Root Growth Dynamics and Structure in Seedlings of Four Shade Tolerant Mediterranean Species Grown under Moderate and Low Light
Abstract
1. Introduction
2. Materials and Methods
2.1. Seedling Cultivation and Plant Material
2.2. Experiment Design
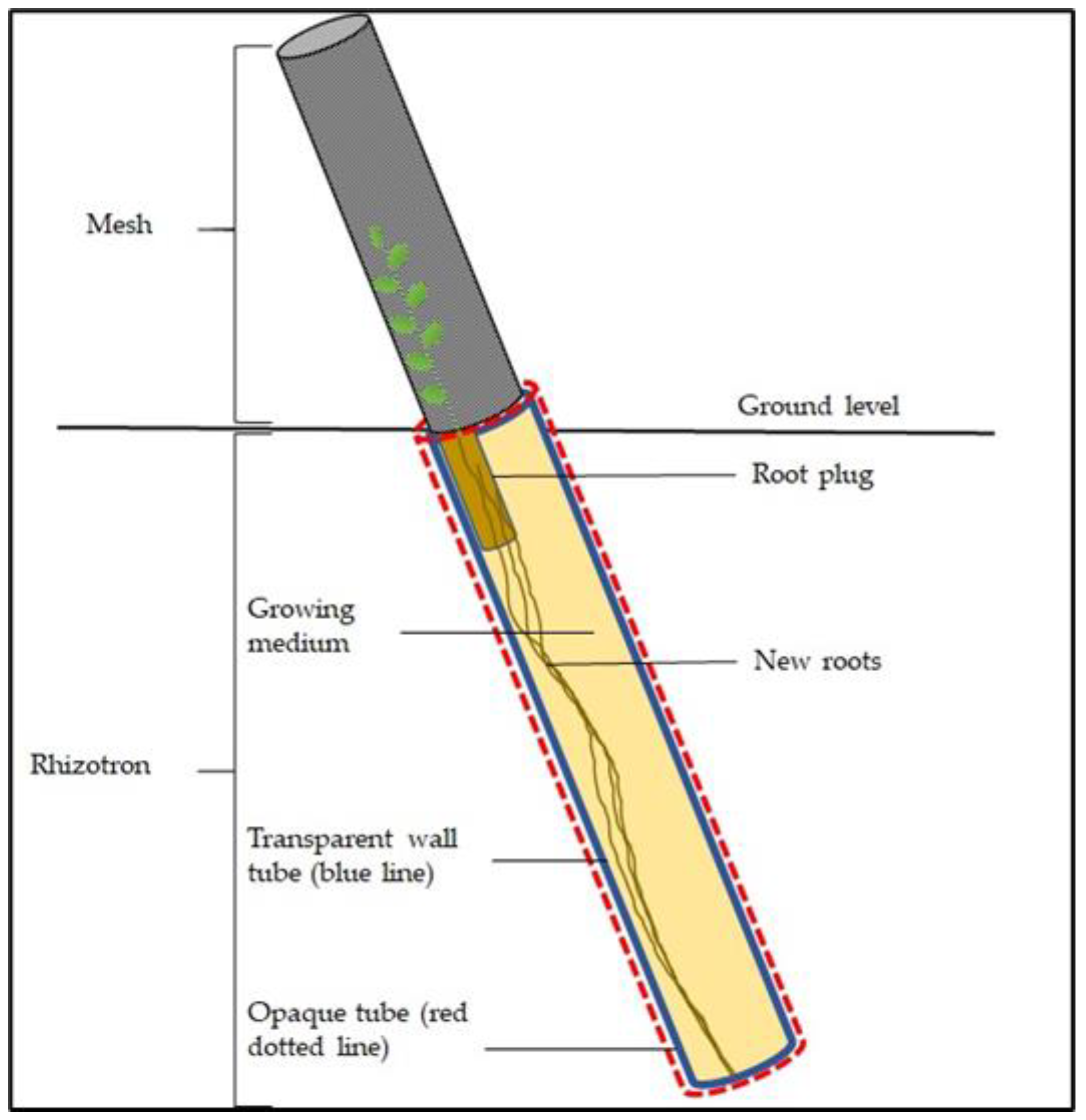
2.3. Root Measurements
2.4. Root and Leaf Morphological Traits
2.5. Physiological Measurements
2.6. Data Analysis
3. Results
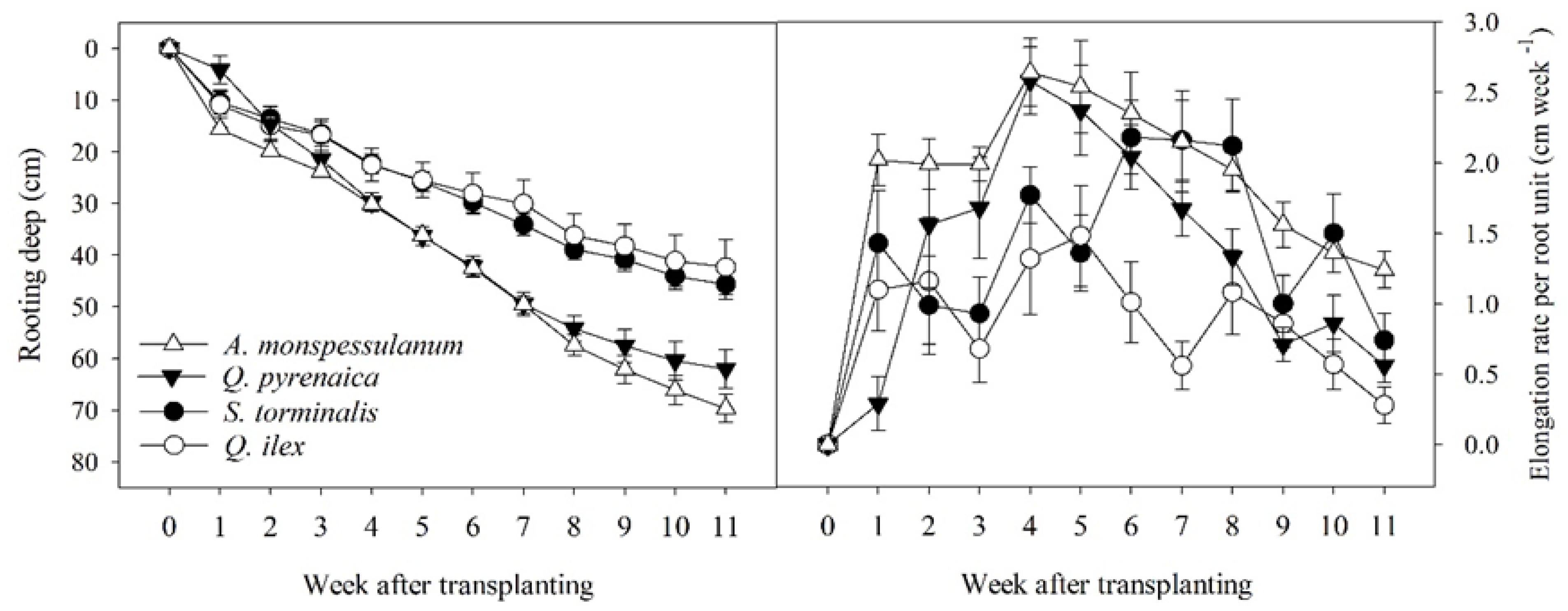
3.1. Root Growth Dynamics
3.2. Plant Morphological Traits at the End of the Experiment
3.3. Physiological Traits
4. Discussion
4.1. Interspecific Differences in Root Development and Its Relationship with Species Functional Attributes
4.2. Responses to Light Gradient
5. Conclusions and Practical Implications
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moles, A.T.; Westoby, M. Seedling Survival and Seed Size: A Synthesis of the Literature. J. Ecol. 2004, 92, 372–383. [Google Scholar] [CrossRef]
- Lucas Borja, M.E. Climate Change and Forest Natural Regeneration in Mediterranean Mountain Areas. Forest Res. 2014, 03, 2–3. [Google Scholar] [CrossRef]
- Pausas, J.G.; Bladé, C.; Valdecantos, A.; Seva, J.P.; Fuentes, D.; Alloza, J.A.; Vilagrosa, A.; Bautista, S.; Cortina, J.; Vallejo, R. Pines and Oaks in the Restoration of Mediterranean Landscapes of Spain: New Perspectives for an Old Practice—A Review. Plant Ecol. 2004, 171, 209–220. [Google Scholar] [CrossRef]
- Grossnickle, S.C. Importance of Root Growth in Overcoming Planting Stress. New For. 2005, 30, 273–294. [Google Scholar] [CrossRef]
- Padilla, F.M.; Pugnaire, F.I. Rooting Depth and Soil Moisture Control Mediterranean Woody Seedling Survival during Drought. Funct. Ecol. 2007, 21, 489–495. [Google Scholar] [CrossRef]
- Padilla, F.M.; de Dios Miranda, J.; Pugnaire, F.I. Early Root Growth Plasticity in Seedlings of Three Mediterranean Woody Species. Plant Soil 2007, 296, 103–113. [Google Scholar] [CrossRef]
- Dhief, A.; Abdellaoui, R.; Tarhouni, M.; Belgacem, A.O.; Smiti, S.A.; Neffati, M. Root and Aboveground Growth of Rhizotron-Grown Seedlings of Three Tunisian Desert Calligonum Species under Water Deficit. Can. J. Soil Sci. 2011, 91, 15–27. [Google Scholar] [CrossRef]
- Schenk, H.J.; Jackson, R.B. Rooting Depths, Lateral Root Spreads and below-Ground / above-Ground Allometries of Plants in Water-Limited Ecosystems. J. Ecol. 2002, 90, 480–494. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root Traits Contributing to Plant Productivity under Drought. Front. Plant Sci. 2013, 4, 1–16. [Google Scholar] [CrossRef]
- Kramer-Walter, K.R.; Bellingham, P.J.; Millar, T.R.; Smissen, R.D.; Richardson, S.J.; Laughlin, D.C. Root Traits Are Multidimensional: Specific Root Length Is Independent from Root Tissue Density and the Plant Economic Spectrum. J. Ecol. 2016, 104, 1299–1310. [Google Scholar] [CrossRef]
- Garbowski, M.; Avera, B.; Bertram, J.H.; Courkamp, J.S.; Gray, J.; Hein, K.M.; Lawrence, R.; McIntosh, M.; McClelland, S.; Post, A.K.; et al. Getting to the Root of Restoration: Considering Root Traits for Improved Restoration Outcomes under Drought and Competition. Restor. Ecol. 2020, 28, 1384–1395. [Google Scholar] [CrossRef]
- Tarshis, L.G. Morphological-Anatomical Features of Underground Organs in Some Higher Plant Species in Relation to Their Adaptation to Ecological Conditions. Russ. J. Ecol. 2005, 36, 81–88. [Google Scholar] [CrossRef]
- Pallardy, S.G. Vegetative Growth. In Physiology of Woody Plants, 3rd ed.; Pallardy, S.G., Ed.; Academic Press: San Diego, CA, USA, 2008; pp. 39–86. ISBN 978-0-12-088765-1. [Google Scholar]
- Mancuso, S. Measuring Roots; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-22066-1. [Google Scholar]
- Lyr, H.; Hoffmann, G. Growth Rates and Growth Periodicity of Tree Roots. In International Review of Forestry Research; Romberger, J.A., Mikola, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1967; Volume 2, pp. 181–236. ISBN 978-1-4831-9976-4. [Google Scholar]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant Physiological Ecology; Springer: New York, NY, USA, 2008; ISBN 978-0-387-78340-6. [Google Scholar]
- Jackson, R.B.; Canadell, J.; Ehleringer, J.R.; Mooney, H.A.; Sala, O.E.; Schulze, E.D. A Global Analysis of Root Distributions for Terrestrial Biomes. Oecologia 1996, 108, 389–411. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; DeForest, J.L.; Burton, A.J.; Allen, M.F.; Ruess, R.W.; Hendrick, R.L. Fine Root Architecture of Nine North American Trees. Ecol. Monogr. 2002, 72, 293–309. [Google Scholar] [CrossRef]
- Andivia, E.; Zuccarini, P.; Grau, B.; de Herralde, F.; Villar-Salvador, P.; Savé, R. Rooting Big and Deep Rapidly: The Ecological Roots of Pine Species Distribution in Southern Europe. Trees 2019, 33, 293–303. [Google Scholar] [CrossRef]
- de la Riva, E.G.; Pérez-Ramos, I.M.; Tosto, A.; Navarro-Fernández, C.M.; Olmo, M.; Marañón, T.; Villar, R. Disentangling the Relative Importance of Species Occurrence, Abundance and Intraspecific Variability in Community Assembly: A Trait-Based Approach at the Whole-Plant Level in Mediterranean Forests. Oikos 2016, 125, 354–363. [Google Scholar] [CrossRef]
- Reich, P.B. The World-Wide ‘Fast–Slow’ Plant Economics Spectrum: A Traits Manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Comas, L.H.; Eissenstat, D.M. Linking Fine Root Traits to Maximum Potential Growth Rate among 11 Mature Temperate Tree Species. Funct. Ecol. 2004, 18, 388–397. [Google Scholar] [CrossRef]
- Gavinet, J.; Prévosto, B.; Fernandez, C. Do Shrubs Facilitate Oak Seedling Establishment in Mediterranean Pine Forest Understory? For. Ecol. Manag. 2016, 381, 289–296. [Google Scholar] [CrossRef]
- Granados, M.E.; Vilagrosa, A.; Chirino, E.; Vallejo, V.R. Reforestation with Resprouter Species to Increase Diversity and Resilience in Mediterranean Pine Forests. For. Eco. Manag. 2016, 362, 231–240. [Google Scholar] [CrossRef]
- Alem, S.; Pavlis, J.; Urban, J.; Kucera, J. Pure and Mixed Plantations of Eucalyptus camaldulensis and Cupressus lusitanica: Their Growth Interactions and Effect on Diversity and Density of Undergrowth Woody Plants in Relation to Light. Open J. For. 2015, 05, 375. [Google Scholar] [CrossRef]
- Löf, M.; Paulsson, R.; Rydberg, D.; Welander, N.T. The Influence of Different Overstory Removal on Planted Spruce and Several Broadleaved Tree Species: Survival, Growth and Pine Weevil Damage during Three Years. Ann. For. Sci. 2005, 62, 237–244. [Google Scholar] [CrossRef]
- Gómez-Aparicio, L.; Zavala, M.A.; Bonet, F.J.; Zamora, R. Are Pine Plantations Valid Tools for Restoring Mediterranean Forests? An Assessment along Abiotic and Biotic Gradients. Ecol. Appl. 2009, 19, 2124–2141. [Google Scholar] [CrossRef]
- Puértolas, J.; Oliet, J.A.; Jacobs, D.F.; Benito, L.F.; Peñuelas, J.L. Is Light the Key Factor for Success of Tube Shelters in Forest Restoration Plantings under Mediterranean Climates? For. Ecol. Manag. 2010, 260, 610–617. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass Allocation to Leaves, Stems and Roots: Meta-Analyses of Interspecific Variation and Environmental Control: Tansley Review. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, E.; Bernal, C.J. Morphological Response and Growth of Cork Oak (Quercus suber L.) Seedlings at Different Shade Levels. For. Ecol. Manag. 2006, 222, 296–301. [Google Scholar] [CrossRef]
- Gómez-Aparicio, L.; Pérez-Ramos, I.M.; Mendoza, I.; Matías, L.; Quero, J.L.; Castro, J.; Zamora, R.; Marañón, T. Oak Seedling Survival and Growth along Resource Gradients in Mediterranean Forests: Implications for Regeneration in Current and Future Environmental Scenarios. Oikos 2008, 117, 1683–1699. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, T.; Ma, W.; Tian, C.; Sha, Z.; Li, J. Morphological and Physiological Response of Acer catalpifolium Rehd. Seedlings to Water and Light Stresses. Glob. Ecol. Conserv. 2019, 19, e00660. [Google Scholar] [CrossRef]
- Sánchez-Gómez, D.; Valladares, F.; Zavala, M.A. Functional Traits and Plasticity in Response to Light in Seedlings of Four Iberian Forest Tree Species. Tree Physiol. 2006, 26, 1425–1433. [Google Scholar] [CrossRef]
- de Castro, A.V.; Oliet, J.A.; Puértolas, J.; Jacobs, D.F. Light Transmissivity of Tube Shelters Affects Root Growth and Biomass Allocation of Quercus ilex L. and Pinus halepensis Mill. Ann. For. Sci. 2014, 71, 91–99. [Google Scholar] [CrossRef][Green Version]
- Valladares, F.; Chico, J.; Aranda, I.; Balaguer, L.; Dizengremel, P.; Manrique, E.; Dreyer, E. The Greater Seedling High-Light Tolerance of Quercus robur over Fagus sylvatica is Linked to a Greater Physiological Plasticity. Trees 2002, 16, 395–403. [Google Scholar] [CrossRef]
- Gomez-Aparicio, L.; Valladares, F.; Zamora, R. Differential Light Responses of Mediterranean Tree Saplings: Linking Ecophysiology with Regeneration Niche in Four Co-Occurring Species. Tree Physiol. 2006, 26, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Quero, J.L.; Villar, R.; Marañón, T.; Zamora, R. Interactions of Drought and Shade Effects on Seedlings of Four Quercus Species: Physiological and Structural Leaf Responses. New Phytol. 2006, 170, 819–834. [Google Scholar] [CrossRef]
- Poorter, L.; McDonald, I.; Alarcón, A.; Fichtler, E.; Licona, J.-C.; Peña-Claros, M.; Sterck, F.; Villegas, Z.; Sass-Klaassen, U. The Importance of Wood Traits and Hydraulic Conductance for the Performance and Life History Strategies of 42 Rainforest Tree Species. New Phytol. 2010, 185, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.A.; Kitajima, K. Carbohydrate Storage Enhances Seedling Shade and Stress Tolerance in a Neotropical Forest. J. Ecol. 2007, 95, 383–395. [Google Scholar] [CrossRef]
- Thyroff, E.C.; Burney, O.T.; Mickelbart, M.V.; Jacobs, D.F. Unraveling Shade Tolerance and Plasticity of Semi-Evergreen Oaks: Insights from Maritime Forest Live Oak Restoration. Front. Plant Sci. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pemán, J.; Navarro C., R.M.; Nicolás Peragón, J.L.; Prada Sáez, M.A.; Serrada Hierro, R. Producción y Manejo de Semillas y Plantas Forestales; Serie Forestal; Organismo Autónomo de Parques Nacionales; Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2013. [Google Scholar]
- Ninemets, Ü.; Valladares, F. Tolerance to Shade, Drought, and Waterlogging of Temperate Northern Hemisphere Trees and Shrubs. Ecol. Monogr. 2006, 76, 521–547. [Google Scholar] [CrossRef]
- Alía M, R.; García del Barrio, J.M.; Iglesias Sauce, S.; Mancha Núñez, J.A.; de Miguel y del Ángel, J.; Nicolás Peragón, J.L.; Pérez Martín, F.; de Ron Martínez, D.S. Regiones de Procedencia de Especies Forestales en España; Organismo Autónomo Parques Nacionales: Madrid, Spain, 2009; ISBN 978-84-8014-759-0. [Google Scholar]
- Cuesta, B.; Vega, J.; Villar-Salvador, P.; Rey-Benayas, J.M. Root Growth Dynamics of Aleppo Pine (Pinus halepensis Mill.) Seedlings in Relation to Shoot Elongation, Plant Size and Tissue Nitrogen Concentration. Trees 2010, 24, 899–908. [Google Scholar] [CrossRef]
- Toca, A.; Oliet, J.A.; Villar-Salvador, P.; Martínez Catalán, R.A.; Jacobs, D.F. Ecologically Distinct Pine Species Show Differential Root Development after Outplanting in Response to Nursery Nutrient Cultivation. For. Ecol. Manag. 2019, 451, 117562. [Google Scholar] [CrossRef]
- Frazer, G.W.; Canham, C.D.; Sallaway, P. Gap Light Analyzer (GLA): Imaging Software to Extract Canopy Structure and Gap Light Transmission Indices from True-Colour Fisheye Photographs, Users Manual and Program Documentation; Simon Fraser University: Burnaby, BC, Canada; The Institute of Ecosystem Studies: Millbrook, NY, USA, 1999. [Google Scholar]
- Serrasoles, I.; Victoria, D.; Bonilla, D. Soil Nitrogen Dynamics. In Ecology of Mediterranean Evergreen Oak Forests; Rodà, F., Retana, J., Gracia, C.A., Bellot, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 131, pp. 223–235. [Google Scholar]
- Lobet, G.; Pagès, L.; Draye, X. A Novel Image Analysis Toolbox Enabling Quantitative Analysis of Root System Architecture. Plant Physiol. 2011, 157, 29–39. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Birouste, M.; Zamora-Ledezma, E.; Bossard, C.; Pérez-Ramos, I.M.; Roumet, C. Measurement of Fine Root Tissue Density: A Comparison of Three Methods Reveals the Potential of Root Dry Matter Content. Plant Soil. 2014, 374, 299–313. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 2nd ed.; SAGE Publications: Thousand Oaks, CA, USA, 2011; ISBN 978-1-4129-7514-8. [Google Scholar]
- Lenth, R.V. Least-Squares Means: The R Package Lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Laureano, R.G.; Lazo, Y.O.; Linares, J.C.; Luque, A.; Martinez, F.; Seco, J.I.; Merino, J. The Cost of Stress Resistance: Construction and Maintenance Costs of Leaves and Roots in Two Populations of Quercus ilex. Tree Physiol. 2008, 28, 1721–1728. [Google Scholar] [CrossRef]
- Villar, R.; Held, A.A.; Merino, J. Dark Leaf Respiration in Light and Darkness of an Evergreen and a Deciduous Plant Species. Plant Physiol. 1995, 107, 421–427. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Puértolas, J.; Cuesta, B.; Peñuelas, J.L.; Uscola, M.; Heredia-Guerrero, N.; Rey Benayas, J.M. Increase in Size and Nitrogen Concentration Enhances Seedling Survival in Mediterranean Plantations. Insights from an Ecophysiological Conceptual Model of Plant Survival. New For. 2012, 43, 755–770. [Google Scholar] [CrossRef]
- Oliet, J.A.; Puértolas, J.; Planelles, R.; Jacobs, D.F. Nutrient Loading of Forest Tree Seedlings to Promote Stress Resistance and Field Performance: A Mediterranean Perspective. New For. 2013, 44, 649–669. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Uscola, M.; Jacobs, D.F. The Role of Stored Carbohydrates and Nitrogen in the Growth and Stress Tolerance of Planted Forest Trees. New For. 2015, 46, 813–839. [Google Scholar] [CrossRef]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How Tree Roots Respond to Drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef]
- Gastón-González, A.; García-Viñas, J.I. El estudio del hábitat climático para la selección de especies en la restauración de la vegetación (Anexo I). In Producción y Manejo de Semillas y Plantas Forestales. Organismo Autónomo Parques Nacionales; Pemán, J., Navarro C, R.M., Nicolás, J.L., Prada, M.A., Serrada, R., Eds.; Serie Forestal; Organismo Autónomo de Parques Nacionales; Revista Ecosistemas: Madrid, Spain, 2013; pp. 615–730. ISBN 978-84-8014-846-7. [Google Scholar]
- Tissier, J.; Lambs, L.; Peltier, J.-P.; Marigo, G. Relationships between Hydraulic Traits and Habitat Preference for Six Acer Species Occurring in the French Alps. Ann. For. Sci. 2004, 61, 81–86. [Google Scholar] [CrossRef]
- Knight, C.A.; Ackerly, D.D. Evolution and Plasticity of Photosynthetic Thermal Tolerance, Specific Leaf Area and Leaf Size: Congeneric Species from Desert and Coastal Environments. New Phytol. 2003, 160, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.-D.; Chen, Y.-J.; Ye, Q.; He, P.-C.; Liu, H.; Li, R.-H.; Fu, P.-L.; Jiang, G.-F.; Cao, K.-F. Leaf Turgor Loss Point Is Correlated with Drought Tolerance and Leaf Carbon Economics Traits. Tree Physiol. 2018, 38, 658–663. [Google Scholar] [CrossRef]
- Bucci, S.J.; Scholz, F.G.; Goldstein, G.; Meinzer, F.C.; Arce, M.E. Soil Water Availability and Rooting Depth as Determinants of Hydraulic Architecture of Patagonian Woody Species. Oecologia 2009, 160, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Fitter, A.H.; Stickland, T.R. Architectural Analysis of Plant Root Systems 2. Influence of Nutrient Supply on Architecture in Contrasting Plant Species. New Phytol. 1991, 118, 383–389. [Google Scholar] [CrossRef]
- Aikio, S.; Markkola, A.M. Optimality and Phenotypic Plasticity of Shoot-to-Root Ratio under Variable Light and Nutrient Availabilities. Evol. Ecol. 2002, 16, 67–76. [Google Scholar] [CrossRef]
- Poorter, H.; Pepin, S.; Rijkers, T.; de Jong, Y.; Evans, J.R.; Körner, C. Construction Costs, Chemical Composition and Payback Time of High- and Low-Irradiance Leaves. J. Exp. Bot. 2006, 57, 355–371. [Google Scholar] [CrossRef]
- Ballaré, C.L.; Pierik, R. The Shade-Avoidance Syndrome: Multiple Signals and Ecological Consequences: Neighbour Detection and Shade Avoidance. Plant Cell Enviro. 2017, 40, 2530–2543. [Google Scholar] [CrossRef]
- Bauhus, J.; Schemerbeck, J. Silvicultural options to enhance and use forest plantation biodiversity. In Ecosystem Goods and Services from Plantation Forests; Bauhus, J., van der Meer, P., Kanninen, M., Eds.; EarthScan: London, UK, 2010; pp. 96–139. ISBN 978-1-84977-641-7. [Google Scholar]




| Traits | Species (S) | Light (L) | Time (T) | S × L | S × T | L × T | S × L × T |
|---|---|---|---|---|---|---|---|
| Rooting depth | 8.8 (<0.001) | 0.17 (0.49) | 307 (<0.001) | 0.41 (0.74) | 7.20 (<0.001) | 0.48 (0.65) | 0.97 (0.50) |
| Number of elongating roots | 24.4 (<0.001) | 1.46 (0.24) | 74.8 (<0.001) | 0.09 (0.96) | 6.93 (<0.001) | 3.37 (0.003) | 1.67 (0.02) |
| Root system elongation rate | 27.9 (<0.001) | 1.10 (0.30) | 52.7 (<0.001) | 0.16 (0.92) | 6.01 (<0.001) | 2.24 (0.016) | 1.95 (0.003) |
| Elongation rate per root unit | 7.10 (<0.001) | 0.008 (0.93) | 13.5 (<0.001) | 0.26 (0.85) | 2.71 (<0.001) | 0.50 (0.89) | 0.86 (0.67) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Pérez, J.L.; Oliet, J.A.; Villar-Salvador, P.; Guzmán, J.E. Root Growth Dynamics and Structure in Seedlings of Four Shade Tolerant Mediterranean Species Grown under Moderate and Low Light. Forests 2021, 12, 1540. https://doi.org/10.3390/f12111540
García-Pérez JL, Oliet JA, Villar-Salvador P, Guzmán JE. Root Growth Dynamics and Structure in Seedlings of Four Shade Tolerant Mediterranean Species Grown under Moderate and Low Light. Forests. 2021; 12(11):1540. https://doi.org/10.3390/f12111540
Chicago/Turabian StyleGarcía-Pérez, José L., Juan A. Oliet, Pedro Villar-Salvador, and Jorge Eduardo Guzmán. 2021. "Root Growth Dynamics and Structure in Seedlings of Four Shade Tolerant Mediterranean Species Grown under Moderate and Low Light" Forests 12, no. 11: 1540. https://doi.org/10.3390/f12111540
APA StyleGarcía-Pérez, J. L., Oliet, J. A., Villar-Salvador, P., & Guzmán, J. E. (2021). Root Growth Dynamics and Structure in Seedlings of Four Shade Tolerant Mediterranean Species Grown under Moderate and Low Light. Forests, 12(11), 1540. https://doi.org/10.3390/f12111540





