ATR-FTIR Study of Alaska Yellow Cedar Extractives and Relationship with Their Natural Durability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Origin and Preparation
2.2. Total Extractives Analysis
2.3. Durability Assessment
2.3.1. Wood Decay Testing
2.3.2. Termite Testing
2.4. ATR-FTIR Spectroscopy
2.5. Statistical Analysis
3. Results and Discussion
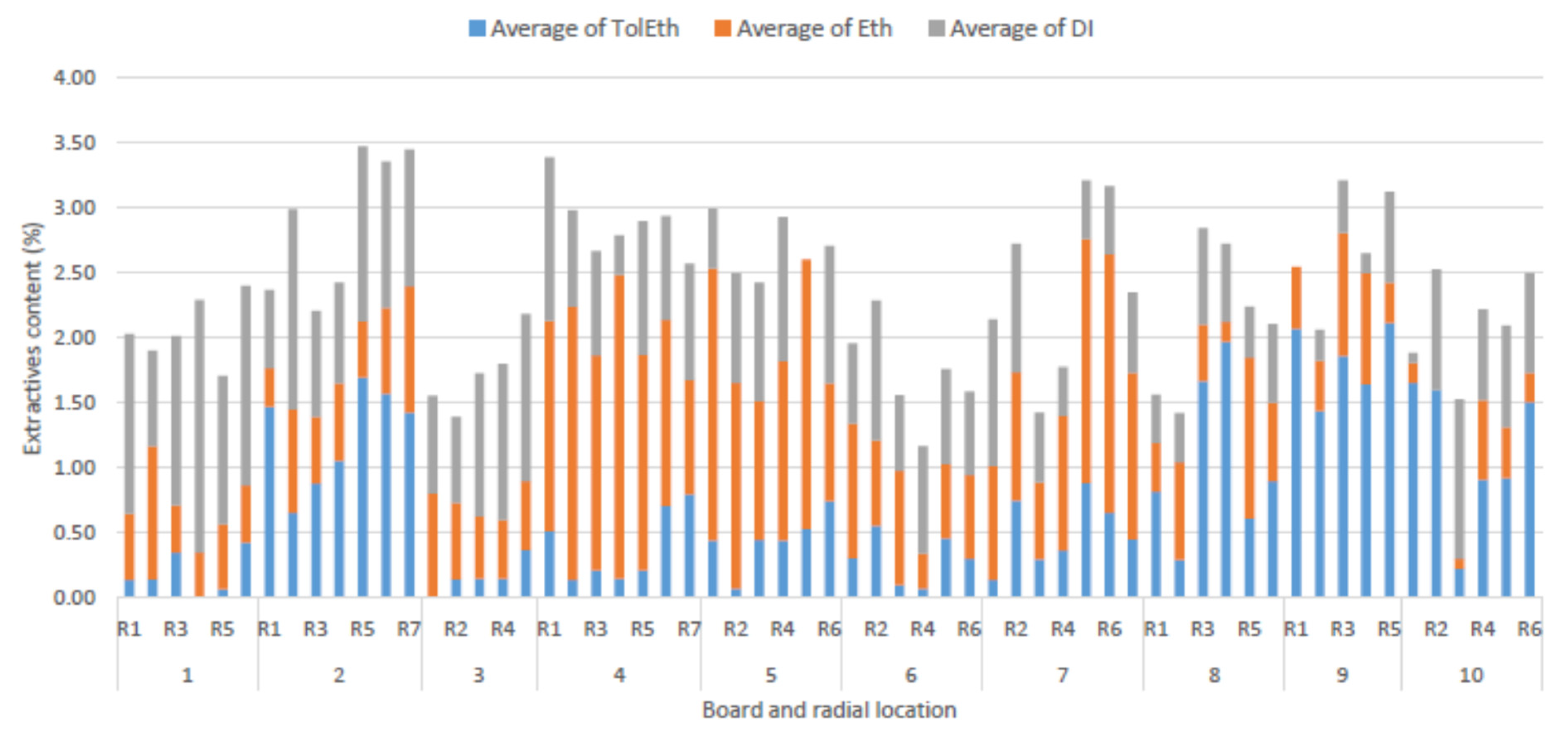
3.1. Extractives Analysis
3.2. Weight Loss to Decay Fungi and Termites
3.3. Relationship between Weight Loss and Extractive Content
3.4. ATR-FTIR Spectra
3.5. ATR-FTIR for Identifying Alaska Yellow Cedar Durability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hillis, W.E. Heartwood and Tree Exudates; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Haupt, M.; Leithoff, H.; Meier, D.; Puls, J.; Richter, H.G.; Faix, O. Heartwood extractives and natural durability of plantation-grown teakwood (Tectona grandis L.)—A case study. Eur. J. Wood Wood Prod. 2003, 61, 473–474. [Google Scholar] [CrossRef]
- França, F.J.N.; França, T.S.F.A.; Arango, R.M.; Woodward, B.M.; Vidaurre, G.B. Variation in natural durability of seven Eucalyptus grandis × Eucalyptus hybrid clones. For. Prod. J. 2017, 67, 230–235. [Google Scholar]
- Scheffer, T.C.; Cowling, E.B. Natural resistance of wood to microbial deterioration. Annu. Rev. Phytopathol. 1966, 4, 147–168. [Google Scholar] [CrossRef]
- Nault, J. Radial distribution of thujaplicins in old growth and second growth western red cedar (Thuja plicata Donn). Wood Sci. Technol. 1988, 22, 73–80. [Google Scholar] [CrossRef]
- Gierlinger, N.; Wimmer, R. Radial distribution of heartwood extractives and lignin in mature European larch. Wood Fiber Sci. 2004, 36, 387–394. [Google Scholar]
- Lukmandaru, G.; Takahashi, K. Radial distribution of quinones in plantation teak (Tectona grandis L.f.). Ann. For. Sci. 2009, 66, 605. [Google Scholar] [CrossRef] [Green Version]
- Hashida, K.; Tabata, M.; Kuroda, K.; Otsuka, Y.; Kubo, S.; Makino, R.; Kubojima, Y.; Tonosaki, M.; Ohara, S. Phenolic extractives in the trunk of Toxicodendron vernicifluum: Chemical characteristics, contents and radial distribution. J. Wood Sci. 2014, 60, 160–168. [Google Scholar] [CrossRef]
- Anderson, A.B.; Scheffer, T.; Duncan, C.G. The chemistry of decay resistance and its decrease with heartwood aging in incense cedar (Libocedrus decurrens Torrey). Holzforschung 1963, 17, 1–5. [Google Scholar] [CrossRef]
- Dizhbite, T. Characterization of the radical scavenging activity of lignins—Natural antioxidants. Bioresour. Technol. 2004, 95, 309–317. [Google Scholar] [CrossRef]
- Gao, H.; Shupe, T.F.; Eberhardt, T.L.; Hse, C.Y. Antioxidant activity of extracts from the wood and bark of Port Orford cedar. J. Wood Sci. 2007, 53, 147–152. [Google Scholar] [CrossRef]
- AWPA. E10-16 Laboratory method for evaluating the decay resistance of wood-based materials against pure basidiomycete cultures: Soil/block test. In Annual Book of AWPA Standards; American Wood Protection Association: Birmingham, AL, USA, 2017; pp. 402–413. [Google Scholar]
- AWPA. E7-15 Standard field test for evaluation of wood preservatives to be used in ground contact (UC4a, UC4b, UC4c): Stake test. In Annual Book of AWPA Standards; American Wood Protection Association: Birmingham, AL, USA, 2015; p. 10. [Google Scholar]
- Stirling, R. Natural durability classification systems used around the world. In Proceedings of the IRG Annual Meeting, Beijing, China, 24–28 May 2009; IRG/WP 09-10694. International Research Group on Wood Protection: Stockholm, Sweden, 2009. [Google Scholar]
- Miller, R.B.; Wiedenhoeft, A.C.; Williams, R.S.; Stockman, W.; Green, F. Characteristics of ten tropical hardwoods from certified forests in Bolivia. Part II, Natural durability to decay fungi. Wood Fiber Sci. 2003, 35, 429–433. [Google Scholar]
- Pandey, K.K. A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. J. Appl. Polym. Sci. 1999, 71, 1969–1975. [Google Scholar] [CrossRef]
- So, C.-L.; Via, B.K.; Groom, L.H.; Schimleck, L.R.; Shupe, T.F.; Kelley, S.S.; Rials, T.G. Near infrared spectroscopy in the forest products industry. For. Prod. J. 2004, 54, 6–16. [Google Scholar]
- Tsuchikawa, S.; Schwanninger, M. A review of recent near-infrared research for wood and paper (Part 2). Appl. Spectrosc. Rev. 2013, 48, 560–587. [Google Scholar] [CrossRef]
- Lipeh, S.; Schimleck, L.R.; Mankowski, M.E.; McDonald, A.G.; Morrell, J.J. Relationship between attenuated total reflectance Fourier transform infrared spectroscopy of western juniper and natural resistance to fungal and termite attack. Holzforschung 2020, 74, 246–259. [Google Scholar] [CrossRef]
- Ajuong, E.M.; Breese, M.C. Fourier transform infrared characterization of Pai wood (Afzelia africana Smith) extractives. Holz Roh Werkst. 1998, 56, 139–142. [Google Scholar] [CrossRef]
- Schwanninger, M.; Hinterstoisser, B. Determination of the lignin content in wood by FT-NIR. In Proceedings of the 11th ISWPC, International Symposium on Wood and Pulping Chemistry, Nice, France, 11–14 June 2001; pp. 641–644. [Google Scholar]
- Jones, R.W.; Meglen, R.R.; Hames, B.R.; McClelland, J.F. Chemical analysis of wood chips in motion using thermal-emission mid-infrared spectroscopy with projection to latent structures regression. Anal. Chem. 2002, 74, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Hodge, G.R.; Woodbridge, W.C. Use of near infrared spectroscopy to predict lignin content in tropical and sub-tropical pines. J. Near Infrared Spectrosc. 2004, 12, 381–390. [Google Scholar] [CrossRef]
- Poke, F.S.; Raymond, C.A. Predicting extractives, lignin, and cellulose contents using near infrared spectroscopy on solid wood in Eucalyptus globulus. J. Wood Chem. Technol. 2006, 26, 187–199. [Google Scholar] [CrossRef]
- Schimleck, L.R.; Evans, R. Estimation of wood stiffness of increment cores by near infrared spectroscopy: The development and application of calibrations based on selected cores. IAWA J. 2002, 23, 217–224. [Google Scholar] [CrossRef]
- Meder, R.; Gallagher, S.; Mackie, K.L.; Böhler, H.; Meglen, R.R. Rapid determination of the chemical composition and density of Pinus radiata by PLS modelling of transmission and diffuse reflectance FTIR spectra. Holzforschung 1999, 53, 261–266. [Google Scholar] [CrossRef]
- Kelley, S.S.; Rials, T.G.; Snell, R.; Groom, L.H.; Sluiter, A. Use of near infrared spectroscopy to measure the chemical and mechanical properties of solid wood. Wood Sci. Technol. 2004, 38, 257–276. [Google Scholar] [CrossRef]
- Schimleck, L.R.; Rezende, G.D.; Demuner, B.J.; Downes, G.M. Estimation of whole-tree wood quality traits using near infrared spectra from increment cores. Appita J. 2006, 59, 231–236. [Google Scholar]
- Gierlinger, N.; Jacques, D.; Schwanninger, M.; Wimmer, R.; Hinterstoisser, B.; Pâques, L.E. Rapid prediction of natural durability of larch heartwood using Fourier transform near-infrared spectroscopy. Can. J. For. Res. 2003, 33, 1727–1736. [Google Scholar] [CrossRef]
- Sykacek, E.; Gierlinger, N.; Wimmer, R.; Schwanninger, M. Prediction of natural durability of commercial available European and Siberian larch by near-infrared spectroscopy. Holzforschung 2006, 60, 643–647. [Google Scholar] [CrossRef]
- Stirling, R.; Trung, T.; Breuil, C.; Bicho, P. Predicting wood decay and density using NIR spectroscopy. Wood Fiber Sci. 2007, 39, 414–423. [Google Scholar]
- Stirling, R.; Morris, P.I.; Grace, J.K. Prediction of the decay and termite resistance of western red cedar heartwood. For. Prod. J. 2015, 65, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Altaner, C. Predicting extractives content of Eucalyptus bosistoana F. Muell. heartwood from stem cores by near infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 198, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Lipeh, S.; Schimleck, L.R.; Morrell, J.J. Determination of carvacrol content in Alaska yellow cedar (Callitropsis nootkatensis) extractives. Bioresources 2019, 14, 9928–9944. [Google Scholar]
- Gierlinger, N.; Schwanninger, M.; Hinterstoisser, B.; Wimmer, R. Rapid determination of heartwood extractives in Larix sp by means of Fourier transform near infrared spectroscopy. J. Near Infrared Spectrosc. 2002, 10, 203–214. [Google Scholar] [CrossRef]
- Schimleck, L.R.; Michell, A.J.; Vinden, P. Eucalypt wood classification by NIR spectroscopy and principal components analysis. Appita J. 1996, 49, 319–324. [Google Scholar]
- Martin, M.; Martin, F.L. Identifying variables responsible for clustering in discriminant analysis of data from infrared microspectroscopy of a biological sample. J. Comput. Biol. 2007, 14, 1176–1184. [Google Scholar] [CrossRef]
- Toscano, G.; Rinnan, A.; Pizzi, A.; Mancini, M. The use of near-infrared (NIR) spectroscopy and principal component analysis (PCA) to discriminate bark and wood of the most common species of the pellet sector. Energy Fuels 2017, 31, 2814–2821. [Google Scholar] [CrossRef]
- Meder, R.; Thumm, A.; Bier, H. Veneer stiffness predicted by NIR spectroscopy calibrated using mini-LVL test panels. Holz Roh Werkst. 2002, 60, 159–164. [Google Scholar] [CrossRef]
- Nascimbem, L.B.L.R.; Rubini, B.R.; Poppi, R.J. Determination of quality parameters in moist wood chips by near infrared spectroscopy combining PLS-DA and Support Vector Machines. J. Wood Chem. Technol. 2013, 33, 247–257. [Google Scholar] [CrossRef]
- Harris, A.S. Chamaecyparis nootkatensis (D.Don) Spach, Alaska-cedar. In Silvics of North America; Burns, R.M., Honkala, B.H., Eds.; Technical Coordinators, Conifers, Agriculture, Handbook 654; USDA-FS: Washington, DC, USA, 1990; Volume 1, pp. 97–102. [Google Scholar]
- Sturrock, R.N. Phellinus weirii and other fungi causing decay in western redcedar and yellow-cedar. In A Tale of Two Cedars—International Symposium on Western Red Cedar and Yellow-Cedar; USDA-FS: Portland, OR, USA, 2010. [Google Scholar]
- Kelsey, R.G.; Hennon, P.E.; Huso, M.; Karchesy, J.J. Changes in heartwood chemistry of dead yellow-cedar trees that remain standing for 80 years or more in southeast Alaska. J. Chem. Ecol. 2005, 31, 2653–2670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karchesy, J.J.; Kelsey, R.G.; González-Hernández, M.P. Yellow-cedar, Callitropsis (Chamaecyparis) nootkatensis, secondary metabolites, biological activities, and chemical ecology. J. Chem. Ecol. 2018, 44, 510–524. [Google Scholar] [CrossRef] [PubMed]
- Jozsa, L.A. Yellow cypress wood quality and the hinoki connection. In Yellow Cypress: Can We Grow It? Can We Sell It? Lousier, J.D., Ed.; Forestry Canada British Columbia Ministry of Forests: Victoria, BC, Canada, 1991; pp. 9–12. [Google Scholar]
- Hennon, P.E.; Shaw, C.G.; Hansen, E.M. Dating decline and mortality of Chamaecyparis nootkatensis in southeast Alaska. For. Sci. 1990, 36, 502–515. [Google Scholar]
- Beier, C.M.; Sink, S.E.; Hennon, P.E.; D’Amore, D.V.; Juday, G.P. Twentieth-century warming and the dendroclimatology of declining yellow-cedar forests in southeastern Alaska. Can. J. For. Res. 2008, 38, 1319–1334. [Google Scholar] [CrossRef] [Green Version]
- Ajuong, E.; Freitag, C.; Morrell, J.J. Decay resistance and extractive content of second growth Port Orford cedar (Chamaecyparis lawsoniana) wood. Wood Fiber Sci. 2014, 46, 502–509. [Google Scholar]
- ASTM. D4442-16 Standard test methods for direct moisture content measurement of wood and wood-based materials. In ASTM Annual Book of Standards; ASTM International: West Conshohocken, PA, USA, 2017; pp. 415–420. [Google Scholar]
- Scheffer, T.C. Microbiological degradation and the causal organisms; Degradation and protection of wood. In Wood Deterioration and Its Prevention by Preservative Treatment; Nicholas, D.D., Ed.; Syracuse University Press: Syracuse, NY, USA, 1973; Volume 1, pp. 31–106. [Google Scholar]
- Taylor, A.M.; Gartner, B.L.; Morrell, J.J.; Tsunoda, K. Effects of heartwood extractive fractions of Thuja plicata and Chamaecyparis nootkatensis on wood degradation by termites or fungi. J. Wood Sci. 2006, 52, 147–153. [Google Scholar] [CrossRef]
- ASTM. D1105-96 Standard test method for preparation of extractive free wood. In ASTM Annual Book of Standards; ASTM International: West Conshohocken, PA, USA, 2017; pp. 151–152. [Google Scholar]
- AWPA. E30-16 Standard method for evaluating natural decay resistance of wood using laboratory decay tests. In Annual Book of AWPA Standards; American Wood Protection Association: Birmingham, AL, USA, 2019; pp. 490–493. [Google Scholar]
- Duncan, C.G.; Lombard, F.F. Fungi Associated with Principal Decays in Wood Products in the United States; USDA-FS Research Paper WO-4; USDA-FS: Washington, DC, USA, 1965.
- AWPA. E1-16 Laboratory method for evaluating the termite resistance of wood-based materials: Choice and no-choice tests. In Annual Book of AWPA Standards; American Wood Protection Association: Birmingham, AL, USA, 2017; pp. 375–380. [Google Scholar]
- RStudio. RStudio: Integrated Development for R; RStudio: Boston, MA, USA, 2016; Available online: http://www.rstudio.com/ (accessed on 24 August 2018).
- Hanson, B.A. ChemoSpec: An R Package for Chemometric Analysis of Spectroscopic Data (Package Version 4.4.85). 2017. Available online: https://mran.microsoft.com/snapshot/2017-08-06/web/packages/ChemoSpec/vignettes/ChemoSpec.pdf (accessed on 24 August 2018).
- Kirker, G.T.; Blodgett, A.B.; Arango, R.A.; Lebow, P.K.; Clausen, C.A. The role of extractives in naturally durable wood species. Int. Biodeterior. Biodegrad. 2013, 82, 53–58. [Google Scholar] [CrossRef]
- Sjöström, E. Wood Chemistry, 2nd ed.; Academic Press: San Diego, CA, USA, 1993; pp. 90–108. [Google Scholar]
- Khasawneh, M.A.; Karchesy, J.J. Terpenoids of the heartwood of Chamaecyparis nootkatensis. Am. J. Org. Chem. 2011, 1, 1–5. [Google Scholar] [CrossRef]
- Kelsey, R.; González-Hernández, M.; Karchesy, J.; Veluthoor, S. Volatile terpenoids and tropolones in heartwood extracts of yellow-cedar, Monterey cypress, and their hybrid Leyland cypress. Ann. For. Sci. 2015, 72, 349–355. [Google Scholar] [CrossRef]
- Bharadwaj, A.; Stafford, K.C.; Behle, R.W. Efficacy and environmental persistence of nootkatone for the control of the blacklegged tick (Acari: Ixodidae) in residential landscapes. J. Med. Entomol. 2012, 49, 1035–1044. [Google Scholar] [CrossRef]
- De Groot, R.C.; Woodward, B.; Hennon, P.E. Natural decay resistance of heartwood from dead, standing yellow-cedar trees: Laboratory evaluations. For. Prod. J. 2000, 50, 53–59. [Google Scholar]
- Grace, J.K.; Yamamoto, R.T. Natural resistance of Alaska-cedar, redwood, and teak to Formosan subterranean termites. For. Prod. J. 1994, 44, 41–45. [Google Scholar]
- Arango, R.A.; Green, F.; Hintz, K.; Lebow, P.K.; Miller, R.B. Natural durability of tropical and native woods against termite damage by Reticulitermes flavipes (Kollar). Int. Biodeterior. Biodegrad. 2006, 57, 146–150. [Google Scholar] [CrossRef]
- Rao, A.; Zhang, Y.; Muend, S.; Rao, R. Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the TOR pathway. Antimicrob. Agents Chemother. 2010, 54, 5062–5069. [Google Scholar] [CrossRef] [Green Version]
- Panella, N.A.; Dolan, M.C.; Karchesy, J.J.; Xiong, Y.; Peralta-Cruz, J.; Khasawneh, M.A.; Montenieri, J.A.; Maupin, G.O. Use of novel compounds for pest control: Insecticidal and acaricidal activity of essential oil components from heartwood of Alaska yellow cedar. J. Med. Entomol. 2018, 42, 352–358. [Google Scholar] [CrossRef]
- Zabel, R.A.; Morrell, J.J. Wood Microbiology: Decay and Its Prevention, 2nd ed.; Academic Press: San Diego, CA, USA, 1992; p. 556. [Google Scholar]
- Kuo, M.; McClelland, J.F.; Luo, S.; Chien, P.-L.; Walker, R.D.; Hse, C.-Y. Applications of infrared photoacoustic spectroscopy for wood samples. Wood Fiber Sci. 2007, 20, 132–145. [Google Scholar]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini-review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Lipeh, S.; Morrell, J.J. Rapid detection of the Alaska yellow cedar, Callitropsis nootkatensis (Cupressaceae) extractives using Fourier transform infrared (FT-IR) spectroscopy. In Proceedings of the IRG Annual Meeting, Ghent, Belgium, 4–8 June 2017; IRG/WP 17-20612. International Research Group on Wood Protection: Stockholm, Sweden, 2017. [Google Scholar]
- Kondal, S.; Cakir, A.; Ozer, H.; Cakmakei, R.; Kesdak, M.; Mete, E. Antifungal, phytotoxic and insecticidal properties of essential oils isolated from Turkish Origenum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar]
- Xie, Y.; Wang, K.; Huang, Q.; Lei, C. Evaluation of toxicity of monoterpenes to subterranean termites, Reticulitermes chinensis Snyder. Ind. Crops Prod. 2014, 53, 163–166. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, T.; Mi, N.; Wang, Y.; Li, G.; Wang, L.; Xi, Y. Antifungal activity of monoterpenes against wood white-rot fungi. Int. Biodeterior. Biodegrad. 2016, 106, 157–160. [Google Scholar] [CrossRef]



| Board | Radial | Extractives Content (%) (n = 3) | Weight Loss (%) | ||
|---|---|---|---|---|---|
| G. trabeum (n = 9) | R. placenta (n = 9) | R. flavipes (n = 9) | |||
| 1 | R1 | 1.96 (0.44) | 12.29 (9.70) | 1.18 (0.86) | 15.32 (4.00) |
| R2 | 1.83 (0.36) | 6.53 (6.48) | 2.01 (0.76) | 16.85 (1.98) | |
| R3 | 1.94 (0.67) | 18.41 (3.69) | 1.01 (0.09) | 18.10 (0.41) | |
| R4 | 1.94 (0.23) | 11.97 (2.77) | 1.03 (0.11) | 15.11 (6.99) | |
| R5 | 1.13 (1.30) | 17.10 (14.14) | 3.02 (2.87) | 26.21 (15.34) | |
| R6 | 2.10 (0.15) | 37.58 (28.73) | 19.01 (19.38) | 33.33 (17.54) | |
| 2 | R1 | 2.37 (0.76) | 28.73 (30.74) | 17.26 (0.62) | 18.17 (2.28) |
| R2 | 2.99 (0.42) | 17.42 (18.79) | 8.96 (1.58) | 8.38 (3.41) | |
| R3 | 2.21 (0.37) | 16.19 (20.50) | 4.80 (3.63) | 9.32 (2.66) | |
| R4 | 2.43 (0.44) | 15.37 (13.35) | 5.47 (4.06) | 9.99 (0.66) | |
| R5 | 2.81 (3.39) | 12.91 (17.70) | 5.49 (2.21) | 9.48 (4.22) | |
| R6 | 2.84 (3.59) | 18.78 (25.77) | 8.36 (1.97) | 12.78 (1.70) | |
| R7 | 2.45 (3.73) | 12.74 (17.45) | 17.17 (15.43) | 10.85 (2.16) | |
| 3 | R1 | 1.48 (0.88) | 0.85 (0.04) | 4.94 (0.50) | 6.70 (2.84) |
| R2 | 1.17 (0.62) | 1.24 (0.38) | 11.87 (9.12) | 18.87 (1.76) | |
| R3 | 1.69 (0.49) | 8.23 (10.51) | 9.17 (12.25) | 14.35 (0.69) | |
| R4 | 1.80 (1.18) | 10.89 (12.11) | 0.62 (0.35) | 14.88 (0.34) | |
| R5 | 1.17 (0.49) | 0.50 (0.08) | 0.73 (0.31) | 19.71 (3.01) | |
| R6 | 0.84 (0.60) | 0.88 (0.33) | 1.07 (0.24) | 17.85 (0.55) | |
| R7 | 2.18 (0.13) | 0.99 (0.44) | 1.34 (0.30) | 12.35 (0.60) | |
| 4 | R1 | 3.39 (1.23) | 3.44 (0.70) | 3.82 (0.29) | 5.07 (1.00) |
| R2 | 2.76 (0.47) | 3.42 (0.33) | 3.75 (0.91) | 11.81 (2.21) | |
| R3 | 2.59 (0.36) | 2.26 (0.00) | 9.06 (0.00) | 6.65 (6.59) | |
| R4 | 2.64 (0.56) | 3.96 (1.05) | 6.10 (4.82) | 5.26 (1.39) | |
| R5 | 2.90 (0.50) | 2.31 (0.66) | 4.43 (1.81) | 7.85 (2.09) | |
| R6 | 2.94 (0.55) | 2.20 (0.90) | 2.23 (0.63) | 8.10 (1.29) | |
| R7 | 2.57 (0.51) | 2.76 (0.06) | 4.15 (1.54) | 6.29 (2.43) | |
| 5 | R1 | 2.99 (0.31) | 0.15 (4.39) | 0.34 (1.87) | 14.21 (3.18) |
| R2 | 2.50 (0.57) | −1.10 (3.51) | −0.53 (0.91) | 10.99 (2.83) | |
| R3 | 2.43 (0.41) | 0.86 (3.83) | −0.52 (1.06) | 12.37 (0.93) | |
| R4 | 2.93 (0.35) | 3.21 (1.02) | −0.25 (2.67) | 13.68 (2.07) | |
| R5 | 2.60 (0.11) | 0.39 (1.93) | −0.97 (1.59) | 12.50 (2.71) | |
| R6 | 2.71 (0.19) | 5.22 (12.11) | −0.21 (1.89) | 10.38 (0.80) | |
| 6 | R1 | 1.89 (0.98) | 1.01 (0.89) | 0.99 (0.86) | 1.06 (0.92) |
| R2 | 2.22 (0.91) | 0.61 (0.53) | 0.87 (0.77) | 2.57 (1.03) | |
| R3 | 1.17 (0.49) | 1.12 (0.97) | 0.95 (0.82) | 1.38 (1.35) | |
| R4 | 0.84 (0.60) | 1.95 (0.36) | 1.49 (0.16) | 1.49 (0.13) | |
| R5 | 1.82 (0.50) | 1.50 (0.09) | 1.79 (0.13) | 2.31 (1.66) | |
| R6 | 1.59 (0.80) | 2.02 (0.10) | 1.38 (0.13) | 1.31 (1.27) | |
| 7 | R1 | 1.92 (0.40) | 33.62 (26.34) | 11.40 (14.02) | 10.74 (2.59) |
| R2 | 2.72 (0.04) | 26.95 (20.26) | 7.93 (7.27) | 12.51 (1.89) | |
| R3 | 1.20 (0.38) | 31.59 (39.52) | 8.36 (8.37) | 10.01 (0.43) | |
| R4 | 1.78 (0.80) | 27.07 (33.63) | 6.22 (5.20) | 7.19 (3.38) | |
| R5 | 3.21 (0.39) | 14.13 (15.63) | 5.66 (3.87) | 3.38 (1.98) | |
| R6 | 3.17 (0.66) | 12.75 (11.86) | 7.60 (6.79) | 10.04 (2.78) | |
| R7 | 2.35 (0.38) | N/a | 6.17 (4.37) | 7.15 (1.40) | |
| 8 | R1 | 1.27 (1.10) | 1.22 (0.23) | 1.52 (0.43) | 6.40 (1.89) |
| R2 | 1.20 (0.90) | 0.30 (0.26) | 4.55 (1.19) | 5.62 (1.00) | |
| R3 | 2.69 (0.27) | −0.76 (3.13) | 3.15 (1.89) | 4.15 (0.92) | |
| R4 | 2.14 (0.73) | 1.54 (0.24) | 1.74 (0.21) | 7.26 (3.76) | |
| R5 | 2.24 (0.52) | 1.50 (0.22) | 1.62 (0.14) | 6.01 (4.90) | |
| R6 | 2.11 (0.33) | 1.82 (0.27) | 1.43 (0.38) | 5.61 (2.34) | |
| 9 | R1 | 2.15 (0.45) | 13.72 (12.81) | 0.46 (0.61) | 21.44 (2.19) |
| R2 | 3.10 (0.22) | −1.20 (1.43) | 1.00 (0.72) | 6.47 (0.99) | |
| R3 | 2.90 (1.01) | 21.22 (5.77) | 1.22 90.60) | 38.62 (4.15) | |
| R4 | 2.65 (0.48) | 6.26 (8.82) | 1.00 (0.79) | 8.43 (1.57) | |
| R5 | 3.12 (0.83) | 10.94 (6.48) | 12.22 (8.21) | 25.90 (2.85) | |
| R6 | N/a | 19.82 (3.41) | 16.85 (11.63) | 30.59 (3.72) | |
| 10 | R1 | 1.80 (0.30) | 13.72 (12.81) | 0.46 (0.61) | 3.29 (0.86) |
| R2 | 2.14 (0.64) | −1.20 (1.43) | 1.00 (0.72) | 5.38 (1.36) | |
| R3 | 1.37 (0.22) | 21.22 (5.77) | 1.22 (0.60) | 7.45 (1.64) | |
| R4 | 2.22 (0.48) | 6.26 (8.82) | 1.00 (0.79) | 3.75 (0.88) | |
| R5 | 2.09 (0.22) | 10.94 (6.48) | 12.22 (8.21) | 6.89 (0.22) | |
| R7 | 2.34 (0.62) | 19.82 (3.41) | 16.85 (11.63) | 4.39 (1.60) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipeh, S.; Schimleck, L.; Mankowski, M.E.; McDonald, A.G.; Morrell, J.J. ATR-FTIR Study of Alaska Yellow Cedar Extractives and Relationship with Their Natural Durability. Forests 2021, 12, 1692. https://doi.org/10.3390/f12121692
Lipeh S, Schimleck L, Mankowski ME, McDonald AG, Morrell JJ. ATR-FTIR Study of Alaska Yellow Cedar Extractives and Relationship with Their Natural Durability. Forests. 2021; 12(12):1692. https://doi.org/10.3390/f12121692
Chicago/Turabian StyleLipeh, Shahlinney, Laurence Schimleck, Mark E. Mankowski, Armando G. McDonald, and Jeffrey J. Morrell. 2021. "ATR-FTIR Study of Alaska Yellow Cedar Extractives and Relationship with Their Natural Durability" Forests 12, no. 12: 1692. https://doi.org/10.3390/f12121692
APA StyleLipeh, S., Schimleck, L., Mankowski, M. E., McDonald, A. G., & Morrell, J. J. (2021). ATR-FTIR Study of Alaska Yellow Cedar Extractives and Relationship with Their Natural Durability. Forests, 12(12), 1692. https://doi.org/10.3390/f12121692








