The Driving Forces of Anoplophora glabripennis Have Spatial Spillover Effects
Abstract
1. Introduction
2. Materials and Methods
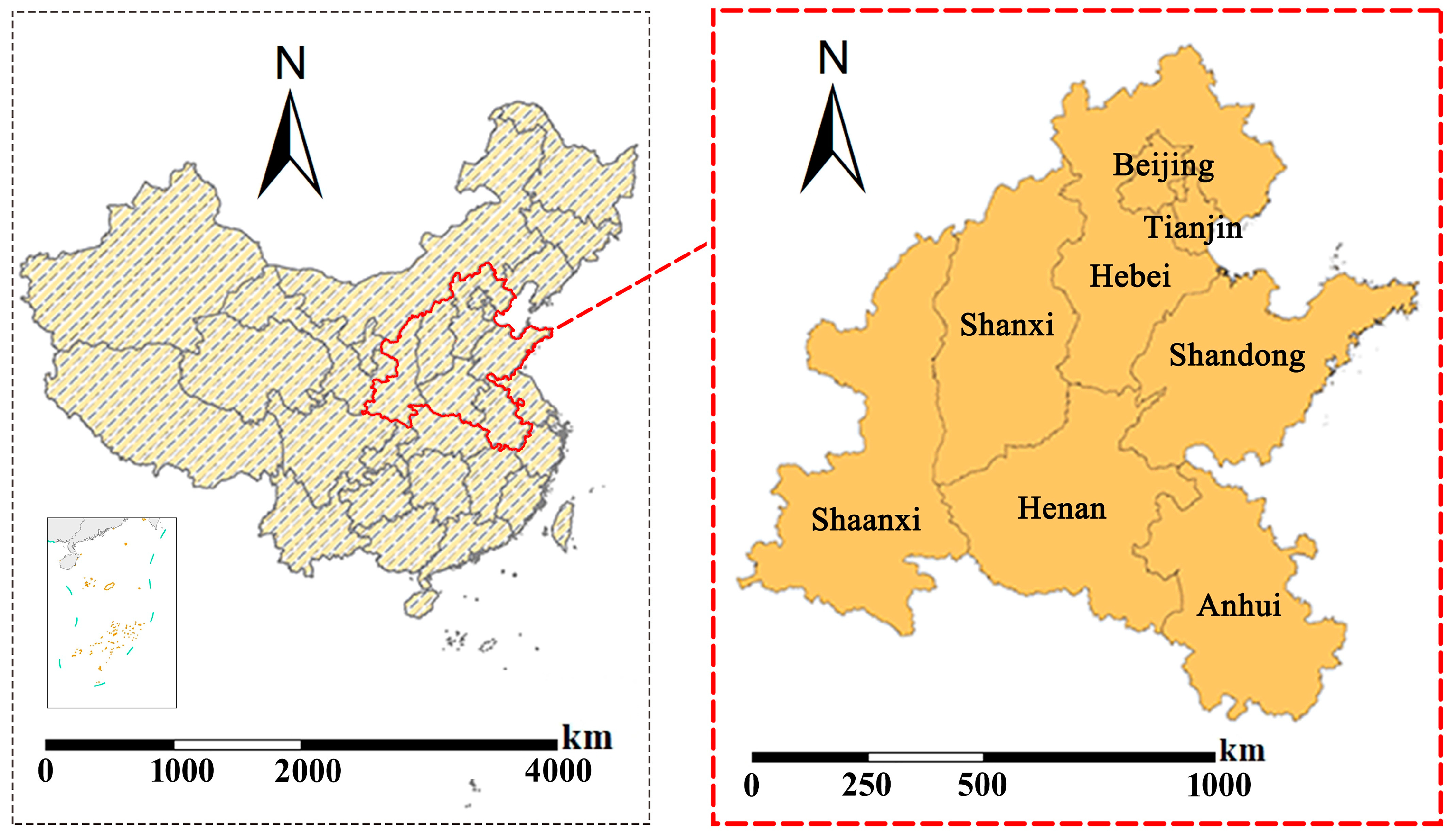
2.1. Study Area
2.2. A. glabripennis Incidence Calculation
2.3. Environmental Factors and Variables
2.3.1. Standardized Precipitation Evapotranspiration Index
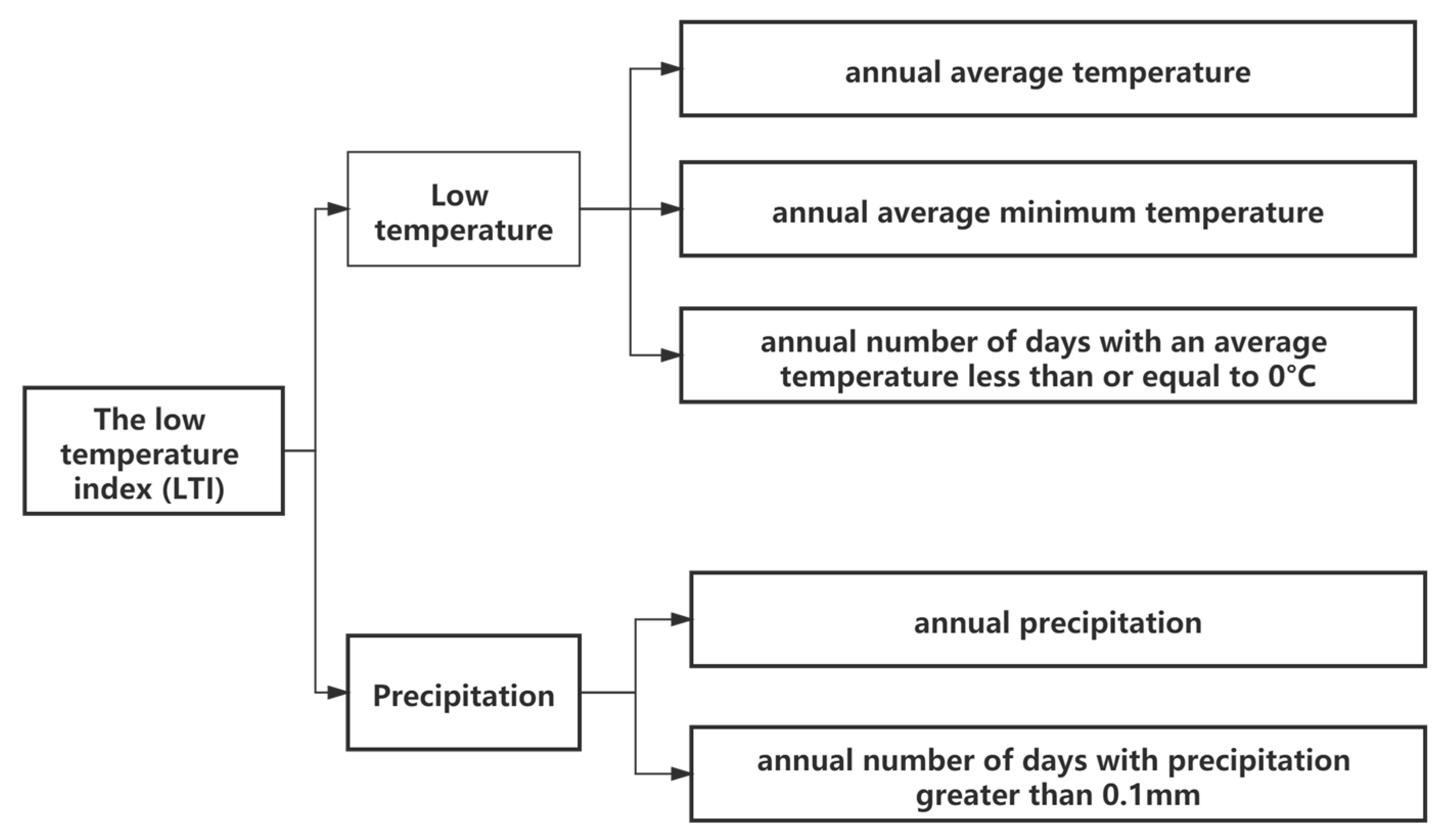
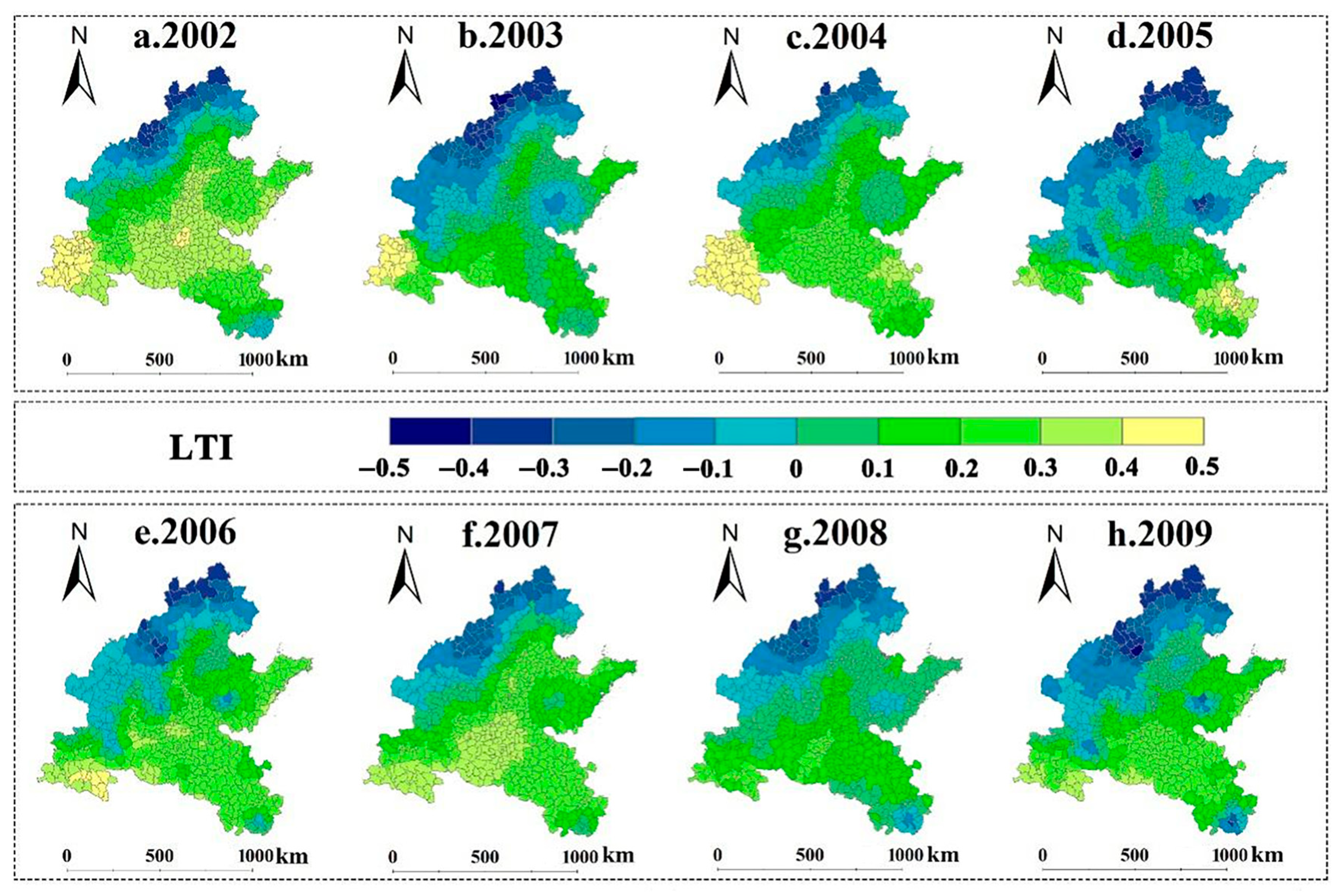
2.3.2. Low Temperature Index
2.3.3. Environmental Factors and Proxy Variables
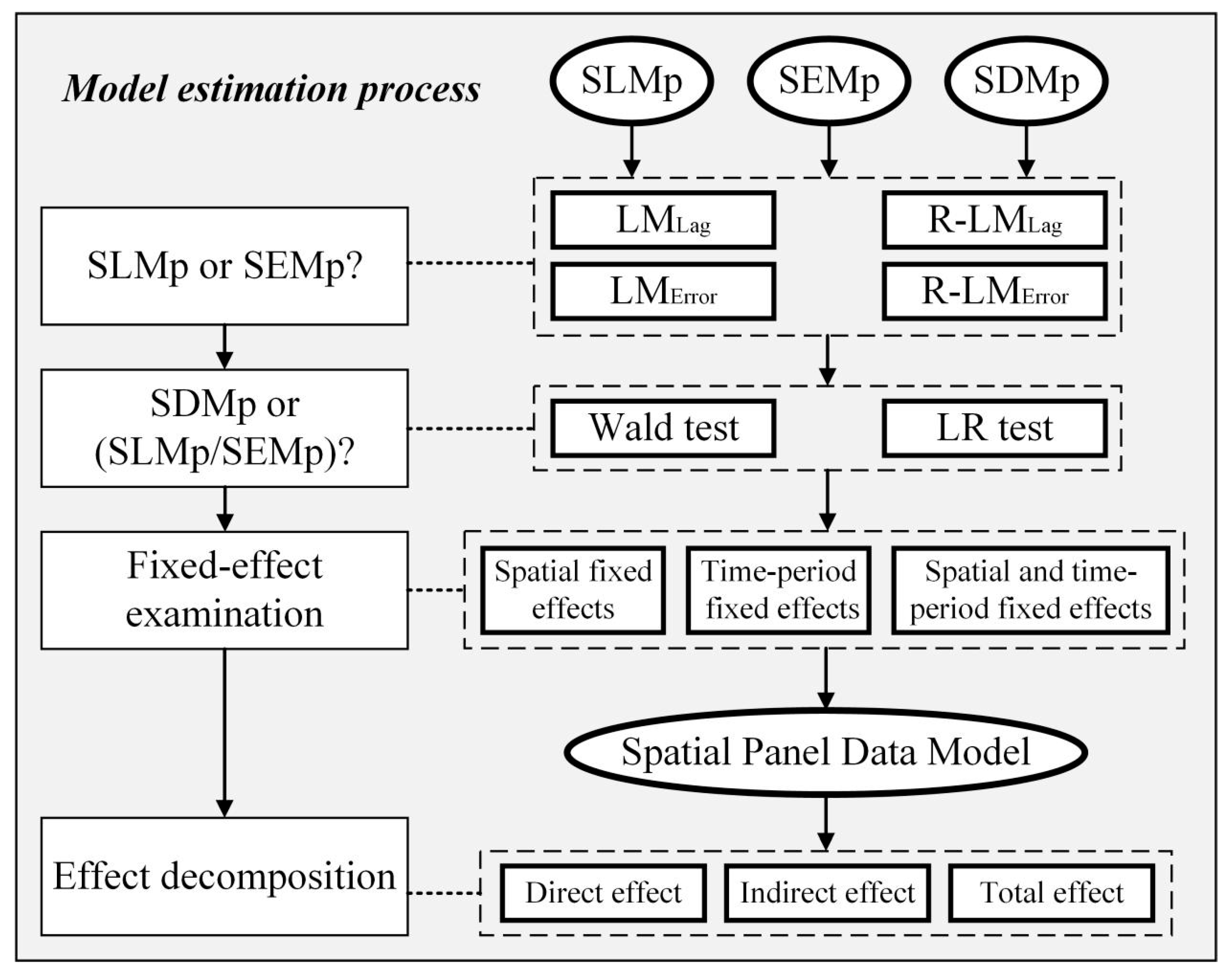
2.4. Spatial Panel Data Models
3. Results
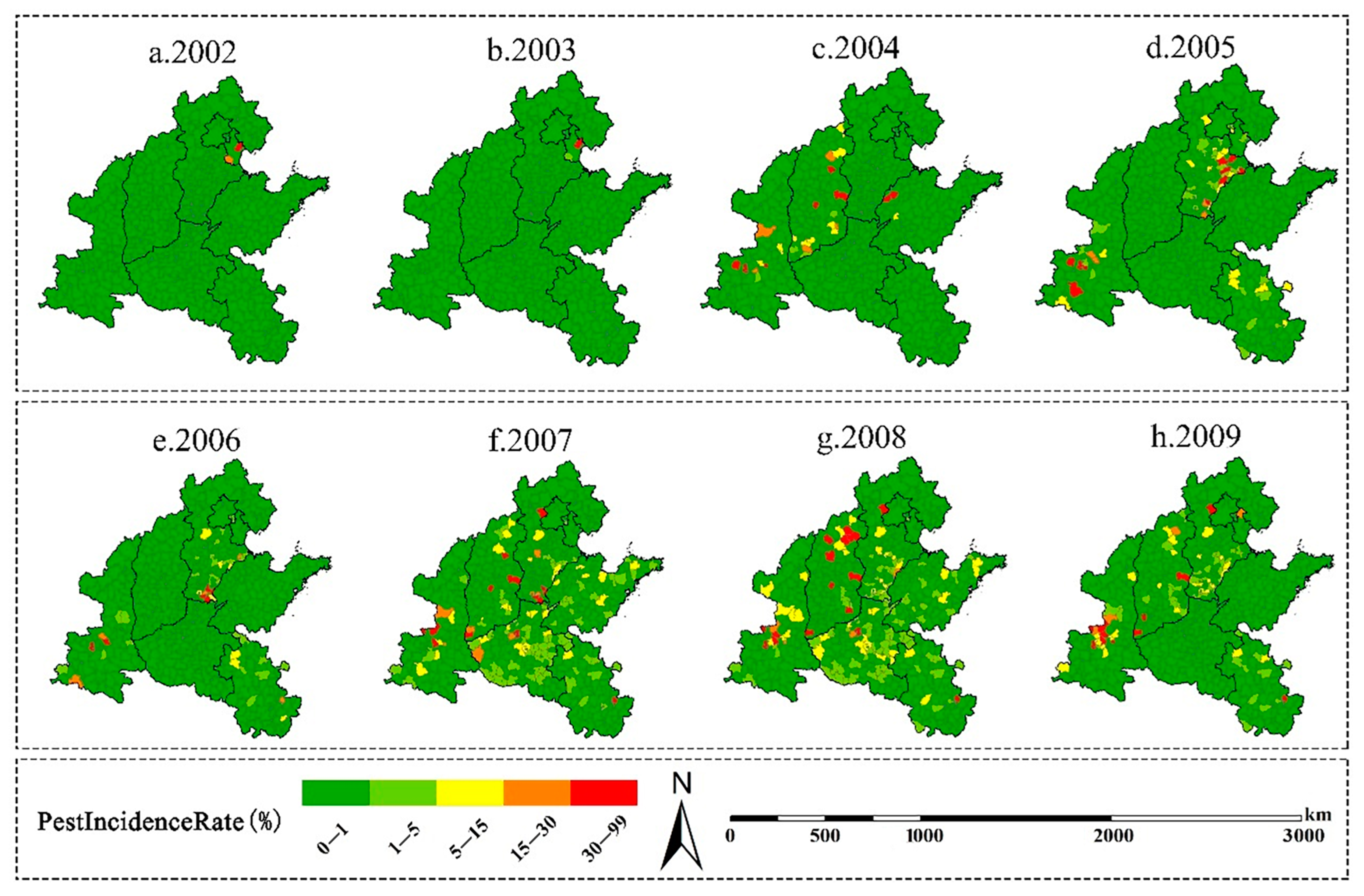
3.1. Spatiotemporal Patterns of the Incidence of A. glabripennis in 2002–2009
3.2. Spatiotemporal Patterns of SPEI and LTI in 2002–2009
3.3. Spatial Spillover Effects of Proxy Variables on A. glabripennis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Numerical Rating | Description |
|---|---|
| 9 | Extreme importance |
| 7 | Very strong importance |
| 5 | Strong importance |
| 3 | Moderate importance |
| 1 | Equal importance |
| 2,4,6,8 | Intermediate values |
| Reciprocals | Values for inverse comparison |
| Low Temperature | Precipitation | |
|---|---|---|
| low temperature | 1 | 2 |
| precipitation | 0.5 | 1 |
| Annual Average Temperature | Annual Average Minimum Temperature | Annual Number of Days with an Average Temperature Less than or Equal to 0 °C | |
|---|---|---|---|
| annual average temperature | 1 | 3 | 4 |
| annual average minimum temperature | 0.67 | 1 | 2 |
| annual number of days with an average temperature less than or equal to 0 °C | 0.25 | 0.5 | 1 |
| Annual Precipitation | Annual Number of Days with Precipitation Greater than 0.1 mm | |
|---|---|---|
| annual precipitation | 1 | 2 |
| annual number of days with precipitation greater than 0.1 mm | 0.5 | 1 |
Appendix B
| Unit Root Test | Variable | LLC | IPS | Fisher ADF | Fisher PP |
|---|---|---|---|---|---|
| PIR | −45,544.7 *** | −2209.28 *** | 3135.87 *** | 3760.99 *** | |
| PCR | −7235.25 *** | −7204.55 *** | 2894.35 *** | 3647.68 *** | |
| LTI | −65.6825 *** | −17.41 *** | 2552.35 *** | 2594.86 *** | |
| SPEI | −57.7655 *** | −17.2849 *** | 2576.30 *** | 2620.81 *** | |
| SH | −78.7047 *** | −19.4279 *** | 2616.19 *** | 2818.68 *** | |
| AWS | −184.383 *** | −27.2237 *** | 2853.90 *** | 3022.43 *** | |
| PD | −66.447 *** | −16.2877 *** | 2458.34 *** | 2718.60 *** | |
| KAO panel ADF | PIGDP | −105.145 *** | −25.5709 *** | 2616.72 *** | 3135.14 *** |
| −91.7544 *** | NDVI | −52.8738 *** | −15.9433 *** | 2480.57 *** | 2842.38 *** |
References
- Lingafelter, S.W.; Hoebeke, E.R. Revision of the Genus Anoplophora (Coleoptera: Cerambycidae); Entomological Society of Washington: Washington, DC, USA, 2002. [Google Scholar]
- Luo, Y.; Li, J. Bionomics and occurrence of Anoplophora glabripennis (Motschulsky). Plant. Quar. 1999, 1, 7–9. [Google Scholar]
- Haack, R.A.; Hérard, F.; Sun, J.; Turgeon, J.J. Managing invasive populations of Asian longhorned beetle and citrus longhorned beetle: A worldwide perspective. Annu. Rev. Entomol. 2010, 55, 521–546. [Google Scholar] [CrossRef]
- Wu, W.W.; Jiang, S.N. The glabripennis species group of the genus Anoplophora in China (Coleoptera: Cerambycidae). Acta Entomol. Sin. 1998, 41, 284–290. [Google Scholar]
- Pan, H.Y. Review of the Asian Longhorned Beetle Research, Biology, Distribution and Management in China; Forest Health and Biosecurity Working Papers; Forest Resources Development Service, Forest Resources Division, FAO: Rome, Italy, 2005; pp. 41–44. [Google Scholar]
- Golec, J.R.; Li, F.; Cao, L.; Wang, X.; Duan, J.J. Mortality factors of Anoplophora glabripennis (Coleoptera: Cerambycidae) infesting Salix and Populus in central, northwest, and northeast China. Biol. Control 2018, 126, 198–208. [Google Scholar] [CrossRef]
- Hu, J.; Angeli, S.; Schuetz, S.; Luo, Y.; Hajek, A.E. Ecology and management of exotic and endemic Asian longhorned beetle Anoplophora glabripennis. Agr. For. Entomol. 2009, 11, 359–375. [Google Scholar] [CrossRef]
- Javal, M.; Roques, A.; Haran, J.; Hérard, F.; Keena, M.; Roux, G. Complex invasion history of the Asian long-horned beetle: Fifteen years after first detection in Europe. J. Pest Sci. 2019, 92, 173–187. [Google Scholar] [CrossRef]
- Xu, T.; Teale, S.A. Chemical Ecology of the Asian Longhorn Beetle, Anoplophora glabripennis. J. Chem. Ecol. 2021, 47, 489–503. [Google Scholar] [CrossRef]
- Nowak, D.J.; Pasek, J.E.; Sequeira, R.A.; Crane, D.E.; Mastro, V.C. Potential effect of Anoplophora glabripennis (Coleoptera: Cerambycidae) on urban trees in the United States. J. Econ. Entomol. 2001, 94, 116–122. [Google Scholar] [CrossRef]
- Wang, Z.G.; Su, Z.; Liu, M.H.; Zhao, Y.M.; Zhang, G.; Cui, Z.R.; Dan, H.L.; Chen, X.M. Comparision of the Resistant Characteristics of Populus alba var. pyramidalis and Populus deltoides cl. Beikang to Damages against Anoplophora glabripennis (Coleoptera: Cerambycidae). Sci. Silvae Sin. 2018, 54, 89–96. [Google Scholar] [CrossRef]
- Haack, R.A.U.F.; Law, K.R.; Mastro, V.C.; Ossenbruggen, H.S.; Raimo, B.J. New York’s battle with the Asian long-horned beetle. J. For. 1997, 95, 11–15. [Google Scholar]
- Li, H.; Yu, F.; Bi, Y.; Wang, Z. Reviews on the serious wood-boring pest Anoplophora glabripennis in forestry of China. For. Ecol. Sci. 2020, 35, 1–9. [Google Scholar]
- MacLeod, A.; Evans, H.; Baker, R. An analysis of pest risk from an Asian longhorn beetle (Anoplophora glabripennis) to hardwood trees in the European community. Crop Prot. 2002, 21, 635–645. [Google Scholar] [CrossRef]
- Keena, M.A. Effects of temperature on Anoplophora glabripennis (Coleoptera: Cerambycidae) adult survival, reproduction, and egg hatch. Environ. Entomol. 2006, 35, 912–921. [Google Scholar] [CrossRef]
- Yang, Z.M.; Wang, X.; Yao, W.S.; Chu, X.M.; Li, P. Generation differentiation and effective accumulated temperature of Anoplophora glabripennis (Motsch.). For. Pest Dis. 2000, 19, 12–14. [Google Scholar] [CrossRef]
- Bancroft, J.; Smith, M. Dispersal and influences on movement for Anoplophora glabripennis calculated from individual mark-recapture. Entomol. Exp. Appl. 2005, 116, 83–92. [Google Scholar] [CrossRef]
- Faccoli, M. Effect of weather on Ips typographus (Coleoptera Curculionidae) phenology, voltinism, and associated spruce mortality in the southeastern Alps. Environ. Entomol. 2009, 38, 307–316. [Google Scholar] [CrossRef]
- Wang, C.Z.; Guo, A.H.; Wang, Y.L.; Zhou, Y.J.; Mao, L.X. Forecasting model of meteorological suitability for occurrence and development of valsa sordida nits in North China. Chin. J. Agrometeorol. 2011, 32, 139. [Google Scholar]
- Zhang, Y.; Tao, B.; Lu, Q.; Shi, Q.; Zhao, M.; Bai, L. A Preliminary Study on the Attraction Effect of Linden Trees on Anoplophora glabripennis. Inn. Mongolia For. Sci. Technol. 1998, 3, 2–3. [Google Scholar]
- Gao, R.; Li, G.; Wang, K.; Sun, J. Studies on the Forecast and Population Dynamics of Adult of Anoplophora glabripennis. For. Res. 1997, 10, 619. [Google Scholar]
- Smith, M.T.; Tobin, P.C.; Bancroft, J.; Li, G.; Gao, R. Dispersal and spatiotemporal dynamics of Asian longhorned beetle (Coleoptera: Cerambycidae) in China. Environ. Entomol. 2004, 33, 435–442. [Google Scholar] [CrossRef]
- Williams, D.W.; Li, G.; Gao, R. Tracking movements of individual Anoplophora glabripennis (Coleoptera: Cerambycidae) adults: Application of harmonic radar. Environ. Entomol. 2004, 33, 644–649. [Google Scholar] [CrossRef]
- Peterson, A.T.; Scachetti-Pereira, R. Potential geographic distribution of Anoplophora glabripennis (Coleoptera: Cerambycidae) in North America. Am. Midl. Nat. 2004, 151, 170–178. [Google Scholar] [CrossRef]
- Shatz, A.J.; Rogan, J.; Sangermano, F.; Ogneva-Himmelberger, Y.; Chen, H. Characterizing the potential distribution of the invasive Asian longhorned beetle (Anoplophora glabripennis) in Worcester County, Massachusetts. Appl. Geogr. 2013, 45, 259–268. [Google Scholar] [CrossRef]
- Shatz, A.J.; Rogan, J.; Sangermano, F.; Miller, J.; Elmes, A. Modeling the risk of spread and establishment for Asian longhorned beetle (Anoplophora glabripennis) in Massachusetts from 2008–2009. Geocarto Int. 2016, 31, 813–831. [Google Scholar] [CrossRef]
- Tobin, P.C.; Parry, D.; Aukema, B.H. The influence of climate change on insect invasions in temperate forest ecosystems. In Challenges and Opportunities for the World’s Forests in the 21st Century; Trevor, F., Ed.; Springer: Dordrecht, The Netherlands, 2014; Volume 81, pp. 267–293. [Google Scholar] [CrossRef]
- Battisti, A.; Larsson, S. Climate change and insect pest distribution range. In Climate Change and Insect Pests; Björkman, C., Niemelä, P., Eds.; CABI: Wallingford, UK, 2015; pp. 1–15. [Google Scholar]
- Shmookler, R.; Rogan, J.; Kulakowski, D. Determining Potential Habitat Suitability for the Invasive Asian Longhorned Beetle Anoplophora glabripennis in Worcester, MA Using the Mahalanobis Typicality Approach. Honors Thesis, Clark University, Worcester, MA, USA, 2008. [Google Scholar]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Miller, J. Species distribution modeling. Geogr. Compass 2010, 4, 490–509. [Google Scholar] [CrossRef]
- Ludwig, S.W.; Lazarus, L.; Mccullough, D.G.; Hoover, K.; Montero, S.; Sellmer, J.C. Methods to evaluate host tree suitability to the Asian longhorned beetle, Anoplophora glabripennis. J. Environ. Hortic. 2002, 20, 175–180. [Google Scholar] [CrossRef]
- Favaro, R.; Wichmann, L.; Ravn, H.P.; Faccoli, M. Spatial spread and infestation risk assessment in the Asian longhorned beetle, Anoplophora glabripennis. Entomol. Exp. Appl. 2015, 155, 95–101. [Google Scholar] [CrossRef]
- Hajek, A.E. Asian longhorned beetle: Ecology and control. Encycl. Pest Manag. 2007, 2, 21–24. [Google Scholar]
- Turgeon, J.; Smith, M. Anoplophora glabripennis (Motschulsky), Asian longhorned beetle (Coleoptera: Cerambycidae). In Biological Control Programmes in Canada 2001–2012; Mason, P.G., Gillespie, D.R., Eds.; CABI: Wallingford, UK, 2013; pp. 82–92. [Google Scholar]
- Wong, W.S.D.; Lee, J. Statistical Analysis of Geographic Information with ArcView GIS and ArcGIS.; Wiley: Hoboken, NJ, USA, 2005; p. 13. [Google Scholar]
- Best, N.; Richardson, S.; Thomson, A. A comparison of Bayesian spatial models for disease mapping. Stat. Methods Med. Res. 2005, 14, 35–59. [Google Scholar] [CrossRef]
- Turgeon, J.J.; Orr, M.; Grant, C.; Wu, Y.; Gasman, B. Decade-old satellite infestation of Anoplophora glabripennis Motschulsky (Coleoptera: Cerambycidae) found in Ontario, Canada outside regulated area of founder population. Coleopt. Bull. 2015, 69, 674–678. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A multiscalar drought index sensitive to global warming: The standardized precipitation evapotranspiration index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Chen, X.; Lei, H.; Xu, J.; Huang, X.; Zhang, Z.; Hu, J.; Shang, C.; Yang, J. Spatial and temporal distribution characteristics of drought during crop growth period in Guizhou province from climate change perspectives. J. Nat. Resour. 2015, 30, 1735–1749. [Google Scholar] [CrossRef]
- Yu, M.; Li, Q.; Hayes, M.J.; Svoboda, M.D.; Heim, R.R. Are droughts becoming more frequent or severe in China based on the standardized precipitation evapotranspiration index: 1951–2010? Int. J. Climatol. 2014, 34, 545–558. [Google Scholar] [CrossRef]
- López-Moreno, J.I.; Vicente-Serrano, S.M.; Zabalza, J.; Beguería, S.; Lorenzo-Lacruz, J.; Azorin-Molina, C.; Morán-Tejeda, E. Hydrological response to climate variability at different time scales: A study in the Ebro basin. J. Hydrol. 2013, 477, 175–188. [Google Scholar] [CrossRef]
- Su, H.; Li, G. Low-frequency drought variability based on SPEI in association with climate indices in Beijing. Acta Ecol. Sin. 2012, 32, 5467–5475. [Google Scholar] [CrossRef]
- Yao, J.; Zhao, Y.; Yu, X. Spatial-temporal variation and impacts of drought in Xinjiang (Northwest China) during 1961–2015. PeerJ 2018, 6, e4926. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, B.; Quan, X.; Liao, Z.; Bai, X. Use of the standardized precipitation evapotranspiration index (SPEI) to characterize the drying trend in southwest China from 1982–2012. Remote Sens. 2015, 7, 10917–10937. [Google Scholar] [CrossRef]
- Hamed, K.H. Trend detection in hydrologic data: The Mann–Kendall trend test under the scaling hypothesis. J. Hydrol. 2008, 349, 350–363. [Google Scholar] [CrossRef]
- Wan, S.; Zhou, Y.; Li, L.; Shi, R.; Guo, G.; Chen, B. A multi-index synthetic assessment method for extreme climate events of sleeting and freezing with low temperature. Meteorol. Mon. 2008, 34, 40–46. [Google Scholar]
- Zhang, R.Q.; Liu, Y.W.; Yao, G.; Zhao, Q.J.; Huang, T.F.; Zhong, J. Cause and Influence Analysis of the Rare Low-temperature Freezing Disaster in Guizhou in 2008. Trop. Geogr. 2009, 29, 319–323. [Google Scholar]
- Yang, K.; Chen, B.B.; Chen, H.; Lin, J.; Wang, J.Y.; Yang, X.Q. Study on Integrated Climatic Index for Low Temperature Injury of Loquat in Putian. Adv. Mater. Res. 2013, 726–731, 4436–4441. [Google Scholar] [CrossRef]
- Gao, J. Analysis and assessment of the risk of snow and freezing disaster in China. Int. J. Disaster Risk Reduct. 2016, 19, 334–340. [Google Scholar] [CrossRef]
- Lesage, J.P.; Pace, R.K. Spatial Econometric Models. In Handbook of Applied Spatial Analysis: Software Tools, Methods and Applications; Fischer, M.M., Getis, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 355–376. [Google Scholar]
- Elhorst, J.P. Matlab software for spatial panels. Int. Reg. Sci. Rev. 2014, 37, 389–405. [Google Scholar] [CrossRef]
- Fei, L.; Dong, S.; Xue, L.; Liang, Q.; Yang, W. Energy consumption-economic growth relationship and carbon dioxide emissions in China. Energy Policy 2011, 39, 568–574. [Google Scholar] [CrossRef]
- Bekun, F.V.; Alola, A.A.; Sarkodie, S.A. Toward a sustainable environment: Nexus between CO2 emissions, resource rent, renewable and nonrenewable energy in 16-EU countries. Sci. Total Environ. 2019, 657, 1023–1029. [Google Scholar] [CrossRef]
- Lu, W.; Hu, M.; Hu, J.J.; Yao, X.R. Discussion on severity and control of Asian longhorned beetle of poplar trees in the Three North Protection Forest Program. For. Sci. Technol. 2004, 58, 39–41. [Google Scholar]
- Gagne, J.A.; Coulson, R.N.; Foltz, J.L.; Wagner, T.L.; Edson, L.J. Attack and survival of Dendroctonus frontalis in relation to weather during three years in east Texas. Environ. Entomol. 1980, 9, 222–229. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Kong, X.; Liu, S.; Shen, Z. Preliminary deduction of potential distribution and alternative hosts of invasive pest, Dendroctonus valens (Coleoptera: Scolytidae). Sci. Silvae Sin. 2007, 43, 71–76. [Google Scholar] [CrossRef]
- White, T. Are outbreaks of cambium-feeding beetles generated by nutritionally enhanced phloem of drought-stressed trees? J. Appl. Entomol. 2015, 139, 567–578. [Google Scholar] [CrossRef]
- Ren, G.; Feng, G.; Yan, Z. Progresses in observation studies of climate extremes and changes in mainland China. Clim. Environ. Res. 2010, 15, 337–353. [Google Scholar]
- Feng, Y.; Tursun, R.; Xu, Z.; Ouyang, F.; Zong, S. Effect of three species of host tree on the cold hardiness of overwintering larvae of Anoplophora glabripennis (Coleoptera: Cerambycidae). Eur. J. Entomol. 2016, 113, 212. [Google Scholar] [CrossRef][Green Version]
- Li, G.; Gao, R.; Smith, M.; Kong, L. Study on dispersal of Anoplophora glabripennis (Motsch.) (Coleoptera: Cerambycidae) population. For. Res. 2010, 23, 678–684. [Google Scholar]
- Keena, M.A. Factors that influence flight propensity in Anoplophora glabripennis (Coleoptera: Cerambycidae). Environ. Entomol. 2018, 47, 1233–1241. [Google Scholar] [CrossRef]
- Brabbs, T.; Collins, D.; Hérard, F.; Maspero, M.; Eyre, D. Prospects for the use of biological control agents against Anoplophora in Europe. Pest Manag. Sci. 2015, 71, 7–14. [Google Scholar] [CrossRef]
- Keena, M.A.; Sánchez, V. Reproductive behaviors of Anoplophora glabripennis (Coleoptera: Cerambycidae) in the laboratory. J. Econ. Entomol. 2018, 111, 620–628. [Google Scholar] [CrossRef]
- Hill, M.P.; Gallardo, B.; Terblanche, J.S. A global assessment of climatic niche shifts and human influence in insect invasions. Glob. Ecol. Biogeogr. 2017, 26, 679–689. [Google Scholar] [CrossRef]
- Swift, R.J.; Rodewald, A.D.; Senner, N.R. Environmental heterogeneity and biotic interactions as potential drivers of spatial patterning of shorebird nests. Landsc. Ecol. 2017, 32, 1689–1703. [Google Scholar] [CrossRef]
- Boesing, A.L.; Nichols, E.; Metzger, J.P. Effects of landscape structure on avian-mediated insect pest control services: A review. Landsc. Ecol. 2017, 32, 931–944. [Google Scholar] [CrossRef]
- Smith, M.J.; Turgeon, J.J.; De Groot, P.; Gasman, B. Asian longhorned beetle Anoplophora glabripennis (Motschulsky): Lessons learned and opportunities to improve the process of eradication and management. Am. Entomol. 2009, 55, 21. [Google Scholar] [CrossRef]
- Barlow, L.-A.; Cecile, J.; Bauch, C.T.; Anand, M. Modelling interactions between forest pest invasions and human decisions regarding firewood transport restrictions. PLoS ONE 2014, 9, e90511. [Google Scholar] [CrossRef]
- Trotter, R.T.; Hull-Sanders, H.M. Quantifying dispersal of the Asian longhorned beetle (Anoplophora glabripennis, Coleoptera) with incomplete data and behavioral knowledge. Biol. Invasions 2015, 17, 3359–3369. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, J. Preliminary studies on the growth-loss of young poplar stands caused by Anoplophora glabripennis motsch. J. Beijing For. Univ. 1993, 75–87. [Google Scholar]
- Bao, Y.Z. Control methods of Anoplophora glabripennis. Anhui For. Sci. Technol. 2004, 5, 36. [Google Scholar]
- Ru, W.D.; Lv, L.G. Distribution and control of Anoplophora glabripennis in the west of Henan Province. In Proceedings of the The eighth Henan Plant Protection Society, the Seventh Henan Entomological Society, and the second member Congress and Symposium of Henan Plant Disease Society, Henan, China, 1 October 2005; pp. 216–219. [Google Scholar]
- Li, T.L.; Ma, Y. Occurrence status and control techniques of willow stem borer in Shijiazhuang city. Pract. For. Technol. 2009, 10, 32–33. [Google Scholar]
- Guo, Q.Y. Occurrence regularity and control techniques of Anoplophora glabripennis in Tianjin area. Modern Landscape Architecture 2010, 10, 66–68. [Google Scholar]
- Zhao, Y.H. Damage characteristics and control measures of Anoplophora glabripennis. J. Bot. 2012, 25, 24–25. [Google Scholar]
- Liang, X.Y. Occurrence and Comprehensive Control measures of Anoplophora glabripennis in Liquan County. Agric. Technol. 2015, 35, 150. [Google Scholar]
- Sun, Q.J. Occurrence and Control of Anoplophora glabripennis in Pingyin County. Agric. Technol. 2015, 2, 84. [Google Scholar]
- Ding, Y.F.; Zhao, X.E.; Dai, B. Occurrence and Control Countermeasures of Anoplophora glabripennis, a pest of willow stem borer in Wugong County. Shaanxi Agric. Sci. 2015, 63, 85. [Google Scholar]
- Qing, Y.L. Risk Assessment and Analysis of the harm of Anoplophora glabripennis in Dali County. Mod. Hortic. 2017, 9, 141–142. [Google Scholar]


| Variable | Mean | StdDev | Min | Max |
|---|---|---|---|---|
| PIR | 1.26 | 7.19 | 0 | 99.64 |
| LTI | 0.11 | 0.19 | −0.44 | 0.45 |
| SPEI | 0.05 | 0.26 | −0.56 | 1.01 |
| AWS | 2.24 | 0.46 | 1.24 | 4.69 |
| SH | 2157.13 | 365.22 | 1129.56 | 2952.18 |
| PIGDP | 9.71 | 11.49 | 0.23 | 126.73 |
| PD | 550.98 | 531.5 | 6.06 | 5324.12 |
| NDVI RD | 0.54 17.19 | 0.1 22.46 | 0.22 0.12 | 0.83 209.34 |
| PCR | 9.41 | 27.91 | 0 | 100 |
| Ordinary Least Square (OLS) | Spatial Fixed Effects | Time-Period Fixed Effects | Spatial and Time-Period Fixed Effects | |
|---|---|---|---|---|
| R2 | 0.0970 | 0.0880 | 0.0899 | 0.0761 |
| σ2 | 46.7350 | 32.5133 | 46.5311 | 32.3688 |
| LMLag | 57.1994 *** | 43.1199 *** | 49.7640 *** | 38.9140 *** |
| R- LMLag | 0.3950 | 2.7550 | 0.7129 | 1.8456 |
| LMError | 65.2111 *** | 51.9631 *** | 57.1980 *** | 45.8077 *** |
| R-LMError | 8.4067 *** | 11.5982 *** | 8.1469 *** | 8.7393 *** |
| Spatial Fixed Effects | Time-Period Fixed Effects | Spatial and Time-Period Fixed Effects | |
|---|---|---|---|
| Wald_spatial_lag | 21.7455 *** (p = 0.0097) | 28.5366 *** (p = 0.0008) | 19.3922 ** (p = 0.0221) |
| Wald_spatial_error | 13.7424 (p = 0.1318) | 22.3532 * (p = 0.0780) | 13.1501 (p = 0.1559) |
| LR_spatial_lag | 21.5087 ** (p = 0.0106) | 28.3853 *** (p = 0.0008) | 19.2121 ** (p = 0.0234) |
| LR_spatial_error | 12.8898 (p = 0.1053) | 22.1075 * (p = 0.0850) | 13.0737 (p = 0.1593) |
| Spatial Fixed Effects | Time-Period Fixed Effects | Spatial and Time-Period Fixed Effects | |
|---|---|---|---|
| LTI | −1.5361 ** | 1.4341 | −1.4658 |
| SPEI | −1.1384 *** | −0.3138 | −1.3558 ** |
| AWS | 1.6551 *** | −0.0062 | 1.6066 ** |
| SH | −0.0046 *** | 0.0010 | −0.0062 *** |
| PIGDP | −0.0100 | −0.0467 *** | −0.0255 * |
| PD | −0.0018 | −0.0002 | −0.0024 * |
| NDVI | 3.5612 | −1.6425 | −7.9150 |
| RD | 5.0770 | 0.0045 | 5.3191 |
| PCR | 0.0727 *** | 0.0794 *** | 0.0730 *** |
| σ2 | 36.5820 | 45.8413 | 36.5246 |
| R2 | 0.3716 | 0.1007 | 0.3745 |
| CorrectedR2 | 0.0899 | 0.0879 | 0.0761 |
| LogL | −16,727.723 | −17,758.275 | −16,795.896 |
| Spat.error. | 0.1521 *** | 0.1550 | 0.1483 *** |
| Variable | Direct Effect | Indirect Effect | Total Effect |
|---|---|---|---|
| LTI | −1.5319 * | −0.2671 * | −1.7990 * |
| SPEI | −1.1484 *** | −0.2003 *** | −1.3487 *** |
| AWS | 1.6507 *** | 0.2878 *** | 1.9358 *** |
| SH | −0.0046 *** | −0.0008 *** | −0.0054 *** |
| PIGDP | −0.0098 | −0.0017 | −0.0115 |
| PD | −0.0018 | −0.0003 | −0.0021 |
| NDVI | 3.5110 | 0.6131 | 4.1254 |
| RD | 5.1350 | 0.9006 | 6.0356 |
| PCR | 0.0730 *** | 0.0127 *** | 0.0857 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Lu, X.; Liu, H.; Zong, S. The Driving Forces of Anoplophora glabripennis Have Spatial Spillover Effects. Forests 2021, 12, 1678. https://doi.org/10.3390/f12121678
Huang J, Lu X, Liu H, Zong S. The Driving Forces of Anoplophora glabripennis Have Spatial Spillover Effects. Forests. 2021; 12(12):1678. https://doi.org/10.3390/f12121678
Chicago/Turabian StyleHuang, Jixia, Xiao Lu, Hengzi Liu, and Shixiang Zong. 2021. "The Driving Forces of Anoplophora glabripennis Have Spatial Spillover Effects" Forests 12, no. 12: 1678. https://doi.org/10.3390/f12121678
APA StyleHuang, J., Lu, X., Liu, H., & Zong, S. (2021). The Driving Forces of Anoplophora glabripennis Have Spatial Spillover Effects. Forests, 12(12), 1678. https://doi.org/10.3390/f12121678






