Micropropagation, Characterization, and Conservation of Phytophthora cinnamomi-Tolerant Holm Oak Mature Trees
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant and Oomycete Material
2.2. In Vitro Culture Establishment
2.3. Shoot Stabilization and Proliferation
2.4. Shoot Rooting
2.5. Cold Storage of Shoot Cultures
2.6. Somatic Embryogenesis
2.7. Dual Cultures
2.8. Total Phenols Determinations
2.9. Gene Expression Analyses
2.10. Statistical Analysis
3. Results
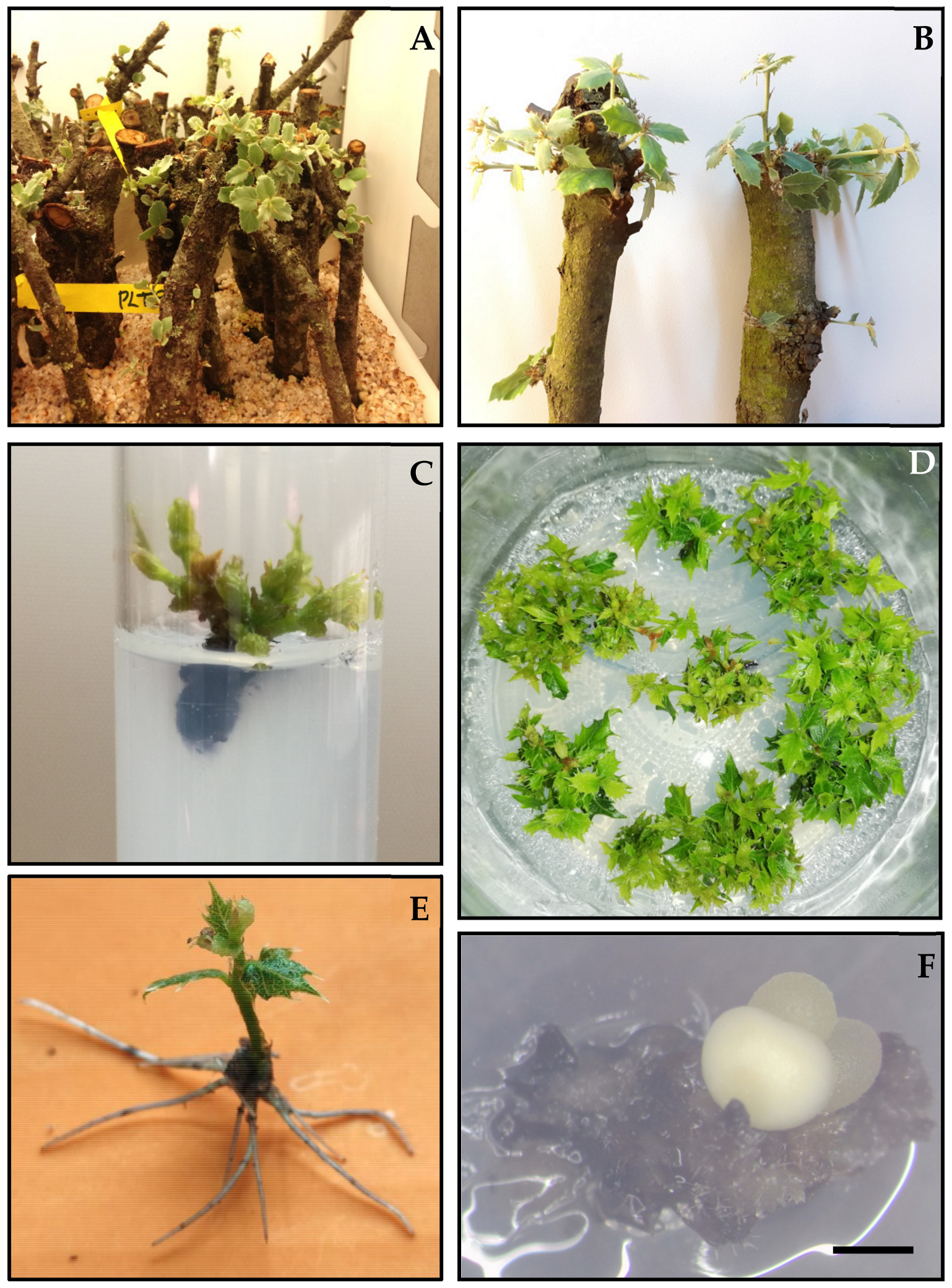
3.1. Micropropagation by Axillary Budding
3.1.1. Establishment, Stabilization, and Proliferation of Axillary Shoot Cultures
3.1.2. Rooting of Axillary Shoots
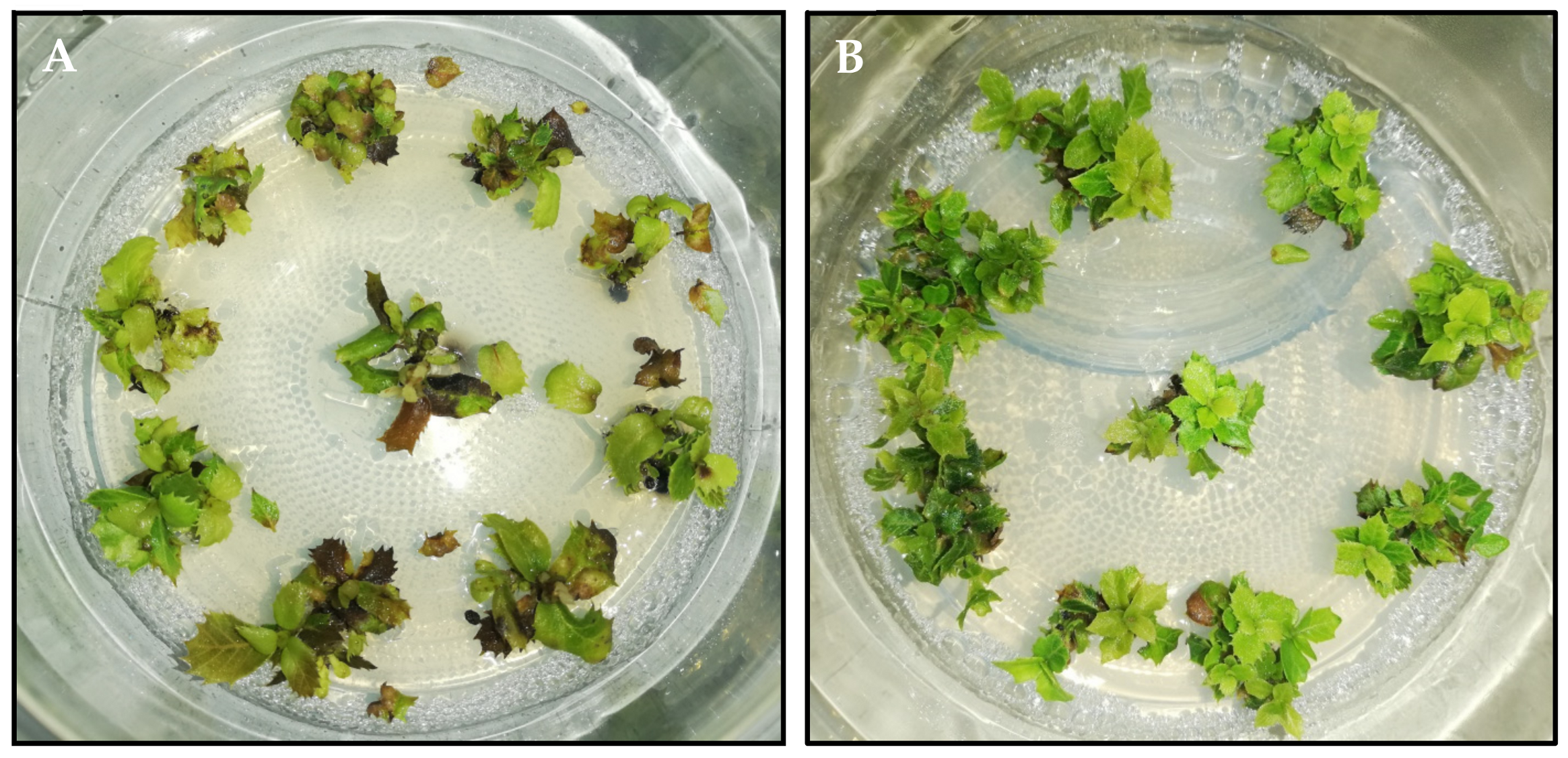
3.1.3. Medium-Term Conservation of Axillary Shoots
3.2. Somatic Embryogenesis
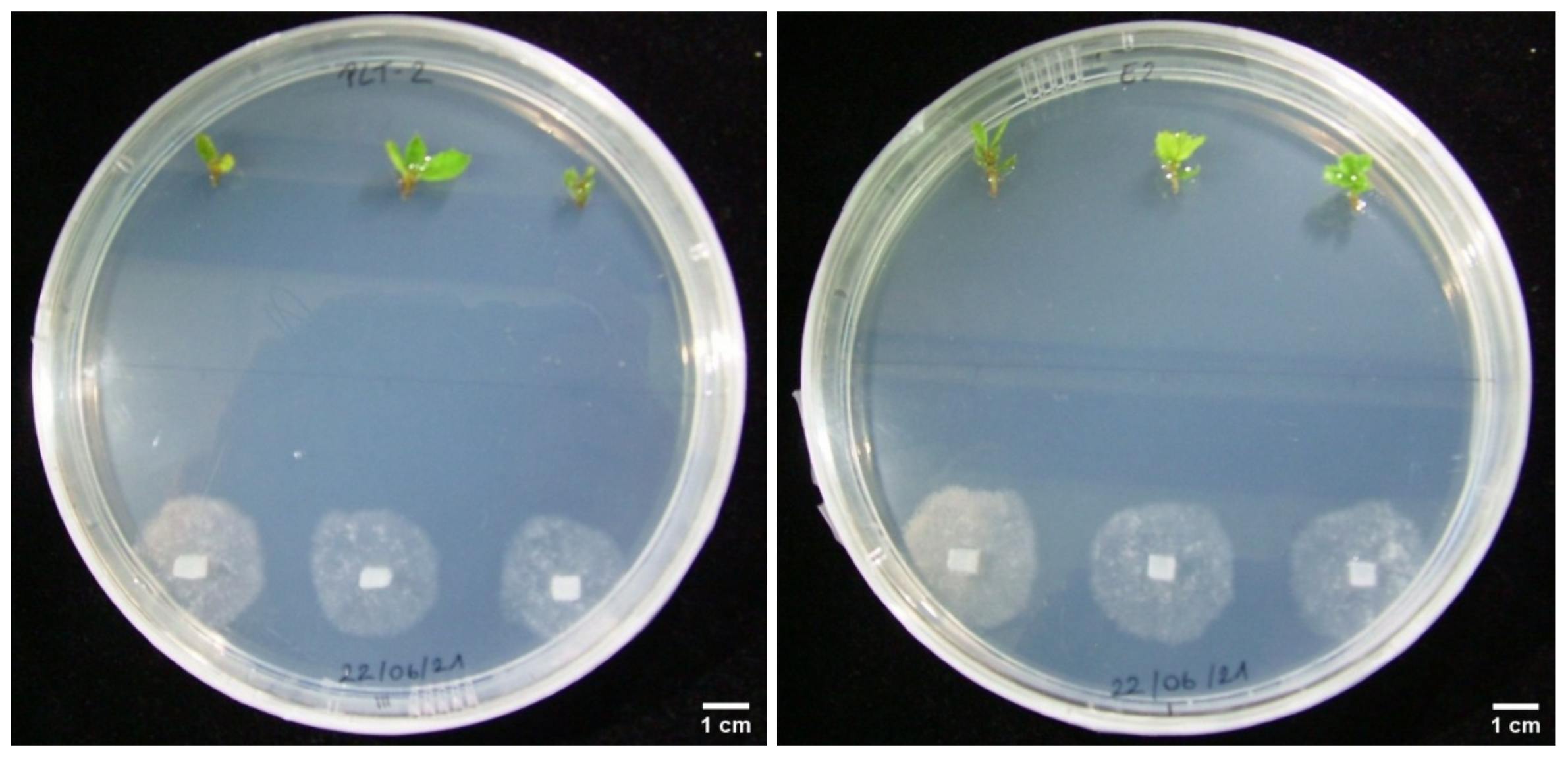
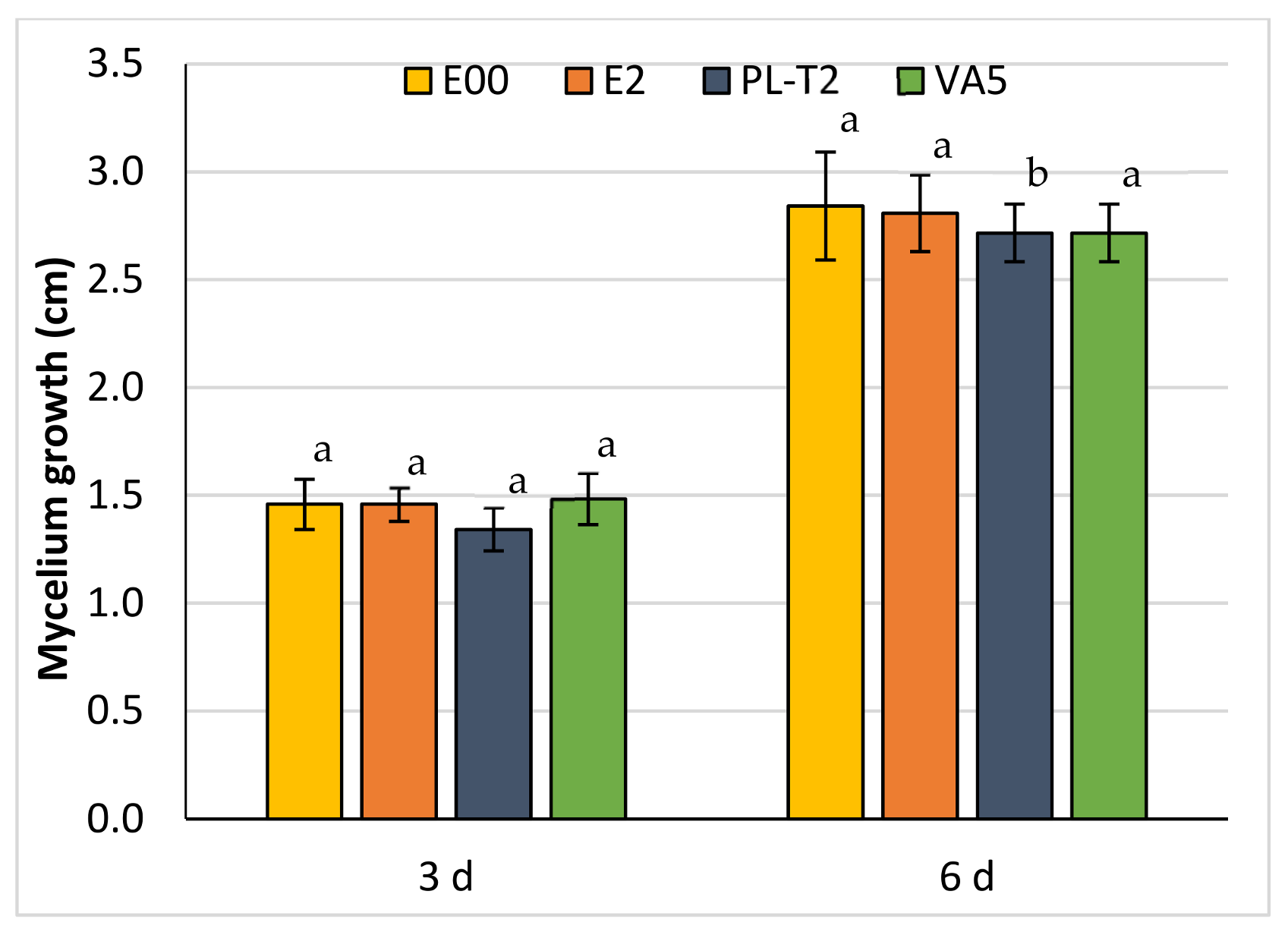
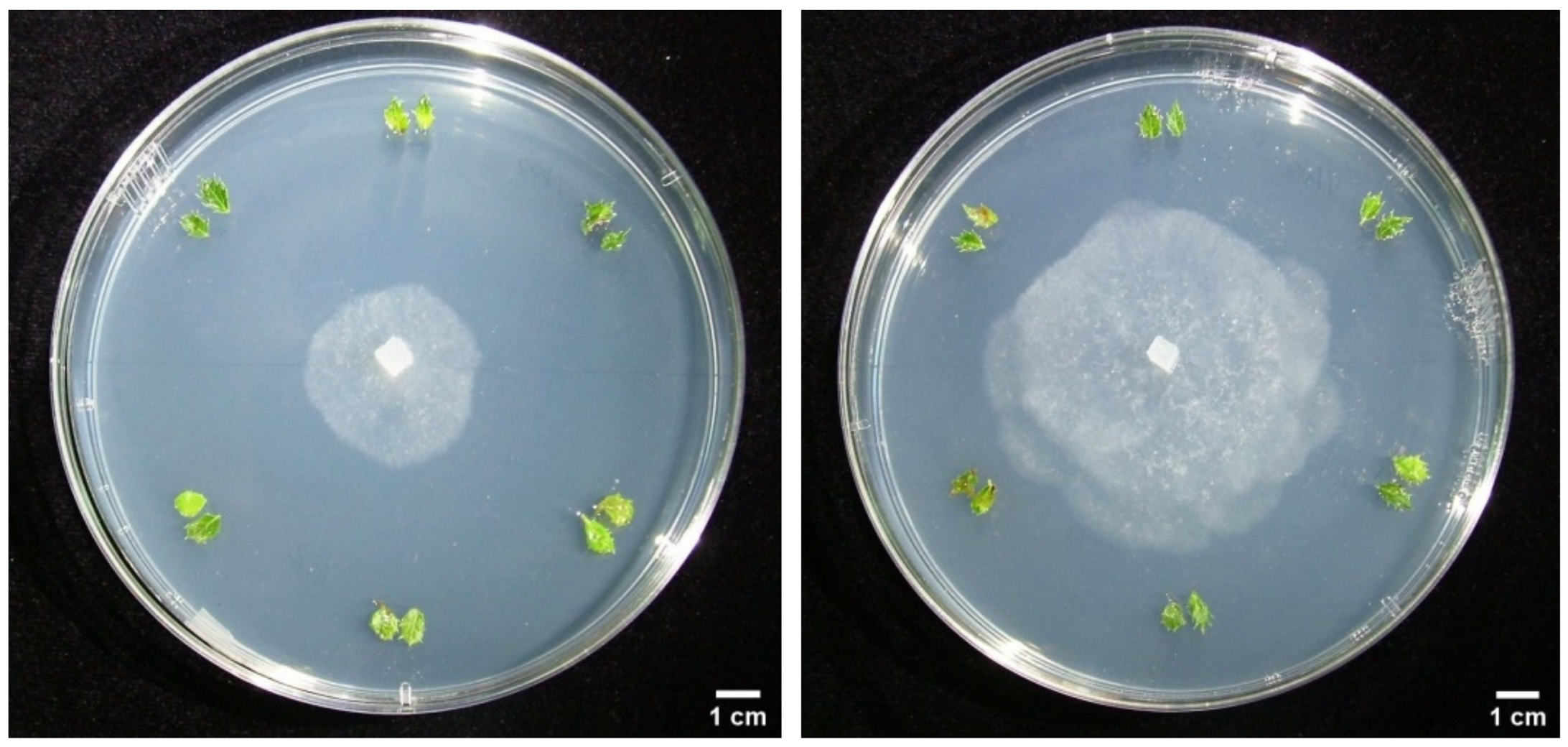
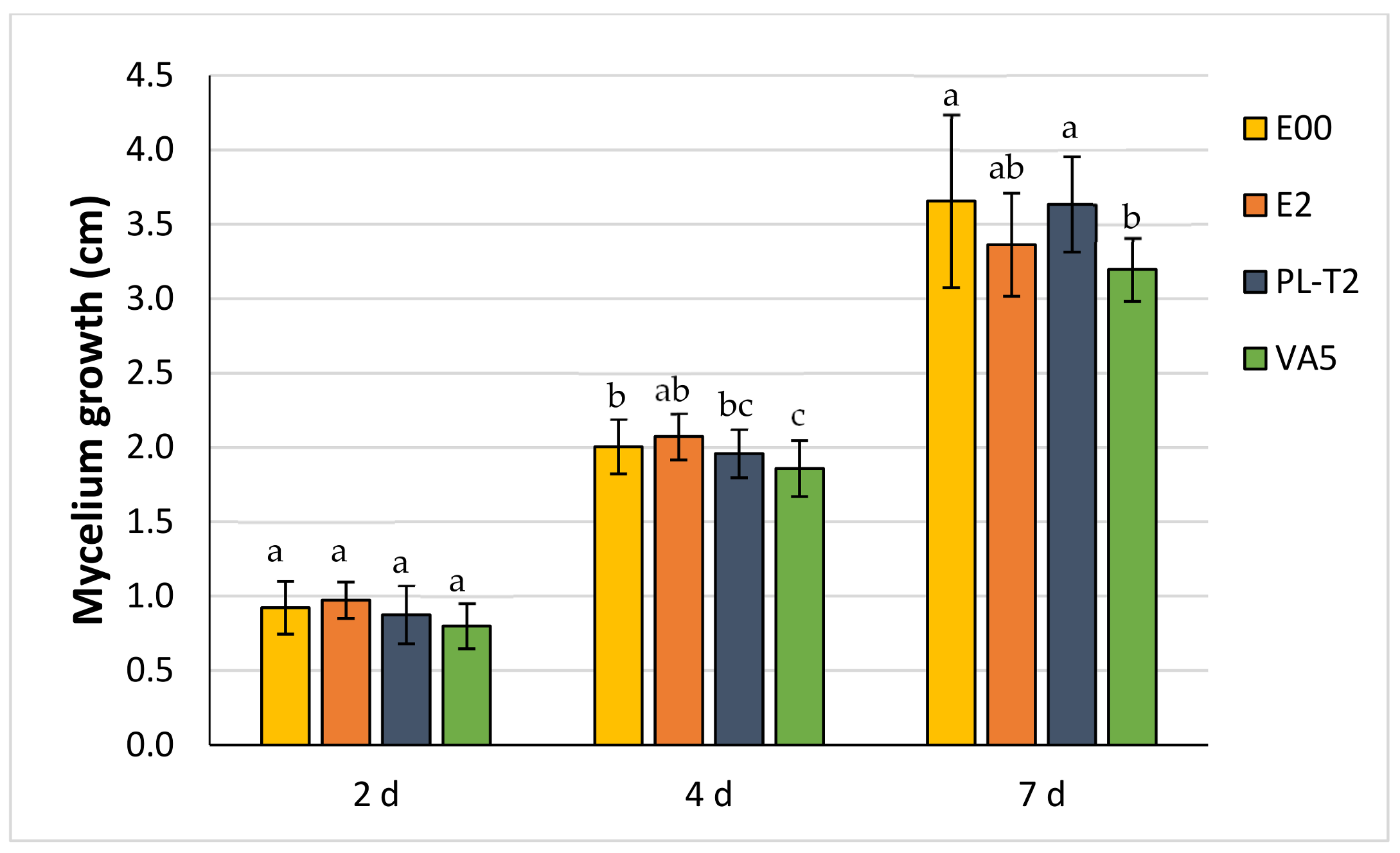
3.3. Dual Cultures
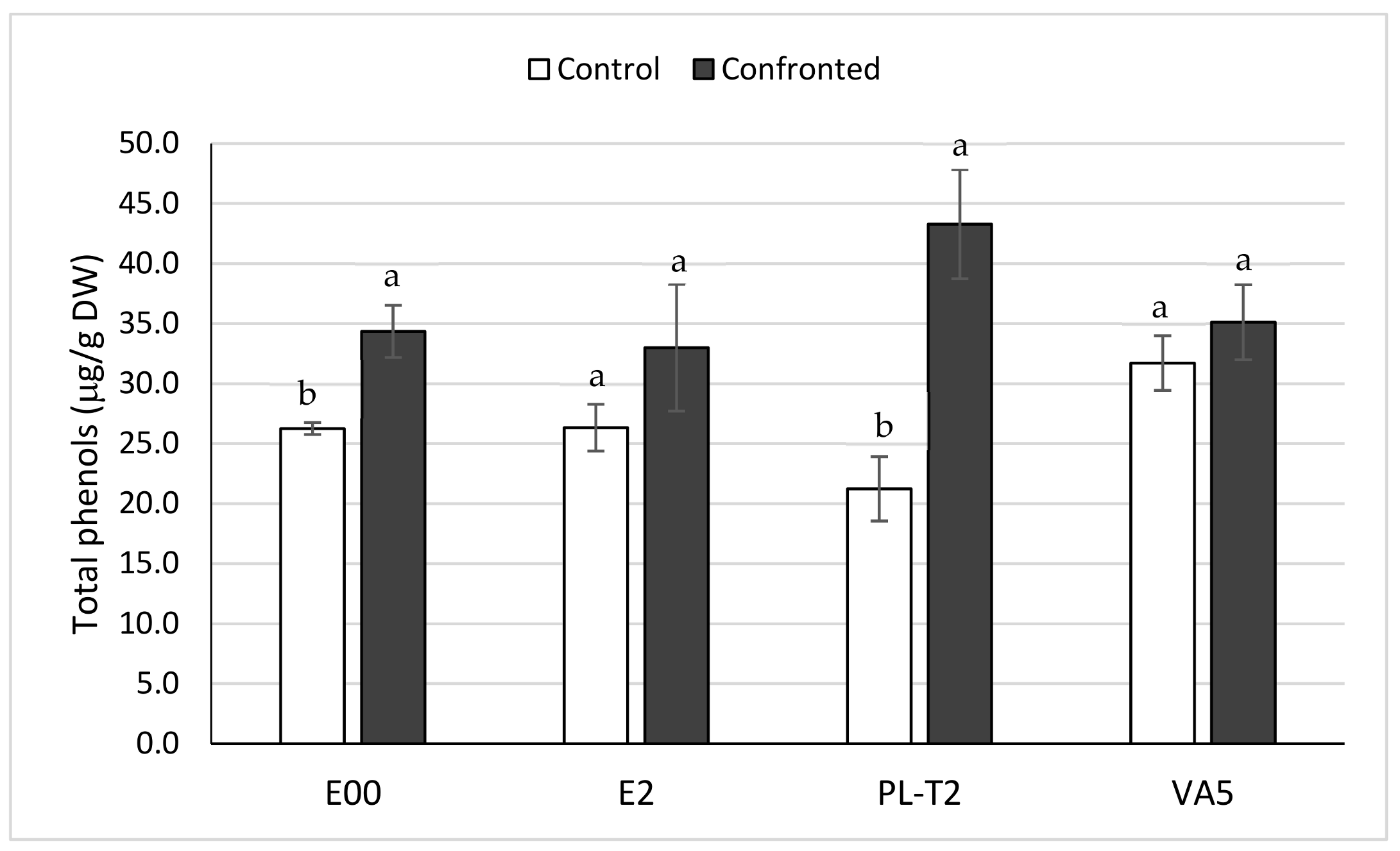
3.4. Total Phenols Content of In Vitro Grown Holm Oak Shoots
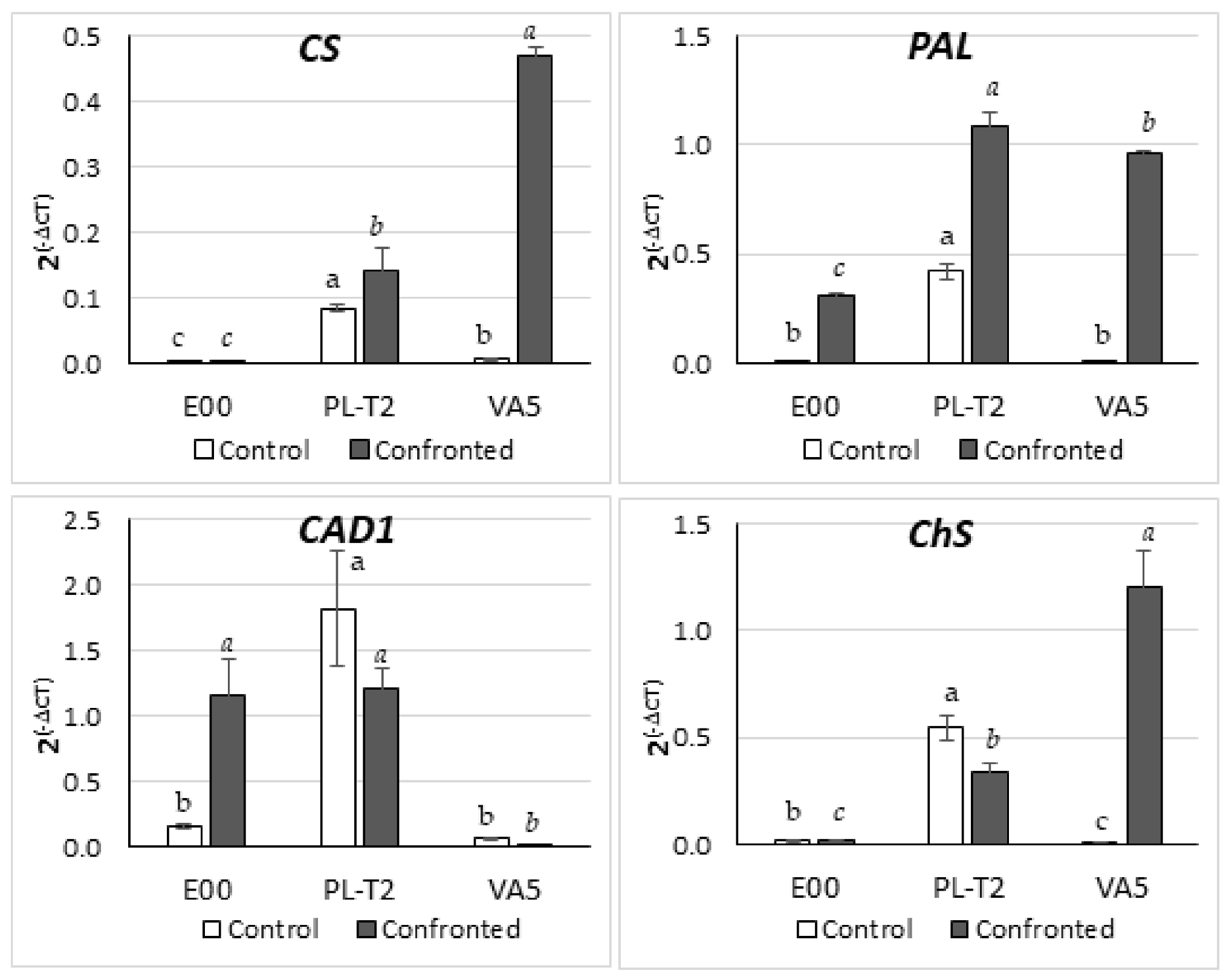
3.5. Gene Expression in Leaves of In Vitro Grown Holm Oak Shoots
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BA | benzyladenine |
| Bs | basal part of the tree |
| C | crown of the tree |
| CAD | NADPH-dependent cinnamyl alcohol dehydrogenase |
| ChS | chalcone synthase |
| CS | chorismate synthase |
| EF | elongation factor |
| GD | Gresshoff and Doy medium |
| IAA | indole-3-acetic acid |
| IBA | indol-3-butyric acid |
| MS | Murashige and Skoog medium |
| NAA | α-naphthalene acetic acid |
| NSs | nodular embryogenic structures |
| PAL | phenylalanineammonia-lyase |
| Pc | Phytophthora cinnamomic |
| PDA | Potato Dextrose Agar medium |
| PGRs | plant growth regulators |
| PPA | Plant Propagation Agar |
| qPCR | quantitative PCR |
| SE | somatic embryogenesis |
| SH | Schenk and Hildebrandt |
| STS | silver thiosulphate |
| WPM | Woody Plant Medium |
References
- Huntsinger, L.; Campos, P.; Starrs, P.F.; Oviedo, J.L.; Díaz, M.; Standiford, R.B.; Montero, G. Working landscapes of the Spanish dehesa and the California oak woodlands: An introduction. In Mediterranean Oak Woodland Working Landscapes; Campos, P., Huntsinger, L., Oviedo, J.L., Starrs, P.F., Diaz, M.M., Standiford, R.B., Montero, G., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 3–23. [Google Scholar] [CrossRef]
- Plieninger, T.; Hartel, T.; Martin-Lopez, B.; Beaufoy, G.; Bergmeier, E.; Kirby, K.; Montero-Parejo, M.J.; Moreno, G.; Oteros-Rozas, E.; Van Uytvanck, J. Wood-pastures of Europe: Geographic coverage, social–ecological values, conservation management, and policy implications. Biol. Conserv. 2015, 190, 70–79. [Google Scholar] [CrossRef]
- Moreno, G.; Aviron, S.; Berg, S.; Crous-Duran, J.; Franca, A.; de Jalón, S.G.; Hartel, T.; Mirck, J.; Pantera, A.; Palma, J.H.N.; et al. Agroforestry systems of high nature and cultural value in Europe: Provision of commercial goods and other ecosystem services. Agrofor. Syst. 2018, 92, 877–891. [Google Scholar] [CrossRef] [Green Version]
- Encinas-Valero, M.; Esteban, R.; Hereş, A.-M.; Becerril, J.M.; García-Plazaola, J.I.; Artexe, U.; Vivas, M.; Solla, A.; Moreno, G.; Yuste, J.C. Photoprotective compounds as early markers to predict holm oak crown defoliation in declining Mediterranean savannahs. Tree Physiol. 2021, tpab006. [Google Scholar] [CrossRef] [PubMed]
- Pulido, F.; McCreary, D.; Cañellas, I.; McClaran, M.; Plieninger, T. Oak Regeneration: Ecological Dynamics and Restoration Techniques; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2013; pp. 123–144. [Google Scholar]
- Natalini, F.; Alejano, R.; Vázquez-Piqué, J.; Cañellas, I.; Gea-Izquierdo, G. The role of climate change in the widespread mortality of holm oak in open woodlands of Southwestern Spain. Dendrochronologia 2016, 38, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Hereş, A.-M.; Kaye, M.W.; Granda, E.; Benavides, R.; Lázaro-Nogal, A.; Rubio-Casal, A.E.; Valladares, F.; Yuste, J.C. Tree vigour influences secondary growth but not responsiveness to climatic variability in Holm oak. Dendrochronologia 2018, 49, 68–76. [Google Scholar] [CrossRef]
- García-Angulo, D.; Hereş, A.-M.; Fernández-López, M.; Flores, O.; Sanz, M.; Rey, A.; Valladares, F.; Yuste, J.C. Holm oak decline and mortality exacerbates drought effects on soil biogeochemical cycling and soil microbial communities across a climatic gradient. Soil Biol. Biochem. 2020, 149, 107921. [Google Scholar] [CrossRef]
- Corcobado, T.; Solla, A.; Madeira, M.A.; Moreno, G. Combined effects of soil properties and Phytophthora cinnamomi infections on Quercus ilex decline. Plant Soil 2013, 373, 403–413. [Google Scholar] [CrossRef]
- Duque-Lazo, J.; Navarro-Cerrillo, R.M.; van Gils, H.; Groen, T.A. Forecasting oak decline caused by Phytophthora cinnamomi in Andalusia: Identification of priority areas for intervention. For. Ecol. Manag. 2018, 417, 122–136. [Google Scholar] [CrossRef] [Green Version]
- Sevillano, E.H.; Contador, J.F.L.; Pulido, M.; Schnabel, S. Spatial patterns of lost and remaining trees in the Iberian wooded rangelands. Appl. Geogr. 2017, 87, 170–183. [Google Scholar] [CrossRef]
- Serrano, M.S.; Fernández-Rebollo, P.; De Vita, P.; Sánchez, M.E. Calcium mineral nutrition increases the tolerance of Quercus ilex to Phytophthora root disease affecting oak rangeland ecosystems in Spain. Agrofor. Syst. 2012, 87, 173–179. [Google Scholar] [CrossRef]
- González, M.; Caetano, P.; Sánchez, M.E. Testing systemic fungicides for control of Phytophthora oak root disease. For. Pathol. 2017, 47, e12343. [Google Scholar] [CrossRef]
- Morales-Rodríguez, C.; Vettraino, A.M.; Vannini, A. Efficacy of Biofumigation with Brassica carinata Commercial Pellets (BioFence) to Control Vegetative and Reproductive Structures of Phytophthora cinnamomi. Plant Dis. 2016, 100, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Kamoun, S.; Furzer, O.; Jones, J.D.G.; Judelson, H.S.; Ali, G.S.; Dalio, R.J.D.; Roy, S.G.; Schena, L.; Zambounis, A.; Panabières, F.; et al. The Top 10 oomycete pathogens in molecular plant pathology. Mol. Plant Pathol. 2015, 16, 413–434. [Google Scholar] [CrossRef]
- Savill, P.; Fennessy, J.; Samuel, C. Approaches in Great Britain and Ireland to the genetic improvement of broadleaved trees. Forests 2005, 78, 163–173. [Google Scholar] [CrossRef]
- Vieitez, A.M.; Corredoira, E.; Martínez, M.T.; José, M.C.S.; Sánchez, C.; Valladares, S.; Vidal, N.; Ballester, A. Application of biotechnological tools to Quercus improvement. Eur. J. For. Res. 2011, 131, 519–539. [Google Scholar] [CrossRef] [Green Version]
- Martínez, M.T.; San-José, M.C.; Arrillaga, I.; Cano, V.; Morcillo, M.; Cernadas, M.J.; Corredoira, E. Holm oak somatic embryogenesis: Current status and future perspectives. Front. Plant Sci. 2019, 10, 239. [Google Scholar] [CrossRef] [Green Version]
- Gaidamashvili, M.; Benelli, C. Threatened Woody Plants of Georgia and Micropropagation as a Tool for In Vitro Conservation. Agronomy 2021, 11, 1082. [Google Scholar] [CrossRef]
- Ngezahayo, F.; Liu, B. Axillary Bud Proliferation Approach for Plant Biodiversity Conservation and Restoration. Int. J. Biodivers. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Martínez, M.T.; Corredoira, E.; Vieitez, A.M.; Cernadas, M.J.; Montenegro, R.; Ballester, A.; Vieitez, F.J.; José, M.C.S. Micropropagation of mature Quercus ilex L. trees by axillary budding. Plant Cell Tissue Organ Cult. 2017, 131, 499–512. [Google Scholar] [CrossRef]
- Corredoira, E.; Merkle, S.A.; Martínez, M.T.; Toribio, M.; Canhoto, J.M.; Correia, S.I.; Ballester, A.; Vieitez, A.M. Non-Zygotic Embryogenesis in Hardwood Species. Crit. Rev. Plant Sci. 2019, 38, 29–97. [Google Scholar] [CrossRef]
- Mauri, P.; Manzanera, J. Induction, maturation and germination of holm oak (Quercus ilex L.) somatic embryos. Plant Cell Tissue Organ Cult. 2003, 74, 229–235. [Google Scholar] [CrossRef]
- Blasco, M.; Barra, A.; Brisa, C.; Corredoira, E.; Segura, J.; Toribio, M.; Arrillaga, I. Somatic embryogenesis in holm oak male catkins. Plant Growth Regul. 2013, 71, 261–270. [Google Scholar] [CrossRef]
- Barra-Jiménez, A.; Blasco, M.; Ruiz-Galea, M.; Celestino, C.; Alegre, J.; Arrillaga, I.; Toribio, M. Cloning mature holm oak trees by somatic embryogenesis. Trees 2014, 28, 657–667. [Google Scholar] [CrossRef]
- Martínez, M.T.; José, M.C.S.; Vieitez, A.M.; Cernadas, M.J.; Ballester, A.; Corredoira, E. Propagation of mature Quercus ilex L. (holm oak) trees by somatic embryogenesis. Plant Cell Tissue Organ Cult. 2017, 131, 321–333. [Google Scholar] [CrossRef]
- Valladares, F.; Benavides, R.; Rabasa, S.G.; Díaz, M.; Pausas, J.G.; Paula, S.; Simonson, W.D. Global change and Mediterranean forests: Current impacts and potential responses. In Forests and Global Change; Cambridge University Press (CUP): Cambridge, UK, 2014; pp. 47–76. [Google Scholar]
- De Rigo, D.; Caudullo, G. Quercus ilex in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; The Publications Office of the European Union: Luxembourg, 2016; pp. 152–153. [Google Scholar]
- Corredoira, E.; Martínez, M.T.; San-José, M.C.; Ballester, A. Conservation of Hardwood Forests species. In Biodiversity and Conservation of Woody Plants; Ahuja, M.R., Jain, S.M., Eds.; Springer: Gewerbestrasse, Switzerland, 2017; pp. 421–453. [Google Scholar]
- Martínez, M.T.; Vieitez, F.J.; Solla, A.; Tapias, R.; Ramírez-Martín, N.; Corredoira, E. Vegetative Propagation of Phytophthora cinnamomi-Tolerant Holm Oak Genotypes by Axillary Budding and Somatic Embryogenesis. Forests 2020, 11, 841. [Google Scholar] [CrossRef]
- Lambardi, M.; De Carlo, A. Application of tissue culture to germplasm conservation of temperate broad-leaf trees. In Micropropagation of Woody Trees and Fruits; Jain, S.M., Ishii, K., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 815–840. [Google Scholar]
- Ballesteros, D.; Pritchard, H.W. The Cryobiotechnology of Oaks: An Integration of Approaches for the Long-Term Ex Situ Conservation of Quercus Species. Forests 2020, 11, 1281. [Google Scholar] [CrossRef]
- Jiménez, A.B.; Aronen, T.S.; Alegre, J.; Toribio, M. Cryopreservation of embryogenic tissues from mature holm oak trees. Cryobiology 2015, 70, 217–225. [Google Scholar] [CrossRef]
- Nawrot-Chorabik, K. The Use of Interactions in Dual Cultures in vitro to evaluate the Pathogenicity of Fungi and Susceptibility of Host Plant Genotypes. In Environmental Biotechnology—New Approaches and Prospective Applications; Intech Open Science Publisher: Rijeka, Croatia, 2013; pp. 287–301. [Google Scholar]
- Morcillo, M.; Sales, E.; Ponce, L.; Guillén, A.; Segura, J.; Arrillaga, I. Effect of elicitors on holm oak somatic embryo development and efficacy inducing tolerance to Phytophthora cinnamomi. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Yadav, V.; Wang, Z.; Wei, C.; Amo, A.; Ahmed, B.; Yang, X.; Zhang, X. Phenylpropanoid Pathway Engineering: An Emerging Approach towards Plant Defense. Pathogens 2020, 9, 312. [Google Scholar] [CrossRef] [Green Version]
- Oßwald, W.; Fleischmann, F.; Rigling, D.; Coelho, A.C.; Cravador, A.; Diez, J.; Dalio, R.J.; Jung, M.H.; Pfanz, H.; Robin, C.; et al. Strategies of attack and defence in woody plant-Phytophthora interactions. For. Pathol. 2014, 44, 169–190. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, C.; Zheng, Y.; Wang, J.; Wang, F. Root Exudates Metabolic Profiling Suggests Distinct Defense Mechanisms Between Resistant and Susceptible Tobacco Cultivars Against Black Shank Disease. Front. Plant Sci. 2020, 11, 559775. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb. Proc. Int. Plant Prop. Soc. 1980, 30, 421–427. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gresshoff, P.M.; Doy, C.H. Development and differentiation of haploid Lycopersicon esculentum (tomato). Planta 1972, 107, 161–170. [Google Scholar] [CrossRef]
- Schenk, R.U.; Hildebrandt, A.C. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Asensio, E.; Vitales, D.; Pérez, I.; Peralba, L.; Viruel, J.; Montaner, C.; Vallès, J.; Garnatje, T.; Sales, E. Phenolic Compounds Content and Genetic Diversity at Population Level across the Natural Distribution Range of Bearberry (Arctostaphylos uva-ursi, Ericaceae) in the Iberian Peninsula. Plants 2020, 9, 1250. [Google Scholar] [CrossRef]
- Chaves, I.; Passarinho, J.A.P.; Capitão, C.; Chaves, M.M.; Fevereiro, P.; Ricardo, C. Temperature stress effects in Quercus suber leaf metabolism. J. Plant Physiol. 2011, 168, 1729–1734. [Google Scholar] [CrossRef]
- Soler, M.; Serra, O.; Molinas, M.; García-Berthou, E.; Caritat, A.; Figueras, M. Seasonal variation in transcript abundance in cork tissue analyzed by real time RT-PCR. Tree Physiol. 2008, 28, 743–751. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Cano, V.; Martínez, M.T.; San José, M.C.; Couselo, J.L.; Varas, E.; Bouza-Morcillo, L.; Toribio, M.; Corredoira, E. Regeneration of transgenic plants by Agrobacterium-mediated transformation of Quercus ilex L. somatic embryos with the gene CsTL1. New For. 2020, 51, 1003–1021. [Google Scholar] [CrossRef]
- Edesi, J.; Tikkinen, M.; Elfstrand, M.; Olson, Å.; Varis, S.; Egertsdotter, U.; Aronen, T. Root Rot Resistance Locus PaLAR3 Is Delivered by Somatic Embryogenesis (SE) Pipeline in Norway Spruce (Picea abies (L.) Karst.). Forests 2021, 12, 193. [Google Scholar] [CrossRef]
- Tapias, R.; Fernández, M.; Moreira, A.C.; Sánchez, E.; Cravador, A. Possibilities of genetic variability in holm and cork oak for conservation and restoration of threatened forest by oak decline. Bol. Inf. CIDEU 2006, 1, 45–51. [Google Scholar]
- Solla, A.; Bohnens, J.; Collin, E.; Diamandis, S.; Franke, A.; Gil, L.; Burón, M.; Santini, A.; Mittempergher, L.; Pinon, J.; et al. Screening European Elms for Resistance to Ophiostoma novo ulmi. For. Sci. 2005, 51, 134–141. [Google Scholar]
- Townsend, A.M.; Bentz, S.E.; Douglass, L.W. Evaluation of 19 American Elm Clones for Tolerance to Dutch Elm Disease. J. Environ. Hortic. 2005, 23, 21–24. [Google Scholar] [CrossRef]
- Martín, J.A.; Sobrino-Plata, J.; Rodríguez-Calcerrada, J.; Collada, C.; Gil, L. Breeding and scientific advances in the fight against Dutch elm disease: Will they allow the use of elms in forest restoration? New For. 2019, 50, 183–215. [Google Scholar] [CrossRef] [Green Version]
- Kräutler, K.; Kirisits, T. The ash dieback pathogen Hymenoscyphus pseudoalbidus is associated with leaf symptoms on ash species (Fraxinus spp.). J. Agric. Ext. Rural Dev. 2012, 4, 261–265. [Google Scholar]
- Muñoz, F.; Marçais, B.; Dufour, J.; Dow, A. Rising Out of the Ashes: Additive Genetic Variation for Crown and Collar Resistance to Hymenoscyphus fraxineus in Fraxinus excelsior. Am. Phytopath. Soc. 2016, 106, 1535–1543. [Google Scholar] [CrossRef] [Green Version]
- Vieitez, A.M.; Sánchez, M.C.; Amomarco, J.B.; Ballester, A. Forced flushing of branch segments as a method for obtaining reactive explants of mature Quercus robur trees for micropropagation. Plant Cell Tissue Organ Cult. 1994, 37, 287–295. [Google Scholar]
- Ballester, A.; Vidal, N.; Vieitez, A.M. Developmental stages during in vitro rooting of hardwood trees from material with juvenile and mature characteristics. In Adventitious Root Formation of Forest Trees and Horticultural Plants-from Genes to Applications; Niemi, K., Scagel, C., Eds.; Research Signpost: Kerala, India, 2009; pp. 277–296. [Google Scholar]
- Vieitez, A.M.; Sánchez, M.C.; García-Nimo, M.L.; Ballester, A. Protocol for micropropagation of Castanea sativa Mill. In Protocols for Micropropagation of Woody Trees and Fruits; Jain, S.M., Häggman, H., Eds.; Springer: Heidelberg, Germany, 2007; pp. 299–312. [Google Scholar]
- Gomes, F.; Simões, M.; Lopes, M.L.; Canhoto, J.M. Effect of plant growth regulators and genotype on the micropropagation of adult trees of Arbutus unedo L. (strawberry tree). New Biotechnol. 2010, 27, 882–892. [Google Scholar] [CrossRef]
- L’Helgoual’ch, M.; Espagnac, H. First observations on the adventitious rhizogenic capacity of holm oak (Quercus ilex L.) (in French). Ann. Sci. For. 1987, 44, 325–334. [Google Scholar]
- Bhau, B.; Wakhlu, A. Rapid Micropropagation of Five Cultivars of Mulberry. Biol. Plant. 2003, 46, 349–355. [Google Scholar] [CrossRef]
- Vieitez, A.M.; Corredoira, E.; Ballester, A.; Muñoz, F.; Duran, J.; Ibarra, M. In vitro regeneration of the important North American oak species Quercus alba, Quercus bicolor and Quercus rubra. Plant Cell Tissue Organ Cult. 2009, 98, 135–145. [Google Scholar] [CrossRef] [Green Version]
- José, M.S.; Janeiro, L.; Corredoira, E. Micropropagation of threatened black alder. Silva Fenn. 2013, 47. [Google Scholar] [CrossRef] [Green Version]
- José, M.C.S.; Martínez, M.T.; Cernadas, M.J.; Montenegro, R.; Mosteiro, F.; Corredoira, E. Biotechnological efforts for the propagation of Quercus lusitanica Lam., an endangered species. Trees 2017, 31, 1571–1581. [Google Scholar] [CrossRef]
- Da Silva, J.A.T.; Dobránszki, J.; Ross, S. Phloroglucinol in plant tissue culture. Vitr. Cell. Dev. Biol. Anim. 2013, 49, 1–16. [Google Scholar] [CrossRef]
- Ozudogru, E.A.; Previati, A.; Lambardi, M. In Vitro Conservation and Cryopreservation of Ornamental Plants. MAP Kinase Signal. Protoc. 2009, 589, 303–324. [Google Scholar] [CrossRef]
- Janeiro, L.; Vieitez, A.; Ballester, A. Cold storage of in vitro cultures of wild cherry, chestnut and oak. Ann. Sci. For. 1995, 52, 287–293. [Google Scholar] [CrossRef]
- Lambardi, M.; Rugini, E. Micropropagation of olive (Olea europaea L.). In Micropropagation of Woody Trees and Fruits; Jain, S.M., Ishii, K., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 621–646. [Google Scholar]
- José, M.C.S.; Janeiro, L.V.; Corredoira, E. Simple strategy for the in vitro conservation of Alnus glutinosa (L.) Gaertn. germplasm. Trees 2014, 29, 539–549. [Google Scholar] [CrossRef]
- Ballester, A.; Corredoira, E.; Vieitez, A.M. Limitations of somatic embryogenesis in hardwood trees. In Vegetative Propagation of Forest Trees; Park, Y.S., Bonga, J.M., Moon, H.K., Eds.; National Institute of Forest Science (NiFos): Seoul, Korea, 2016; pp. 56–74. [Google Scholar]
- San José, M.C.; Corredoira, E.; Martínez, M.T.; Vidal, N.; Valladares, S.; Mallón, R.; Vieitez, A.M. Shoot apex explants for in-duction of somatic embryogenesis in mature Quercus robur L. trees. Plant Cell Rep. 2010, 29, 661–671. [Google Scholar] [CrossRef]
- Corredoira, E.; San-José, M.C.; Vieitez, A.M. Induction of somatic embryogenesis from different explants of shoot cultures derived from young Quercus alba trees. Trees 2012, 26, 881–891. [Google Scholar] [CrossRef]
- Mallón, R.; Martínez, M.T.; Corredoira, E.; Vieitez, A.M. The positive effect of arabinogalactan on induction of somatic embryogenesis in Quercus bicolor followed by embryo maturation and plant regeneration. Trees 2013, 27, 1285–1296. [Google Scholar] [CrossRef]
- Martínez, M.T.; Vieitez, A.M.; Corredoira, E. Improved secondary embryo production in Quercus alba and Q. rubra by activated charcoal, silver thiosulphate and sucrose: Influence of embryogenic explant used for subculture. Plant Cell Tissue Organ Cult. 2015, 121, 531–546. [Google Scholar] [CrossRef]
- Corredoira, E.; Ballester, A.; Ibarra, M.; Vieitez, A.M. Induction of somatic embryogenesis in leaf and shoot apex explants of shoot cultures derived from adult Eucalyptus globulus and Eucalyptus saligna × E. maidenii trees. Tree Physiol. 2015, 35, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Sablowski, R. Plant stem cell niches: From signalling to execution. Curr. Opin. Plant Biol. 2011, 14, 4–9. [Google Scholar] [CrossRef]
- Fehér, A. Somatic embryogenesis—Stress-induced remodeling of plant cell fate. Biochim. Biophys. Acta Bioenerg. 2015, 1849, 385–402. [Google Scholar] [CrossRef]
- Nawrot-Chorabik, K.; Grad, B.; Kowalski, T. Interactions between callus cultures of Pinus sylvestris and pine fungi with different trophic properties. For. Pathol. 2015, 46, 179–186. [Google Scholar] [CrossRef]
- Marčiulynas, A.; Sirgedaitė-Šėžienė, V.; Žemaitis, P.; Baliuckas, V. The Resistance of Scots Pine (Pinus sylvestris L.) Half-sib Families to Heterobasidion annosum. Forests 2019, 10, 287. [Google Scholar] [CrossRef] [Green Version]
- Francini, A.; Giro, A.; Ferrante, A. Biochemical and Molecular Regulation of Phenylpropanoids Pathway Under Abiotic Stresses. In Plant Signaling Molecules; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 183–192. [Google Scholar]
- Top, S.M.; Preston, C.M.; Dukes, J.S.; Tharayil, N. Climate Influences the Content and Chemical Composition of Foliar Tannins in Green and Senesced Tissues of Quercus rubra. Front. Plant Sci. 2017, 8, 423. [Google Scholar] [CrossRef] [Green Version]
- Sghaier-Hammami, B.; Galván, J.V.; Romero-Rodríguez, M.C.; Navarro-Cerrillo, R.M.; Abdelly, C.; Novo, J.V.J. Physiological and proteomics analyses of Holm oak (Quercus ilex subsp. ballota [Desf.] Samp.) responses to Phytophthora cinnamomi. Plant Physiol. Biochem. 2013, 71, 191–202. [Google Scholar] [CrossRef]
- Cahill, D.M. Resistance of Micropropagated Eucalyptus marginata to Phytophthora cinnamomi. Plant Dis. 1992, 76, 630–632. [Google Scholar] [CrossRef]
- Ebadzad, G.; Cravador, A. Quantitative RT-PCR analysis of differentially expressed genes in Quercus suber in response to Phytophthora cinnamomi infection. SpringerPlus 2014, 3, 613. [Google Scholar] [CrossRef] [Green Version]
- Escandón, M.; Castillejo, M.; Jorrín-Novo, J.; Rey, M.-D. Molecular Research on Stress Responses in Quercus spp.: From Classical Biochemistry to Systems Biology through Omics Analysis. Forests 2021, 12, 364. [Google Scholar] [CrossRef]
- Tohge, T.; Watanabe, M.; Hoefgen, R.; Fernie, A.R. Shikimate and Phenylalanine Biosynthesis in the Green Lineage. Front. Plant Sci. 2013, 4, 62. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Vásquez, R.; Day, R.; Buschmann, H.; Randles, S.; Beeching, J.R.; Cooper, R.M. Phenylpropanoids, Phenylalanine Ammonia Lyase and Peroxidases in Elicitor-challenged Cassava (Manihot esculenta) Suspension Cells and Leaves. Ann. Bot. 2004, 94, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Kavil, S.; Otti, G.; Bouvaine, S.; Armitage, A.; Maruthi, M.N. PAL1 gene of the phenylpropanoid pathway increases resistance to the Cassava brown streak virus in cassava. Virol. J. 2021, 18, 1–10. [Google Scholar] [CrossRef]
- Coelho, A.; Horta, M.; Neves, D.; Cravador, A. Involvement of a cinnamyl alcohol dehydrogenase of Quercus suber in the defence response to infection by Phytophthora cinnamomi. Physiol. Mol. Plant Pathol. 2006, 69, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Dao, T.T.H.; Linthorst, H.J.M.; Verpoorte, R. Chalcone synthase and its functions in plant resistance. Phytochem. Rev. 2011, 10, 397–412. [Google Scholar] [CrossRef] [Green Version]
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| CAD | CAGATGATAAGCCATTTGCG | AGGAACTTCAGGGTGCTAC |
| ChS | TGAGATCACAGCAGTTAC | CAAGTTGAAACAGTGGAC |
| CS | TGGATTGATTGGAAACAGATTAC | CAAGGAAGCAGCACACAG |
| PAL | ATTAGCAGGGATTGATGG | CAAGTGGTCTGTAAATTCG |
| EF | TTGTGCCGTCCTCATTATTGACT | TCACGGGTCTGACCATCCTT |
| Clones | Flushing Capacity of Branch Segments (4 w) 1 | Response to In Vitro Establishment (8 w) 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Branch Segment Number | Branch with Sprouting (%) | Total Number Shoots | Length of Shoots (mm) | Number of Initial Explants | Contamination Rate (%) | Response Rate (%) 3 | Shoot Establishment | |
| Mediterranean | ||||||||
| H1 | 30 | 86.7 | 48 | 16.2 ± 5.8 | 66 | 18.3 | 21.8 | No |
| H5 | 22 | 68.2 | 58 | 20.1 ± 5.8 | 94 | 27.7 | 26.5 | No |
| VA5 | 32 | 96.9 | 109 | 30.1 ± 10.5 | 206 | 2.4 | 30.9 | Yes |
| VA11-Bs 4 | 21 | 100.0 | 131 | 43.7 ± 20.2 | 225 | 15.6 | 15.8 | Yes |
| VA11-C 4 | 12 | 75.0 | 33 | 18.4 ± 7.2 | 51 | 2.0 | 42.0 | Yes |
| South-West | ||||||||
| PL-T1 | 26 | 30.8 | 36 | 14.7 ± 5.2 | 41 | 61.0 | 100.0 | Yes |
| PL-T2 | 31 | 70.9 | 57 | 15.4 ± 7.7 | 96 | 52.1 | 15.2 | Yes |
| PL-T3 | 23 | 65.2 | 40 | 13.9 ± 6.4 | 59 | 25.4 | 11.4 | No |
| PL-T4 | 32 | 31.3 | 19 | 12.9 ± 4.8 | 26 | 34.6 | 11.8 | No |
| PL-T5 | 12 | 33.3 | 6 | 12.5 ± 3.7 | 6 | 33.3 | 0.0 | - |
| PL-T6 | 32 | 0.0 | 0 | - | - | - | - | - |
| Clone | Responsive Explants (%) | Total Shoots (Nº) | Shoots 0.5–1.0 (Nº) | Shoots ≥ 1 cm (Nº) | Longest Shoot Length (mm) |
|---|---|---|---|---|---|
| VA11-Bs | 100 ± 0.0 | 7.82 ± 0.38 | 2.53 ± 0.27 | 5.29 ± 0.21 | 19.33 ± 0.48 |
| VA11-C | 100 ± 0.0 | 8.30 ± 0.17 | 4.96 ± 0.15 | 3.34 ± 0.12 | 15.57 ± 0.39 |
| VA5 | 100 ± 0.0 | 17.76 ± 0.56 | 10.33 ± 0.52 | 7.43 ± 0.31 | 22.40 ± 0.83 |
| PL-T2 | 100 ± 0.0 | 5.34 ± 0.17 | 1.96 ± 0.18 | 3.38 ± 0.16 | 13.30 ± 0.52 |
| ANOVA I | 1.0 ns | 0.001 *** | 0.001 *** | 0.001 *** | 0.001 *** |
| Clone | Rooting (%) | Root Number | Root Length (mm) |
|---|---|---|---|
| VA5 | 36.0 ± 9.3 | 4.4 ± 0.7 | 47.5 ± 5.7 |
| VA11-Bs | 12.0 ± 3.1 | 1.2 ± 0.1 | 17.3 ± 2.1 |
| VA11-C | 8.0 ± 3.1 | 1.0 ± 0.0 | 40.0 ± 7.8 |
| ANOVA I | 0.012 * | 0.004 ** | 0.028 * |
| CLONE | Storage Period (Months) | Recovery (%) 1 | Total Shoots (Nº) | Shoots 0.5–1.0 cm (Nº) | Shoots ≥1 cm (Nº) | Longest Shoot Length (mm) |
|---|---|---|---|---|---|---|
| Q10 | 0 | 100 ± 0.0 | 3.2 ± 0.2 | 1.3 ± 0.1 | 1.9 ± 0.2 | 16.2 ± 0.5 |
| 6 | 100 ± 0.0 | 4.8 ± 0.3 | 1.8 ± 0.2 | 3.0 ± 0.2 | 14.7 ± 1.4 | |
| 9 | 97.1 ± 2.5 | 4.3 ± 0.3 | 1.5 ± 0.2 | 2.8 ± 0.2 | 12.8 ± 1.1 | |
| 12 | 100 ± 0.0 | 3.7 ± 0.2 | 2.0 ± 0.2 | 1.7 ± 0.2 | 15.7 ± 0.6 | |
| ANOVA I | 0.418 ns | 0.008 ** | 0.045 * | 0.003 ** | 0.81 ns | |
| Q3 | 0 | 100 ± 0.0 | 2.7 ± 0.1 | 1.7 ± 0.2 | 1.0 ± 0.1 | 16.2 ± 0.5 |
| 6 | 78.6 ± 4.0 | 2.5 ± 0.4 | 1.4 ± 0.2 | 1.1 ± 0.2 | 14.5 ± 0.3 | |
| 9 | 64.3 ± 6.3 | 2.8 ± 0.1 | 1.6 ± 0.2 | 1.2 ± 0.1 | 11.2 ± 0.5 | |
| 12 | 100 ± 0.0 | 2.3 ± 0.3 | 1.2 ± 0.2 | 1.1 ± 0.2 | 11.2 ± 2.4 | |
| ANOVA I | 0.001 *** | 0.523 ns | 0.386 ns | 0.818 ns | 0.62 ns | |
| E00 | 0 | 100 ± 0.0 | 4.4 ± 0.3 | 1.8 ± 0.1 | 2.6 ± 0.1 | 23.3 ± 1.0 |
| 6 | 100 ± 0.0 | 3.1 ± 0.1 | 1.5 ± 0.1 | 1.6 ± 0.2 | 15.5 ± 0.2 | |
| 9 | 71.4 ± 13.3 | 2.5 ± 0.5 | 1.4 ± 0.1 | 1.1 ± 0.4 | 12.7 ± 2.9 | |
| 12 | 68.6 ± 8.4 | 1.8 ± 0.1 | 1.3 ± 0.1 | 0.5 ± 0.1 | 10.3 ± 0.9 | |
| ANOVA I | 0.012 * | 0.001 *** | 0.082 ns | 0.001 *** | 0.001 *** | |
| PL-T2 | 0 | 100 ± 0.0 | 5.5 ± 0.3 | 2.1 ± 0.3 | 3.4 ± 0.3 | 13.4 ± 0.9 |
| 6 | 84.0 ± 3.4 | 4.0 ± 0.1 | 1.7 ± 0.1 | 2.3 ± 0.1 | 10.0 ± 0.8 | |
| 9 | 54.3 ± 15.8 | 1.9 ± 0.2 | 1.4 ± 0.3 | 0.5 ± 0.1 | 7.4 ± 0.9 | |
| 12 | - | - | - | - | - | |
| ANOVA I | 0.018 * | 0.001 *** | 0.207 ns | 0.001 *** | 0.006 ** | |
| VA11-C | 0 | 100 ± 0.0 | 8.4 ± 0.2 | 4.9 ± 0.1 | 3.5 ± 0.1 | 16.4 ± 0.3 |
| 6 | 100 ± 0.0 | 9.2 ± 0.5 | 5.8 ± 0.6 | 3.4 ± 0.2 | 19.7 ± 0.6 | |
| 9 | 100 ± 0.0 | 5.8 ± 0.3 | 3.1 ± 0.3 | 2.7 ± 0.2 | 18.4 ± 0.7 | |
| 12 | 100 ± 0.0 | 6.7 ± 0.2 | 4.0 ± 0.2 | 2.7 ± 0.2 | 18.7 ± 0.8 | |
| ANOVA I | 1.0 ns | 0.001 *** | 0.002 ** | 0.007 ** | 0.026 * | |
| VA11-Bs | 0 | 100 ± 0.0 | 8.3 ± 0.4 | 2.9 ± 0.3 | 5.4 ± 0.1 | 19.3 ± 0.28 |
| 6 | 100 ± 0.0 | 9.6 ± 0.5 | 5.7 ± 0.4 | 3.9 ± 0.2 | 19.7 ± 0.6 | |
| 9 | 100 ± 0.0 | 7.1 ± 0.2 | 4.4 ± 0.1 | 2.7 ± 0.2 | 17.9 ± 0.6 | |
| 12 | 100 ± 0.0 | 6.0 ± 0.5 | 3.1 ± 0.3 | 2.9 ± 0.3 | 19.2 ± 0.9 | |
| ANOVA I | 1.0 ns | 0.001 *** | 0.001 *** | 0.001 *** | 0.378 ns | |
| VA5 | 0 | 100 ± 0.0 | 18.5 ± 0.6 | 10.5 ± 0.6 | 8.0 ± 0.3 | 20.2 ± 0.6 |
| 6 | 100 ± 0.0 | 15.3 ± 0.8 | 9.2 ± 0.3 | 6.1 ± 0.6 | 24.3 ± 0.9 | |
| 9 | 100 ± 0.0 | 10.2 ± 1.4 | 3.4 ± 0.6 | 6.8 ± 0.9 | 23.5 ± 0.7 | |
| 12 | 97.1 ± 2.5 | 5.2 ± 0.8 | 4.6 ± 0.7 | 0.6 ± 0.2 | 10.3 ± 0.7 | |
| ANOVA I | 0.418 ns | 0.001 *** | 0.001 *** | 0.001 *** | 0.001 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, M.T.; Arrillaga, I.; Sales, E.; Pérez-Oliver, M.A.; González-Mas, M.d.C.; Corredoira, E. Micropropagation, Characterization, and Conservation of Phytophthora cinnamomi-Tolerant Holm Oak Mature Trees. Forests 2021, 12, 1634. https://doi.org/10.3390/f12121634
Martínez MT, Arrillaga I, Sales E, Pérez-Oliver MA, González-Mas MdC, Corredoira E. Micropropagation, Characterization, and Conservation of Phytophthora cinnamomi-Tolerant Holm Oak Mature Trees. Forests. 2021; 12(12):1634. https://doi.org/10.3390/f12121634
Chicago/Turabian StyleMartínez, Mª Teresa, Isabel Arrillaga, Ester Sales, María Amparo Pérez-Oliver, Mª del Carmen González-Mas, and Elena Corredoira. 2021. "Micropropagation, Characterization, and Conservation of Phytophthora cinnamomi-Tolerant Holm Oak Mature Trees" Forests 12, no. 12: 1634. https://doi.org/10.3390/f12121634
APA StyleMartínez, M. T., Arrillaga, I., Sales, E., Pérez-Oliver, M. A., González-Mas, M. d. C., & Corredoira, E. (2021). Micropropagation, Characterization, and Conservation of Phytophthora cinnamomi-Tolerant Holm Oak Mature Trees. Forests, 12(12), 1634. https://doi.org/10.3390/f12121634






