Effect of an Ectomycorrhizal Fungus on the Growth of Castanea henryi Seedlings and the Seasonal Variation of Root Tips’ Structure and Physiology
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Site
2.2. Seedlings Production in the Nursery
2.3. Inoculum Production and Seedling Inoculation
2.4. ECM Colonization, Plant Growth, Root Architecture, and N and P Content
2.5. Soil Sample Preparation and Enzymatic Activity Assays
2.6. Microscopic Observation
2.7. Root Tip Physiological Characteristics
2.7.1. Root Tip Activity and Antioxidant Enzyme Analysis
2.7.2. Oxidative Damage and Protein Measurement
2.8. Statistical Analysis
3. Results
3.1. Root Colonization, Plant Growth, Biomass, and Root Architecture
3.2. Nutrient Content
3.3. Enzymatic Activity in the Rhizospheric Soil of Seedlings
3.4. Root Tips Structure of Each Season
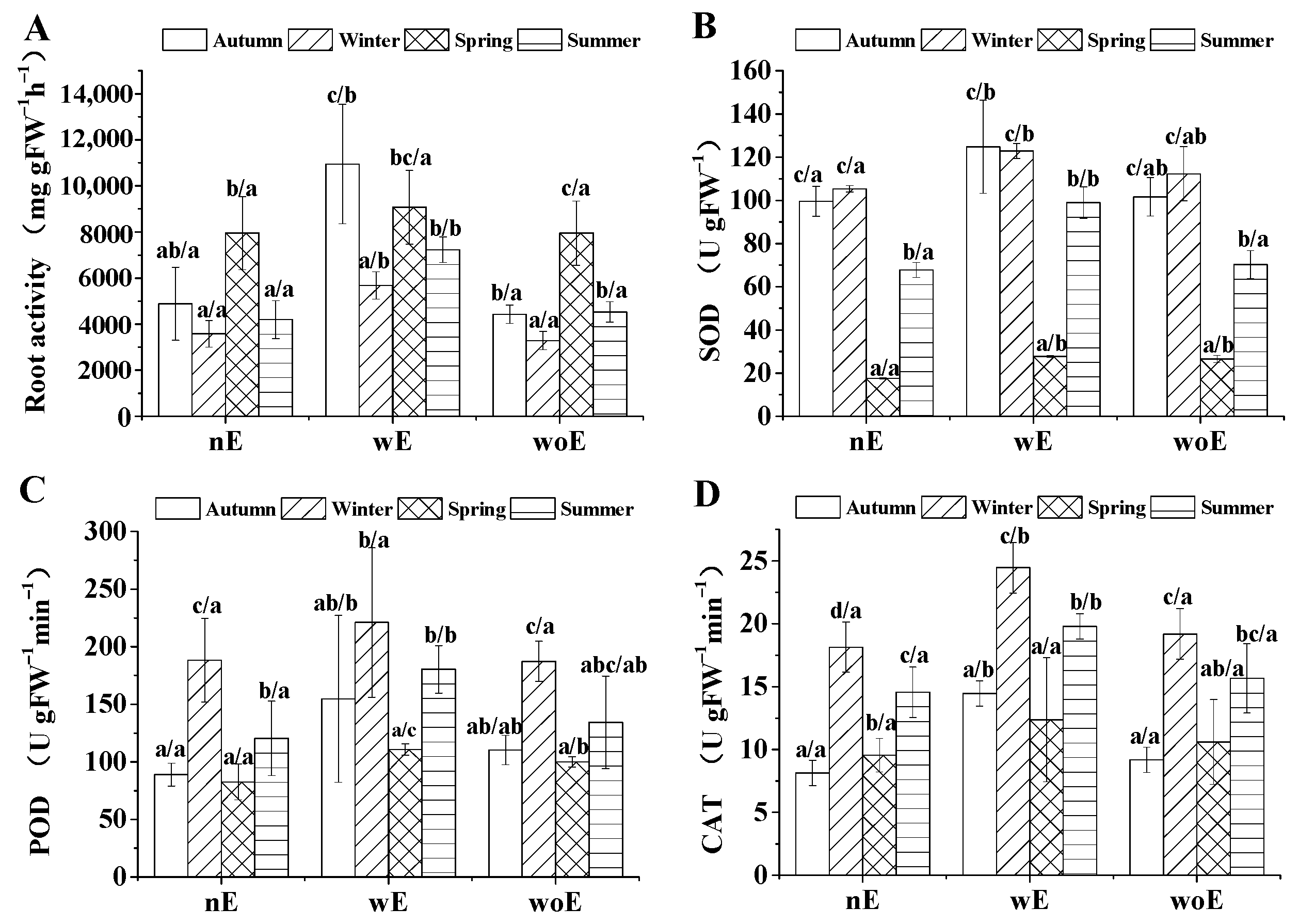
3.5. Root Tips Physiology in Each Season
4. Discussion
4.1. Growth Responses of Inoculation
4.2. Variation of ECM Root Tip Structure and Physiology in Different Seasons
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xiong, H.; Sun, H.; Zou, F.; Fan, X.M.; Niu, G.H.; Yuan, D.Y. Micropropagation of chinquapin (Castanea henryi) using axillary shoots and cotyledonary nodes. HortScience 2018, 53, 1482–1486. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.M.; Yuan, D.Y.; Tian, X.M.; Zhu, Z.J.; Liu, M.L.; Cao, H.P. Comprehensive transcriptome analysis of phytohormone biosynthesis and signaling genes in the flowers of Chinese chinquapin (Castanea henryi). J. Agr. Food Chem. 2017, 65, 10332–10349. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.M.; Yuan, D.Y.; Tang, J.; Tian, X.M.; Zhang, L.; Zou, F.; Tan, X.F. Sporogenesis and gametogenesis in Chinese chinquapin (Castanea henryi (Skam) Rehder & Wilson) and their systematic implications. Trees 2015, 29, 1713–1723. [Google Scholar]
- Yuan, J.; Huang, L.Y.; Zhou, N.F.; Wang, H.; Niu, G.H. Fractionation of Inorganic Phosphorus and Aluminum in Red Acidic Soil and the Growth of Camellia oleifera. HortScience 2017, 52, 1293–1297. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.L.; Xiong, H.; Yuan, D.Y.; Zhang, X.; Zou, F. Effect of temperature stress on leaf structure and photosynthesis of chinquapin (Castanea henryi). Acta Agric. Univ. Jiangxiensis 2020, 42, 692–699. [Google Scholar]
- Liu, D.M.; Yuan, D.Y.; Zou, F.; Zhang, X.H.; Zhu, Z.J.; Tan, L.M. Optimization of culture conditions for 3 Castanea henryi ectomycorrhizal fungi. J. Northw. Fore. Univ. 2016, 31, 195–200. [Google Scholar]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press Elsevier: Cambridge, UK, 2008; pp. 126–160. [Google Scholar]
- Martin, F.; Kohler, A.; Murat, C.; Veneault-Fourrey, C.; Hibbett, D.S. Unearthing the roots of ectomycorrhizal symbioses. Nat. Rev. Microbiol. 2016, 14, 760–773. [Google Scholar] [CrossRef]
- Colpaert, J.V.; Wevers, J.H.L.; Krznaric, E.; Adriaensen, K. How metal-tolerant ecotypes of ectomycorrhizal fungi protect plants from heavy metal pollution. Ann. For. Sci. 2011, 68, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.L.; He, J.L.; Ma, C.F.; Luo, J.; Li, H.; Liu, T.X.; Polle, A.; Peng, C.H.; Luo, Z.B. Ectomycorrhizas with Paxillus involutus enhance cadmium uptake and tolerance in Populus × canescens. Plant Cell Environ. 2014, 37, 627–642. [Google Scholar] [CrossRef]
- Yin, D.C.; Halifu, S.; Song, R.Q.; Qi, J.Y.; Deng, X.; Deng, J.F. Effects of an ectomycorrhizal fungus on the growth and physiology of Pinus sylvestris var. mongolica seedlings subjected to saline-alkali stress. J. For. Res. 2020, 31, 781–788. [Google Scholar] [CrossRef]
- Nardini, A.; Salleo, S.; Tyree, M.T.; Vertovec, M. Influence of the ectomycorrhizas formed by Tuber melanosporum Vitt. on hydraulic conductance and water relations of Quercus ilex L. seedlings. Ann. For. Sci. 2000, 57, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Feng, G.; Xu, B.; Qin, L.; Li, X.L. Effects of ectomycorrhizal fungi on the growth and nutrient uptake of chestnut (Castanea mollissima BL.). Acta Horticult. Sin. 2003, 30, 311–313. [Google Scholar]
- Gu, X.R.; Wang, X.L.; Li, J.; He, X.H. Accumulation and translocation of phosphorus, calcium, magnesium, and aluminum in Pinus massoniana Lamb. seedlings inoculated with Laccaria bicolor growing in an acidic yellow soil. Forests 2019, 10, 1153. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Núñez, J.A.; Serrano, J.S.; Rodríguez Barreal, J.A.; Saiz de Omeñaca, J.A. The influence of mycorrhization with Tuber melanosporum in the afforestation of a Mediterranean site with Quercus ilex and Quercus faginea. For. Ecol. Manag. 2006, 231, 226–233. [Google Scholar] [CrossRef]
- Dulmer, K.M.; LeDuc, S.D.; Horton, T.R. Ectomycorrhizal inoculum potential of northeastern US forest soils for American chestnut restoration: Results from filed and laboratory bioassays. Mycorrhiza 2014, 24, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Lafuente, A.; Benito-Matías, L.F.; Peñuelas-Rubira, J.L.; Suz, L.M. Multi-cropping edible truffles and sweet chestnuts: Production of high-quality Castanea sativa seedlings inoculated with Tuber aestivum, its ecotype T. uncinatum, T. brumale, and T. macrosporum. Mycorrhiza 2018, 28, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Zong, K.; Huang, J.; Nara, K.; Chen, Y.H.; Shen, Z.G.; Lian, C.L. Inoculation of ectomycorrhizal fungi contributes to the survival of tree seedlings in a copper mine tailing. J. For. Res. 2015, 20, 493–500. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.; Martín-Pinto, P.; Oria-de-Rueda, J.A.; Diaz-Balteiro, L. Optimal management of Cistus ladanifer shrublands for biomass and Boletus edulis mushroom production. Agroforest. Syst. 2017, 91, 663–676. [Google Scholar] [CrossRef]
- Conjeaud, C.; Scheromm, P.; Mousain, D. Effects of phosphorus and ectomycorrhiza on maritime pine seedlings (Pinus pinaster). New Phytol. 1996, 133, 345–351. [Google Scholar] [CrossRef]
- Kytöviita, M.M. Role of nutrient level and defoliation on symbiotic function: Experimental evidence by tracing 14C/15N exchange in mycorrhizal birch seedlings. Mycorrhiza 2005, 15, 65–70. [Google Scholar] [CrossRef]
- Lu, N.; Yu, M.; Cui, M.; Luo, Z.J.; Feng, Y.; Cao, S.; Sun, Y.H.; Li, Y. Effect of different ectomycorrhizal fungal inoculates on the growth of Pinus tabulaeformis seedlings under greenhouse conditions. Forests 2016, 7, 316. [Google Scholar] [CrossRef] [Green Version]
- Dosskey, M.G.; Linderman, R.G.; Boersma, L. Carbon-sink stimulation of photosynthesis in Douglas fir seedlings by some ectomycorrhizas. New Phytol. 1990, 115, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Ekwebelam, S.A.; Reid, C.P.P. Effect of light, nitrogen fertilization, and mycorrhizal fungi on growth and photosynthesis of lodgepole pine seedlings. Can. J. For. Res. 1983, 13, 1099–1106. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, X.Y.; Liang, Z.C.; Li, T. Effect of ectomycorrhizal fungi on growth and physiology of Pinus tabulaeformis plants to saline environment. J. Agro-Environ. Sci. 2006, 25, 1475–1480. [Google Scholar]
- Harley, J.L. Mycorrhizal studies: Past and future. In Physiological and Genetical Aspects of Mycorrhizae, 1st ed.; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA: Paris, France, 1986; pp. 25–33. [Google Scholar]
- Tagu, D.; Lapeyrie, F.; Martin, F. The ectomycorrhizal symbiosis: Genetics and development. Plant Soil 2002, 244, 97–105. [Google Scholar] [CrossRef]
- Acioli-Santos, B.; Sebastiana, M.; Pessoa, F.; Sousa, L.; Figueiredo, A.; Fortes, A.M.; Baldé, A.; Maia, L.C.; Pais,, M.S. Fungal transcript pattern during the preinfection stage (12 h) of ectomycorrhiza formed between Pisolithus tinctorius and Castanea sativa roots, identified using cDNA microarrays. Curr. Microbiol. 2008, 57, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Schubert, B.A.; Jahren, A.H. Seasonal temperature and precipitation recorded in the intra-annual oxygen isotope pattern of meteoric water and tree-ring cellulose. Quat. Sci. Rev. 2015, 125, 1–14. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Bergamini, C.M.; Gambetti, S.; Dondi, A.; Cervellati, C. Oxygen, reactive oxygen species and tissue damage. Curr. Pharm. Des. 2004, 10, 1611–1626. [Google Scholar] [CrossRef]
- Perez-Perez, M.E.; Lemaire, S.D.; Crespo, J.L. Reactive oxygen species and autophagy in plants and algae. Plant Physiol. 2012, 160, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.Z.; He, L.B.; Tian, S.Y.; Masabni, J.; Zhang, R.Q.; Zou, F.; Yuan, D.Y. Combined addition of bovine bone and cow manure: Rapid composting of chestnut burrs and production of a high-quality chestnut seedling substrate. Agronomy 2020, 10, 288. [Google Scholar] [CrossRef] [Green Version]
- Baptista, P.; Martins, A.; Pais, M.S.; Tavares, R.M.; Lino-Neto, T. Involvement of reactive oxygen species during early stages of ectomycorrhiza establishment between Castanea sativa and Pisolithus tinctorius. Mycorrhiza 2007, 17, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, H.; Masabni, J.; Zou, F.; Yuan, D.Y. Castanea henryi roots serve as host for Ganoderma lucidum. Int. J. Agric. Biol. 2019, 22, 420–426. [Google Scholar]
- Gao, J.F. Experimental Guidance for Plant Physiology, 1st ed.; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Hall, I.R.; Lyon, A.J.E.; Wang, Y.; Sinclair, L. Ectomycorrhizal fungi with edible fruiting bodies 2.Boletus edulis. Econ. Bot. 1998, 52, 44–56. [Google Scholar] [CrossRef]
- Xu, B.; Feng, G.; Pan, J.R.; Qin, L.; Li, X.L. Transferring of phosphorus between chestnut seedlings via ectomycorrhizal hyphal links. Acta Ecol. Sin. 2003, 23, 765–770. [Google Scholar]
- Endo, N.; Kawamura, F.; Kitahara, R.; Sakuma, D.; Fukuda, M.; Yamada, A. Synthesis of Japanese Boletus edulis ectomycorrhizae with Japanese red pine. Mycoscience 2014, 55, 405–416. [Google Scholar] [CrossRef]
- Ruess, R.W.; Swanson, M.M.; Kielland, K.; McFarland, J.W.; Olson, K.D.; Taylor, D.L. Phosphorus mobilizing enzymes of Alnus-associated ectomycorrhizal fungi in an Alaskan boreal floodplain. Forests 2019, 10, 554. [Google Scholar] [CrossRef] [Green Version]
- García-Montero, L.G.; Valverde-Asenjo, I.; Moreno, D.; Díaz, P.; Hernando, I.; Menta, C.; Tarasconi, K. Influence of edaphic factors on edible ectomycorrhizal mushrooms: New hypotheses on soil nutrition and C sinks associated to ectomycorrhizae and soil fauna using the Tuber brulé model. In Edible Ectomycorrhizal Mushrooms: Current Knowledge and Future Prospects, 1st ed.; Zambonelli, A., Bonito, G.M., Eds.; Springer: Berlin, Germany, 2012; Volume 34, pp. 83–104. [Google Scholar]
- Felten, J.; Kohler, A.; Morin, E.; Bhalerao, R.P.; Palme, K.; Martin, F.; Ditengou, F.A.; Legué, V. The ectomycorrhizal fungus Laccaria bicolor stimulates lateral root formation in poplar and Arabidopsis through auxin transport and signaling. Plant Physiol. 2009, 151, 1991–2005. [Google Scholar] [CrossRef] [Green Version]
- Splivallo, R.; Fischer, U.; Göbel, C.; Feussner, I.; Karlovsky, P. Truffles regulate plant root morphogenesis via the production of auxin and ethylene. Plant Physiol. 2009, 150, 2018–2029. [Google Scholar] [CrossRef] [Green Version]
- Vayssières, A.; Pencík, A.; Felten, J.; Kohler, A.; Ljung, K.; Martin, F.; Legué, V. Development of the poplar-Laccaria bicolor ectomycorrhizal modifies root auxin metabolism, signaling, and response. Plant Physiol. 2015, 169, 890–902. [Google Scholar] [CrossRef] [Green Version]
- Schimel, J.P.; Bennett, J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Chen, S.S.; Xing, Y.; Wang, T.; Zhang, Q.; Yu, W.Y.; Fang, K.F.; Newhouse, A.E.; McGuigan, L.D.; Stewart, K.R.; Maynard, C.A.; et al. Ectomycorrhizae symbiosis in Castanea mollissima improves phosphate acquisition through activating gene expression and H+ efflux. Sci. Hortic. 2016, 210, 99–107. [Google Scholar] [CrossRef]
- Martin, F.; Nehls, U. Harnessing ectomycorrhizal genomics for ecological insights. Curr. Opin. Plant Biol. 2009, 12, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.F.; Chai, R.S.; Jin, G.L.; Wang, H.; Tang, C.X.; Zhang, Y.S. Responses of root architecture development to low phosphorus availability: A review. Ann. Bot. 2013, 112, 391–408. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Ezawa, T.; Cheng, W.G.; Tawaraya, K. Release of acid phosphatase from extraradical hyphae of arbuscular mycorrhizal fungus Rhizophagus clarus. Soil Sci. Plant Nutr. 2015, 61, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Lindahl, B.D.; Ihrmark, K.; Boberg, J.; Trumbore, S.E.; Högberg, P.; Stenlid, J.; Finlay, R.D. Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. New Phytol. 2007, 173, 611–620. [Google Scholar] [CrossRef] [Green Version]
- Talbot, J.M.; Allison, S.D.; Treseder, K.K. Decomposers in disguise: Mycorrhizal fungi as regulators of soil C dynamics in ecosystems under global change. Funct. Ecol. 2008, 22, 955–963. [Google Scholar] [CrossRef] [Green Version]
- Talbot, J.M.; Bruns, T.D.; Smith, D.P.; Branco, S.; Glassman, S.I.; Erlandson, S.; Vilgalys, R.; Peay, K.G. Independent roles of ectomycorrhizal and saprotrophic communities in soil organic matter decomposition. Soil Biol. Biochem. 2013, 57, 282–291. [Google Scholar] [CrossRef]
- Phillips, L.A.; Ward, V.; Jones, M.D. Ectomycorrhizal fungi contribute to soil organic matter cycling in sub-boreal forests. ISME J. 2013, 8, 699–713. [Google Scholar] [CrossRef] [Green Version]
- Bödeker, I.T.M.; Clemmensen, K.E.; Boer, W.; Martin, F.; Olson, Å.; Lindahl, B.D. Ectomycorrhizal Cortinarius species participate in enzymatic oxidation of humus in northern forest ecosystems. New Phytol. 2014, 203, 245–256. [Google Scholar] [CrossRef]
- Wang, J.L.; Li, T.; Wu, X.G.; Zhao, Z.W. Molecular cloning and functional analysis of H+-dependent phosphate transporter gene from the ectomycorrhizal fungus Boletus edulis in Southwest China. Fungal Biol. 2014, 118, 453–461. [Google Scholar] [CrossRef]
- De la Varga, H.; Águeda, B.; Ágreda, T.; Martínez-Peña, F.; Parladé, J.; Pera, J. Seasonal dynamics of Boletus edulis and Lactarius deliciosus extraradical mycelium in pine forests of central Spain. Mycorrhiza 2013, 23, 391–402. [Google Scholar] [CrossRef]
- Queralt, M.; Parladé, J.; Pera, J.; De Miguel, A.M. Seasonal dynamics of extraradical mycelium and mycorrhizas in black truffle (Tuber melanosporum) plantation. Mycorrhiza 2017, 27, 565–576. [Google Scholar] [CrossRef]
- Balestrini, R.; Kottke, I. Structure and development of ectomycorrhizal roots. In Molecular Mycorrhizal Symbiosis, 1st ed.; Martin, F., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2017; pp. 47–61. [Google Scholar]
- De la Varga, H.; Águeda, B.; Martínez-Peña, F.; Parladé, J.; Pera, J. Quantification of extraradical soil mycelium and ectomycorrhizas of Boletus edulis in a Scots pine forest with variable sporocarp productivity. Mycorrhiza 2012, 22, 59–68. [Google Scholar] [CrossRef]
- Parladé, J.; Hortal, S.; Pera, J.; Galipienso, L. Quantitative detection of Lactarius deliciosus extraradical soil mycelium by real-time PCR and its application in the study of fungal persistence and interspecific competition. J. Biotechnol. 2007, 128, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Hortal, S.; Pera, J.; Parladé, J. Tracking mycorrhizas and extraradical mycelium of the edible fungus Lactarius deliciosus under field competition with Rhizopogon spp. Mycorrhiza 2008, 18, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signaling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Wang, W.B.; Kim, Y.H.; Lee, H.S.; Kim, K.Y.; Deng, X.P.; Kwak, S.S. Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol. Biochem. 2009, 47, 570–577. [Google Scholar] [CrossRef]








| Treatment | Total Length (cm) | Surface Area (cm2) | Volume (cm3) | Average Diameter (mm) | Number of Root Tips |
|---|---|---|---|---|---|
| JG | 3374 ± 102 a | 531 ± 44 a | 6.69 ± 0.94 a | 0.51 ± 0.04 a | 16,880 ± 439 a |
| CK | 1533 ± 53 b | 208 ± 22 b | 2.30 ± 0.51 b | 0.44 ± 0.04 b | 10,295 ± 233 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, H.; Chen, P.; Chen, W.; Yang, Y.; Jin, Y.; Tian, S.; Masabni, J.; Yuan, D.; Zou, F. Effect of an Ectomycorrhizal Fungus on the Growth of Castanea henryi Seedlings and the Seasonal Variation of Root Tips’ Structure and Physiology. Forests 2021, 12, 1643. https://doi.org/10.3390/f12121643
Xiong H, Chen P, Chen W, Yang Y, Jin Y, Tian S, Masabni J, Yuan D, Zou F. Effect of an Ectomycorrhizal Fungus on the Growth of Castanea henryi Seedlings and the Seasonal Variation of Root Tips’ Structure and Physiology. Forests. 2021; 12(12):1643. https://doi.org/10.3390/f12121643
Chicago/Turabian StyleXiong, Huan, Ping Chen, Wangzun Chen, Yinghui Yang, Yijia Jin, Shiyi Tian, Joseph Masabni, Deyi Yuan, and Feng Zou. 2021. "Effect of an Ectomycorrhizal Fungus on the Growth of Castanea henryi Seedlings and the Seasonal Variation of Root Tips’ Structure and Physiology" Forests 12, no. 12: 1643. https://doi.org/10.3390/f12121643
APA StyleXiong, H., Chen, P., Chen, W., Yang, Y., Jin, Y., Tian, S., Masabni, J., Yuan, D., & Zou, F. (2021). Effect of an Ectomycorrhizal Fungus on the Growth of Castanea henryi Seedlings and the Seasonal Variation of Root Tips’ Structure and Physiology. Forests, 12(12), 1643. https://doi.org/10.3390/f12121643







