Directional Variability in Response of Pinus koraiensis Radial Growth to Climate Change
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tree Rings and Climate Data
2.2. Radial Growth Process Analysis
2.3. Radial Growth–Climate Analysis
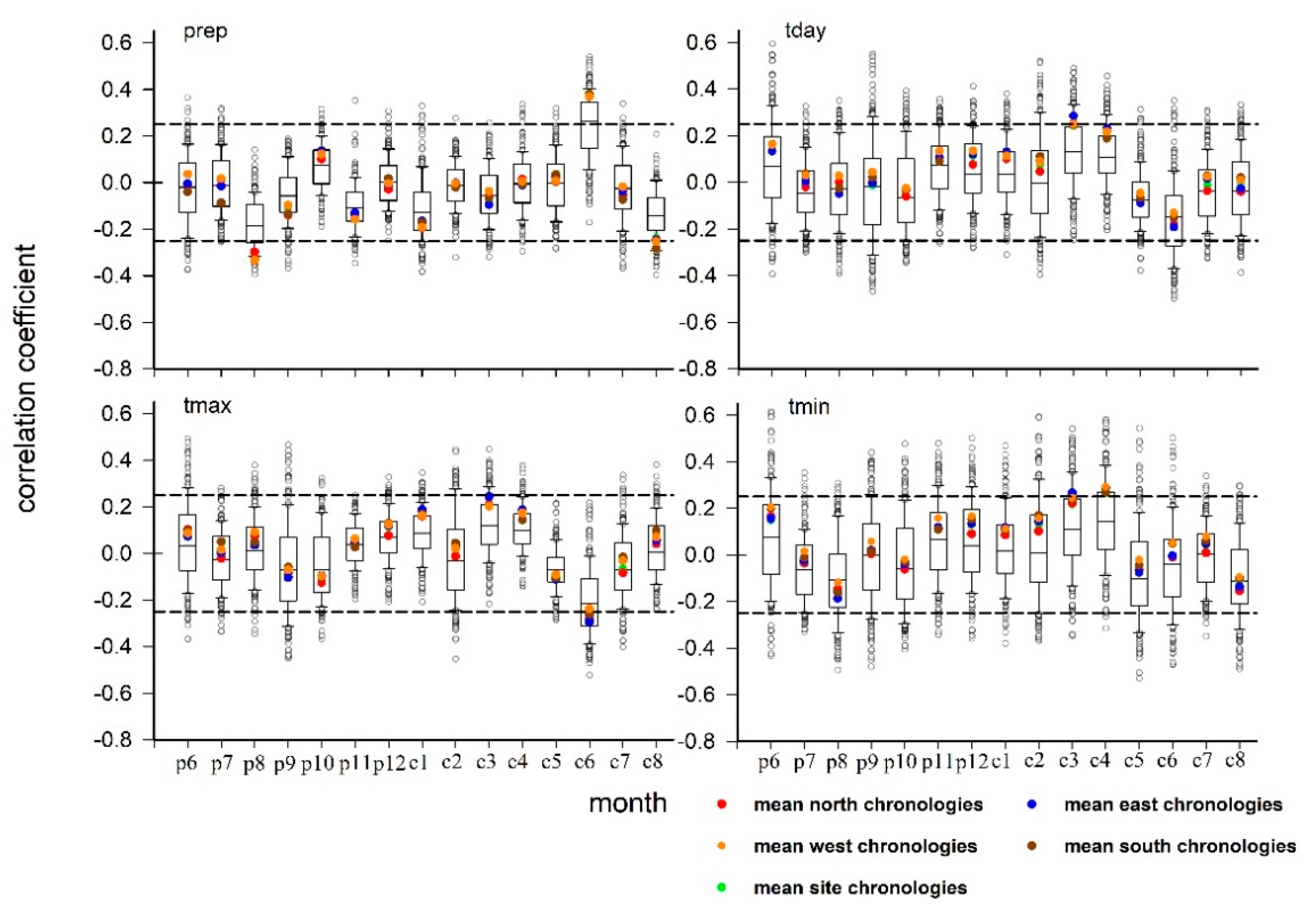
3. Results
3.1. Directional Differences of Tree Ring Indices
3.2. Direction Differences in Climate–Growth Relationship
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [Green Version]
- Matías, L.; Linares, J.C.; Sánchez-Miranda, Á.; Jump, A.S. Contrasting growth forecasts across the geographical range of scots pine due to altitudinal and latitudinal differences in climatic sensitivity. Glob. Chang. Biol. 2017, 23, 4106. [Google Scholar] [CrossRef]
- Babst, F.; Bodesheim, P.; Charney, N.; Friend, A.D.; Girardin, M.P.; Klesse, S. When tree rings go global: Challenges and opportunities for retro- and prospective insight. Quat. Sci. Rev. 2018, 197, 1–20. [Google Scholar] [CrossRef]
- Yuan, D.Y.; Zhao, H.Y.; Li, Z.S.; Zhu, L.J.; Guo, M.; Zhang, Y.D.; Wang, X.C. Radial growth of Pinus koraiensis and Picea koraiensis response to climate change in Yichun City, Heilongjiang Province. Acta Ecol. Sin. 2020, 40, 1150–1160. [Google Scholar]
- Liang, H.; Huang, J.G.; Ma, Q.; Li, J.; Wang, Z.; Guo, X.; Zhu, H.; Jiang, S.; Zhou, P.; Yu, B.; et al. Contributions of competition and climate on radial growth of Pinus massoniana in subtropics of China. Agric. For. Meteorol. 2019, 274, 7–17. [Google Scholar] [CrossRef]
- Huang, J.; Tardif, J.C.; Bergeron, Y.; Denneler, B.; Berninger, F.; Girardin, M.P. Radial growth response of four dominant boreal tree species to climate along a latitudinal gradient in the eastern canadian boreal forest. Glob. Chang. Biol. 2010, 16, 711–731. [Google Scholar] [CrossRef]
- Nicault, A.; Boucher, E.; Tapsoba, D.; Arseneault, D.; Berninger, F.; Bégin, C.; DesGranges, J.L.; Guiot, J.; Marion, J.; Wicha, S.; et al. Spatial analysis of black spruce, Picea mariana, Mill. (B.S.P.) radial growth response to climate in northern Québec-Labrador Peninsula, Canada. Can. J. For. Res. 2015, 45, 343–352. [Google Scholar] [CrossRef] [Green Version]
- Lempereur, M.; Limousin, J.M.; Guibal, F.; Ourcival, J.M.; Mouillot, F. Recent climate hiatus revealed dual control by temperature and drought on the stem growth of mediterranean Quercus ilex. Glob. Chang. Biol. 2017, 23, 42–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozas, V. Individual-based approach as a useful tool to disentangle the relative importance of tree age, size and inter-tree competition in dendroclimatic studies. iForest 2014, 8, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Clark, J.S.; Bell, D.M.; Kwit, M.; Stine, A.; Vierra, B.; Zhu, K. Individual-scale inference to anticipate climate-change vulnerability of biodiversity. Philos. Trans. R. Soc. B 2012, 367, 236. [Google Scholar] [CrossRef] [Green Version]
- Abiyu, A.; Mokria, M.; Gebrekirstos, A.; Braüning, A. Tree-ring record in ethiopian church forests reveals successive generation differences in growth rates and disturbance events. For. Ecol. Manag. 2018, 409, 835–844. [Google Scholar] [CrossRef]
- Latreille, A.; Davi, H.; Huard, F.; Pichot, C. Variability of the climate-radial growth relationship among Abies alba trees and populations along altitudinal gradients. For. Ecol. Manag. 2017, 396, 150–159. [Google Scholar] [CrossRef]
- Sullivan, P.F.; Ellison, S.B.; Mcnown, R.W.; Brownlee, A.H.; Sveinbjornsson, B. Evidence of soil nutrient availability as the proximate constraint on growth of treeline trees in northwest Alaska. Ecology 2015, 97, 803. [Google Scholar] [CrossRef] [PubMed]
- Ettl, G.J.; Peterson, D.L. Extreme climate and variation in tree growth: Individualistic response in subalpine fir, Abies lasiocarpa. Glob. Chang. Biol. 1995, 1, 231–241. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Academic: San Diego, CA, USA, 1976. [Google Scholar] [CrossRef]
- Speer, J.H. Fundamentals of Tree-Ring Research; University of Arizona: Tucson, AZ, USA, 2010. [Google Scholar] [CrossRef]
- Kelly, D. The evolutionary ecology of mast seeding. Trends Ecol. Evol. 1994, 9, 465–470. [Google Scholar] [CrossRef]
- Chhin, S.; Hogg, E.H.; Lieffers, V.J.; Huang, S. Growth-climate relationships vary with height along the stem in lodgepole pine. Tree Physiol. 2010, 30, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Gao, L.; Qiu, Y.; Guo, J. Characteristics of Korean pine, Pinus koraiensis radial growth at different heights and its response to climate change on Changbai Mountain. Acta Ecol. Sin. 2015, 35, 2978–2984. [Google Scholar]
- Zhang, T.; Huang, L.; Zhang, R.; Gao, Y.; Hu, D.; Yu, S.; Jiang, S. The impacts of climatic factors on radial growth patterns at different stem heights in Schrenk spruce, Piceas chrenkiana. Trees 2020, 34, 163–175. [Google Scholar] [CrossRef]
- Liu, K.X.; Zhang, T.W.; Zhang, R.B.; Yu, S.L.; Hu, D.Y. Characteristics of tree-ring density at different stem heights and their climatic responses. Chin. J. Appl. Ecol. 2021, 32, 503–512. [Google Scholar] [CrossRef]
- Buras, A.; Wilmking, M. Straight lines or eccentric eggs? A comparison of radial and spatial ring width measurements and its implications for climate transfer functions. Dendrochronologia 2014, 32, 313–326. [Google Scholar] [CrossRef]
- Shetti, R.; Buras, A.; Smiljanic, M.; Wilmking, M. Climate sensitivity is affected by growth differentiation along the length of Juniperus communis L. shrub stems in the Ural Mountains. Dendrochronologia 2018, 49, 29–35. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, H.; Zhang, Y.; Li, Z.; Wang, X. Climate–growth relationship for different directions of Pinus pumila radial growth at the treeline of northern Daxing’an Mountains, China. Trees 2018, 32, 311–322. [Google Scholar] [CrossRef]
- Fang, K.; Chen, D.; Gou, X.; D’Arrigo, R.; Davi, N. Influence of non-climatic factors on the relationships between tree growth and climate over the chinese loess plateau. Glob. Planet. Chang. 2015, 132, 54–63. [Google Scholar] [CrossRef]
- Gut, U.; Árvai, M.; Bijak, S.; Camarero, J.J. No systematic effects of sampling direction on climate-growth relationships in a large-scale, multi-species tree-ring data set. Dendrochronologia 2019, 57, 125624. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Ji, Y. Temperature signals in tree-ring width and divergent growth of Korean pine response to recent climate warming in northeast Asia. Trees 2017, 31, 415–427. [Google Scholar] [CrossRef]
- Stokes, M.A.; Smiley, T.L. An Introduction to Tree-Ring Dating; University of Arizona: Tucson, AZ, USA, 1968. [Google Scholar]
- Holmes, R.L. User Manual for Program COFECHA; Laboratory of Tree-Ring Research, University of Arizona: Tucson, AZ, USA, 1999. [Google Scholar]
- Cook, E.R.; Holmes, R.L. User Manual for Program ARSTAN; Laboratory of Tree-Ring Research, University of Arizona: Tucson, AZ, USA, 1999. [Google Scholar]
- Dormann, C.F.; Elith, J.; Bacher, S. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2012, 36, 27–46. [Google Scholar] [CrossRef]
- Harrison, X.A. A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 2018, 6, e4794. [Google Scholar] [CrossRef] [Green Version]
- Buras, A.; van der Maaten-Theunissen, M.; van der Maaten, E.; Ahlgrimm, S.; Hermann, P.; Simard, S.; Heinrich, I.; Helle, G.; Unterseher, M.; Schnittler, M.; et al. Tuning the voices of a choir: Detecting ecological gradients in time-series populations. PLoS ONE 2016, 11, e0158346. [Google Scholar] [CrossRef]
- Mašek, J.; Tumajer, J.; Rydval, M.; Lange, J.; Treml, V. Age and size outperform topographic effects on growth-climate responses of trees in two Central European coniferous forest types. Dendrochronologia 2021, 68, 125845. [Google Scholar] [CrossRef]
- Stock, J.H.; Watson, M.W. Introduction to Econometrics; Prentice Hall: New York, NY, USA, 2003. [Google Scholar]
- Linares, J.C.; Camarero, J.J.; Carreira, J.A. Competition modulates the adaptation capacity of forests to climatic stress: Insights from recent growth decline and death in relict stands of the Mediterranean fir Abies pinsapo. J. Ecol. 2010, 98, 592–603. [Google Scholar] [CrossRef]
- Liu, M.; Mao, Z.J.; Li, Y.; Sun, T.; Li, X.H.; Huang, W.; Liu, R.P.; Li, Y.H. Response of radial growth of Pinus koraiensis in broad-leaved Korean pine forests with different latitudes to climatical factors. Chin. J. Appl. Ecol. 2016, 27, 1341–1352. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, B.; Ren, J.Y.; Tan, K.L. Effect of Competitionon Tree Ring Growth and Climate Factors of Pinus koraiensis. J. Northeast For. Univ. 2019, 47, 6–10, 22. [Google Scholar] [CrossRef]
- Sidder, A. Topography and microclimate shape tree ring growth. Biogeosciences 2019, 100. [Google Scholar] [CrossRef]
- Andreu, L.; Gutiérrez, E.; Ribas, M.; Camarero, J.J. Climate increases regional tree-growth variability in Iberian pine forests. Glob. Chang. Biol. 2007, 13, 804–815. [Google Scholar] [CrossRef]
- Zhang, T.; Niinemets, Ü.; Sheffield, J.; Lichstein, J.W. Shifts in tree functional composition amplify the response of forest biomass to climate. Nature 2018, 556, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Čermák, P.; Rybníček, M.; Žid, T.; Steffenrem, A.; Kolář, T. Site and age-dependent responses of picea abies growth to climate variability. Eur. J. For. Res. 2019, 138, 445–460. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Mcrae, D.J. Spatial and age-dependent tree-ring growth responses of Larix gmelinii to climate in northeastern China. Trees 2009, 23, 875–885. [Google Scholar] [CrossRef]
- Coomes, D.A. Effects of size, competition and altitude on tree growth. J. Ecol. 2007, 95, 1084–1097. [Google Scholar] [CrossRef]
- Rabbel, I.; Neuwirth, B.; Bogena, H.; Diekkrüger, B. Exploring the growth response of Norway spruce (Picea abies) along a small-scale gradient of soil water supply. Dendrochronologia 2018, 52, 123–130. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Zhou, P.; Huang, J.G.; Liang, H.; Rossi, S. Radial growth of Larix sibirica was more sensitive to climate at low than high altitudes in the Altai Mountains, China. Agric. For. Meteorol. 2021, 304–305, 108392. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Henderson, M.; Liu, B.; Yan, H. Directional Variability in Response of Pinus koraiensis Radial Growth to Climate Change. Forests 2021, 12, 1684. https://doi.org/10.3390/f12121684
Sun Y, Henderson M, Liu B, Yan H. Directional Variability in Response of Pinus koraiensis Radial Growth to Climate Change. Forests. 2021; 12(12):1684. https://doi.org/10.3390/f12121684
Chicago/Turabian StyleSun, Yingjie, Mark Henderson, Binhui Liu, and Hong Yan. 2021. "Directional Variability in Response of Pinus koraiensis Radial Growth to Climate Change" Forests 12, no. 12: 1684. https://doi.org/10.3390/f12121684
APA StyleSun, Y., Henderson, M., Liu, B., & Yan, H. (2021). Directional Variability in Response of Pinus koraiensis Radial Growth to Climate Change. Forests, 12(12), 1684. https://doi.org/10.3390/f12121684







