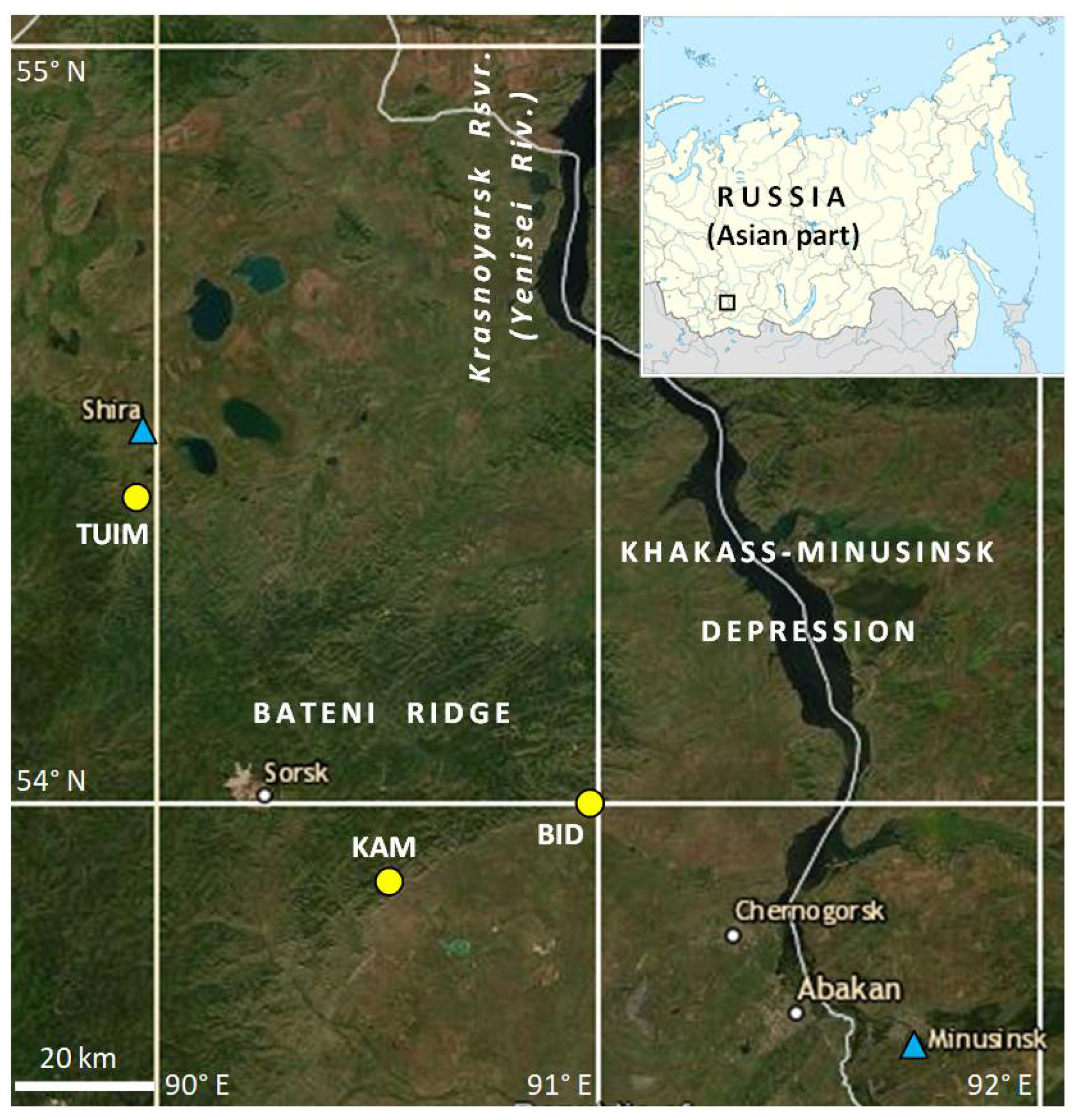
2.1. Geographical Setting, Climatic Data Sources and Climate of Study Region
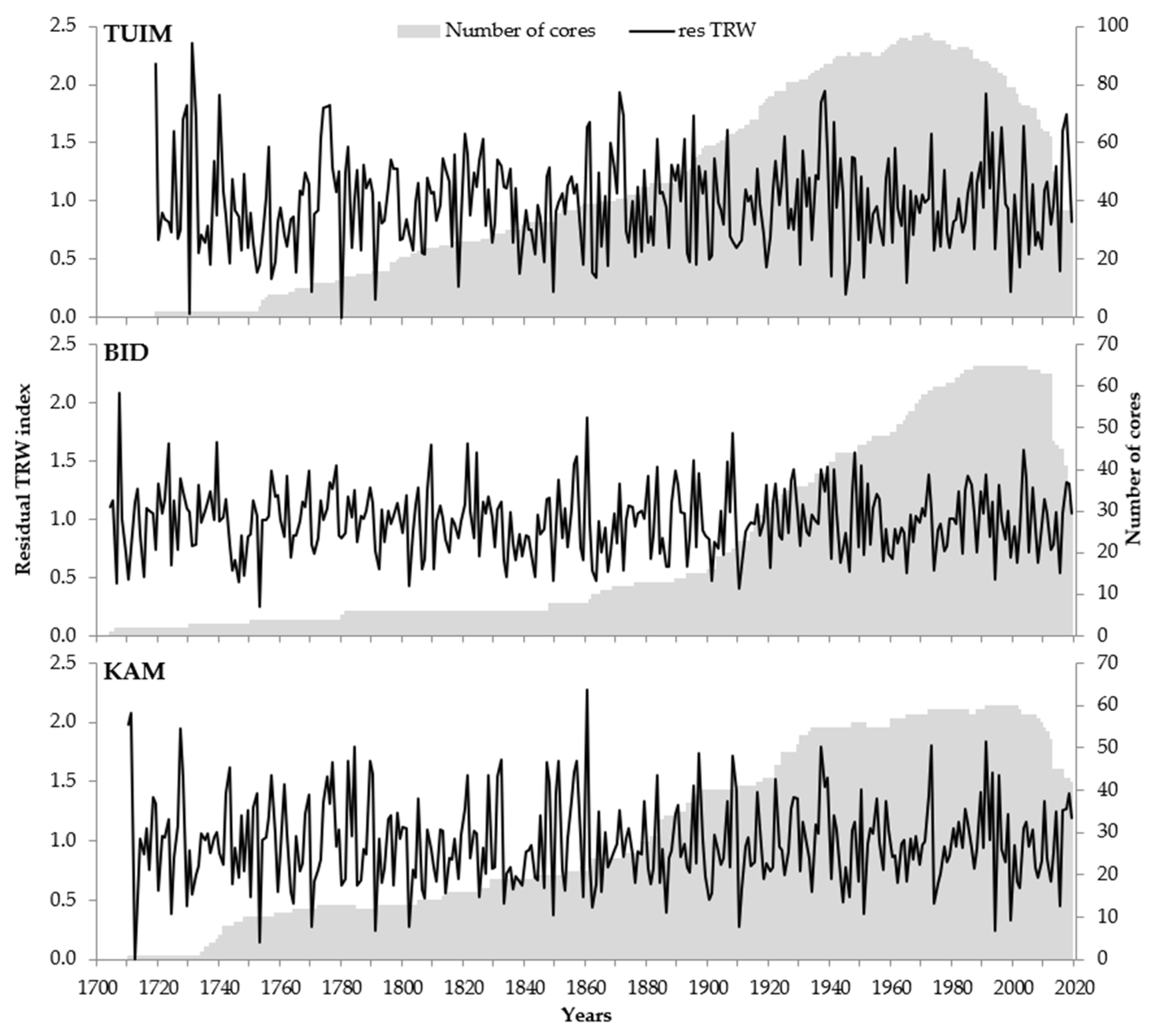
2.2. Tree-Ring Sites, Sampling, Measurement, and Data Processing
2.3. Statistical Analysis
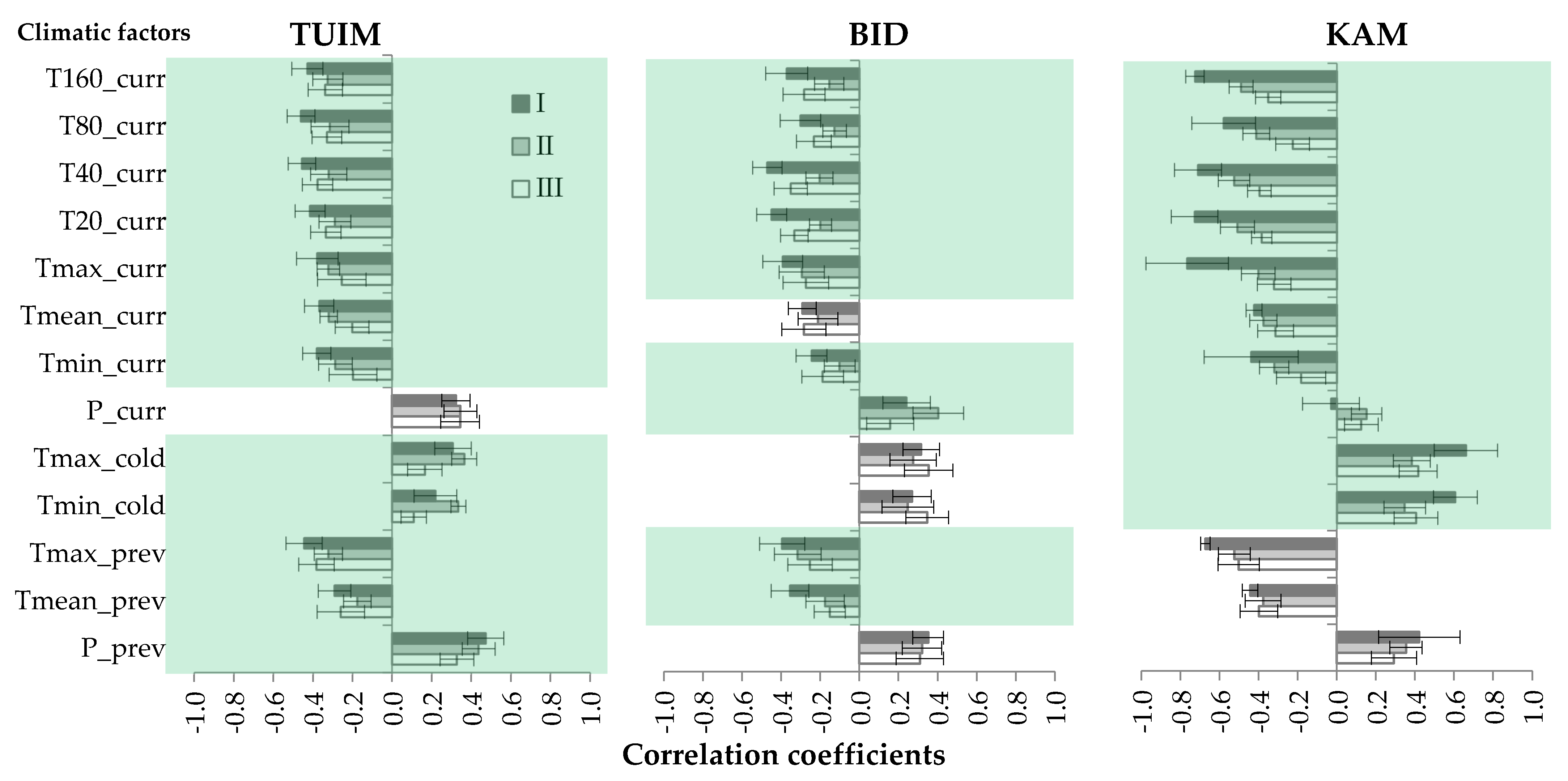
3. Results
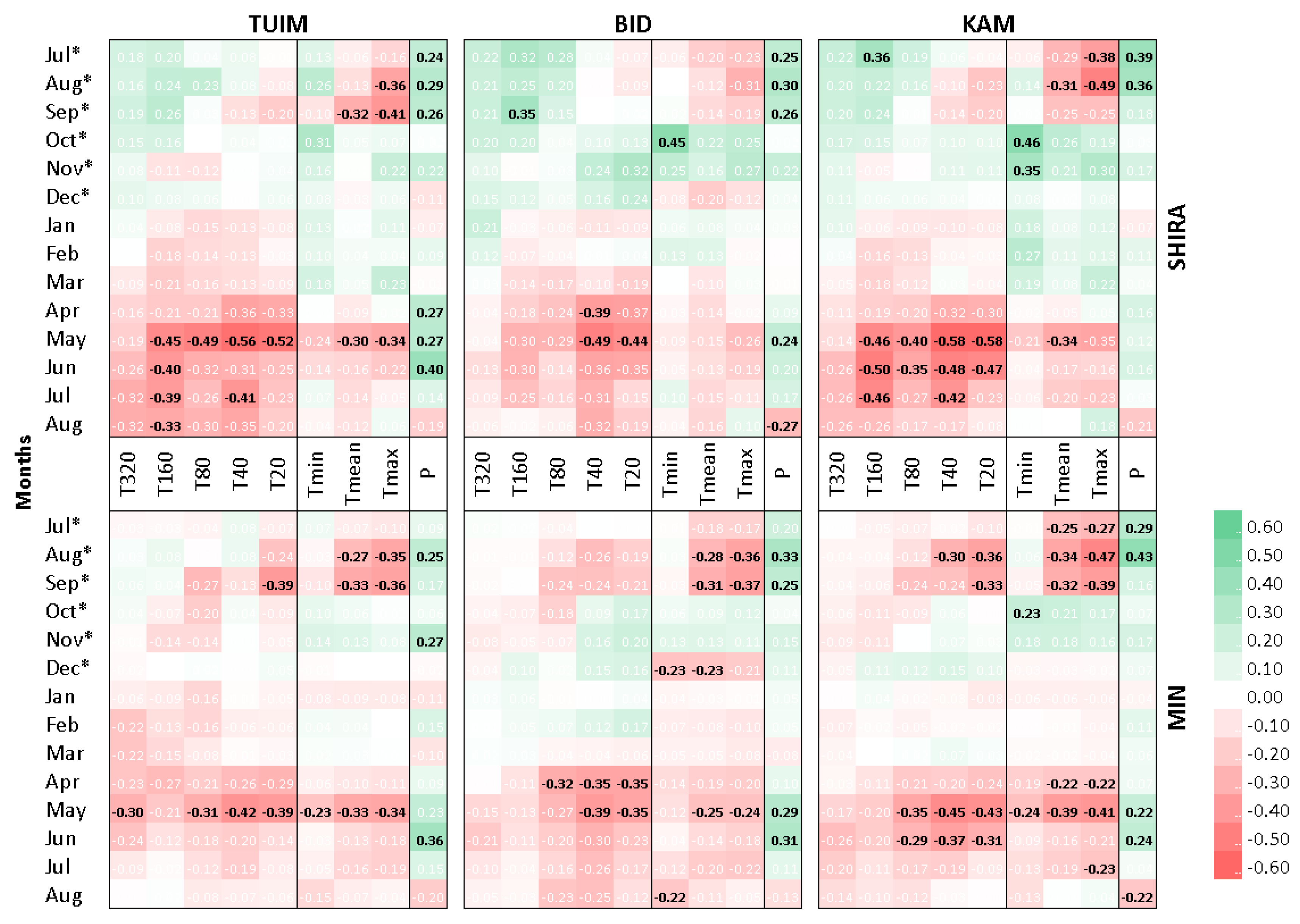
3.1. Climatic Response of Tree-Ring Chronologies
3.2. Cluster Analysis of Individual Trees’ Climatic Reactions
4. Discussion
4.1. Physical Basis for Climate-Growth Correlations
4.2. Dependence of Climatic Response on Individual Characteristics of Trees
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK, 1976. [Google Scholar] [CrossRef]
- Hughes, M.K.; Swetnam, T.W.; Diaz, H.F. Dendroclimatology: Progress and Prospects; Springer: Dordrecht, Germany, 2010. [Google Scholar] [CrossRef]
- Eshel, A.; Beeckman, T. Plant Roots: The Hidden Half, 4th ed.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2013. [Google Scholar] [CrossRef]
- Alvarez-Uria, P.; Körner, C. Low temperature limits of root growth in deciduous and evergreen temperate tree species. Funct. Ecol. 2007, 21, 211–218. [Google Scholar] [CrossRef]
- Veihmeyer, F.J.; Hendrickson, A.H. Soil moisture in relation to plant growth. Ann. Rev. Plant Physiol. 1950, 1, 285–304. [Google Scholar] [CrossRef]
- Babst, F.; Alexander, M.R.; Szejner, P.; Bouriaud, O.; Klesse, S.; Roden, J.; Ciais, P.; Poulter, B.; Frank, D.; Moore, D.J.P.; et al. A tree-ring perspective on the terrestrial carbon cycle. Oecologia 2014, 176, 307–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, K.; Wang, X.; Liang, P.; An, H.; Sun, H.; Han, W.; Li, Q. Tree-ring widths are good proxies of annual variation in forest productivity in temperate forests. Sci. Rep. 2017, 7, 1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Franklin, J.F. Growing-season microclimate variability within an old-growth Douglas-fir forest. Clim. Res. 1997, 8, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Morecroft, M.D.; Taylor, M.E.; Oliver, H.R. Air and soil microclimates of deciduous woodland compared to an open site. Agric. For. Meteorol. 1998, 90, 141–156. [Google Scholar] [CrossRef]
- Daly, C.; Halbleib, M.; Smith, J.I.; Gibson, W.P.; Doggett, M.K.; Taylor, G.H.; Pasteris, P.P. Physiographically sensitive mapping of climatological temperature and precipitation across the conterminous United States. Int. J. Climatol. 2008, 28, 2031–2064. [Google Scholar] [CrossRef]
- Meko, D.M.; Stahle, D.W.; Griffin, D.; Knight, T.A. Inferring precipitation–anomaly gradients from tree rings. Quatern. Int. 2011, 235, 89–100. [Google Scholar] [CrossRef]
- Harris, I.; Jones, P.D.; Osborn, T.J.; Lister, D.H. Updated high-resolution grids of monthly climatic observations—the CRU TS3.10 Dataset. Int. J. Climatol. 2014, 34, 623–642. [Google Scholar] [CrossRef] [Green Version]
- Touchan, R.; Anchukaitis, K.J.; Shishov, V.V.; Sivrikayan, F.; Attieh, J.; Ketmen, M.; Meko, D.M. Spatial patterns of eastern Mediterranean climate influence on tree growth. Holocene 2014, 24, 381–392. [Google Scholar] [CrossRef]
- Porte, A.; Huard, F.; Dreyfus, P. Microclimate beneath pine plantation, semi-mature pine plantation and mixed broadleaved-pine forest. Agric. For. Meteorol. 2004, 126, 175–182. [Google Scholar] [CrossRef]
- Geiger, R.; Aron, R.H.; Todhunter, P. The Climate near the Ground, 7th ed.; Rowman & Littlefield: Lanham, MD, USA, 2009. [Google Scholar]
- von Arx, G.; Graf Pannatier, E.; Thimonier, A.; Rebetez, M. Microclimate in forests with varying leaf area index and soil moisture: Potential implications for seedling establishment in a changing climate. J. Ecol. 2013, 101, 1201–1213. [Google Scholar] [CrossRef]
- Qian, B.; Gregorich, E.G.; Gameda, S.; Hopkins, D.W.; Wang, X.L. Observed soil temperature trends associated with climate change in Canada. J. Geophys. Res. Atmos. 2011, 116, D02106. [Google Scholar] [CrossRef]
- Onwuka, B.; Mang, B. Effects of soil temperature on some soil properties and plant growth. Adv. Plants Agric. Res. 2018, 8, 34–37. [Google Scholar] [CrossRef]
- Wu, J.; Nofziger, D.L. Incorporating temperature effects on pesticide dug radiation into a management model. J. Environ. Qual. 1999, 28, 92–100. [Google Scholar] [CrossRef]
- Elias, E.A.; Cichota, R.; Torraiani, H.H.; De Jong Van Lier, Q. Analytical soil temperature model: Correction for temporal variation of daily amplitude. Soil Sci. Soc. Am. J. 2004, 68, 784–788. [Google Scholar] [CrossRef]
- Nwankwo, C.; Ogagarue, D. An Investigation of Temperature Variation at Soil Depths in Parts of Southern Nigeria. Am. J. Environ. Eng. 2012, 2, 142–147. [Google Scholar] [CrossRef] [Green Version]
- Vaganov, E.A.; Hughes, M.K.; Kirdyanov, A.V.; Schweingruber, F.H.; Silkin, P.P. Influence of snowfall and melt timing on tree growth in subarctic Eurasia. Nature 1999, 400, 149–151. [Google Scholar] [CrossRef]
- Zhang, T.; Barry, R.; Gilichinsky, D.; Bykhovets, S.; Sorokovikov, V.A.; Ye, J. An Amplified Signal of Climatic Change in Soil Temperatures during the Last Century at Irkutsk, Russia. Clim. Chang. 2001, 49, 41–76. [Google Scholar] [CrossRef]
- Kirdyanov, A.; Hughes, M.; Vaganov, E.; Schweingruber, F.; Silkin, P. The importance of early summer temperature and date of snow melt for tree growth in the Siberian Subarctic. Trees 2003, 17, 61–69. [Google Scholar] [CrossRef]
- Sanmiguel-Vallelado, A.; Camarero, J.J.; Morán-Tejeda, E.; Gazol, A.; Colangelo, M.; Alonso-González, E.; López-Moreno, J.I. Snow dynamics influence tree growth by controlling soil temperature in mountain pine forests. Agric. For. Meteorol. 2021, 296, 108205. [Google Scholar] [CrossRef]
- García-Suárez, A.M.; Butler, C.J. Soil temperatures at Armagh observatory, Northern Ireland, from 1904 to 2002. Int. J. Climatol. 2006, 26, 1075–1089. [Google Scholar] [CrossRef] [Green Version]
- Osawa, A.; Zyryanova, O.A.; Matsuura, Y.; Kajimoto, T.; Wein, R.W. Permafrost Ecosystems: Siberian Larch Forests; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Bryukhanova, M.V.; Kirdyanov, A.V.; Prokushkin, A.S.; Silkin, P.P. Specific features of xylogenesis in Dahurian larch, Larix gmelinii (Rupr.) Rupr., growing on permafrost soils in Middle Siberia. Russ. J. Ecol. 2013, 44, 361–366. [Google Scholar] [CrossRef]
- Dashtseren, A.; Ishikawa, M.; Iijima, Y.; Jambaljav, Y. Temperature regimes of the active layer and seasonally frozen ground under a forest-steppe mosaic, Mongolia. Permafr. Periglac. Process. 2014, 25, 295–306. [Google Scholar] [CrossRef]
- Park, H.; Sherstiukov, A.B.; Fedorov, A.N.; Polyakov, I.V.; Walsh, J.E. An observation-based assessment of the influences of air temperature and snow depth on soil temperature in Russia. Environ. Res. Lett. 2014, 9, 064026. [Google Scholar] [CrossRef]
- Wang, X.; Chen, R.; Han, C.; Yang, Y.; Liu, J.; Liu, Z.; Guo, S.; Song, Y. Response of shallow soil temperature to climate change on the Qinghai–Tibetan Plateau. Int. J. Clim. 2020, 41, 1–16. [Google Scholar] [CrossRef]
- Abu-Hamdeh, N.H.; Reeder, R.C. Soil thermal conductivity effects of density, moisture, salt concentration, and organic matter. Soil Sci. Soc. Am. J. 2000, 64, 1285–1290. [Google Scholar] [CrossRef]
- Elizbarashvili, E.S.; Urushadze, T.F.; Elizbarashvili, M.E.; Elizbarashvili, S.E.; Schaefer, M.K. Temperature regime of some soil types in Georgia. Eurasian Soil Sci. 2010, 43, 427–435. [Google Scholar] [CrossRef]
- Alem, S.; Madera, P.; Pavlis, J. Soil temperature in an open site and below two plantation forest canopies in a tropical highland area, southern Ethiopia. Theor. Appl. Climatol. 2020, 139, 907–914. [Google Scholar] [CrossRef]
- Chen, J.Q.; Franklin, J.F.; Spies, T.A. Contrasting microclimates among clear-cut, edge, and interior of old-growth Douglas-fir forest. Agric. For. Met. 1993, 63, 219–237. [Google Scholar] [CrossRef]
- Breshears, D.D.; Rich, P.M.; Barnes, F.J.; Campbell, K. Overstory-imposed heterogeneity in solar radiation and soil moisture in a semiarid woodland. Ecol. Appl. 1997, 7, 1201–1215. [Google Scholar] [CrossRef]
- Holst, T.; Mayer, H.; Schindler, D. Microclimate within beech stands part II: Thermal conditions. Eur. J. Forest Res. 2004, 123, 13–28. [Google Scholar] [CrossRef]
- Matthias, A.D.; Musil, S. Temperatures and thermal diffusivity within a rangeland soil near oracle, Arizona. J. Arizona-Nevada Acad. Sci. 2012, 44, 15–21. [Google Scholar] [CrossRef]
- García-Suárez, A.M.; Butler, C.J.; Baillie, M.G.L. Climate signal in tree-ring chronologies in a temperate climate: A multi-species approach. Dendrochronologia 2009, 27, 183–198. [Google Scholar] [CrossRef] [Green Version]
- Nikolaev, A.N.; Fedorov, P.P.; Desyatkin, A.R. Influence of climate and soil hydrothermal regime on radial growth of Larix cajanderi and Pinus sylvestris in Central Yakutia, Russia. Scand. J. For. Res. 2009, 24, 217–226. [Google Scholar] [CrossRef]
- Brédoire, F.; Kayler, Z.E.; Dupoueyl, J.-L.; Derrien, D.; Zeller, B.; Barsukov, P.A.; Rusalimova, O.; Nikitich, P.; Bakker, M.R.; Legout, A. Limiting factors of aspen radial growth along a climatic and soil water budget gradient in south-western Siberia. Agric. For. Meteorol. 2020, 282, 107870. [Google Scholar] [CrossRef]
- Yuan, S.; Zheng, Y.; Qi, Y.; Kong, F.; Wang, D.; Zhang, F. A method for reconstructing the past soil temperature based on tree-ring widths. For. Sci. 2020, 66, 393–402. [Google Scholar] [CrossRef]
- Belokopytova, L.V.; Babushkina, E.A.; Zhirnova, D.F.; Panyushkina, I.P.; Vaganov, E.A. Climatic response of conifer radial growth in forest-steppes of South Siberia: Comparison of three approaches. Contemp. Probl. Ecol. 2018, 11, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Babushkina, E.A.; Zhirnova, D.F.; Belokopytova, L.V.; Tychkov, I.I.; Vaganov, E.A.; Krutovsky, K.V. Response of four tree species to changing climate in a moisture-limited area of South Siberia. Forests 2019, 10, 999. [Google Scholar] [CrossRef] [Green Version]
- Zhirnova, D.F.; Babushkina, E.A.; Belokopytova, L.V.; Vaganov, E.A. To which side are the scales swinging? Growth stability of Siberian larch under permanent moisture deficit with periodic droughts. For. Ecol. Manag. 2020, 459, 117841. [Google Scholar] [CrossRef]
- Zhirnova, D.F.; Belokopytova, L.V.; Meko, D.M.; Babushkina, E.A.; Vaganov, E.A. Climate change and tree growth in the Khakass-Minusinsk Depression (South Siberia) impacted by large water reservoirs. Sci. Rep. 2021, 11, 14266. [Google Scholar] [CrossRef]
- Alisov, B.P. Climate of the USSR; Moscow State University: Moscow, Russia, 1956. (In Russian) [Google Scholar]
- Cook, E.R.; Kairiukstis, L.A. Methods of Dendrochronology: Applications in the Environmental Sciences; Springer: Dordrecht, The Netherlands, 1990. [Google Scholar] [CrossRef]
- Pirie, M.R.; Fowler, A.M.; Triggs, C.M. Assessing the accuracy of three commonly used pith offset methods applied to Agathis australis (Kauri) incremental cores. Dendrochronologia 2015, 36, 60–68. [Google Scholar] [CrossRef]
- Rinn, F. TSAP-Win: Time Series Analysis and Presentation for Dendrochronology and Related Applications: User Reference; RINNTECH: Heidelberg, Germany, 2003. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree Ring Bull. 1983, 43, 68–78. [Google Scholar]
- Cook, E.R.; Krusic, P.J. Program ARSTAN: A Tree-Ring Standardization Program Based on Detrending and Autoregressive Time Series Modeling, with Interactive Graphics; Lamont-Doherty Earth Observatory, Columbia University: New York, NY, USA, 2005. [Google Scholar]
- Cook, E.R. A Time Series Analysis Approach to Tree-Ring Standardization. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 1985. [Google Scholar]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Appl. Meteorol. Climatol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Wilks, D.S. Statistical Methods in the Atmospheric Sciences, 4th ed.; Elsevier: Cambridge, UK, 2019. [Google Scholar]
- Velisevich, S.N.; Khutornoi, O.V. Effects of climatic factors on radial growth of Siberian stone pine and Siberian larch in sites with different soil humidity in the south of western Siberia. J. Sib. Fed. Univ. Biol. 2009, 2, 117–132. (In Russian) [Google Scholar]
- Urban, J.; Rubtsov, A.V.; Urban, A.V.; Shashkin, A.V.; Benkova, V.E. Canopy transpiration of a Larix sibirica and Pinus sylvestris forest in Central Siberia. Agric. For. Meteorol. 2019, 271, 64–72. [Google Scholar] [CrossRef]
- Liu, H.; Park Williams, A.; Allen, C.D.; Guo, D.; Wu, X.; Anenkhonov, O.A.; Liang, E.; Sandanov, D.V.; Yin, Y.; Qi, Z.; et al. Rapid warming accelerates tree growth decline in semi-arid forests of Inner Asia. Glob. Chang. Biol. 2013, 19, 2500–2510. [Google Scholar] [CrossRef]
- Belokopytova, L.; Zhirnova, D.; Kostyakova, T.; Babushkina, E. Dynamics of moisture regime and its reconstruction from a tree-ring width chronology of Pinus sylvestris in the downstream basin of the Selenga River, Russia. J. Arid Land 2018, 10, 877–891. [Google Scholar] [CrossRef] [Green Version]
- Ermida, S.L.; Trigo, I.F.; DaCamara, C.C.; Göttsche, F.M.; Olesen, F.S.; Hulley, G. Validation of remotely sensed surface temperature over an oak woodland landscape—The problem of viewing and illumination geometries. Remote Sens. Environ. 2014, 148, 16–27. [Google Scholar] [CrossRef]
- Jackson, R.D.; Idso, S.B.; Reginato, R.J.; Pinter, P.J., Jr. Canopy temperature as a crop water stress indicator. Water Resour. Res. 1981, 17, 1133–1138. [Google Scholar] [CrossRef]
- Williams, A.P.; Allen, C.D.; Macalady, A.K.; Griffin, D.; Woodhouse, C.A.; Meko, D.M.; Swetnam, T.W.; Rauscher, S.A.; Seager, R.; Grissino-Mayer, H.D.; et al. Temperature as a potent driver of regional forest drought stress and tree mortality. Nat. Clim. Chang. 2013, 3, 292–297. [Google Scholar] [CrossRef]
- Sorokina, O.A. (Krasnoyarsk State Agrarian University, Krasnoyarsk, Russian Federation). Personal communication. 2021. [Google Scholar]
- Vaganov, E.A.; Hughes, M.K.; Shashkin, A.V. Growth Dynamics of Conifer Tree Rings: Images of Past and Future Environments; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Carbohydrate sources and sinks in woody plants. Bot. Rev. 1992, 58, 107–222. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef] [Green Version]
- Pallardy, S.G. Physiology of Woody Plant, 3rd ed.; Academic Press: San-Diego, CA, USA, 2010. [Google Scholar]
- Piper, F.I.; Fajardo, A. Foliar habit, tolerance to defoliation and their link to carbon and nitrogen storage. J. Ecol. 2014, 102, 1101–1111. [Google Scholar] [CrossRef]
- Danek, M.; Chuchro, M.; Walanus, A. tree-ring growth of larch (Larix decidua Mill.) in the Polish Sudetes—the influence of altitude and site-related factors on the climate–growth relationship. Forests 2018, 9, 663. [Google Scholar] [CrossRef] [Green Version]
- Gurskaya, M.A.; Shiyatov, S.G. Distribution of frost injuries in the wood of conifers. Russ. J. Ecol. 2006, 37, 7–12. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Griçar, J.; Seo, J.W.; Rathgeber, C.B.K.; Anfodillo, T.; Morin, H.; Levanic, T.; Oven, P.; Jalkanen, R. Critical temperatures for xylogenesis in conifers of cold climates. Glob. Ecol. Biogeogr. 2008, 17, 696–707. [Google Scholar] [CrossRef]
- Migliavacca, M.; Cremonese, E.; Colombo, R.; Busetto, L.; Galvagno, M.; Ganis, L.; Meroni, M.; Pari, E.; Rossini, M.; Siniscalco, C.; et al. European larch phenology in the Alps: Can we grasp the role of ecological factors by combining field observations and inverse modelling? Int. J. Biometeorol. 2008, 52, 587–605. [Google Scholar] [CrossRef]
- Lukkarinen, A.J.; Ruotsalainen, S.; Peltola, H.; Nikkanen, T. Bud set and autumn coloration of Larix sibirica and Larix gmelinii provenances in a field trial in southern Finland. Scand. J. For. Res. 2014, 29, 27–40. [Google Scholar] [CrossRef]
- Bílek, L.; Remes, J.; Podrazsky, V.; Rozenbergar, D.; Diaci, J.; Zahradník, D. Gap regeneration in near-natural European beech forest stands in Central Bohemia-the role of heterogeneity and micro-habitat factors. Dendrobiology 2014, 71, 59–71. [Google Scholar] [CrossRef] [Green Version]
- García de Jalón, L.; Limousin, J.M.; Richard, F.; Gessler, A.; Peter, M.; Hättenschwiler, S.; Milcu, A. Microhabitat and ectomycorrhizal effects on the establishment, growth and survival of Quercus ilex L. seedlings under drought. PLoS ONE 2020, 15, e0229807. [Google Scholar] [CrossRef]
- Sandoval, S.; Cancino, J. Modeling the edge effect in even-aged Monterrey pine (Pinus radiata D. Don) stands incorporating a competition index. For. Ecol. Manag. 2008, 256, 78–87. [Google Scholar] [CrossRef]
- Albiero-Júnior, A.; Venegas-González, A.; Rodríguez-Catón, M.; Oliveira, J.M.; Longhi-Santos, T.; Galvão, F.; Temponi, L.G.; Botosso, P.C. Edge effects modify the growth dynamics and climate sensitivity of Araucaria angustifolia trees. Tree-Ring Res. 2020, 76, 11–26. [Google Scholar] [CrossRef]
- Rozas, V.; DeSoto, L.; Olano, J.M. Sex-specific, age-dependent sensitivity of tree-ring growth to climate in the dioecious tree Juniperus thurifera. New Phytol. 2009, 182, 687–697. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; McRae, D.J. Spatial and age-dependent tree-ring growth responses of Larix gmelinii to climate in northeastern China. Trees 2009, 23, 875–885. [Google Scholar] [CrossRef]
- Martín-Benito, D.; Cherubini, P.; Del Río, M.; Cañellas, I. Growth response to climate and drought in Pinus nigra Arn. trees of different crown classes. Trees 2008, 22, 363–373. [Google Scholar] [CrossRef]
- Zang, C.; Pretzsch, H.; Rothe, A. Size-dependent responses to summer drought in Scots pine, Norway spruce and common oak. Trees 2012, 26, 557–569. [Google Scholar] [CrossRef]
- Comas, L.; Becker, S.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.S.; Chhetri, P.K.; Krutovsky, K.V.; Cairns, D.M. Growth and its relationship to individual genetic diversity of mountain hemlock (Tsuga mertensiana) at alpine treeline in Alaska: Combining dendrochronology and genomics. Forests 2017, 8, 418. [Google Scholar] [CrossRef] [Green Version]
- Fasanella, M.; Suarez, M.L.; Hasbun, R.; Premoli, A.C. Individual-based dendrogenomic analysis of forest dieback driven by extreme droughts. Can. J. For. Res. 2021, 51, 420–432. [Google Scholar] [CrossRef]
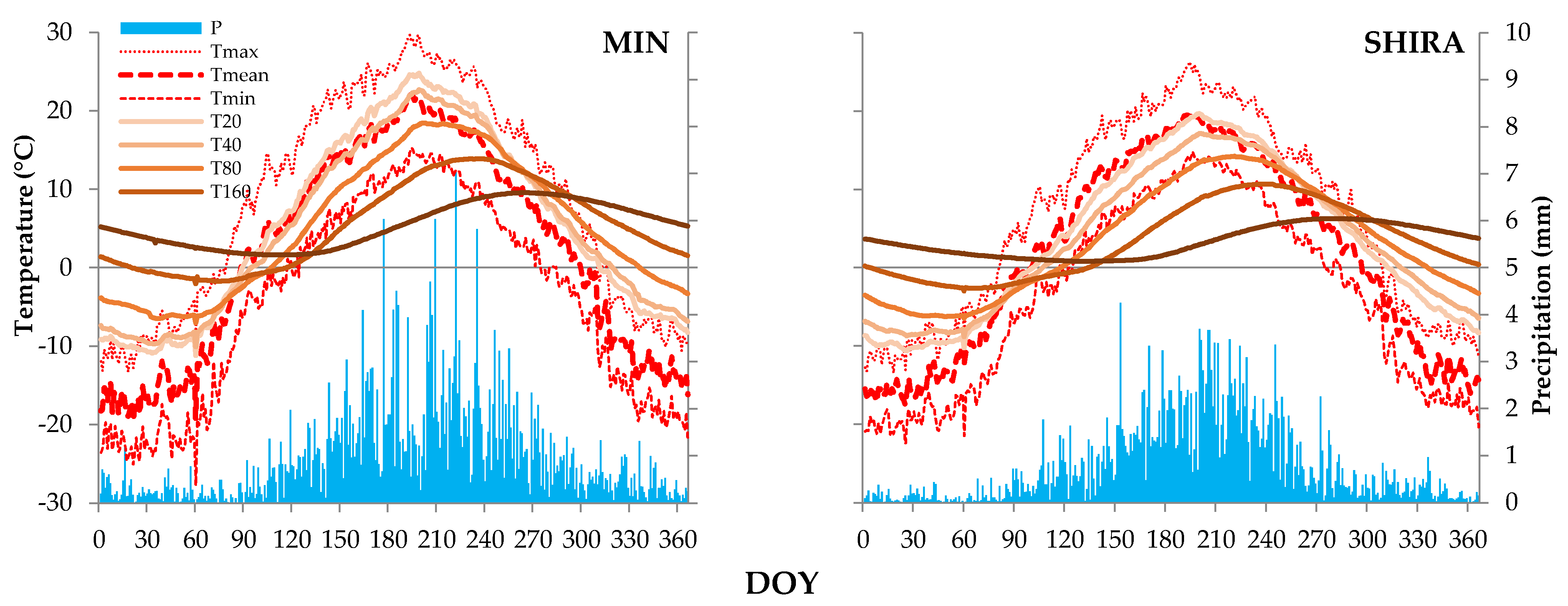
| Depth, cm | Seasonal Range, °C * | Cross-Correlation of | |||||
|---|---|---|---|---|---|---|---|
| Average Seasonal Curves | Long-Term Daily Series, Mean | Long-Term 21-Day Series, Mean | |||||
| Delay, Days | R ** | Delay, Days | R | Delay, Days | R | ||
| MIN | |||||||
| 20 | 36.0 (−11.2–+24.8) | 5 | 0.990 | 1 | 0.68 | 2 | 0.72 |
| 40 | 32.7 (−10.1–+22.7) | 10 | 0.989 | 2 | 0.63 | 4 | 0.75 |
| 80 | 25.5 (−7.0–+18.5) | 22 | 0.985 | 4 | 0.46 | 9 | 0.64 |
| 160 | 15.9 (−2.0–+13.9) | 45 | 0.979 | insignificant R | 21 | 0.40 | |
| 320 | 7.9 (+1.6–+9.6) | 81 | 0.985 | insignificant R | insignificant R | ||
| SHIRA | |||||||
| 20 | 30.3 (−10.6–+19.7) | 9 | 0.990 | 1 | 0.67 | 3 | 0.69 |
| 40 | 26.0 (−8.8–+17.1) | 16 | 0.989 | 2 | 0.57 | 5 | 0.66 |
| 80 | 20.7 (−6.5–+14.2) | 28 | 0.986 | 5 | 0.42 | 10 | 0.58 |
| 160 | 13.7(−3.0–+10.7) | 49 | 0.977 | insignificant R | 19 | 0.43 | |
| 320 | 5.5 (+0.8–+6.3) | 98 | 0.975 | insignificant R | insignificant R | ||
| Statistics | Sampling Sites | ||
|---|---|---|---|
| TUIM | BID | KAM | |
| Sample | |||
| Number of trees * | 84 (35) | 68 (35) | 61 (43) |
| Cover period, years | 1719–2019 | 1704–2019 | 1710–2019 |
| Average TRW, cm | 1.265 | 1.228 | 0.924 |
| Residual chronology | |||
| Mean inter-series correlation | 0.496 | 0.469 | 0.451 |
| Standard deviation | 0.399 | 0.283 | 0.357 |
| Mean sensitivity coefficient | 0.462 | 0.330 | 0.380 |
| Climatic Variable | TUIM | BID | KAM |
|---|---|---|---|
| Previous vegetative season | |||
| Pprev | 21 Jul *–1 Oct */0.48 † | 16 Jul *–1 Oct */0.47 † | 19 Jul *–1 Oct */0.49 † |
| Tmean_prev | 22 Jul *–21 Sep */−0.35 † | 9 Jul *–22 Sep */−0.33 † | 1 Jul *–21 Sep */−0.49 † |
| Tmax_prev | 18 Jul *–21 Sep */−0.58 † | 6 Jul *–22 Sep */−0.46 † | 1 Jul *–22 Sep */−0.64 † |
| Cold season | |||
| Tmin_cold | 12 Nov *–5 Dec */0.34 † | 12 Nov *–7 Dec */0.36 † | 12 Nov *–7 Dec */0.47 † |
| Tmax_cold | 11 Nov *–8 Dec */0.42 † | 12 Nov *–7 Dec */0.39 † | 11 Nov *–8 Dec */0.52 † |
| Current vegetative season | |||
| Pcurr | 28 Mar–21 Jul/0.47 † | 28 Mar–21 Jul/0.38 † | 28 Mar–21 Jul/0.21 |
| Tmin_curr | 22 Apr–10 Jun/−0.41 † | 18 Apr–10 Jun/−0.24 | 19 Apr–10 Jun/−0.29 |
| Tmean_curr | 22 Apr–21 Jul/−0.42 † | 11 Apr–22 Jul/−0.33 † | 19 Apr–23 Jul/−0.45 † |
| Tmax_curr | 21 Apr–22 Jul/−0.45 † | 17 Apr–23 Jul/−0.43 † | 18 Apr–23 Jul/−0.47 † |
| T20_curr | 24 Apr–18 Jul/−0.47 † | 21 Apr–17 Jul/−0.47 † | 25 Apr–18 Jul/−0.58 † |
| T40_curr | 26 Apr–12 Aug/−0.51 † | 23 Apr–13 Aug/−0.49 † | 27 Apr–9 Aug/−0.59 † |
| T80_curr | 6 May–31 May/−0.50 † | 25 Apr–1 Jun/−0.31 | 27 Apr–1 Jun/−0.41 † |
| T160_curr | 1 Jun–4 Aug/−0.48 † | 1 Jun–21 Jul/−0.35 † | 1 Jun–21 Jul/−0.57 † |
| Cluster | Characteristics | TUIM | BID | KAM |
|---|---|---|---|---|
| I | N | 16 | 14 | 2 |
| min–max | 62–220 | 41–119 | 34–37 | |
| mean ± SD | 130 ± 47 a | 83 ± 27 a | 36 ± 2 a | |
| II | N | 11 | 11 | 25 |
| min–max | 67–186 | 42–247 | 101–288 | |
| mean ± SD | 119 ± 36 a | 124 ± 63 ab | 186 ± 69 b | |
| III | N | 8 | 10 | 16 |
| min–max | 79–174 | 51–319 | 68–286 | |
| mean ± SD | 120 ± 36 a | 168 ± 89 b | 132 ± 51 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belokopytova, L.V.; Zhirnova, D.F.; Meko, D.M.; Babushkina, E.A.; Vaganov, E.A.; Krutovsky, K.V. Tree Rings Reveal the Impact of Soil Temperature on Larch Growth in the Forest-Steppe of Siberia. Forests 2021, 12, 1765. https://doi.org/10.3390/f12121765
Belokopytova LV, Zhirnova DF, Meko DM, Babushkina EA, Vaganov EA, Krutovsky KV. Tree Rings Reveal the Impact of Soil Temperature on Larch Growth in the Forest-Steppe of Siberia. Forests. 2021; 12(12):1765. https://doi.org/10.3390/f12121765
Chicago/Turabian StyleBelokopytova, Liliana V., Dina F. Zhirnova, David M. Meko, Elena A. Babushkina, Eugene A. Vaganov, and Konstantin V. Krutovsky. 2021. "Tree Rings Reveal the Impact of Soil Temperature on Larch Growth in the Forest-Steppe of Siberia" Forests 12, no. 12: 1765. https://doi.org/10.3390/f12121765
APA StyleBelokopytova, L. V., Zhirnova, D. F., Meko, D. M., Babushkina, E. A., Vaganov, E. A., & Krutovsky, K. V. (2021). Tree Rings Reveal the Impact of Soil Temperature on Larch Growth in the Forest-Steppe of Siberia. Forests, 12(12), 1765. https://doi.org/10.3390/f12121765








