The Mediterranean Old-Growth Forests: Anomalies or Relicts? The Contribution of Soil Charcoal Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Area
2.2. Local History
2.3. Sampling Strategy and Soil Description
2.4. Soil Charcoal Identification
2.5. Charcoal Data Handling
2.6. Chronological Analysis
3. Results
3.1. Soil Description and Charcoal Richness
3.2. Taxanomical Analysis
3.3. Chronological Analysis
4. Discussion
4.1. Origin of the Sainte-Baume Forest
4.2. The Long-Term Trajectory of the Sainte-Baume Forest and Its Surroundings
4.3. The Mechanism(s) of Survival of the Old-Growth Sainte-Baume Forest
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hilbert, J.; Wiensczyk, A. Old-growth definitions and management: A Literature review. J. Ecosyst. Manag. 2007, 8, 15–31. [Google Scholar]
- Wirth, C.; Gleixner, G.; Heimann, M. Old-Growth Forests: Function, Fate and Value. In Ecological Studies 207; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Mansourian, S.; Rossi, M.; Vallauri, D. Ancient Forests in the Northern Mediterranean: Neglected High Conservation Value Areas; WWF: Marseille, France, 2013. [Google Scholar]
- Luyssaert, S.; Detlef, S.E.; Börner, A.; Knohl, A.; Hessenmöller, D.; Law, B.E.; Ciais, P.; Grace, J. Old-growth forests as global carbon sinks. Nature 2008, 455, 213–215. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, J.C.; Thompson, J.R.; Epstein, H.E.; Shugart, H.H., Jr. Carbon storage in old-growth forests of the Mid-Atlantic: Toward better understanding the eastern forest carbon sink. Ecology 2015, 96, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.; Bell, D.M.; Kim, H.; Nelson, P.N.; Schulze, M.; Betts, M.G. Temporal consistency of undercanopy thermal refugia in old-growth forest. Agric. For. Meteorol. 2021, 307, 108520. [Google Scholar] [CrossRef]
- Spies, T.A.; Hemstrom, M.A.; Youngblood, A.; Hummel, S. Conserving Old-Growth Forest Diversity in Disturbance-Prone Landscapes. Conserv. Biol. 2006, 20, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, C.E.; Friederici, P.; Petruncio, M. Monitoring old growth in frequent-fire landscapes. Ecol. Soc. 2007, 12, 22. Available online: http://www.ecologyandsociety.org/vol12/iss2/art22 (accessed on 5 November 2021). [CrossRef][Green Version]
- De Assis Barros, L.; Elkin, C. An index for tracking old-growth value in disturbance-prone forest landscapes. Ecol. Indic. 2021, 121, 107175. [Google Scholar] [CrossRef]
- Quézel, P.; Médail, F. Écologie et Biogéographie des Forêts du Bassin Méditerranéen; Elsevier: Paris, France, 2003. [Google Scholar]
- Grove, A.T.; Rackham, O. The Nature of Mediterranean Europe: An Ecological History; Yale University Press: New Haven, UK, 2003. [Google Scholar]
- Blondel, J.; Aronson, J.; Bodiou, J.-Y.; Bœuf, G. The Mediterranean Region. Biological Diversity in Space and Time; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Mercuri, A.M.; Florenzano, A.; Burjachs, F.; Giardini, M.; Kouli, K.; Masi, A.; Picornell-Gelabert, L.; Revelles, J.; Sadori, L.; Servera-Vives, G.; et al. From influence to impact: The multifunctional land use in Mediterranean prehistory emerging from palynology of archaeological sites (8.0–2.8 ka BP). Holocene 2019, 29, 830–846. [Google Scholar] [CrossRef]
- Chirici, G.; Nocentini, S. Old-growth forests in Italy: Recent research developments and future perspectives. L’Italia For. E Mont. 2010, 65, 475–480. [Google Scholar] [CrossRef]
- Lombardi, F.; Chirici, G.; Marchetti, M.; Tognetti, R.; Lasserre, B.; Corona, P.; Barbati, A.; Ferrari, B.; Di Paolo, S.; Giuliarelli, D.; et al. Deadwood in forest stands close to old-growthness under Mediterranean conditions in the Italian Peninsula. L’Italia For. E Mont. 2010, 65, 481–584. [Google Scholar] [CrossRef][Green Version]
- Badalamenti, E.; La Mantia, T.; La Mantia, G.; Cairone, A.; Veca, D.S.L.M. La Mantiving and Dead Aboveground Biomass in Mediterranean Forests: Evidence of Old-Growth Traits in a Quercus pubescens Willd. s.l. Stand. Forests 2017, 8, 187. [Google Scholar] [CrossRef]
- Dutoit, T.; Thinon, M.; Talon, B.; Buisson, E.; Alard, D. Sampling soil wood charcoals at a high spatial resolution: A new methodology to investigate the origin of grassland plant communities. J. Veg. Sci. 2009, 20, 349–358. [Google Scholar] [CrossRef]
- Touflan, P.; Talon, B.; Walsh, K. Soil charcoal analysis: A reliable tool for spatially precise studies of past forest dynamics: A case study in the French southern Alps. Holocene 2010, 20, 45–52. [Google Scholar] [CrossRef]
- Feiss, T.; Horen, H.; Brasseur, B.; Lenoir, J. Optimal sampling design and minimal effort for soil charcoal analyses considering the soil type and forest history. Veg. Hist. Archaeobotany 2017, 26, 627–637. [Google Scholar] [CrossRef]
- Gavin, D.G. Estimation of inbuilt age in radiocarbon ages of soil charcoal for fire history studies. Radiocarbon 2001, 43, 27–44. [Google Scholar] [CrossRef]
- Fesenmyer, K.A.; Christensen, N.L. Reconstructing Holocene fire history in a southern Appalachian forest using soil charcoal. Ecology 2010, 91, 662–670. [Google Scholar] [CrossRef]
- Robin, V.; Bork, H.-R.; Nadeau, M.-J.; Nelle, O. Fire and forest history of central European low mountain forest sites based on soil charcoal analysis: The case of the eastern Harz. Holocene 2014, 24, 35–47. [Google Scholar] [CrossRef]
- IFN. Une nouvelle partition écologique et forestière du territoire métropolitain : Les sylvoécorégions (SER). Bull. L’if 2011, 26, 1–8. [Google Scholar]
- Darras, C.; Tresmontant, D.; Lemaire, C. La Forêt Sacrée de la Sainte-Baume; Naturalia Publications: Turriers, France, 2017. [Google Scholar]
- Blanc, J.-J. Histoire des creusements karstiques et des surfaces d’érosion en Provence occidentale. Physio Géo. 2010, 4, 1–26. [Google Scholar] [CrossRef]
- Mazet, J.; Nicod, J. Les bassins supérieurs du Cauron et du Caramy, au nord-est du massif de la Sainte Baume (Var, Provence): Des hydrosystèmes karstiques complexes. Études Géographie Phys. 2012, 39, 21–51. [Google Scholar]
- Vila, B.; Vennetier, M.; Ripert, C.; Chandioux, O.; Liang, E.; Guibal, F.; Torre, F. Has global change induced divergent trends in radial growth of Pinus sylvestris and Pinus halepensis at their bioclimatic limit? The example of the Sainte-Baume forest (south-east France). Ann. For. Sci. 2008, 65, 709. [Google Scholar] [CrossRef]
- Charles, J.P. Une hêtraie à Androsace chaixii dans le massif de la Sainte-Baume. Bull. Soc. Linn. Provence 1991, 42, 59–70. [Google Scholar]
- Cattenoz, D. Dynamiques Passée et Actuelle de la Hêtraie de la Sainte-Baume (Var). Master’s Thesis, Aix-Marseille University, Aix-en-Provence, France, 2009. [Google Scholar]
- Molinier, R. Flore de la forêt domaniale de la Sainte Baume (Var). Catalogue des espèces présentes dans les limites de la forêt. Ann. Soc. Nat. Archéol. ToulonVar. 1950, 3, 45–66. [Google Scholar]
- Bonin, G.; Gamisans, J.; Gruber, M. Etude des successions dynamiques de la végétation de la Sainte Baume. Ecol. Mediterr. 1983, 9, 129–171. [Google Scholar] [CrossRef]
- Bonin, G.; Sandoz, H.; Thinon, M.; Vedrenne, G. Relations entre la dynamique de la végétation (chênaie hêtraie) et les caractéristiques édaphiques dans le massif de la Sainte-Baume (Provence). Ecol. Mediterr. 1984, 9, 193–210. [Google Scholar] [CrossRef]
- Quertier, P.; Guicheteau, D. Site Natura 2000 PR 110–FR 9301606 Massif de la Sainte Baume—Document d’Objectifs Natura 2000; Muséum National d’Histoire Naturelle: Paris, France, 2001. [Google Scholar]
- SRA. Bilan Scientifique de la Région Provence-Alpes-Côte d’Azur; Ministère de la Culture et de la Communication, Direction Générale des Patrimoines, Sous-Direction de l’Archéologie, Service Regional d’Archeologie: Aix-en-Provence, France, 2013. [Google Scholar]
- Servan, P. Voies antiques de la moyenne vallée de l’Huveaune. Rev. Provence Hist. 1960, 10, 189–221. [Google Scholar]
- Brun, J.-P.; Conges, G.; Geraba, C.; Pasqualini, M. L’habitat rural dans le Var à l’époque romaine: Données archéologiques récentes. Rev. Provence Hist. 1985, 35, 233–251. [Google Scholar]
- Fedele, A. From Christian religion to feminist spirituality: Mary Magdalene pilgrimages to La Sainte-Baume, France. Cult. Relig. 2009, 10, 243–261. [Google Scholar] [CrossRef]
- Chalvet, M. La forêt domaniale de la Sainte-Baume : Un espace exceptionnel et protégé en Provence. Cahiers Framespa 2013, 13, 1–19. [Google Scholar] [CrossRef]
- Hervé, P. La forêt domaniale de la Sainte-Baume (Var)—Problèmes posés par sa gestion. Rev. For. Française 1953, 9, 557–564. [Google Scholar] [CrossRef]
- Dugelay, A. La Sainte-Baume. Esquisse d’histoire d’une relique forestière. Rev. Bois mars. 1957. [Google Scholar]
- Nédonsel, Y. Le massif de la Sainte Baume et la production de glace naturelle: Les glacières de Fontfrège. Rev. Régionale D’ethnologie 1981, 2–3, 103–125. [Google Scholar] [CrossRef]
- Munsell. Soil Color Charts; Gretag Macbeth: New Windsor, NY, USA, 2000. [Google Scholar]
- Schoeneberger, P.J.; Wysocki, D.A.; Benham, E.C. Soil Survey Staff. Field Book for Describing and Sampling Soils, Version 2.0; U.S. Department of Agriculture, Natural Resources Conservation Service, National Soil Survey Center: Lincoln, NE, USA, 2002. [Google Scholar]
- Sponagel, H.; Grottenthaler, W.; Hartmann, K.J.; Eckelmann, W. Bodenkundliche Kartieranleitung (5th verbesserte und erweiterte Auflage); Bundesanstalt für Geowissenschaften und Rohstoffe: Hannover, Germany, 2005. [Google Scholar]
- Robin, V.; Nadeau, M.-J.; Grootes, P.M.; Bork, H.; Nelle, O. Too early and too northerly: Evidence of temperate trees in northern Central Europe during the Younger Dryas. New Phytol. 2016, 212, 259–268. [Google Scholar] [CrossRef]
- Carcaillet, C.; Thinon, M. Pedoanthracological contribution to the study of the evolution of the upper treeline in the Maurienne Valley (North French Alps): Methodology and preliminary data. Rev. Palaeobot. Palynol. 1996, 91, 399–416. [Google Scholar] [CrossRef]
- Talon, B.; Carcaillet, C.; Thinon, M. Études pédoanthracologiques des variations de la limite supérieure des arbres au cours de l’Holocène dans les Alpes Françaises. Géographie Phys. Quat. 1998, 52, 195–208. [Google Scholar] [CrossRef]
- McParland, L.C.; Collinson, M.E.; Scott, A.C.; Campbell, G.; Veal, R. Is vitrification in charcoal a result of high temperature burning of wood? J. Archaeol. Sci. 2010, 37, 2679–2687. [Google Scholar] [CrossRef]
- Wheeler, E.A.; Baas, P.; Gasson, P.E. IAWA list of microscopic features for hardwood identification. IAWA Bull. 1989, 10, 219–332. [Google Scholar] [CrossRef]
- Schweingruber, F.H. Anatomy of European Woods; Haupt Verlag AG: Bern, Switzeralnd,, 1990. [Google Scholar]
- Schweingruber, F.H. Microscopic Wood Anatomy; Swiss Federal Institute for Forest, Snow and Landscape Research: Birmensdorf, Switzeralnd, 1990. [Google Scholar]
- Darwin, C. The Formation of Vegetable Mould, Through the Action of Worms, with Observations on their Habits. John Murray: London, UK, 1881. [Google Scholar]
- Carcaillet, C. Are Holocene wood-charcoal fragments stratified in alpine and subalpine soils? Evidence from the Alps based on AMS 14C dates. Holocene 2001, 11, 231–242. [Google Scholar] [CrossRef]
- Šamonil, P.; Král, K.; Hort, L. The role of tree uprooting in soil formation: A critical literature review. Geoderma 2010, 157, 65–79. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Juggins, S. User Guide C2—Software for Ecological and Palaeoecological. Data Analysis and Visualization User Guide, version 1.5; University of Newcastle: Newcastle, NWS, Australia, 2007. [Google Scholar]
- Bork, H.-R.; Lang, A. Quantification of past erosion and land use/land cover changes in Germany. In Long Term Hillslope and Fluvial System Modelling; Lang, A., Hennrich, K., Dikan, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 231–239. [Google Scholar]
- Leopold, M.; Völkel, J. Colluvium: Definition, differentiation, and possible suitability for reconstructing Holocene climate data. Quat. Int. 2007, 162–163, 133–140. [Google Scholar] [CrossRef]
- Dreibrodt, S.; Lubos, C.; Terhorst, B.; Damm, B.; Bork, H.-R. Historical soil erosion by water in Germany: Scales and archives, chronology, research perspectives. Quat. Int. 2010, 222, 80–95. [Google Scholar] [CrossRef]
- Bronk-Ramsey, C.; Lee, S. Recent and Planned Developments of the Program OxCal. Radiocarbon 2013, 55, 720–730. [Google Scholar] [CrossRef]
- Reimer, P.J.; Bard, E.; Bayliss, A.; Beck, J.W.; Blackwell, P.G.; Ramsey, C.B.; Buck, C.E.; Cheng, H.; Edwards, R.L.; Friedrich, M.; et al. INTCAL13 and MARINE13 radiocarbon age calibration curves 0-50,000 years cal BP. Radiocarbon 2013, 55, 1869–1887. [Google Scholar] [CrossRef]
- Whitlock, C.; Higuera, P.; McWethy, D.B.; Briles, C.E. Paleoecological Perspectives on Fire Ecology: Revisiting the Fire-Regime Concept. Open Ecol. J. 2010, 3, 6–23. [Google Scholar] [CrossRef]
- Gavin, D.G. Forest soil disturbance intervals inferred from soil charcoal radiocarbon dates. Can. J. For. Res. 2003, 33, 2514–2518. [Google Scholar] [CrossRef]
- Jégou, D.; Cluzeau, D.; Balesdent, J.; Tréhen, P. Effects of four ecological categories of earthworms on carbon transfer in soil. Appl. Soil Ecol. 1998, 9, 249–255. [Google Scholar] [CrossRef]
- Talon, B. Reconstruction of Holocene high altitude vegetation cover in the French Southern Alps: Evidence from soil charcoal. Holocene 2010, 20, 34–44. [Google Scholar] [CrossRef]
- Giesecke, T.; Hickler, T.; Kunkel, T.; Sykes, M.T.; Bradshaw, R.H.; Hickler, T.; Kunkel, T.; Sykes, R.H.; Bradshaw, R.H. Towards an understanding of the Holocene distribution of Fagus sylvatica L. J. Biogeogr. 2007, 34, 118–131. [Google Scholar] [CrossRef]
- Magri, D. Patterns of post-glacial spread and the extent of glacial refugia of European beech (Fagus sylvatica). J. Biogeogr. 2008, 35, 450–463. [Google Scholar] [CrossRef]
- Bradshaw, R.H.W.; Kito, N.; Giesecke, T. Factors influencing the Holocene history of Fagus. For. Ecol. Manag. 2010, 259, 2204–2212. [Google Scholar] [CrossRef]
- Laval, H.; Medus, J.; Roux, M. Palynological and sedimentological records of Holocene human impact from the Etang de Berre, southeastern France. Holocene 1991, 1, 269–272. [Google Scholar] [CrossRef]
- Delhon, C.; Thiébault, S.; Brochier, J.-L.; Berger, J.-F. Dynamiques de végétation au Tardiglaciaire et à l’Holocène ancien en moyenne vallée du Rhône d’après les données anthracologiques. Quaternaire 2010, 21, 281–293. [Google Scholar] [CrossRef]
- Azuara, J.; Lebreton, V.; Peyron, O.; Mazier, F.; Combourieu-Nebout, N. The Holocene history of low altitude Mediterranean Fagus sylvatica forests in southern France. J. Veg. Sci. 2018, 29, 438–449. [Google Scholar] [CrossRef]
- Delhon, C.; Thiébault, S. The migration of beech (Fagus sylvatica L.) up the Rhone: The Mediterranean history of a "mountain" species. Veg. Hist. Archaeobotany 2005, 14, 119–132. [Google Scholar] [CrossRef]
- De Lafontaine, G.; Guerra, C.A.A.; Ducousso, A.; Petit, R.J. Cryptic no more: Soil macrofossils uncover Pleistocene forest microrefugia within a periglacial desert. New Phytol. 2014, 204, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Robin, V.; Knapp, H.; Rickert, B.; Talon, B.; Nelle, O. Comparaison de signaux anthracologiques Holocènes issus de différents types d’archives en Allemagne vers une reconstitution plus précise de l’histoire des incendies ? Quaternaire 2013, 24, 167–177. [Google Scholar] [CrossRef]
- Stewart, J.R.; Lister, A.M. Cryptic northern refugia and the origins of the modern biota. Trends Ecol. Evol. 2001, 16, 608–613. [Google Scholar] [CrossRef]
- Henry, F.; Talon, B.; Dutoit, T. The age and the history of the French Mediterranean steppe revisited by soil wood charcoal analysis. Holocene 2010, 20, 25–34. [Google Scholar] [CrossRef]
- Casals, P.; Camprodon, J.; Caritat, A.; Rios, A.I.; Guixé, D.; Garcia-Marti, X.; Martin-Alcon, S.; Coll, L. Forest structure of Mediterranean yew (Taxus baccata L.) populations and neighbor effects on juvenile yew performance in the NE Iberian Peninsula. For. Syst. 2015, 24, e042. [Google Scholar] [CrossRef]
- Iszkuło, G.; Pers-Kamczyc, E.; Nalepka, D.; Rabska, M.; Walas, Łukasz; Dering, M. Postglacial migration dynamics helps to explain current scattered distribution of Taxus baccata. Dendrobiology 2016, 76, 81–89. [Google Scholar] [CrossRef]
- Guinier, P. Plaidoyer pour la Sainte-Baume auprès de Monsieur le Juge de Paix de Saint-Maximin. Dacty 1944. [Google Scholar]
- Molinier, R. La hêtraie de la forêt domaniale de la Sainte-Baume. Bull. MNHN Marseille 1952, 12, 63–85. [Google Scholar]
- Thiébaut, B. Étude Ecologique de la Hêtraie dans l’Arc Montagneux Nord Méditerranéen de la Vallée du Rhône à Celle de l’Ebre. PhD Thesis, Languedoc University, Montpellier, France, 1976. [Google Scholar]
- Nicol-Pichard, S. Analyse pollinique d’une séquence Tardiet Postglaciaire à Tourves (var, France). Ecol. Med. 1987, 13, 29–42. [Google Scholar]
- Capitanio, R.; Carcaillet, C. Post-fire Mediterranean vegetation dynamics and diversity: A discussion of succession models. For. Ecol. Manag. 2008, 255, 431–439. [Google Scholar] [CrossRef]
- Barbero, M.; Bonin, G.; Loisel, R.; Quézel, P. Changes and disturbances of forest ecosystems caused by human activities in the western part of the Mediterranean basin. Vegetatio 1990, 87, 151–173. [Google Scholar] [CrossRef]
- Roberts, C.N.; Woodbridge, J.; Palmisano, A.; Bevan, A.; Fyfe, R.; Shennan, S. Mediterranean landscape change during the Holocene: Synthesis, comparison and regional trends in population, land cover and climate. Holocene 2019, 25, 923–937. [Google Scholar] [CrossRef]
- Dumé, G.; Gauberville, C.; Mansion, D.; Rameau, J.-C. Flore Forestière Française; Institut pour le développement forestier, Centre national de la propriété forestière: Paris, France, 2003. [Google Scholar]
- Jalut, G.; Dedoubat, J.J.; Fontugne, M.; Otto, T. Holocene circum-Mediterranean vegetation changes: Climate forcing and human impact. Quat. Int. 2009, 200, 4–18. [Google Scholar] [CrossRef]
- Berger, J.-F.; Shennan, S.; Woodbridge, J.; Palmisano, A.; Mazier, F.; Nuninger, L.; Guillon, S.; Doyen, E.; Begeot, C.; Andrieu-Ponel, V.; et al. Holocene land cover and population dynamics in Southern France. Holocene 2019, 25, 776–798. [Google Scholar] [CrossRef]
- Butzer, K.W. Environmental history in the Mediterranean world: Cross-disciplinary investigation of cause-and-effect for degradation and soil erosion. J. Archaeol. Sci. 2005, 32, 1773–1800. [Google Scholar] [CrossRef]
- Vannière, B.; Colombaroli, D.; Chapron, E.; Leroux, A.; Tinner, W.; Magny, M. Climate versus human-driven fire regimes in Mediterranean landscapes: The Holocene record of Lago dell’Accesa (Tuscany, Italy). Quat. Sci. Rev. 2008, 27, 1181–1196. [Google Scholar] [CrossRef]
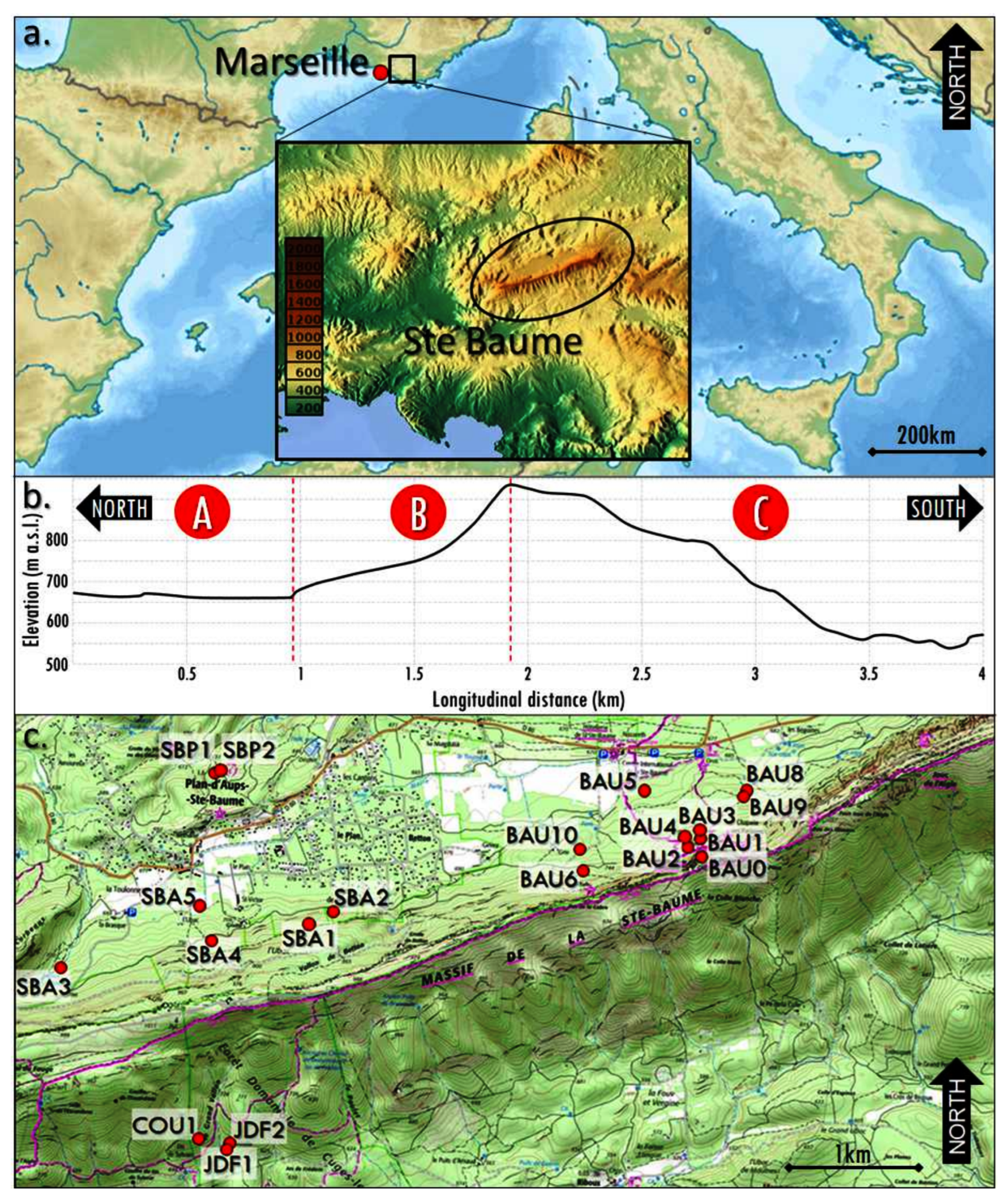
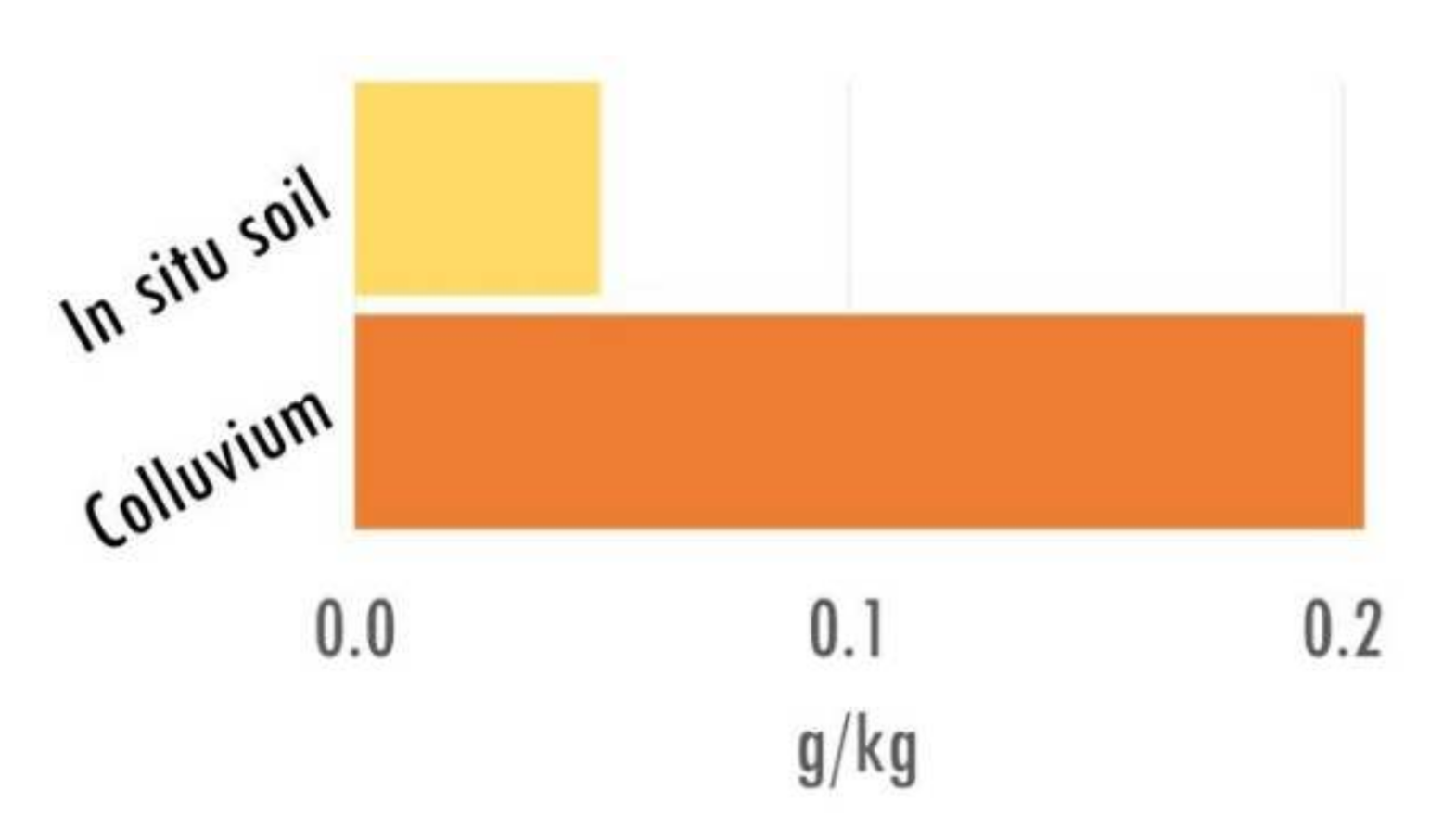
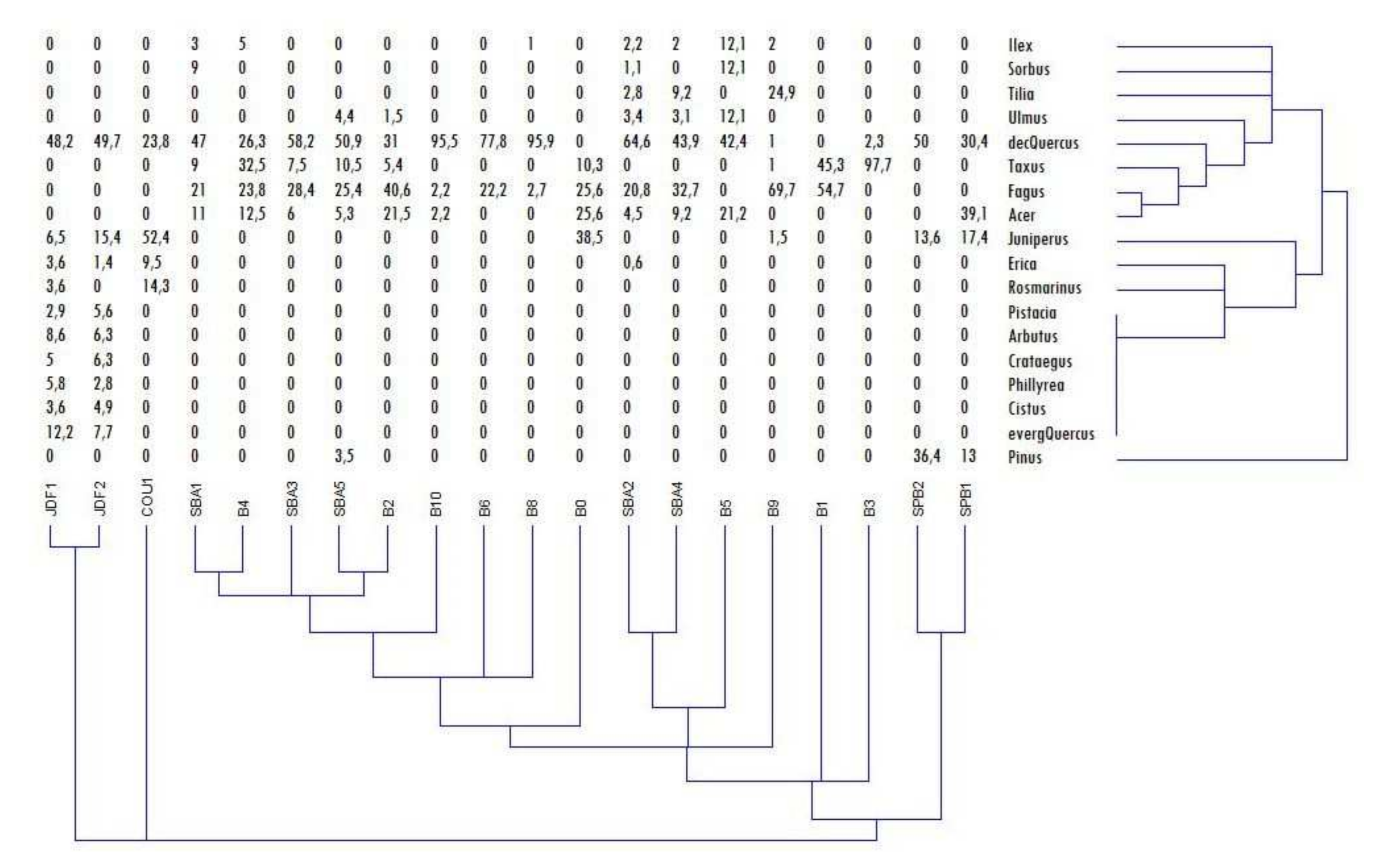
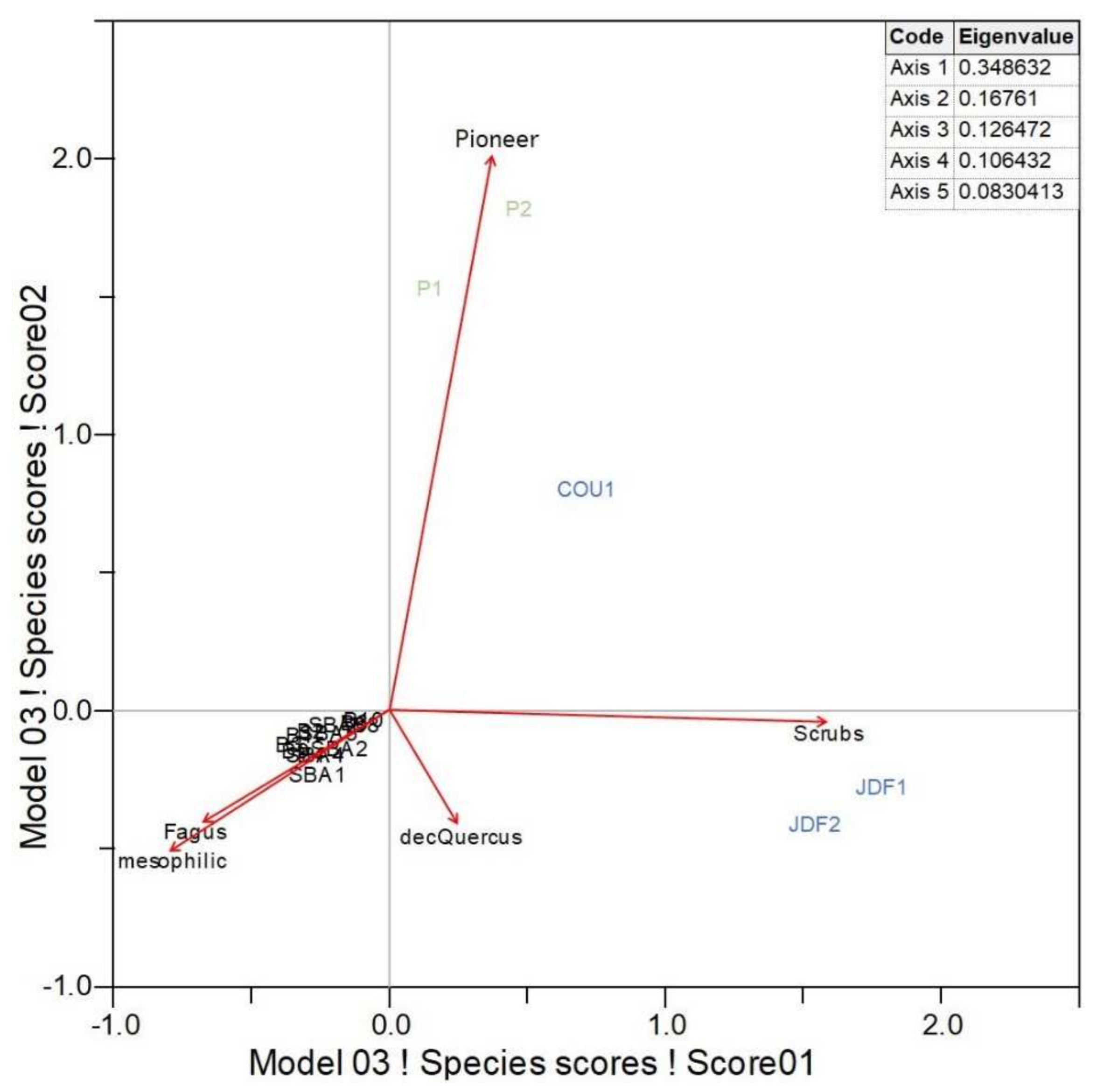
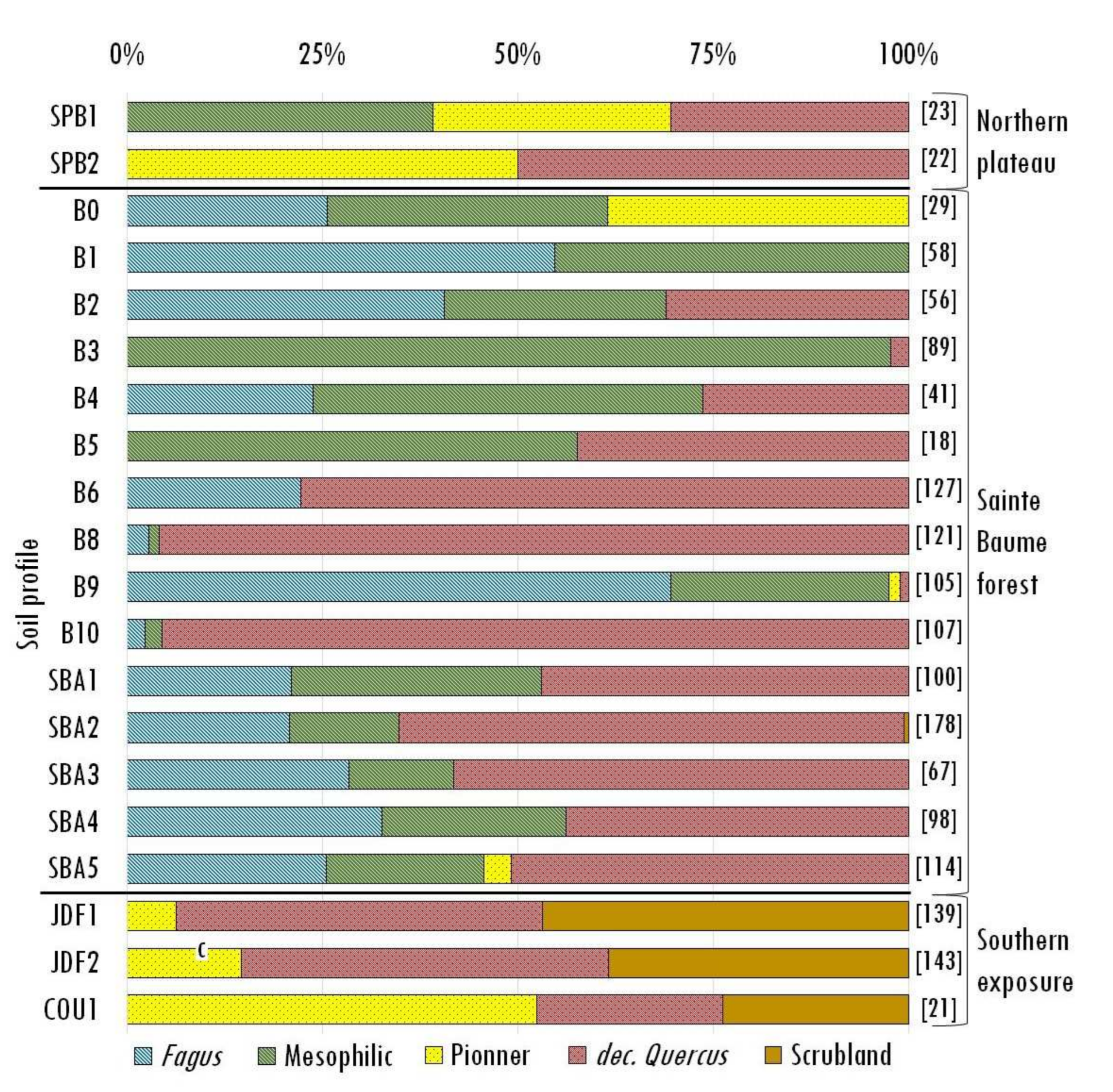
| Units of Investigation | Profile | Type of Soil * | Type of Archive | Max Depth (cm) | Nbr Samples | |
|---|---|---|---|---|---|---|
| Coll. | In Situ | |||||
| Northern plateau | SBP1 | sk-rz-RG | x | x | 50 | 3 |
| SBP2 | x | x | 55 | 3 | ||
| Sainte-Baume forest | SBA1 | co-CM | x | x | 60 | 5 |
| SBA2 | x | x | 70 | 5 | ||
| SBA3 | x | - | 85 | 4 | ||
| SBA4 | x | x | 70 | 4 | ||
| SBA5 | x | x | 140 | 4 | ||
| BAU0 | x | - | 30 | 2 | ||
| BAU1 | x | - | 30 | 2 | ||
| BAU2 | x | - | 30 | 2 | ||
| BAU3 | x | - | 30 | 2 | ||
| BAU4 | x | - | 30 | 2 | ||
| BAU5 | x | - | 30 | 2 | ||
| BAU6 | x | - | 50 | 5 | ||
| BAU8 | x | - | 50 | 5 | ||
| BAU9 | x | - | 50 | 5 | ||
| BAU10 | x | - | 50 | 5 | ||
| Southern exposure | JDF1 | co-rz-RG | x | - | 50 | 5 |
| JDF2 | x | x | 145 | 6 | ||
| COU1 | sk-rz-RG | x | - | 50 | 2 | |
| Units of Investigation | Profile of Sampling | Nbr of Ident. | Fagus | Juniperus | Acer | Taxus | Dec. Quercus | Everg. Quercus | Ulmus | Ilex | Sorbus | Tilia | Cistus | Pinus | Erica | Phillyrea | Crataegus | Rosmarinus | Arbutus | Pistacia |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Northern plateau | SPB1 | 23 | - | 17 | 39 | - | 30 | - | - | - | - | - | - | 13 | - | - | - | - | - | - |
| SPB2 | 22 | - | 14 | - | - | 50 | - | - | - | - | - | - | 36 | - | - | - | - | - | - | |
| Sainte-Baume forest | SBA1 | 100 | 21 | - | 11 | 9 | 47 | - | - | 3 | 9 | - | - | - | - | - | - | - | - | - |
| SBA2 | 178 | 21 | - | 5 | - | 65 | - | 3 | 2 | 1 | 3 | - | - | 1 | - | - | - | - | - | |
| SBA3 | 67 | 28 | - | 6 | 8 | 58 | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| SBA4 | 98 | 33 | - | 9 | - | 44 | - | 3 | 2 | - | 9 | - | - | - | - | - | - | - | - | |
| SBA5 | 114 | 25 | - | 5 | 11 | 51 | - | 4 | - | - | - | - | 4 | - | - | - | - | - | - | |
| B0 | 29 | 26 | 39 | 26 | 10 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| B1 | 58 | 55 | - | - | 45 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| B2 | 56 | 41 | - | 22 | 5 | 31 | - | 2 | - | - | - | - | - | - | - | - | - | - | - | |
| B3 | 89 | - | - | - | 98 | 2 | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| B4 | 41 | 24 | - | 13 | 33 | 26 | - | - | 5 | - | - | - | - | - | - | - | - | - | - | |
| B5 | 18 | - | - | 21 | - | 42 | - | 12 | 12 | 12 | - | - | - | - | - | - | - | - | - | |
| B6 | 127 | 22 | - | - | - | 78 | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| B8 | 121 | 3 | - | - | - | 96 | - | - | 1 | - | - | - | - | - | - | - | - | - | - | |
| B9 | 105 | 70 | 2 | - | 1 | 1 | - | - | 2 | - | 25 | - | - | - | - | - | - | - | - | |
| B10 | 107 | 2 | - | 2 | - | 96 | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Southern exposure | JDF1 | 139 | - | 7 | - | - | 48 | 12 | - | - | - | - | 4 | - | 4 | 6 | 5 | 4 | 9 | 3 |
| JDF2 | 143 | - | 15 | - | - | 50 | 8 | - | - | - | - | 5 | - | 1 | 3 | 6 | - | 6 | 6 | |
| COU1 | 21 | - | 52 | - | - | 24 | - | - | - | - | - | - | - | 10 | - | - | 14 | - | - |
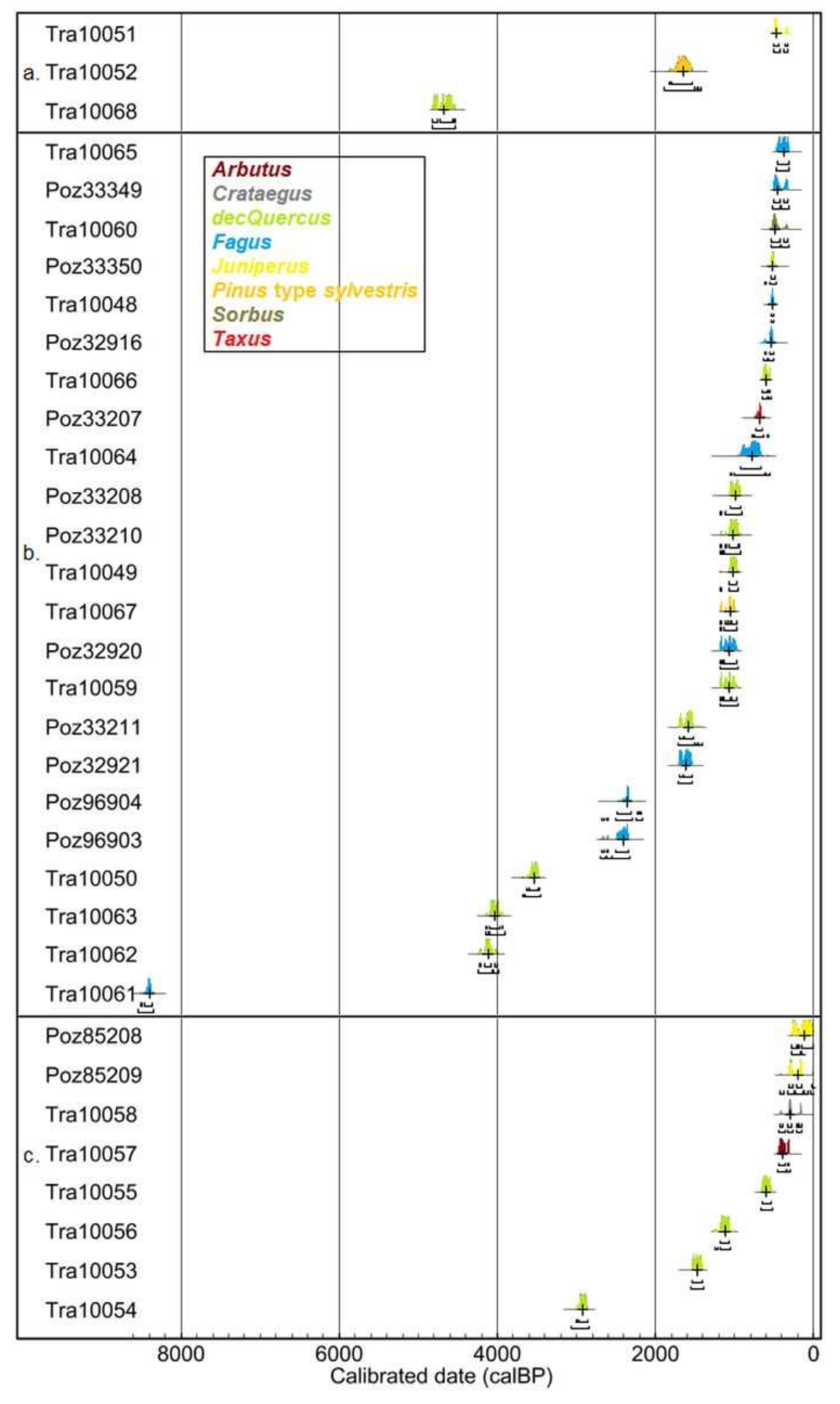
| Units of Investig. | Lab. Ref. | Profile | Taxa | Conv. Age BP ± Error | Cal. Age BP | Cal. Age BCE/CE |
|---|---|---|---|---|---|---|
| Northern plateau | Tra10051 | SBP1 | Juniperus | 389 ± 16 | 504-334 | 1446-1616 CE |
| Tra10052 | SBP1 | Pinus type sylvestris | 1770 ± 59 | 1825-1553 | 125-398 CE | |
| Tra10068 | PDA1 | deciduous Quercus | 4129 ± 21 | 4815-4549 | BCE 2866-2600 | |
| Sainte-Baume forest | Tra10060 | SBA1 | Sorbus | 426 ± 40 | 534-325 | 1416-1625 CE |
| Tra10059 | SBA1 | deciduous Quercus | 1162 ± 26 | 1177-987 | 774-963 CE | |
| Tra10062 | SBA1 | deciduous Quercus | 3755 ± 20 | 4224-4000 | BCE 2275-2051 | |
| Tra10063 | SBA1 | deciduous Quercus | 3692 ± 20 | 4090-3973 | BCE 2141-2024 | |
| Tra10061 | SBA1 | Fagus | 7620 ± 31 | 8509-8372 | BCE 6560-6423 | |
| Tra10065 | SBA3 | Fagus | 333 ± 18 | 465-311 | 1486-1639 CE | |
| Tra10066 | SBA3 | deciduous Quercus | 600 ± 15 | 646-548 | 1304-1403 CE | |
| Tra10064 | SBA3 | Fagus | 851 ± 85 | 930-666 | 1021-1285 CE | |
| Tra10067 | SBA3 | Pinus type sylvestris | 1155 ± 14 | 1174-987 | 776-963 CE | |
| Tra10048 | SBA4 | Fagus | 494 ± 16 | 537-510 | 1414-1441 CE | |
| Tra10049 | SBA4 | deciduous Quercus | 1126 ± 15 | 1062-979 | 889-971 CE | |
| Tra10050 | SBA4 | deciduous Quercus | 3329 ± 23 | 3632-3480 | BCE 1683-1531 | |
| Poz32916 | BAU1 | Fagus | 525 ± 30 | 629-509 | 1322-1442 CE | |
| Poz33207 | BAU1 | Taxus | 750 ± 35 | 733-658 | 1217-1292 CE | |
| Poz33208 | BAU2 | deciduous Quercus | 1085 ± 35 | 1060-932 | 891-1019 CE | |
| Poz32920 | BAU2 | Fagus | 1160 ± 30 | 1177-983 | 773-968 CE | |
| Poz33210 | BAU3 | deciduous Quercus | 1115 ± 35 | 1173-935 | 778-1015 CE | |
| Poz32921 | BAU6 | Fagus | 1725 ± 30 | 1706-1562 | 245-389 CE | |
| Poz96903 | BAU7 | Fagus | 2385 ± 35 | 2680-2342 | BCE 731-393 | |
| Poz33349 | BAU8 | Fagus | 390 ± 30 | 510-320 | 1441-1631 CE | |
| Poz33350 | BAU8 | deciduous Quercus | 480 ± 30 | 543-498 | 1408-1452 CE | |
| Poz33211 | BAU8 | deciduous Quercus | 1690 ± 35 | 1698-1531 | 253-419 CE | |
| Poz96904 | BAU10 | Fagus | 2340 ± 35 | 2485-2213 | BCE 536-264 | |
| Southern exposure | Tra10058 | JDF1 | Crataegus | 249 ± 26 | 426-0 | 1524-0 CE |
| Tra10057 | JDF1 | Arbutus | 309 ± 14 | 432-306 | 1519-1645 CE | |
| Tra10055 | JDF1 | deciduous Quercus | 599 ± 39 | 655-539 | 1295-1411 CE | |
| Tra10056 | JDF1 | deciduous Quercus | 1208 ± 18 | 1219-1066 | 732-884 CE | |
| Poz85209 | JDF2 | Juniperus | 225 ± 30 | 310-0 | 1640-0 CE | |
| Tra10053 | JDF2 | deciduous Quercus | 1608 ± 23 | 1553-1415 | 397-536 CE | |
| Tra10054 | JDF2 | deciduous Quercus | 2821 ± 21 | 2976-2861 | BCE 1027-912 | |
| Poz85208 | COU1 | Juniperus | 120 ± 30 | 272-11 | 1679-1940 CE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robin, V.; Dreibrodt, S.; Talon, B. The Mediterranean Old-Growth Forests: Anomalies or Relicts? The Contribution of Soil Charcoal Analysis. Forests 2021, 12, 1541. https://doi.org/10.3390/f12111541
Robin V, Dreibrodt S, Talon B. The Mediterranean Old-Growth Forests: Anomalies or Relicts? The Contribution of Soil Charcoal Analysis. Forests. 2021; 12(11):1541. https://doi.org/10.3390/f12111541
Chicago/Turabian StyleRobin, Vincent, Stefan Dreibrodt, and Brigitte Talon. 2021. "The Mediterranean Old-Growth Forests: Anomalies or Relicts? The Contribution of Soil Charcoal Analysis" Forests 12, no. 11: 1541. https://doi.org/10.3390/f12111541
APA StyleRobin, V., Dreibrodt, S., & Talon, B. (2021). The Mediterranean Old-Growth Forests: Anomalies or Relicts? The Contribution of Soil Charcoal Analysis. Forests, 12(11), 1541. https://doi.org/10.3390/f12111541






