Birds and the Fire Cycle in a Resilient Mediterranean Forest: Is There Any Baseline?
Abstract
1. Introduction
2. Material and Methods
2.1. Study Site
2.2. Sampling Design
2.3. Sampling Methods
2.3.1. Avifauna
2.3.2. Vegetation
2.4. Statistical Analyses
2.4.1. Gradient Analyses
2.4.2. Alpha Diversity
2.4.3. Indexation of Avifauna on a Reference Gradient
2.4.4. Analysis of the Vegetation
3. Results
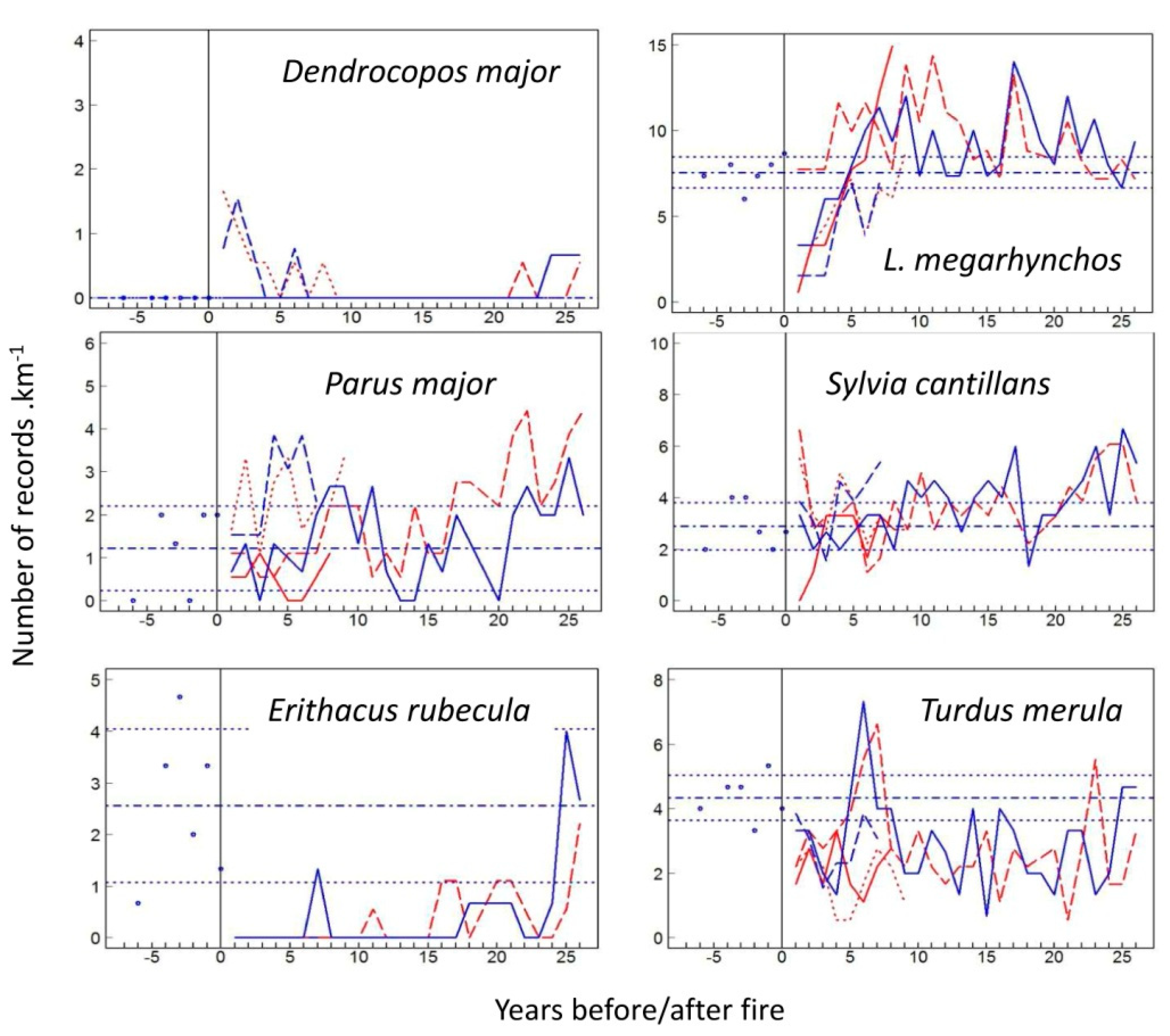
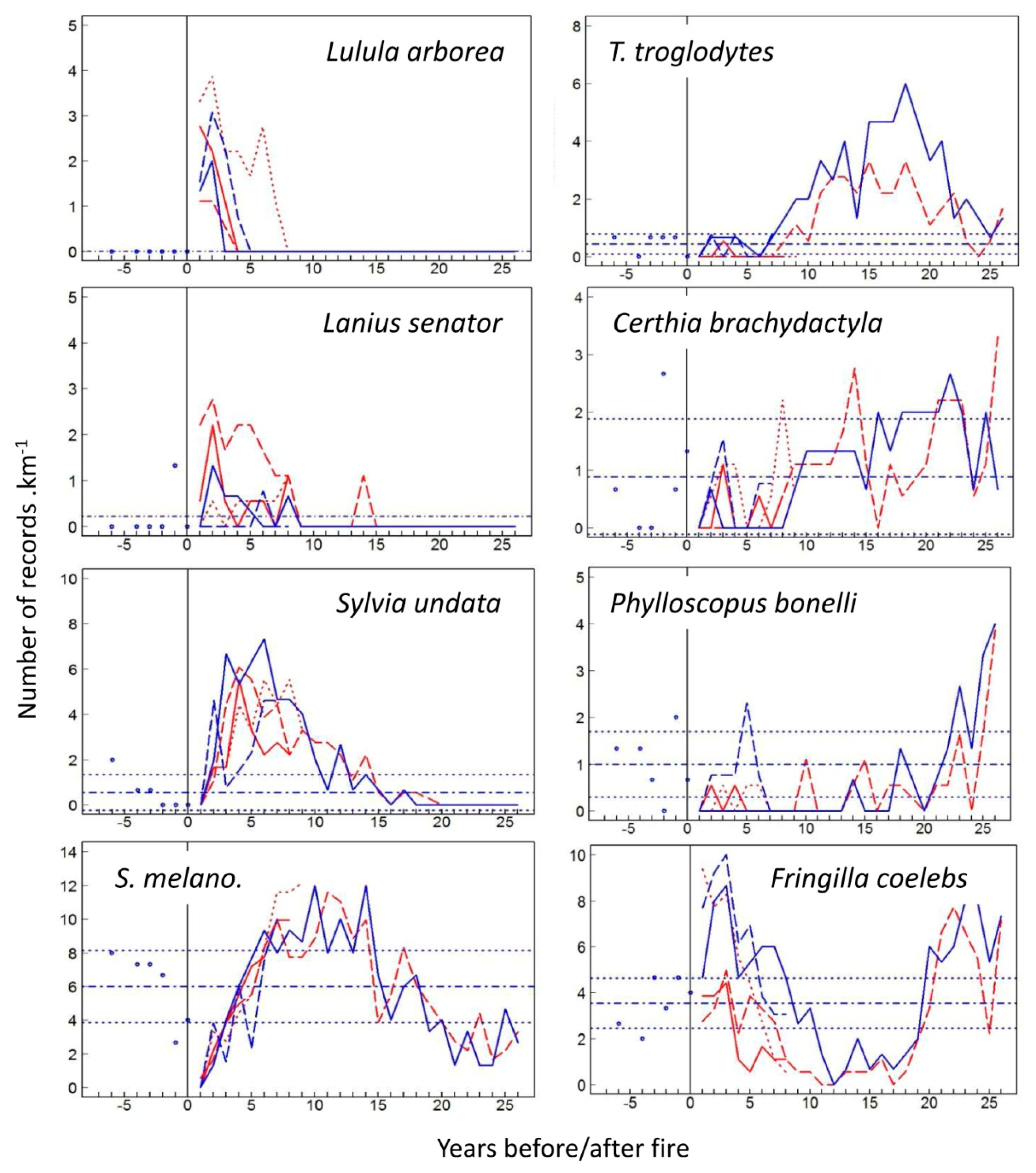
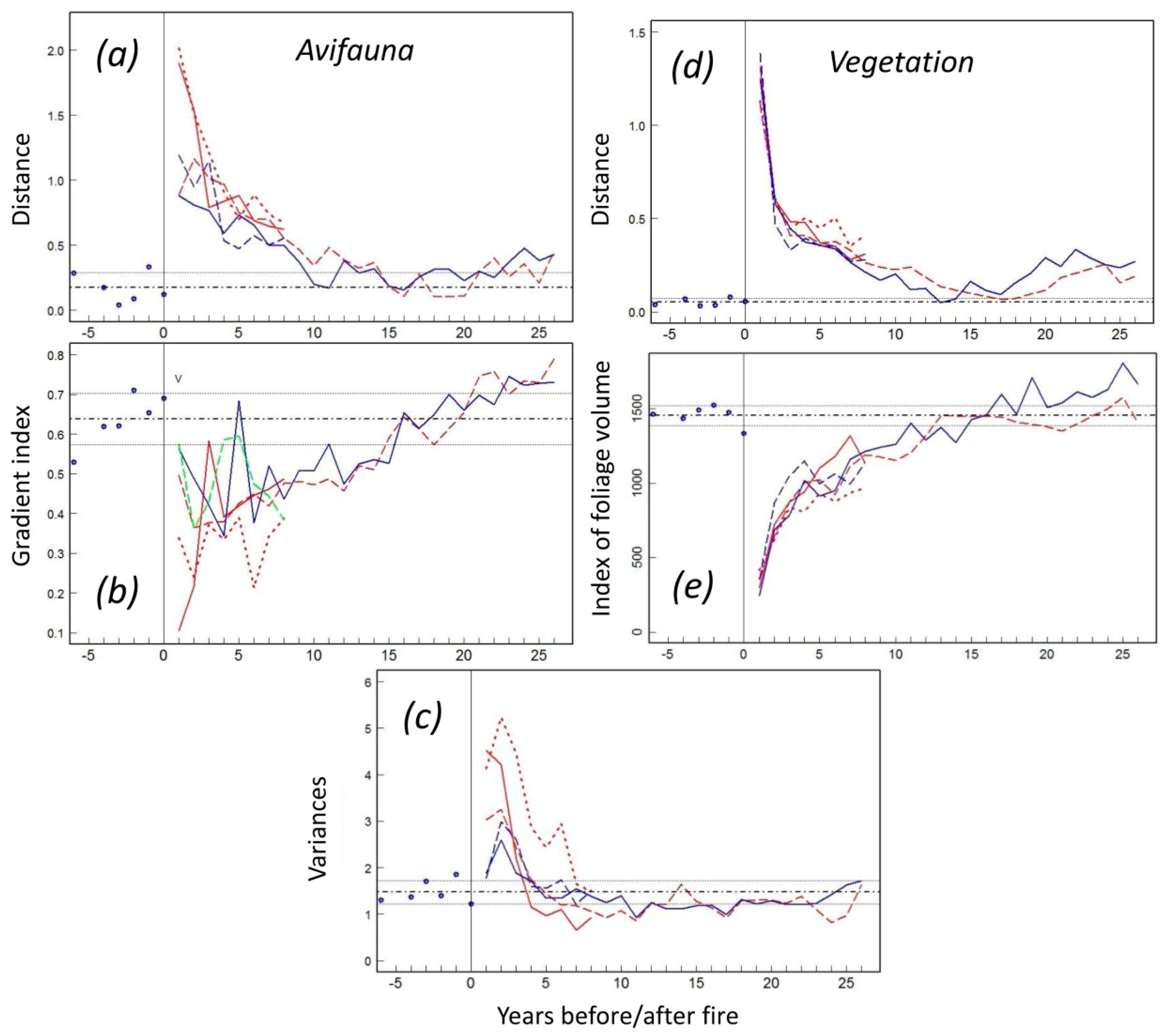
3.1. Species-Specific Postfire Responses of Birds
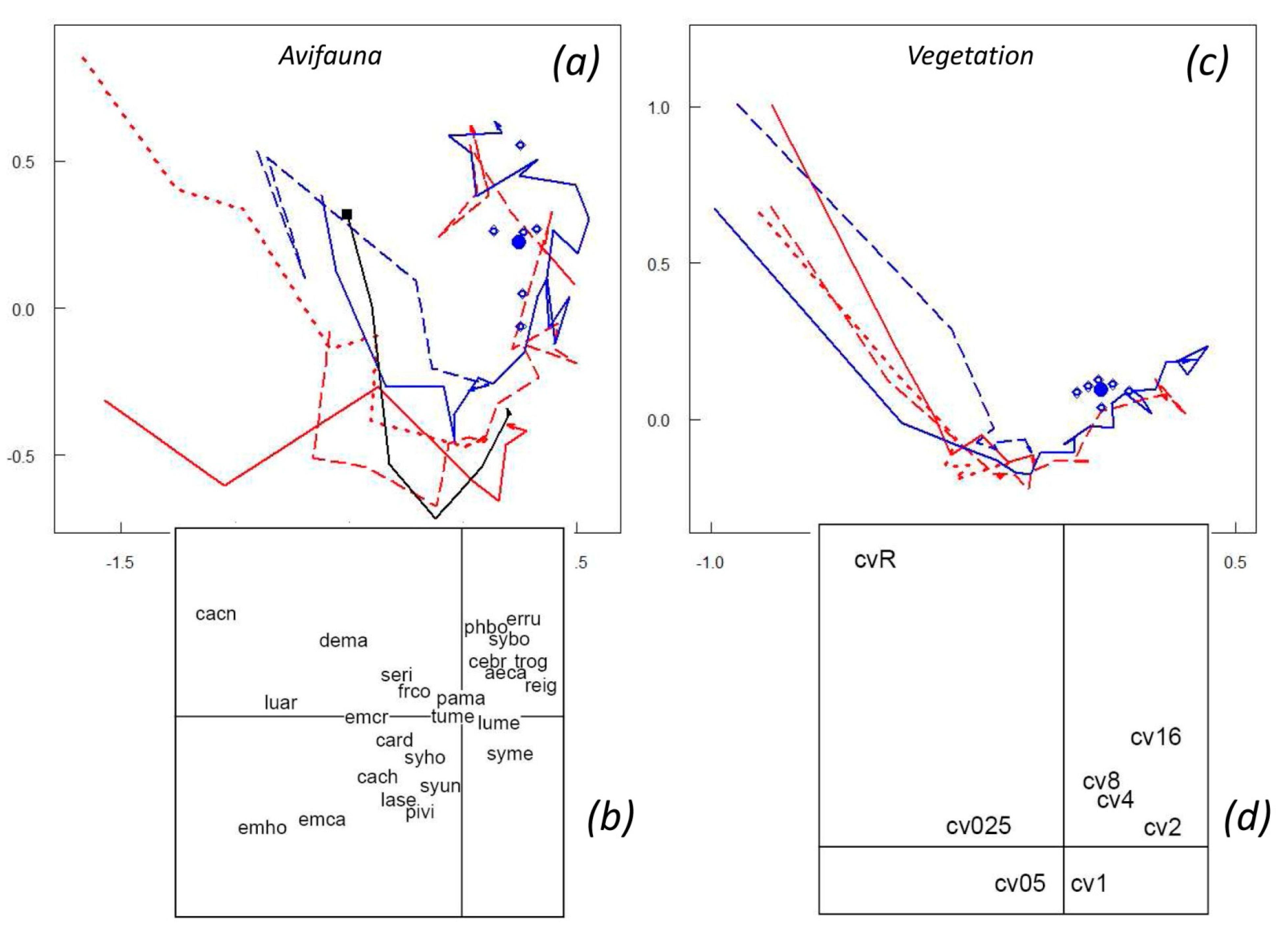
3.2. The Postfire Successional Gradient
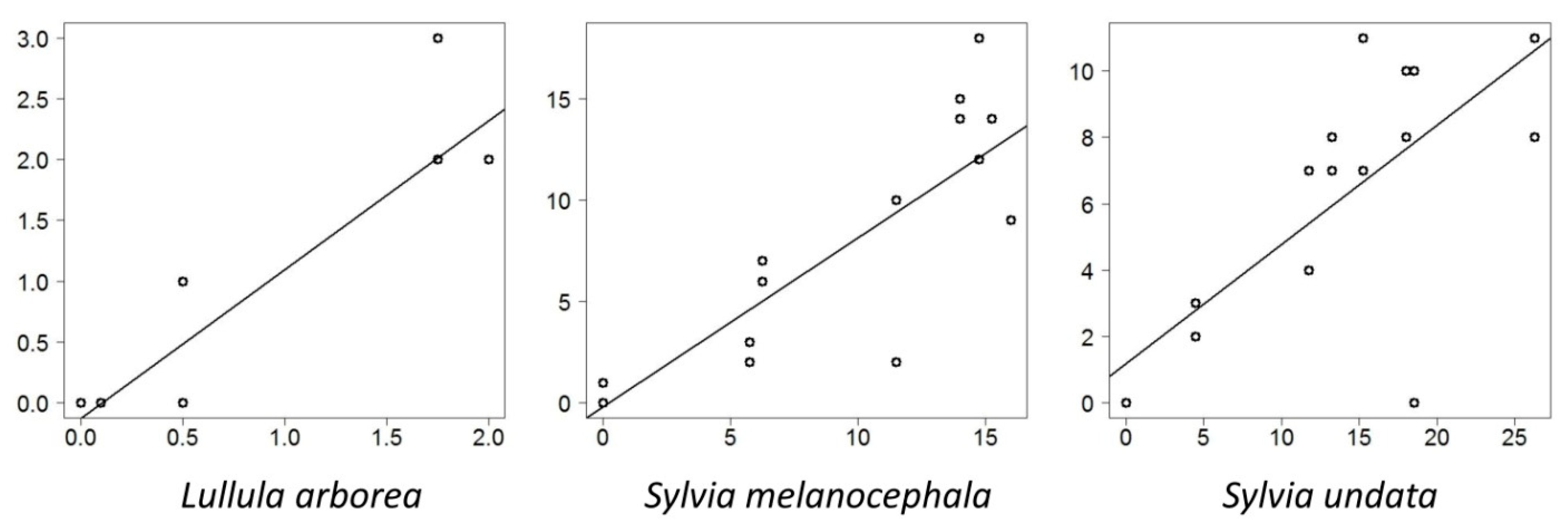
3.3. Indexation on a Reference Structure Gradient
3.4. Migratory versus Sedentary Bird Species
3.5. Bird Richness and Diversity
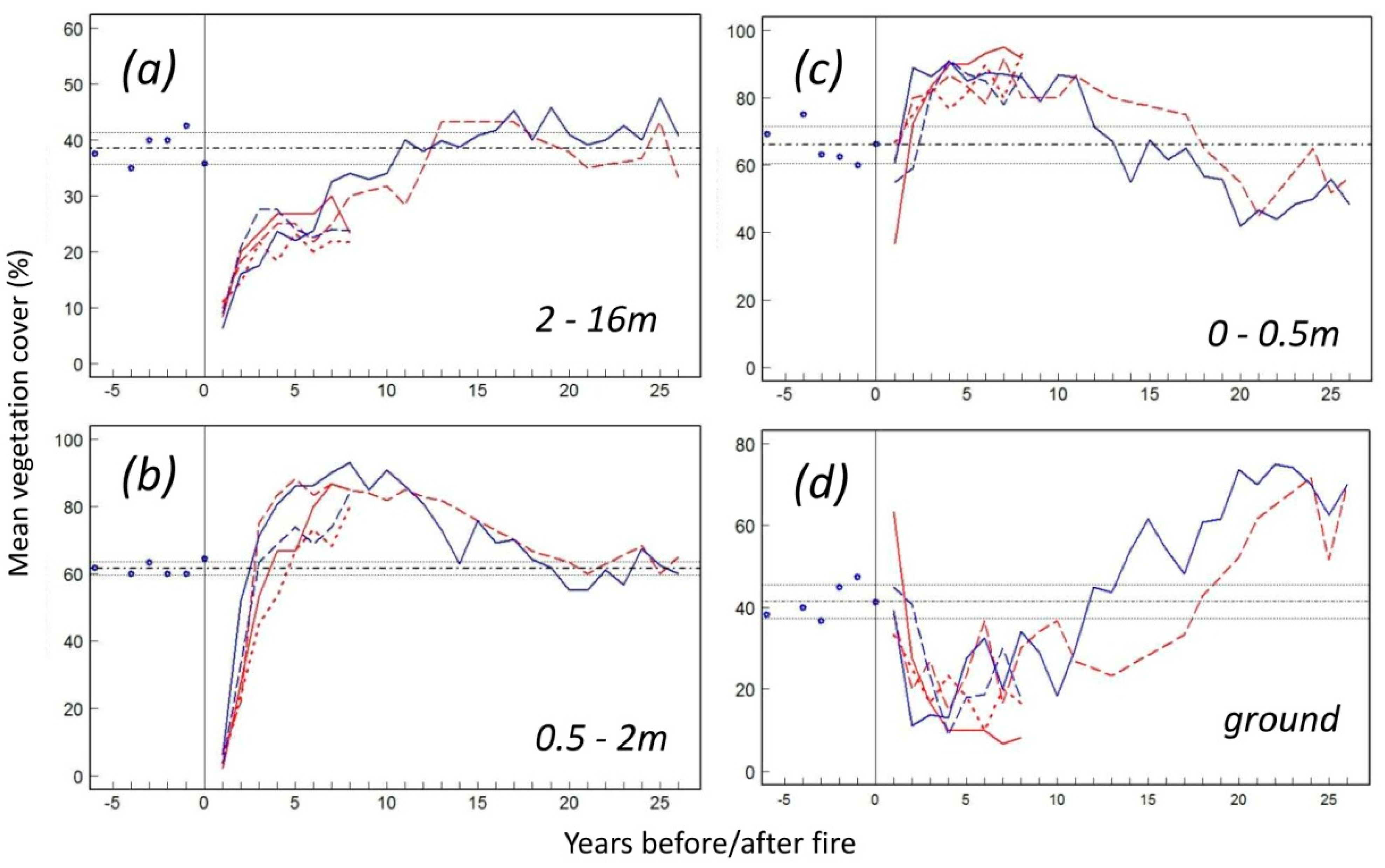
3.6. Vegetation Response
4. Discussion
4.1. Postfire Bird Response Models: Colonization vs. Site Fidelity
4.2. The Four Phases of Postfire Succession
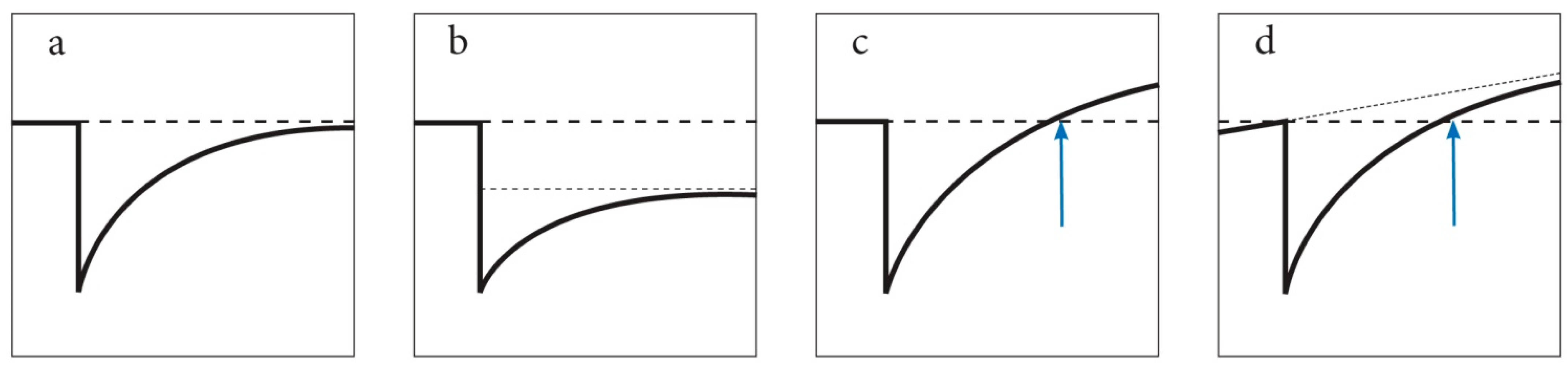
4.3. Resilience after Repeated Disturbance
4.4. Questioning the Control as a Benchmark
4.5. Asymptotic Resilience vs. Overcompensation Hypothesis
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Abbreviation |
|---|---|
| Aegithalos caudatus | aeca |
| Carduelis cachinans | cach |
| Carduelis cannabina | cacn |
| Carduelis carduelis | card |
| Certhia brachydactyla | cebr |
| Columba palombus | copa |
| Cuculus canorus | cuca |
| Cyanistes [Parus] caeruleus | cyca |
| Dendrocopos major | dema |
| Emberiza [Miliaria] calandra | emca |
| Emberiza cirlus | emcr |
| Emberiza hortulana | emho |
| Erithacus rubecula | erru |
| Fringilla coelebs | frco |
| Garrulus glandarius | gagl |
| Hippolais polyglotta | hipo |
| Jynx torquilla | jyto |
| Lanius senator | lase |
| Lophophanes [Parus] cristatus | locr |
| Lullula arborea | luar |
| Luscinia megarhynchos | lume |
| Oriolus oriolus | orio |
| Parus major | pama |
| Phylloscopus bonelli | phbo |
| Picus viridis | pivi |
| Regulus ignicapillus | reig |
| Serinus serinus | seri |
| Streptopelia turtur | sttu |
| Sylvia borin | sybo |
| Sylvia cantillans | syca |
| Sylvia hortensis | syho |
| Sylvia melanocephala | syme |
| Sylvia undata | syun |
| Troglodytes troglodytes | trog |
| Turdus merula | tume |
| Upupa epops | upep |



References
- Ratajczak, Z.; Carpenter, S.R.; Ives, A.R.; Kucharik, C.J.; Ramiadantsoa, T.; Stegner, M.A.; Williams, J.W.; Zhang, J.; Turner, M.G. Abrupt change in ecological systems: Inference and diagnosis. Trends Ecol. Evol. 2018, 33, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, K.; Jucker, T.; Svenning, J.-C. Unifying the concepts of stability and resilience in ecology. J. Ecol. 2021. Available online: https://besjournals.onlinelibrary.wiley.com/doi/abs/10.1111/1365-2745.13651 (accessed on 5 April 2021).
- Holling, C.S. Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef]
- Holling, C.S. Engineering resilience. In Engineering within Ecological Constraints; Schulze, P.C., Ed.; National Academy of Engineering, National Academy Press: Washington, DC, USA, 1996; pp. 31–43. [Google Scholar]
- Westman, W.E. Resilience: Concepts and measures. In Resilience in Mediterranean-Type Ecosystems; Dell, B., Hopkins, A.J.M., Lamont, B.B., Eds.; Tasks for Vegetation Science; Springer: Dordrecht, The Netherlands, 1986; Volume 16. [Google Scholar] [CrossRef]
- Standish, R.J.; Hobbs, R.J.; Mayfield, M.M. Resilience in ecology: Abstraction, distraction, or where the action is? Biol. Conserv. 2014, 177, 43–51. [Google Scholar] [CrossRef]
- Hodgson, D.; McDonald, J.; Hosken, D.J. What do you mean, resilient? Trends Ecol. Evol. 2015, 30, 503–506. [Google Scholar] [CrossRef]
- Van de Leemput, I.A.; Dakos, V.; Scheffer, M.; van Nes, E.H. Slow recovery from local disturbances as an indicator for loss of ecosystem resilience. Ecosystems 2018, 21, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Ingrisch, J.; Bahn, M. Toward a comparable quantification of resilience. Trends Ecol. Evol. 2018, 33, 251–259. [Google Scholar] [CrossRef]
- Arnoldi, J.-F.; Bideault, A.; Loreau, M.; Haegeman, B. How ecosystem recover from pulse perturbations: A theory of short- to long-term responses. J. Theor. Biol. 2018, 7, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Viedma, O.; Meliá, J.; Segarra, D.; Garcia-Haro, J. Modeling rates of ecosystem recovery after fires by using landsat TM data. Remote Sens. Environ. 1997, 61, 383–398. [Google Scholar] [CrossRef]
- Christakopoulos, P.; Hatzopoulos, I.; Kalabokidis, K.; Paronis, D.; Filintas, A. Assessment of the response of a Mediterranean-type forest ecosystem to recurrent wildfires and to different restoration practices using Remote Sensing and GIS techniques. In Advances in Remote Sensing and GIS Applications in Forest Fire Management, Proceedings of the 6th International Workshop of the EARSeL, Thessaloniki, Greece, 27–29 September 2007; Gitas, I., Carmona-Moreno, C., Eds.; European Commission: Thessaloniki, Greece, 2007; pp. 213–216. [Google Scholar]
- Bastos, A.; Gouveia, C.M.; DaCamara, C.C.; Trigo, R.M. Modelling post-fire vegetation recovery in Portugal. Biogeosciences 2011, 8, 3593–3607. [Google Scholar] [CrossRef]
- Rydgren, K.; Halvorsen, R.; Töpper, J.P.; Auestad, I.; Hamre, L.N.; Jongejans, E.; Sulavik, J. Advancing restoration ecology: A new approach to predict time to recovery. J. Appl. Ecol. 2019, 56, 225–234. [Google Scholar] [CrossRef]
- Prodon, R.; Diaz-Delgado, R. Assessing the postfire resilience of a Mediterranean forest from satellite and ground data (NDVI, vegetation structure, avifauna). Écoscience 2021, 28, 81–91. [Google Scholar] [CrossRef]
- Jentsch, A.; White, P. A theory of pulse dynamics and disturbance in ecology. Ecology 2019, 100, e02734. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, N.P. Unifying ecosystem responses to disturbance into a single statistical framework. Oikos 2021, 130, 408–421. [Google Scholar] [CrossRef]
- Jacquet, K.; Prodon, R. Measuring the postfire resilience of a bird-vegetation system: A 28yr study in a Mediterranean oak woodland. Oecologia 2009, 161, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Malak, D.A.; Pausas, J.G. Fire regime and post-fire Normalized Difference Vegetation Index changes in the eastern Iberian peninsula (Mediterranean basin). Int. J. Wildland Fire 2006, 15, 407–413. [Google Scholar] [CrossRef]
- Del Moral, R.; Thomason, L.A.; Wenke, A.C.; Lozanoff, N.; Abata, M.D. Primary succession trajectories on pumice at Mount St. Helens, Washington. J. Veg. Sci. 2012, 23, 73–85. [Google Scholar] [CrossRef]
- Turner, M.; Braziunas, K.H.; Hansen, W.D.; Harvey, B.J. Short-term severe fire erodes the resilience of subalpine lodgepole pine forests. Proc. Natl. Acad. Sci. USA 2019, 116, 11319–11328. [Google Scholar] [CrossRef]
- Zedler, P.H.; Gautier, C.R.; McMaster, G.S. Vegetation Change in Response to Extreme Events: The Effect of a Short Interval between Fires in California Chaparral and Coastal Scrub. Ecology 1983, 64, 809–818. [Google Scholar] [CrossRef]
- Moreno, J.M.; Vázquez, A.; Vélez, R. Recent history of forest fires in Spain. In Large Forest Fires; Moreno, J.M., Ed.; Backhuys Publishers: Leiden, The Netherlands, 1998; pp. 159–185. [Google Scholar]
- Baeza, M.J.; Valdecantos, A.; Alloza, J.A.; Vallejo, V.R. Human disturbance and environmental factors as drivers of long-term post-fire regeneration pattern in Mediterranean forests. J. Veg. Sci. 2007, 18, 243–252. [Google Scholar] [CrossRef]
- Acacio, V.; Holmgren, M.; Jansen, P.A.; Schrotter, O. Multiple recruitment limitation causes arrested succession in Mediterranean cork oak systems. Ecosystems 2007, 10, 1220–1230. [Google Scholar] [CrossRef]
- Tessler, N.; Wittenberg, L.; Provizor, E.; Greenbaum, N. The influence of short-interval recurrent forest fires on the abundance of Aleppo pine (Pinus halepensis Mill.) on Mount Carmel, Israel. For. Ecol. Manag. 2014, 324, 109–116. [Google Scholar] [CrossRef]
- Vilà-Cabrera, A.; Saura-Mas, S.; Lloret, F. Effects of fire frequency on species composition in a Mediterranean shrubland. Écoscience 2008, 15, 519–528. [Google Scholar] [CrossRef]
- Paula, S.; Ojeda, F. Belowground starch consumption after recurrent severe disturbance in three resprouter species of the genus Erica. Botany 2009, 87, 253–259. [Google Scholar] [CrossRef]
- Eugenio, M.; Lloret, F. Effects of repeated burning on Mediterranean communities of the northeastern Iberian Peninsula. J. Veg. Sci. 2006, 17, 755–764. [Google Scholar] [CrossRef]
- Dugy, B.; Paula, S.; Pausas, J.G.; Alloza, J.A.; Gimeno, T.; Vallejo, R.V. Effects of climate and extreme events on wildfire regime and their ecological impacts. In Regional Assessment of Climate Change in the Mediterranean; Navarra, A., Tubiana, L., Eds.; Springer: Dordrecht, The Netherland, 2013; Volume 2, pp. 101–134. [Google Scholar]
- Mouillot, F.; Ratte, J.-P.; Joffre, R.; Mouillot, D.; Rambal, S. Long-term forest dynamic after land abandonment in a fire prone Mediterranean landscape (central Corsica, France). Landsc. Ecol. 2005, 20, 101–112. [Google Scholar] [CrossRef]
- Diaz-Delgado, R.; Lloret, F.; Pons, X.; Terradas, J. Satellite evidence of decreasing resilience in Mediterranean plant communities after recurrent wildfires. Ecology 2002, 83, 2293–2303. [Google Scholar] [CrossRef]
- Schaffhauser, A.; Curt, T.; Véla, E.; Tatoni, T. Recurrent fires and environment shape the vegetation in Quercus suber L. woodlands and maquis. Comptes Rendus Biol. 2012, 335, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Wittenberg, L.; Malkinson, D.; Beeri, O.; Halutzy, A.; Tesler, N. Spatial and temporal patterns of vegetation recovery following sequences of forest fires in a Mediterranean landscape, Mt. Carmel Israel. Catena 2007, 71, 76–83. [Google Scholar] [CrossRef]
- Manning, A.D.; Fischer, J.; Lindenmayer, D.B. Scattered trees are keystone structures—Implications for conservation. Biol. Conserv. 2006, 132, 311–321. [Google Scholar] [CrossRef]
- Barton, P.S.; Ikin, K.; Smith, A.L.; MacGregor, C.; Lindenmayer, D.B. Vegetation structure moderates the effect of fire on bird assemblages in a heterogeneous landscape. Landsc. Ecol. 2014, 29, 703–714. [Google Scholar] [CrossRef]
- Pons, P.; Henry, P.-Y.; Gargallo, G.; Prodon, R.; Lebreton, J.D. Local survival after fire in Mediterranean shrublands: Combining capture-recapture data over several bird species. Popul. Ecol. 2003, 45, 187–196. [Google Scholar] [CrossRef]
- Prodon, R.; Pons, P. Postfire bird studies, methods, questions, and perspectives. In Fire in Mediterranean Ecosystems; Trabaud, L., Prodon, R., Eds.; Ecosystem Research Reports; ECSC-EEC-EAEC: Luxembourg, 1993; Volume 5, pp. 332–344. [Google Scholar]
- Pons, P.; Prodon, R. Short-term temporal pattern in a Mediterranean shrubland bird community after wildfire. Acta Oecol. 1996, 17, 29–41. [Google Scholar]
- Herrando, S.; Brotons, L. Forest bird diversity in Mediterranean areas affected by wildfires: A multi-scale approach. Ecography 2002, 25, 161–172. [Google Scholar] [CrossRef]
- Zozaya, E.L.; Brotons, L.; Vallecillo, S. Bird community response to vegetation heterogeneity following non-direct regeneration of Mediterranean forest after fire. Ardea 2011, 99, 73–84. [Google Scholar] [CrossRef]
- Pausas, J.G.; Fernández-Muñoz, S. Fire regime changes in the Western Mediterranean Basin: From fuel-limited to drought-driven fire regime. Clim. Chang. 2012, 110, 215–226. [Google Scholar] [CrossRef]
- Moriondo, M.; Good, P.; Durao, R.; Bindi, M.; Giannakopoulos, C.; Corte-Real, J. Potential impact of forest change on fire risk in the Mediterranean area. Clim. Res. 2006, 31, 85–95. [Google Scholar] [CrossRef]
- Ruffault, J.; Curt, T.; Moron, V.; Trigo, R.M.; Mouillot, F.; Koutsias, N.; Pimont, F.; Martin-StPaul, N.; Barbero, R.; Dupuy, J.-L.; et al. Increased likelihood of heat-induced large wilfires in the Mediterranean Basin. Sci. Rep. 2020, 10, 13790. Available online: https://10.1038/s41598-020-70069-z (accessed on 1 August 2021). [CrossRef] [PubMed]
- Mouillot, F.; Ratte, J.-P.; Joffre, R.; Moreno, J.M.; Rambal, S. Some determinant of the spatio-temporal fire cycle in a Mediterranean landscape (Corsica, France). Landsc. Ecol. 2003, 18, 665–674. [Google Scholar] [CrossRef]
- Slik, J.W.F.; Van Balen, S. Bird community changes in response to single and repeated fires in a lowland tropical rainforest of eastern Borneo. Biodivers. Conserv. 2006, 15, 4425. [Google Scholar] [CrossRef]
- Fontaine, J.B.; Donato, D.C.; Robinson, W.D.; Law, B.E.; Kauffman, J.B. Bird communities following high-severity fire: Response to single and repeat fires in a mixed-evergreen forest, Oregon, U.S.A. For. Ecol. Manag. 2009, 257, 1496. [Google Scholar] [CrossRef]
- Greenberg, C.H.; Waldrop, T.A.; Tomcho, J.; Phillips, R.J.; Simon, D. Bird response to fire severity and repeated burning in upland hardwood forest. For. Ecol. Manag. 2013, 304, 80–88. [Google Scholar] [CrossRef][Green Version]
- Cusser, S.; Helms IV, J.; Bahlai, C.A.; Haddad, N.M. How long do population level field experiments need to be? Utilising data from the 40-year-old LTER network. Ecol. Lett. 2021, 24, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Nadal, N. Reconstrucció D’incendis Històrics. 2012. Seminari PrincAlb, Mollet, 13–14 December 2012. Available online: http://agricultura.gencat.cat/web/.content/06-medi-natural/gestio-forestal/enllacos-documents/princalb/fitxers-binaris/PRINCALB_Presentacio_I-D_Bombers_incendis_historics.pdf (accessed on 7 January 2020).
- Díaz-Delgado, R.; Lloret, F.; Pons, X. Spatial patterns of fire occurrence in Catalonia, NE, Spain. Landsc. Ecol. 2004, 19, 731–745. [Google Scholar] [CrossRef]
- Nadal Saleillas, N. Prise en Compte de L’historique des feux dans la Planification DFCI. Séminaire sur le Retour D’expérience en Matière de Prévention des Incendies de Forêts dans la zone Méditerranéenne, 17–18 Novembre 2016. Available online: https://www.prevention-incendie66.com/sites/default/files/documents/seminaire_rex/intervention_forestal_catalana_nn.pdf (accessed on 7 January 2020).
- Dubois, C. Comportement du chêne-liège après incendie; Graduation Report; Ecole Nationale des Ingénieurs des Travaux des Eaux et Forêts: Nogent-sur-Vernisson, France, 1990; Available online: https://catalogue.cefe.cnrs.fr/index.php?lvl=notice_display&id=13778 (accessed on 18 March 2021).
- Dubois, C.; Prodon, R. Survie du Chêne-liège (Quercus suber) après incendie. Nat. Monspel. 1991, 596–597. [Google Scholar]
- Catry, F.X.; Moreira, F.; Pausas, J.G.; Fernandes, P.; Rego, F.; Cardillo, E.; Curt, T. Cork oak vulnerability to fire: The role of bark harvesting, tree characteristics and abiotic factors. PLoS ONE 2012, 7, e39810. [Google Scholar] [CrossRef]
- Pons, P. Dynamique de l’avifaune après incendie et brûlage dirigé en zone méditerranéenne: Importance de la territorialité. Ph.D. Thesis, University of Paris, Paris, France, 18 October 1996. [Google Scholar]
- Pons, P.; Clavero, M.; Bas, J.M.; Prodon, R. Time-window of occurrence and vegetation cover preferences of dartford and Sardinian warblers after fire. J. Ornith. 2012, 153, 921–930. [Google Scholar] [CrossRef]
- Prodon, R.; Lebreton, J.D. Breeding avifauna of a Mediterranean succession—The holm oak and cork oak series in the eastern Pyrenees.1. Analysis and Modeling of the Structure Gradient. Oikos 1981, 37, 21–38. [Google Scholar] [CrossRef]
- Kéfi, S.; Domínguez-García, V.; Donohue, I.; Fontaine, C.; Thébault, E.; Dakos, V. Advancing our understanding of ecological stability. Ecol. Lett. 2019, 22, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.P. Use of ordination and other multivariate descriptive methods to study succession. Plant Ecol. 1977, 35, 168–175. [Google Scholar] [CrossRef]
- Halpern, C.B. Early successional pathways and the resistance and resilience of forest communities. Ecology 1988, 69, 1703–1715. [Google Scholar] [CrossRef]
- Malanson, G.P.; Trabaud, L. Ordination Analysis of Components of Resilience of Quercus coccifera Garrigue. Ecology 1987, 68, 463–472. [Google Scholar] [CrossRef]
- Bloom, S.A. Multivariate quantification of community recovery. In The Recovery Process in Damaged Ecosystems; Cairns, J., Jr., Ed.; Ann Arbor Science Publishers: Ann Arbor, MI, USA, 1980; pp. 141–151. [Google Scholar]
- Day, N.J.; Carrière, S.; Baltzer, J.L. Annual dynamics and resilience in post-fire boreal understory vascular plant communities. For. Ecol. Manag. 2017, 401, 264–272. [Google Scholar] [CrossRef]
- Prodon, R. Animal communities and vegetation dynamics: Measuring and modelling animal community dynamics along forest successions. In Responses of Forest Ecosystems to Environmental Changes; Teller, A., Mathy, P., Jeffers, J.N.R., Eds.; Elsevier: Amsterdam, The Netherlands, 1992; pp. 126–141. [Google Scholar]
- Thioulouse, J.; Dray, S.; Dufour, A.-B.; Siberchicot, A.; Jombart, T.; Pavoine, S. Multivariate Analysis of Ecological Data; Springer Nature: New York, NY, USA, 2018. [Google Scholar]
- Chessel, D.; Lebreton, J.-D.; Prodon, R. Mesures symétriques d’amplitude d’habitat et de diversité intra-échantillon dans un tableau espèces-relevés. Cas d’un gradient simple. C. R. Acad. Sci. 1982, 295, 83–88. [Google Scholar]
- Thioulouse, J.; Chessel, D. A Method for Reciprocal Scaling of Species Tolerance and Sample Diversity. Ecology 1992, 73, 670–680. [Google Scholar] [CrossRef]
- Watson, S.J.; Taylor, R.T.; Nimmo, D.G.; Kelly, L.T.; Haslem, A.; Clarke, M.F.; Bennett, A.F. Effect of time since burn: How informative are generalized fire response curves for conservation management. Ecol. Appl. 2012, 22, 685–696. [Google Scholar] [CrossRef]
- Bas, J.M.; Pons, P.; Gómez, C. Home range and territory of the Sardinian warbler Sylvia melanocephala in Mediterranean shrubland. Bird Study 2005, 52, 137–144. [Google Scholar] [CrossRef]
- Creegan, H.P.; Osborne, P.E. Gap-crossing decisions of woodland songbirds in Scotland: An experimental approach. J. Appl. Ecol. 2005, 42, 678–687. [Google Scholar] [CrossRef]
- Brotons, L.; Pons, P.; Herrando, S. Colonisation of dynamic Mediterranean landscapes: Where do birds come from after fire. J. Biogeogr. 2005, 32, 789–798. [Google Scholar] [CrossRef]
- Llimona, E.; Matheu, F.; Prodon, R. Role of standing snags and of tree regeneration in postfire bird succession: Comparison of pine and oak forests of Montserrat (Catalonia). In Fire in Mediterranean Ecosystems; Trabaud, L., Prodon, R., Eds.; Ecosystem Research Reports; ECSC-EEC-EAEC: Luxembourg, 1993; Volume 5, pp. 315–331. [Google Scholar]
- Franklin, J.F.; Lindenmayer, D.; MacMahon, J.A.; McKee, A.; Magnuson, J.; Perry, D.A.; Waide, R.; Foster, D. Threads of Continuity, Ecological Disturbance, Biological Legacy and Recovery. Conserv. Biol. Pract. 2000, 1, 8–16. Available online: https://d1wqtxts1xzle7.cloudfront.net/44840112/ (accessed on 26 March 2021).
- Seidl, R.; Rammer, W.; Spies, T.A. Disturbance legacies increase the resilience of forest ecosystem structure, composition, and functioning. Ecol. Appl. 2014, 24, 2063–2077. [Google Scholar] [CrossRef]
- Swanson, M.E.; Franklin, J.F.; Beschta, R.L.; Crisafulli, C.M.; DellaSala, D.A.; Hutto, R.L.; Lindenmayer, D.B.; Swanson, F.J. The forgotten stage of forest succession: Early-successional ecosystems on forest sites. Front. Ecol. Environ. 2010, 9, 117–125. [Google Scholar] [CrossRef]
- Tingley, M.W.; Ruiz-Gutiérrez, V.; Wilkerson, R.L.; Howell, C.A.; Siegel, R.B. Pyrodiversity promotes avian diversity over the decade following fire. Proc. R. Soc. B 2016, 283, 20161703. [Google Scholar] [CrossRef] [PubMed]
- Trabaud, L.; Oustric, J. Heat requirements for seed germination of three Cistus species in the garrigue of southern France. Flora 1989, 183, 321–325. [Google Scholar] [CrossRef]
- Baeza, M.J.; Vallejo, V.R. Ecological mechanisms involved in dormancy breakage in Ulex parviflorus seeds. Plant Ecol. 2006, 183, 191–205. [Google Scholar] [CrossRef]
- Roy, J.; Sonie, L. Germination and Population Dynamics of Cistus Species in Relation to Fire. J. Appl. Ecol. 1992, 29, 647–655. [Google Scholar] [CrossRef]
- Nascimento Franco, J.D.; Rubido-Bará, M.; Horjales-Luaces, M.; Reigosa, M.J. Allelopathy between seeds of the Ulex L. and Genista L. genera. J. Allelochem. Interact. 2017, 3, 9–17. [Google Scholar]
- Pardo-Muras, M.; Puig, C.G.; López-Nogueira, A.; Cavaleiro, C.; Pedrol, N. On the herbicide potential of Ulex europeus and Cytisus scoparius. Profiles of volatile organic compounds and their phytotoxic effects. PLoS ONE 2018, 13, e0205997. [Google Scholar] [CrossRef]
- Clemente, A.S.; Rego, F.C.; Correia, O.A. Seed bank dynamics of two obligate seeders, Cistus monspeliensis and Rosmarinus officinalis in relation to time since fire. Plant Ecol. 2007, 190, 175–188. [Google Scholar] [CrossRef]
- Baeza, M.; Roy, J. Germination of an obligate seeder (Ulex parviflorus) and consequences for wildfire management. For. Ecol. Manag. 2008, 256, 685–693. [Google Scholar] [CrossRef]
- De Luis, M.; Baeza, M.J.; Raventos, J.; Gonzalez-Hidalgo, J.C. Fuel characteristics and fire behaviour in mature Mediterranean gorse shrublands. Int. J. Wildland Fire 2004, 13, 79–87. [Google Scholar] [CrossRef]
- Parks, S.A.; Miller, C.; Nelson, C.R.; Holden, Z.A. Previous fires moderate burn severity of subsequent wildland fires in two large western US wilderness areas. Ecosystems 2014, 17, 29–42. [Google Scholar] [CrossRef]
- Mokaddem, H. Contribution à l’étude de la relation indice d’accroissement du liège/paramètres pluviométriques de deux subéraies oranaises (M’Sila (W. Oran) et Hafir (W. Tlemcen)); Graduation Report; University of Tlemcen: Chetouane, Algeria, 2012; Available online: https://docplayer.fr/42801326-Memoire-de-fin-d-etudes-pour-l-obtention-du-diplome-d-ingenieur-d-etat-en-foresterie.html (accessed on 9 April 2021).
- Poizaridis, K.; Chaideftou, E.; Martinis, A.; Bontzorlos, V.; Galani, P.; Kalivas, D. Temporal shifts in floristic and avian diversity in Mediterranean pine forest ecosystems under different fire pressure: The island of Zakynthos as a case study. Ann. For. Res. 2018, 61, 19–36. [Google Scholar]
- Ovenden, T.S.; Perks, M.P.; Clarke, T.-K.; Mencuccini, M.; Jump, A.S. Life after recovery: Increased resolution of forest resilience assessment sheds new light on post-drought compensatory growth and recovery dynamics. J. Ecol. 2021, 109, 3157–3170. [Google Scholar] [CrossRef]
- Kampicher, C.; Angeler, D.G.; Holmes, R.T.; Leito, A.; Svensson, S.; Van der Jeugd, H.; Wesolowski, T. Temporal dynamics of bird community composition: An analysis of baseline conditions from long-term data. Oecologia 2014, 175, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, R.A.; Condit, R.; Rahman, K.A.; Baker, P.J.; Bunyavejchewin, S.; Chen, Y.-Y.; Chuyong, G.; Dattaraja, H.S.; Davies, S.; Corneille, E.N.; et al. Temporal variability of forest communities: Empirical estimates of population change in 4000 tree species. Ecol. Lett. 2014, 17, 855–865. [Google Scholar] [CrossRef]
- Pimm, S.L.; Donohue, I.; Montoya, J.M.; Loreau, M. Measuring resilience is essential if we are to understand it. Nat. Sustain. 2019, 2, 895–897. [Google Scholar] [CrossRef] [PubMed]
- Pimm, S.L. The complexity and stability of ecosystems. Nature 1984, 307, 321–326. [Google Scholar] [CrossRef]
- Manobens, J.B.; Gimeno, V.A.; Serrat, J.G. L’evolució del paisatge forestal (1950–2013) a l’Alt Empordà. Ann. Inst. Estud. Empord. 2015, 46, 343–368. [Google Scholar]
- Prodon, R. Une alternative aux “types biogéographiques” de Voous: La mesure des distributions latitudinales. Alauda 1993, 62, 83–90. [Google Scholar]
- Prodon, R. Consequences for avifauna of landscape encroachment by woody vegetation in northern Catalonia. Rev. Catalana d’Ornitol. 2020, 36, 1–9. [Google Scholar]
- Fu, Z.; Li, D.; Hararuk, O.; Schwalm, C.; Luo, Y.; Yan, L.; Niu, S. Recovery time and state change of terrestrial carbon cycle after disturbance. Environ. Res. Lett. 2017, 12, 104004. [Google Scholar] [CrossRef]
- Molinari, R.L.; Bishop, T.B.B.; Bekker, M.F.; Kitchen, S.G.; Allphin, L.; St. Clair, S.B. Creosote growth rate and reproduction increase in postfire environments. Ecol. Evol. 2019, 9, 12897–12905. [Google Scholar] [CrossRef]
- Mutch, L.S.; Swetnam, T.W. Effect of fire severity and climate on ring-width growth of Giant Sequoia after fire. In Proceedings of the Symposium on Fire in Wilderness and Park Management: Past Lessons and Future Opportunities, Missoula, MT, USA, 30 March–1 April 1993; Brown, J.K., Mutch, R.W., Spoon, C.W., Wakimoto, R.H., Eds.; Intermountain Research Station, Gen. Tech. Rep. INT-GTR-320. U.S. Department of Agriculture, Forest Service: Ogden, UT, USA, 1995. Available online: https://www.ltrr.arizona.edu/~tswetnam/tws-pdf/mutch.pdf (accessed on 11 February 2021).
- Hoffman, W.A. Direct and indirect effects of fire on radial growth of cerrado savanna trees. J. Trop. Ecol. 2002, 18, 137–142. [Google Scholar] [CrossRef]
- Wheylan, R.J. The Ecology of Fire; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]

| 16 m | 8 m | 4 m | 2 m | 1 m | 0.5 m | 0.25 m | R | |
|---|---|---|---|---|---|---|---|---|
| (a) L80–86 | 5.5 | 31.9 | 38.5 | 53.0 | 61.6 | 66.0 | 58.5 | 2.5 |
| (b) L98–03 | 4.0 | 27.8 | 40.7 | 62.1 | 72.0 | 64.6 | 48.9 | 2.6 |
| (c) L07–12 | 10.0 | 34.5 | 41.7 | 58.5 | 58.8 | 48.9 | 29.7 | 2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prodon, R. Birds and the Fire Cycle in a Resilient Mediterranean Forest: Is There Any Baseline? Forests 2021, 12, 1644. https://doi.org/10.3390/f12121644
Prodon R. Birds and the Fire Cycle in a Resilient Mediterranean Forest: Is There Any Baseline? Forests. 2021; 12(12):1644. https://doi.org/10.3390/f12121644
Chicago/Turabian StyleProdon, Roger. 2021. "Birds and the Fire Cycle in a Resilient Mediterranean Forest: Is There Any Baseline?" Forests 12, no. 12: 1644. https://doi.org/10.3390/f12121644
APA StyleProdon, R. (2021). Birds and the Fire Cycle in a Resilient Mediterranean Forest: Is There Any Baseline? Forests, 12(12), 1644. https://doi.org/10.3390/f12121644






