Effects of NaCl and CaCl2 Salinization on Morpho-Anatomical and Physiological Traits of Potted Callistemon citrinus Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions and Saline Treatments
2.2. Biometry and Leaf Traits
2.3. Leaf Gas-Exchanges and Chlorophyll a Fluorescence Emission
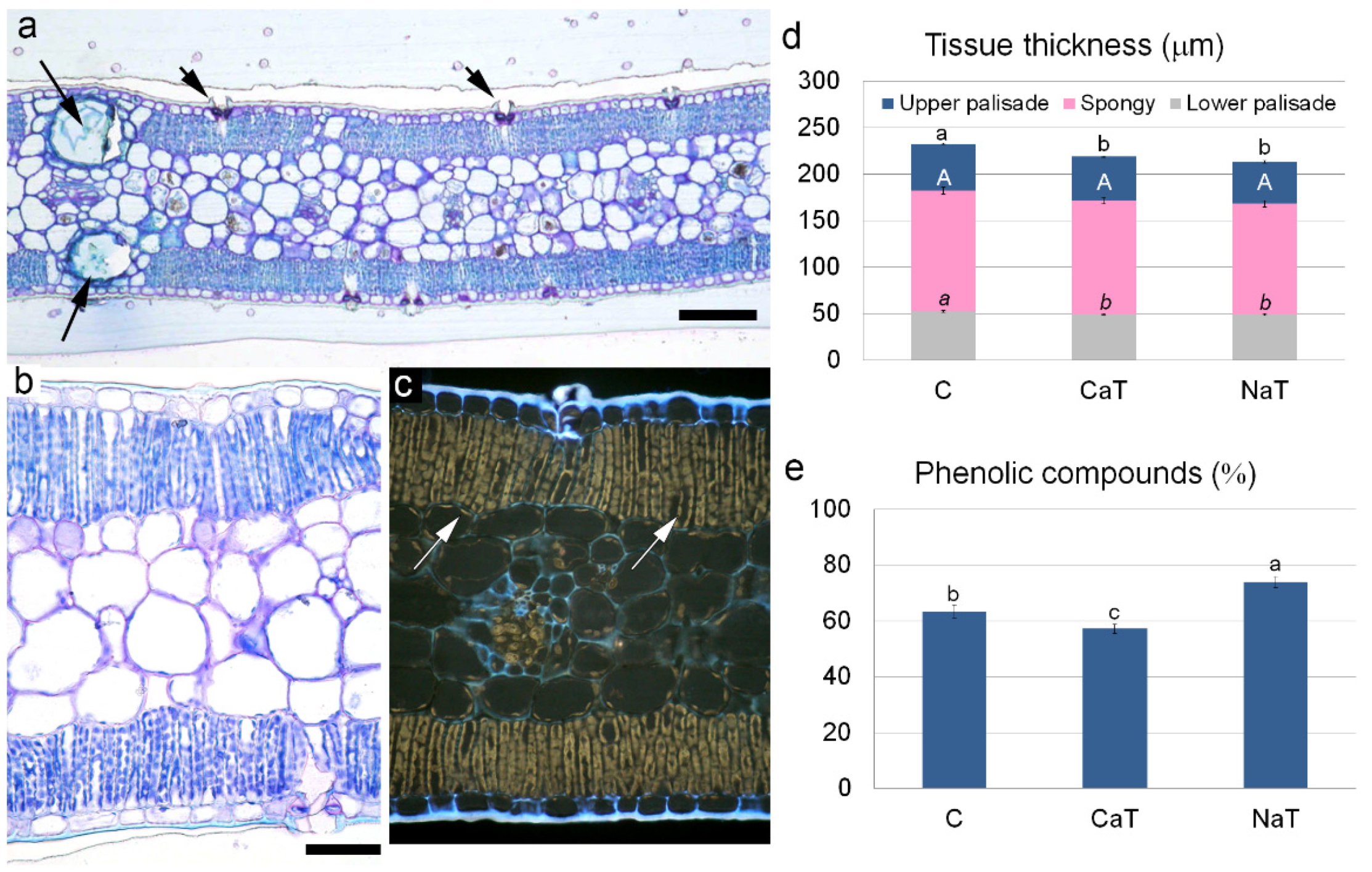
2.4. Microscopy Analyses and Quantification of Leaf Anatomical Traits
2.5. Ion and Organic Acid Analyses
2.6. Statistical Analysis
3. Results
3.1. Growth and Morphology
3.2. Leaf Gas-Exchanges and Photochemistry
3.3. Quantitative Leaf Anatomy
3.4. Mineral and Organic Acids Profiling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, M. Xeriscape Plants; Cooperative Extension Service, College of Tropical Agriculture and Human Resources, University of Hawai’i at Manoa: Honolulu, HI, USA, 2008. [Google Scholar]
- Giordano, M.; Petropoulos, S.A.; Cirillo, C.; Rouphael, Y. Biochemical, physiological, and molecular aspects of ornamental plants adaptation to deficit irrigation. Horticulturae 2021, 7, 107. [Google Scholar] [CrossRef]
- Cirillo, C.; Rouphael, Y.; Caputo, R.; Raimondi, G.; Sifola, M.; De Pascale, S. Effects of high salinity and the exogenous application of an osmolyte on growth, photosynthesis, and mineral composition in two ornamental shrubs. J. Hortic. Sci. Biotechnol. 2016, 91, 14–22. [Google Scholar] [CrossRef]
- Rizzo, V.; Toscano, S.; Farieri, E.; Romano, D. Antioxidative defense mechanism in Callistemon citrinus (Curtis) Skeels and Viburnum tinus L. ‘Lucidum’ in response to seawater aerosol and surfactants. J. Agric. Sci. Technol. 2019, 21, 911–925. [Google Scholar]
- Álvarez, S.; Sánchez-Blanco, M.J. Changes in growth rate, root morphology and water use efficiency of potted Callistemon citrinus plants in response to different levels of water deficit. Sci. Hortic. 2013, 156, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Colla, G.; Rouphael, Y.; Leonardi, C.; Bie, Z. Role of grafting in vegetable crops grown under saline conditions. Sci. Hortic. 2010, 127, 147–155. [Google Scholar] [CrossRef]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of Salt-Induced Land Degradation and Restoration. In Proceedings of Natural Resources Forum; Wiley Online Library: Hoboken, NJ, USA, 2014; pp. 282–295. [Google Scholar]
- Hasanuzzaman, M.; Shabala, L.; Zhou, M.; Brodribb, T.J.; Corkrey, R.; Shabala, S. Factors determining stomatal and non-stomatal (residual) transpiration and their contribution towards salinity tolerance in contrasting barley genotypes. Environ. Exp. Bot. 2018, 153, 10–20. [Google Scholar] [CrossRef]
- Gallé, A.; Haldimann, P.; Feller, U. Photosynthetic performance and water relations in young pubescent oak (Quercus pubescens) trees during drought stress and recovery. New Phytol. 2007, 174, 799–810. [Google Scholar] [CrossRef] [PubMed]
- America, I.; Zhang, C.; Werner, A.D.; van der Zee, S.E. Evaporation and salt accumulation effects on riparian freshwater lenses. Water Resour. Res. 2020, 56, e2019WR026380. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Romano, D. Response of Mediterranean ornamental plants to drought stress. Horticulturae 2019, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Yeager, T.H.; von Merveldt, J.K.; Larsen, C.A. Ornamental plant response to percentage of reclaimed water irrigation. HortScience 2010, 45, 1610–1615. [Google Scholar] [CrossRef] [Green Version]
- Grieve, C.M. Irrigation of floricultural and nursery crops with saline wastewaters. Isr. J. Plant Sci. 2011, 59, 187–196. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, T.; Cheng, Y.; Wang, F.; Zhao, X. Morphological and metabolic responses of four Iris germanica cultivars under salinity stress. Sci. Hortic. 2021, 281, 109960. [Google Scholar] [CrossRef]
- Niu, G.; Cabrera, R.I. Growth and physiological responses of landscape plants to saline water irrigation: A review. HortScience 2010, 45, 1605–1609. [Google Scholar] [CrossRef]
- Cai, X.; Sun, Y.; Starman, T.; Hall, C.; Niu, G. Response of 18 Earth-Kind® rose cultivars to salt stress. HortScience 2014, 49, 544–549. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Her, Y.; Chang, A.; Song, J.-H.; Campoverde, E.V.; Schaffer, B. Assessing the Effects of Irrigation Water Salinity on Two Ornamental Crops by Remote Spectral Imaging. Agronomy 2021, 11, 375. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Jagendorf, A.; Zhu, J.K. Understanding and improving salt tolerance in plants. Crop Sci. 2005, 45, 437–448. [Google Scholar] [CrossRef] [Green Version]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Maximova, E.; Fuggi, A.; Carillo, P. Durum wheat roots adapt to salinity remodeling the cellular content of nitrogen metabolites and sucrose. Front. Plant Sci. 2017, 7, 2035. [Google Scholar] [CrossRef] [Green Version]
- De Micco, V.; Aronne, G. Morpho-Anatomical Traits for Plant Adaptation to Drought. In Plant Responses to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 37–61. [Google Scholar] [CrossRef]
- Shabala, S.; Munns, R. Salinity stress: Physiological constraints and adaptive mechanisms. Plant Stress Physiol. 2012, 1, 59–93. [Google Scholar]
- Shabala, S.; Hariadi, Y.; Jacobsen, S.-E. Genotypic difference in salinity tolerance in quinoa is determined by differential control of xylem Na+ loading and stomatal density. J. Plant Physiol. 2013, 170, 906–914. [Google Scholar] [CrossRef]
- Zhu, M.; Zhou, M.; Shabala, L.; Shabala, S. Linking osmotic adjustment and stomatal characteristics with salinity stress tolerance in contrasting barley accessions. Funct. Plant Biol. 2014, 42, 252–263. [Google Scholar] [CrossRef]
- Hughes, J.; Hepworth, C.; Dutton, C.; Dunn, J.A.; Hunt, L.; Stephens, J.; Waugh, R.; Cameron, D.D.; Gray, J.E. Reducing stomatal density in barley improves drought tolerance without impacting on yield. Plant Physiol. 2017, 174, 776–787. [Google Scholar] [CrossRef] [Green Version]
- Rahnama, A.; James, R.A.; Poustini, K.; Munns, R. Stomatal conductance as a screen for osmotic stress tolerance in durum wheat growing in saline soil. Funct Plant Biol. 2010, 37, 255–263. [Google Scholar] [CrossRef]
- Pagter, M.; Bragato, C.; Malagoli, M.; Brix, H. Osmotic and ionic effects of NaCl and Na2SO4 salinity on Phragmites australis. Aquat. Bot. 2009, 90, 43–51. [Google Scholar] [CrossRef]
- Cirillo, C.; De Micco, V.; Arena, C.; Carillo, P.; Pannico, A.; De Pascale, S.; Rouphael, Y. Biochemical, Physiological and Anatomical Mechanisms of Adaptation of Callistemon citrinus and Viburnum lucidum to NaCl and CaCl2 Salinization. Front. Plant Sci. 2019, 10, 742. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Kumar, P.; Pathan, S.K.; Sharma, K.P. Urban Neighborhood Green Index–A measure of green spaces in urban areas. Landsc. Urban Plan. 2012, 105, 325–335. [Google Scholar] [CrossRef]
- Cornelissen, J.; Lavorel, S.; Garnier, E.; Diaz, S.; Buchmann, N.; Gurvich, D.; Reich, P.B.; Ter Steege, H.; Morgan, H.; Van Der Heijden, M. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef] [Green Version]
- Arena, C.; Conti, S.; Francesca, S.; Melchionna, G.; Hájek, J.; Barták, M.; Barone, A.; Rigano, M.M. Eco-physiological screening of different tomato genotypes in response to high temperatures: A combined field-to-laboratory approach. Plants 2020, 9, 508. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K.; Babani, F. Light adaptation and senescence of the photosynthetic apparatus. Changes in pigment composition, chlorophyll fluorescence parameters and photosynthetic activity. In Chlorophyll a Fluorescence; Springer: Berlin/Heidelberg, Germany, 2004; pp. 713–736. [Google Scholar]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta BBA Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef]
- De Micco, V.; Arena, C.; Vitale, L.; Aronne, G.; Virzo De Santo, A. Anatomy and photochemical behaviour of Mediterranean Cistus incanus winter leaves under natural outdoor and warmer indoor conditions. Botany 2011, 89, 677–688. [Google Scholar] [CrossRef]
- Reale, L.; Gigante, D.; Landucci, F.; Ferranti, F.; Venanzoni, R. Morphological and histo-anatomical traits reflect die-back in Phragmites australis (Cav.) Steud. Aquat. Bot. 2012, 103, 122–128. [Google Scholar] [CrossRef]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press New York: New York, NY, USA, 1999; p. 322. [Google Scholar]
- Fukazawa, K. Ultraviolet Microscopy. In Methods in Lignin Chemistry; Springer: Berlin/Heidelberg, Germany, 1992; pp. 110–121. [Google Scholar]
- Van Buggenhout, S.; Grauwet, T.; Van Loey, A.; Hendrickx, M. Structure/processing relation of vacuum infused strawberry tissue frozen under different conditions. Eur. Food Res. Technol. 2008, 226, 437–448. [Google Scholar] [CrossRef]
- De Micco, V.; Aronne, G. Combined histochemistry and autofluorescence for identifying lignin distribution in cell walls. Biotech. Histochem. 2007, 82, 209–216. [Google Scholar] [CrossRef] [PubMed]
- De Micco, V.; Arena, C.; Aronne, G. Anatomical alterations of Phaseolus vulgaris L. mature leaves irradiated with X-rays. Plant Biol. 2014, 16, 187–193. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Lao, M.T. The effects of salt stress on ornamental plants and integrative cultivation practices. Sci. Hortic. 2018, 240, 430–439. [Google Scholar] [CrossRef]
- Geilfus, C.M. Chloride: From nutrient to toxicant. Plant Cell Phys. 2018, 59, 877–886. [Google Scholar] [CrossRef]
- Rabhi, M.; Farhat, N.; Msilini, N.; Rajhi, H.; Smaoui, A.; Abdelly, C.; Lachaâl, M.; Karray-Bouraoui, N. Physiological responses of Carthamus tinctorius to CaCl2 salinity under Mg-sufficient and Mg-deficient conditions. Flora 2018, 246, 96–101. [Google Scholar] [CrossRef]
- Moriana, A.; Pérez-López, D.; Prieto, M.; Ramírez-Santa-Pau, M.; Pérez-Rodriguez, J. Midday stem water potential as a useful tool for estimating irrigation requirements in olive trees. Agric. Water Manag. 2012, 112, 43–54. [Google Scholar] [CrossRef]
- Amitrano, C.; Rouphael, Y.; Pannico, A.; De Pascale, S.; De Micco, V. Reducing the Evaporative Demand Improves Photosynthesis and Water Use Efficiency of Indoor Cultivated Lettuce. Agronomy 2021, 11, 1396. [Google Scholar] [CrossRef]
- Marino, G.; Caruso, T.; Ferguson, L.; Marra, F.P. Gas exchanges and stem water potential define stress thresholds for efficient irrigation management in olive (Olea europea L.). Water 2018, 10, 342. [Google Scholar] [CrossRef] [Green Version]
- Hepworth, C.; Doheny-Adams, T.; Hunt, L.; Cameron, D.D.; Gray, J.E. Manipulating stomatal density enhances drought tolerance without deleterious effect on nutrient uptake. New Phytol. 2015, 208, 336–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, W.; Struik, P.C.; Yin, X.; Yang, J. Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought. J. Exp. Bot. 2017, 68, 5191–5205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roelfsema, M.R.G.; Hedrich, R. Studying guard cells in the intact plant: Modulation of stomatal movement by apoplastic factors. New Phytol. 2002, 153, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Trankner, M.; Tavakol, E.; Jakli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant 2018, 163, 414–431. [Google Scholar] [CrossRef] [Green Version]
- Erel, R.; Yermiyahu, U.; Ben-Gal, A.; Dag, A.; Shapira, O.; Schwartz, A. Modification of non-stomatal limitation and photoprotection due to K and Na nutrition of olive trees. J. Plant Phys. 2015, 177, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jákli, B.; Tavakol, E.; Tränkner, M.; Senbayram, M.; Dittert, K. Quantitative limitations to photosynthesis in K deficient sunflower and their implications on water-use efficiency. J. Plant Physiol. 2017, 209, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Cirillo, C.; De Micco, V.; Arena, C.; De Pascale, S.; Rouphael, Y. Morpho-anatomical, physiological and biochemical adaptive responses to saline water of Bougainvillea spectabilis Willd. trained to different canopy shapes. Agric. Water Manag. 2019, 212, 12–22. [Google Scholar] [CrossRef]
- Amitrano, C.; Arena, C.; Cirillo, V.; De Pascale, S.; De Micco, V. Leaf morpho-anatomical traits in Vigna radiata L. affect plant photosynthetic acclimation to changing vapor pressure deficit. Environ. Exp. Bot. 2021, 104453. [Google Scholar] [CrossRef]
- Arena, C.; De Micco, V.; De Maio, A.; Mistretta, C.; Aronne, G.; Vitale, L. Winter and summer leaves of Cistus incanus: Differences in leaf morphofunctional traits, photosynthetic energy partitioning, and poly (ADP-ribose) polymerase (PARP) activity. Botany 2013, 91, 805–813. [Google Scholar] [CrossRef]
- Vitale, L.; Arena, C.; De Santo, A.V. Seasonal changes in hotosynthetic activity and photochemical efficiency of the Mediterranean shrub Phillyrea angustifolia L. Plant Biosyst. Int. J. Deal. Aspects Plant Biol. 2012, 146, 443–450. [Google Scholar]
- Gao, L.; Lu, Z.; Ding, L.; Xie, K.; Wang, M.; Ling, N.; Guo, S. Anatomically induced changes in rice leaf mesophyll conductance explain the variation in photosynthetic nitrogen use efficiency under contrasting nitrogen supply. BMC Plant Biol. 2020, 20, 527. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, C.; Arena, C.; Rouphael, Y.; Caputo, R.; Amitrano, C.; Petracca, F.; De Francesco, S.; Vitale, E.; Erbaggio, A.; Bonfante, A. Counteracting the negative effects of copper limitations through the biostimulatory action of a tropical plant extract in grapevine under pedo-climatic constraints. Front. Environ. Sci. 2021, 9, 76. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Quideau, S.; Treutter, D. Plant phenolics—secondary metabolites with diverse functions. Recent Adv. Polyphen. Res. 2008, 1, 1–35. [Google Scholar]
- De Micco, V.; Amitrano, C.; Stinca, A.; Izzo, L.G.; Zalloni, E.; Balzano, A.; Barile, R.; Conti, P.; Arena, C. Dust accumulation due to anthropogenic impact induces anatomical and photochemical changes in leaves of Centranthus ruber growing on the slope of the Vesuvius volcano. Plant Biol. 2019, 22, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A. Physiological significance of morpho-anatomical features of halophytes with particular reference to Cholistan flora. Int. J. Agric. Biol. 2003, 5, 207–212. [Google Scholar]
- Rozentsvet, O.; Nesterov, V.; Bogdanova, E. Structural, physiological, and biochemical aspects of salinity tolerance of halophytes. Rus. J. Plant Phys. 2017, 64, 464–477. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Motos, J.-R.; Diaz-Vivancos, P.; Álvarez, S.; Fernández-García, N.; Sanchez-Blanco, M.J.; Hernández, J.A. Physiological and biochemical mechanisms of the ornamental Eugenia myrtifolia L. plants for coping with NaCl stress and recovery. Planta 2015, 242, 829–846. [Google Scholar] [CrossRef] [Green Version]
- Longstreth, D.J.; Nobel, P.S. Salinity effects on leaf anatomy: Consequences for photosynthesis. Plant Physiol. 1979, 63, 700–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco-Navarro, J.D.; Brumós, J.; Rosales, M.A.; Cubero-Font, P.; Talón, M.; Colmenero-Flores, J.M. Chloride regulates leaf cell size and water relations in tobacco plants. J. Exp. Bot. 2016, 67, 873–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aasamaa, K.; Sõber, A.; Rahi, M. Leaf anatomical characteristics associated with shoot hydraulic conductance, stomatal conductance and stomatal sensitivity to changes of leaf water status in temperate deciduous trees. Funct. Plant Biol. 2001, 28, 765–774. [Google Scholar] [CrossRef]
- Sack, L.; Cowan, P.; Jaikumar, N.; Holbrook, N. The ‘hydrology’of leaves: Co-ordination of structure and function in temperate woody species. Plant Cell Environ. 2003, 26, 1343–1356. [Google Scholar] [CrossRef] [Green Version]
- Aasamaa, K.; Sõber, A. Responses of stomatal conductance to simultaneous changes in two environmental factors. Tree Physiol. 2011, 31, 855–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| CV (m3 plant−1) | TLA/H (cm2 cm−1) | LDW (g plant−1) | SDW (g plant−1) | ALA (cm2) | SLW (g DW cm−2) | |
|---|---|---|---|---|---|---|
| Salt treatment | ||||||
| C | 9.44 ± 0.61 a | 1.47 ± 0.04 a | 162.3 ± 7.61 a | 107.5 ± 3.96 a | 4.39 ± 0.22 a | 98.36 ± 3.59 c |
| CaT | 4.38 ± 0.30 c | 0.79 ± 0.06 c | 95.56 ± 5.52 c | 85.34 ± 2.44 b | 3.86 ± 0.20 a | 149.5 ± 4.93 a |
| NaT | 6.90 ± 0.37 b | 1.23 ± 0.04 b | 131.2 ± 4.0 b | 93.77 ± 2.80 b | 4.28 ± 0.19 a | 114.3 ± 1.93 b |
| Significance | *** | *** | *** | *** | NS | *** |
| PN (µmol CO2 m−2 s−1) | Ci (µmol mol−1) | gs (mmol m−2 s−1) | E (mol H2O m−2 s−1) | iWUE (µmol CO2 mmol−1 H2O) | |
|---|---|---|---|---|---|
| Salt treatment | |||||
| C | 7.55 ± 0.26 a | 210.3 ± 7.63 b | 119.4 ± 4.94 a | 2.23 ± 0.07 a | 72.92 ± 4.83 b |
| CaT | 1.96 ± 0.26 c | 228.9 ± 7.63 a | 28.89 ± 4.94 c | 0.83 ± 0.07 c | 73.46 ± 4.83 b |
| NaT | 4.75 ± 0.25 b | 189.8 ± 7.33 b | 61.41 ± 4.71 b | 1.36 ± 0.06 b | 94.41 ± 4.61 a |
| DAT | |||||
| 93 (12 June) | 4.86 ± 0.31 b | 209.5 ± 6.61 b | 92.18 ± 5.87 a | 2.05 ± 0.08 a | 104.1 ± 4.19 a |
| 106 (25 June) | 5.25 ± 0.22 a | 175.4 ± 6.68 c | 73.62 ± 4.28 b | 1.21 ± 0.06 b | 80.76 ± 4.19 b |
| 121 (10 July) | 4.16 ± 0.22 b | 244.2 ± 9.06 a | 43.92 ± 4.28 c | 1.15 ± 0.06 b | 55.91 ± 5.74 c |
| Significance | |||||
| S | *** | *** | *** | *** | ** |
| D | ** | *** | *** | *** | *** |
| S × D | *** | NS | * | ** | * |
| ΦPSII | qP | qN | Fv/Fo | |
|---|---|---|---|---|
| Salt treatment | ||||
| C | 0.652 ± 0.012 a | 0.917 ± 0.012 a | 0.443 ± 0.035 b | 2.082 ± 0.138 a |
| CaT | 0.525 ± 0.014 b | 0.874 ± 0.013 b | 0.515 ± 0.035 a | 1.161 ± 0.156 b |
| NaT | 0.658 ± 0.015 a | 0.917 ± 0.011 a | 0.379 ± 0.036 b | 2.176 ± 0.165 a |
| DAT | ||||
| 93 (12 June) | 0.605 ± 0.018 a | 0.886 ± 0.011 b | 0.498 ± 0.034 a | 1.759 ± 0.135 a |
| 106 (25 June) | 0.609 ± 0.024 a | 0.909 ± 0.016 a | 0.403 ± 0.044 b | 1.753 ± 0.184 a |
| 121 (10 July) | 0.620 ± 0.022 a | 0.905 ± 0.013 ab | 0.426 ± 0.037 b | 1.908 ± 0.205 a |
| Significance | ||||
| S | *** | *** | *** | *** |
| D | NS | * | ** | NS |
| S × D | NS | NS | NS | * |
| Area (μm2) | MaFD (μm) | MeFD (μm) | MiFD (μm) | Elongation | Sphericity | Convexity | |
|---|---|---|---|---|---|---|---|
| Salt treatment | |||||||
| C | 736.9 ± 1.04 a | 39.72 ± 1.02 a | 34.47 ± 1.02 a | 27.57 ± 1.02 a | 1.50 ± 0.044 a | 0.535 ± 0.019 a | 0.905 ± 0.006 a |
| CaT | 649.3 ± 1.04 b | 37.29 ± 1.02 b | 32.31 ± 1.02 b | 25.77 ± 1.02 b | 1.47 ± 0.029 a | 0.530 ± 0.018 a | 0.904 ± 0.005 a |
| NaT | 488.0 ± 1.05 c | 33.39 ± 1.03 c | 28.71 ± 1.03 c | 22.62 ± 1.03 b | 1.53 ± 0.038 a | 0.513 ± 0.019 a | 0.880 ± 0.007 b |
| Significance | *** | *** | *** | *** | NS | NS | ** |
| Na+ (mg plant−1) | K+ (mg plant−1) | Ca2+ (mg plant−1) | Mg2+ (mg plant−1) | Cl− (mg plant−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| shoot | root | shoot | root | shoot | root | shoot | root | shoot | root | |
| Salt treatment | ||||||||||
| C | 120.4 ± 11.40 b | 81.88 ± 19.24 b | 1803 ± 143.4 a | 533.0 ± 77.49 a | 115.9 ± 7.550 b | 77.4 ± 11.03 b | 178.0 ± 18.81 a | 52.12 ± 8.77 a | 518.8 ± 56.00 c | 86.42 ± 13.97 b |
| CaT | 83.21 ± 7.46 b | 64.27 ± 11.14 b | 1446 ± 142.9 ab | 342.3 ± 42.36 c | 434.6 ± 46.53 a | 159.2 ± 21.24 a | 150.1 ± 13.45 a | 59.05 ± 9.33 a | 2137 ± 316.6 a | 292.7 ± 43.10 a |
| NaT | 589.2 ± 65.72 a | 372.5 ± 60.97 a | 1232 ± 82.23 b | 389.7 ± 34.95 b | 87.0 ± 4.34 b | 55.45 ± 8.33 b | 132.4 ± 10.46 a | 50.37 ± 5.13 a | 1242 ± 210.3 b | 356.6 ± 46.01 a |
| Significance | *** | *** | * | NS | *** | *** | NS | NS | *** | *** |
| Quinic ac. (g/kg DW) | Acetic ac. (g/kg DW) | Formic ac. (g/kg DW) | Malic ac. (g/kg DW) | Tartaric ac. (g/kg DW) | Ossalic ac. (g/kg DW) | Citric ac. (g/kg DW) | |
|---|---|---|---|---|---|---|---|
| Salt treatment | |||||||
| C | 15.24 ± 1.38 a | 3.88 ± 0.37 a | 0.17 ± 0.01 a | 1.49 ± 0.16 a | 0.23 ± 0.02 a | 1.41 ± 0.07 a | 1.30 ± 0.11 a |
| CaT | 2.160 ± 0.67 c | 1.58 ± 0.43 b | 0.15 ± 0.02 a | 0.56 ± 0.07 b | 0.32 ± 0.04 a | 0.73 ± 0.10 b | 0.29 ± 0.04 c |
| NaT | 10.70 ± 1.51 b | 3.73 ± 0.41 a | 0.16 ± 0.02 a | 0.83 ± 0.13 b | 0.25 ±0.03 a | 1.17 ± 0.11 a | 0.70 ± 0.07 b |
| Significance | *** | ** | NS | *** | NS | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Micco, V.; Arena, C.; Amitrano, C.; Rouphael, Y.; De Pascale, S.; Cirillo, C. Effects of NaCl and CaCl2 Salinization on Morpho-Anatomical and Physiological Traits of Potted Callistemon citrinus Plants. Forests 2021, 12, 1666. https://doi.org/10.3390/f12121666
De Micco V, Arena C, Amitrano C, Rouphael Y, De Pascale S, Cirillo C. Effects of NaCl and CaCl2 Salinization on Morpho-Anatomical and Physiological Traits of Potted Callistemon citrinus Plants. Forests. 2021; 12(12):1666. https://doi.org/10.3390/f12121666
Chicago/Turabian StyleDe Micco, Veronica, Carmen Arena, Chiara Amitrano, Youssef Rouphael, Stefania De Pascale, and Chiara Cirillo. 2021. "Effects of NaCl and CaCl2 Salinization on Morpho-Anatomical and Physiological Traits of Potted Callistemon citrinus Plants" Forests 12, no. 12: 1666. https://doi.org/10.3390/f12121666
APA StyleDe Micco, V., Arena, C., Amitrano, C., Rouphael, Y., De Pascale, S., & Cirillo, C. (2021). Effects of NaCl and CaCl2 Salinization on Morpho-Anatomical and Physiological Traits of Potted Callistemon citrinus Plants. Forests, 12(12), 1666. https://doi.org/10.3390/f12121666











