Abstract
Austroboletusbrunneisquamus (Boletaceae/Boletales), an ectomycorrhizal fungus, is described as a new species from a tropical rainforest in China based on morphological and molecular evidence. It is morphologically characterized by a subtomentose pileal surface when young, which cracks into areolae, having large, pale brown and brown to dark brown scales, a stipe with yellowish brown reticulation, basidiospores measuring (11–)12–14.5(–15) × 6–8(–8.5) μm, with fine cristate to subreticulate ornamentation, and a pileipellis in the form of a cutis. A detailed description, color photographs of fresh basidiomata, and line drawings of microscopic features of the new species are presented.
1. Introduction
Austroboletus (Corner) Wolfe (Boletaceae, Boletales), typified by A. dictyotus (Boedijn) Wolfe, is characterized by a pinkish pore surface at maturity, context usually unchanging in color when injured, a stipe, often with conspicuous reticulation, fusiform or amygdaliform basidiospores with verrucose, warted, irregularly pitted, cristate, subcylindrical, ridged or subreticulate ornamentations, and forming ectomycorrhizae with Fagaceae, Pinaceae and Dipterocarpaceae [1,2,3,4,5]. Species of Austroboletus are mostly distributed in tropical regions [6,7,8,9,10,11]. In China, five taxa of the genus, viz. A. dictyotus, A. fusisporus (Kawam. ex Imazeki & Hongo) Wolfe, A. gracilis (Peck) Wolfe, A. malaccensis (Pat. & C.F. Baker) Wolfe and A. subvirens (Hongo) Wolfe were reported in previous studies [1,12,13,14,15]. Hainan, a tropical area covered mostly by rainforests, is considered as a hotspot of biodiversity, and many new taxa of macrofungi were described from the region in the past [16,17,18,19,20,21,22,23,24,25]. With further investigations, more new taxa are expected to be uncovered. During a fieldtrip to the area, we encountered a few collections of Austroboletus. Subsequent morphological and molecular phylogenetic analyses confirmed that it is different from all known species of Austroboletus, and thus we describe these collections as a new species.
2. Materials and Methods
2.1. Morphological Studies
Collections were described and photographed in the field and deposited in the Fungal Herbarium of Hainan Medical University, Haikou City, Hainan Province of China (FHMU). Color codes are from Kornerup and Wanscher [26]. Sections of the pileipellis were cut radial-perpendicularly and halfway between the center and the margin of the pileus. Sections of the stipitipellis were taken from the middle part along the longitudinal axis of the stipe. As a mounting medium for microscopic studies, 5% KOH was used. All microscopic structures were drawn freehand from rehydrated material. The number of measured basidiospores is given as n/m/p, where n represents the total number of basidiospores measured from m basidiomata of p collections. Dimensions of basidiospores are given as (a–)b–c(–d), where the range b–c represents a minimum of 90% of the measured values (5th to 95th percentile), and extreme values (a and d), whenever present (a < 5th percentile, d > 95th percentile), are in parentheses. Q refers to the length/width ratio of basidiospores; Qm refers to the average Q of basidiospores and is given with a sample standard deviation [19,20]. Basidiospores from dried specimens were also examined with a FEI Quanta 250 (USA) scanning electron microscope (SEM) [27].
2.2. Molecular Procedures
Total genomic DNA was obtained with the Plant Genomic DNA Kit (KANGWEI Company, China) from materials dried with silica gel according to the manufacturer’s instructions. Primer pairs used for amplification were: LR0R/LR5 [28,29] for the nuclear ribosomal large subunit RNA (28S), the nuclear rDNA region encompassing the internal transcribed spacers 1 and 2, along with the 5.8S rDNA (ITS), with ITS5/ITS4 [30], and EF1-2F/EF1-2R [27] for the translation elongation factor 1-α gene (TEF1). PCR was performed in a total volume of 25 μL containing 13 μL 2 × Taq PCR MasterMix (KANGWEI Company, China), 2 μL per primer (10 mM), 2 μL DNA template and 8 μL nuclease-free water. PCR reactions were performed with 4 min initial denaturation at 95°, followed by 35 cycles of denaturation at 94° for 30 s, annealing at an appropriate temperature (50° for 28S and ITS, 53° for TEF1) for 30 s, extension at 72° for 120 s and a final extension at 72° for 7 min. PCR products were checked in 1% (w/v) agarose gels, and positive reactions with a bright single band were purified and directly sequenced using an ABI 3730xl DNA Analyzer (Guangzhou Branch of BGI, China) with the same primers used for PCR amplifications [19,20]. BioEdit [31] was used to compile forward or reverse sequences.
2.3. Dataset Assembly
Five DNA sequences (two of 28S, two of ITS, and one of TEF1) from two specimens were newly generated. Mucilopilus castaneiceps Hongo was chosen as an outgroup following Wu et al. [1]. To test for phylogenetic conflict among the different genes in the combined dataset, the single-gene phylogenetic trees based on 28S, ITS and TEF1, respectively, were analyzed and conducted using the ML method to detect the topologies of genes used. The results of analyses showed that the different gene fragments were not in conflict. Then, the sequences of different genes in the combined dataset were aligned using MUSCLE. The sequences of the different genes were concatenated using Phyutility v. 2.2 for further analyses [19,20] (Table 1).

Table 1.
List of collections used in this study.
2.4. Phylogenetic Analyses
The combined nuclear dataset (28S + ITS + TEF1) was analyzed with maximum likelihood (ML) and Bayesian Inference (BI). Maximum likelihood tree generation and bootstrap analyses were performed with the program RAxML 7.2.6 running 1000 replicates combined with an ML search. Bayesian analysis with MrBayes 3.1 implementing the Markov Chain Monte Carlo (MCMC) technique and parameters predetermined with MrModeltest 2.3 was performed [19,20]. The best-fit likelihood models for the combined dataset were GTR + I + G, GTR + I + G and SYM + G for 28S, ITS and TEF1, respectively. Bayesian analysis of the combined nuclear dataset (28S + ITS + TEF1) was repeated for 10 million generations. Trees sampled from the first 25% of generations were discarded as burn-in, and Bayesian posterior probabilities (PP) were then calculated for a majority consensus tree of the retained Bayesian trees [19,20].
3. Results
3.1. Molecular Data
The combined dataset (28S + ITS + TEF1) included 81 taxa with 2225 nucleotide sites, and the alignment was deposited in TreeBASE (S27641). Bayesian analyses resulted in identical topologies to the ML analysis, while statistical supports showed slight differences. Figure 1 is a branch-length phylogram with the support values, and inferred with RAxML. The molecular phylogenetic analyses show that the collections numbered FHMU5875 and FHMU5876 grouped together with a strong statistical support (BS = 99, PP = 1.0), forming an independent lineage within Austroboletus (Figure 1).

Figure 1.
Phylogram inferred from a combined dataset (28S, ITS and TEF1) using RAxML. RAxML likelihood bootstrap (BS ≥ 50%) and Bayesian posterior probabilities (PP ≥ 0.95) are indicated above or below the branches as RAxML BS/PP.
3.2. Taxonomy
Austroboletus brunneisquamus N.K. Zeng, Chang Xu and S. Jiang, sp. nov. (Figure 2, Figure 3 and Figure 4).

Figure 2.
Basidiomata of Austroboletus brunneisquamus ((a) from FHMU5875; (b,c) from FHMU5876, holotype). Photos by Y.G. Fan.

Figure 3.
Microscopic features of Austroboletus brunneisquamus (FHMU5876, holotype). (a) Basidiospores. (b) Basidia. (c) Cheilocystidia. (d) Pleurocystidia. (e) Pileipellis. (f) Stipitipellis. Bars = 10 μm. Drawings by C. Xu.
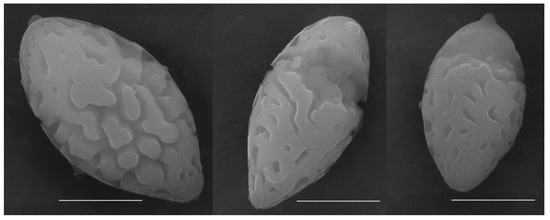
Figure 4.
SEM of basidiospores from a dried specimen of Austroboletus brunneisquamus (FHMU5876, holotype). Bars = 5 μm.
Mycobank: MB838577
Etymology—Latin “brunneisquamus” refers to the pileus of new species displaying distinctive brown scales.
Diagnosis—Characterized by a subtomentose pileal surface when young, which cracks into into areolae, with large, pale brown and brown to dark brown scales, a stipe with yellowish brown reticulation, basidiospores measuring (11–)12–14.5(–15) × 6–8(–8.5) μm, with fine cristate to subreticulate ornamentation, and a cutis in form of pileipellis.
Holotype—CHINA. Hainan Province: Ledong County, Yinggeling of Hainan Tropical Rainforest National Park, 109°17′17″ E, 18°51′6″ N, elev. 650 m, 2 July 2020, N.K. Zeng4294 (FHMU5876). GenBank accession numbers: 28S = MW506829, ITS = MZ855495, TEF1 = MW512637.
Description—Basidiomata: small to medium-sized. Pileus: 3–6.5 cm in diam, subhemispherical when young, then convex to applanate; margin: decurved; surface: dry, subtomentose when young, which cracks into areolae, with large, pale brown (3C7–8) and brown to dark brown (5E7–8) scales; context: 0.5–1.2 cm thick in the center of the pileus, white (1A1), unchanging in color when injured. Hymenophore: poroid, depressed around the stipe; pores: subrounded, white (1A1) when young, then pink (8A2), unchanging in color when injured; tubes: 0.6–1.5cm in length, pinkish (9A2), unchanging in color when injured. Stipe: 4.5–9 × 0.7–1.4 cm, central, subcylindrical, solid; surface: distinctly covered with a yellowish brown (4B5–6) reticulation; context: white (1A1), unchanging in color when injured; basal mycelium: white (1A1). Odor: indistinct.
Basidiospores: [60/3/3] (11–)12–14.5(–15) × 6–8(–8.5) μm, Q = (1.57–)1.60–2.00 (–2.17), Qm = 1.80 ± 0.14 (including ornamentation), [60/3/3] (9–)11–13.5(–14.5) × 4–6 (–6.5) μm, Q = (1.8–)1.92–2.78 (–3.25), Qm = 2.37 ± 0.29 (excluding ornamentation), fusiform or amygdaliform, with a fine cristate to subreticulate ornamentation, pale yellowish brown in KOH. Basidia: 18–26 × 11–15 μm, broadly clavate, thin to slightly thick-walled (up to 0.7 μm), 4-spored, colorless in KOH; sterigmata: 4–6 μm long. Cheilocystidia: 27–54 × 7–14 μm, abundant, fusiform or subfusiform, slightly thick-walled (up to 0.9 μm), colorless in KOH. Pleurocystidia: 27–62 × 4–12 μm, mostly fusiform, occasionally subclavate or subcylindrical, slightly thick-walled (up to 0.9 μm), colorless in KOH. Pileipellis: a cutis up to 315 μm thick, composed of cylindrical hyphae 6.5–13 μm wide, occasionally branched, slightly thick-walled (up to 0.9 μm), colorless in KOH; terminal cells: 44–64 × 6–10.5 μm, subcylindrical, with obtuse apex. Pileal trama: made up of hyphae 4–12 μm in diam, slightly thick-walled (up to 0.9 μm), colorless in KOH. Stipitipellis: a trichoderm-like structure, 70–105 μm thick, composed of slightly thick-walled (up to 0.9 μm) hyphae, 5.5–7.5 μm wide, colorless in KOH; terminal cells: 24.5–53 × 6–6.5 μm, clavate, subclavate or subcylindrical. Stipe trama: composed of parallel hyphae, 4–15 μm wide, cylindrical, slightly thick-walled (up to 0.9 μm), colorless in KOH. Clamp connections: absent in all tissues.
Habitat—solitary on the ground in tropical rainforests dominated by fagaceous trees.
Known distribution—Southern China (Hainan Province).
Additional specimens examined—CHINA. Hainan Province: Ledong County, Yinggeling of Hainan Tropical Rainforest National Park, elev. 650 m, 2 July 2020, N.K.Zeng4291, 4292 (FHMU5874, 5875).
4. Discussion
In the present study, our newly collected specimens were placed into the genus Austroboletus with high statistical support (Figure 1), and morphological features of A. brunneisquamus are highly compatible with the typical characteristics of the genus.
Phylogenetically, A. brunneisquamus is related to A. appendiculatus Semwal, D. Chakr., K. Das, Indoliya, D. Chakrabarty and S. Adhikari & Karun. However, A. appendiculatus, a species originally described from India [42], has a distinctively larger pileus (7.5–9 cm) with a yellowish orange to grayish yellow surface, a longer stipe (8–12 cm), and large basidiospores measuring 14.2–16.5 × 7.3–9.1 μm, and it is associated with Dipterocarpaceae trees [42]. Moreover, A. brunneisquamus is also morphologically similar to A. dictyotus, A. fusisporus and A. subflavidus (Murrill) Wolfe. However, A. dictyotus, originally discovered in Indonesia, has a larger basidioma (pileus up to 11 cm), larger basidiospores measuring (11–)13–16 × (6–)7–8.5 μm with reticulations, and a pileipellis in the form of a trichoderm [1,2]; A. fusisporus, originally discovered in Japan, has a smaller pileus with a viscid surface, large basidiospores measuring 13.5–18.5 × 8–11 μm with subcylindrical ornamentation, and a trichodermium pileipellis [1,43]; A. subflavidus has longer basidiospores measuring 13.1–19.5 × 5.5–8.7 μm with Qm = 2.26, a pileipellis in the form of a trichoderm, and a distribution in North America–Central America–northern South America [5]. Molecular evidence provided in this study also indicated that A. brunneisquamus is genetically distant from A. dictyotus and A. fusisporus, respectively, and it is somewhat related to A. subflavidus, as these two taxa belong to the same clade (not species level) (Figure 1).
Author Contributions
Conceptualization, Z.-Q.L. and N.-K.Z.; Methodology, C.X. and M.-S.S.; Performing the experiment, C.X.; Formal analysis, C.X. and Z.-Q.L.; Resources, N.-K.Z., S.J., Y.C. and Y.-G.F.; Writing—original draft preparation, C.X.; Writing—review and editing, Z.-Q.L. and N.-K.Z.; Supervision, N.-K.Z.; Project administration, N.-K.Z.; Funding acquisition, N.-K.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Hainan Provincial Natural Science Foundation of China (no. 820RC633), and the National Natural Science Foundation of China (nos. 31760008 and 31560005).
Data Availability Statement
The sequence data generated in this study are deposited in NCBI GenBank.
Acknowledgments
The first author is very grateful to Hui-Jing Xie, Xu Zhang, and Yu-Zhuo Zhang, Hainan Medical University, for their help with molecular data analyses, and the forest rangers, Yinggeling Branch of Hainan Tropical Rainforest National Park, China, for their kind help during the field investigations.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Wu, G.; Li, Y.C.; Zhu, X.T.; Zhao, K.; Han, L.H.; Cui, Y.Y.; Li, F.; Xu, J.P.; Yang, Z.L. One hundred noteworthy boletes from China. Fungal Divers. 2016, 81, 25–188. [Google Scholar] [CrossRef]
- Corner, E.J.H. Boletus in Malaysia; Government Printing Office: Singapore, 1972; p. 263.
- Wolfe, C.B. Austroboletus and Tylopilus subgenus. Porphyrellus with emphasis on North American taxa. Bibl. Haematol. 1979, 69, 1–148. [Google Scholar]
- Crous, P.W.; Cowan, D.A.; Maggs-Kölling, G.; Yilmaz, N.; Larsson, E.; Angelini, C.; Brandrud, T.E.; Dearnaley, J.D.W.; Dima, B.; Dovana, F.; et al. Fungal Planet description sheets: 1112–1181 (Austroboletus asper K. Syme, Bonito, T. Lebel, Fechner & Halling, sp. nov.). Persoonia 2020, 45, 251–409. [Google Scholar] [PubMed]
- Gelardi, M.; Angelini, C.; Costanzo, F.; Ercole, E.; Ortiz-Santana, B.; Vizzini, A. Outstanding pinkish brown-spored neotropical boletes: Austroboletus subflavidus and Fistulinella gloeocarpa (Boletaceae, Boletales) from the Dominican Republic. Mycobiology 2020, 49, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Horak, E. Boletellus and Porphyrellus in Papua New Guinea. Kew Bull. 1977, 31, 645–652. [Google Scholar] [CrossRef]
- Fulgenzi, T.D.; Halling, R.E.; Henkel, T.W. Fistulinella cinereoalba sp. nov. and new distribution records for Austroboletus from Guyana. Mycologia 2010, 102, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Vasco-Palacios, A.D.M.; López-Quintero, C.; Franco-Molano, A.E.; Boekhout, T. Austroboletus amazonicus sp. nov. and Fistulinella campinaranae var. scrobiculata, two commonly occurring boletes from a forest dominated by Pseudomonotes tropenbosii (Dipterocarpaceae) in Colombian Amazonia. Mycologia 2014, 106, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Dentinger, B.T.M. Austroboletus olivaceoglutinosus, a new mushroom species from Sikkim, India with a distinctive green, glutinous pileus. Kew Bull. 2015, 70, 15. [Google Scholar] [CrossRef]
- Fechner, N.; Bonito, G.; Bougher, N.L.; Lebel, T.; Halling, R.E. New species of Austroboletus (Boletaceae) in Australia. Mycol. Prog. 2017, 16, 769–775. [Google Scholar] [CrossRef]
- García-Jiménez, J. Austroboletus neotropicalis Singer, J. Garcíay L. D. Gómez (Boletaceae), an interesting fungus associated to montane cloud forest and oak forest in Mexico. Árido-Ciencia 2019, 4, 3–8. [Google Scholar]
- Horak, E. Supplementary remarks to Austroboletus (Corner) Wolfe (Boletaceae). Sydowia 1980, 33, 71–87. [Google Scholar]
- Mao, X.L. The resources of macrofungi from the Mt. Namjagbarwa region in Xizang (Tibet), China. Acta Mycol. Sinica. 1985, 4, 197–207. [Google Scholar]
- Li, T.H.; Song, B. Bolete species known from China. Guizhou Sci. 2003, 21, 78–86. [Google Scholar]
- Hosen, M.I.; Feng, B.; Wu, G.; Zhu, X.T.; Li, Y.C.; Yang, Z.L. Borofutus, a new genus of Boletaceae from tropical Asia: Phylogeny, morphology and taxonomy. Fungal Divers. 2013, 58, 215–226. [Google Scholar] [CrossRef]
- Liang, Z.Q.; An, D.Y.; Jiang, S.; Su, M.S.; Zeng, N.K. Butyriboletus hainanensis (Boletaceae, Boletales), a new species from tropical China. Phytotaxa 2016, 267, 256–262. [Google Scholar] [CrossRef]
- Liang, Z.Q.; Chai, H.; Jiang, S.; Ye, Z.K.; Zeng, N.K. The genus Xanthoconium (Boletaceae, Boletales) in tropical China. Phytotaxa 2017, 295, 246–254. [Google Scholar] [CrossRef]
- An, D.Y.; Liang, Z.Q.; Jiang, S.; Su, M.S.; Zeng, N.K. Cantharellus hainanensis, a new species with a smooth hymenophore from tropical China. Mycoscience 2017, 58, 438–444. [Google Scholar] [CrossRef]
- Zeng, N.K.; Liang, Z.Q.; Tang, L.P.; Li, Y.C.; Yang, Z.L. The genus Pulveroboletus (Boletaceae, Boletales) in China. Mycologia 2017, 109, 422–442. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.K.; Chai, H.; Jiang, S.; Xue, R.; Wang, Y.; Hong, D.; Liang, Z.Q. Retiboletus nigrogriseus and Tengioboletus fujianensis, two new boletes from the south of China. Phytotaxa 2018, 367, 45–54. [Google Scholar] [CrossRef]
- Chai, H.; Liang, Z.Q.; Jiang, S.; Fu, X.L.; Zeng, N.K. Lanmaoa rubriceps, a new bolete from tropical China. Phytotaxa 2018, 347, 71–80. [Google Scholar] [CrossRef]
- Chai, H.; Liang, Z.Q.; Xue, R.; Jiang, S.; Luo, S.H.; Wang, Y.; Wu, L.L.; Tang, L.P.; Chen, Y.; Hong, D.; et al. New and noteworthy boletes from subtropical and tropical China. MycoKeys 2019, 46, 55–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, R.; Wu, L.L.; Jiang, S.; Hao, Y.J.; Chai, H.; Liang, Z.Q.; Zeng, N.K.; Su, M.S. Two new species of the genus Leccinellum (Boletaceae, Boletales) from the south of China. Phytotaxa 2019, 411, 93–104. [Google Scholar] [CrossRef]
- Wang, Y.; Su, M.S.; Jiang, S.; Xue, R.; Wu, L.L.; Xie, H.J.; Zhang, Y.Z.; Liang, Z.Q.; Zeng, N.K. The genus Hourangia in China and a description of Aureoboletus erythraeus sp. nov. Phytotaxa 2020, 472, 87–106. [Google Scholar] [CrossRef]
- Xie, H.J.; Lin, W.F.; Jiang, S.; Xue, R.; Wu, L.L.; Zhang, Y.Z.; Liang, Z.Q.; Zeng, N.K.; Su, M.S. Two new species of Hortiboletus (Boletaceae, Boletales) from China. Mycol. Prog. 2020, 19, 1377–1386. [Google Scholar] [CrossRef]
- Kornerup, A.; Wanscher, J.H. Taschenlexikon der Farben. 3. Aufl.; Muster-Schmidt Verlag: Göttingen, Germany, 1981; p. 242. [Google Scholar]
- Zeng, N.K.; Tang, L.P.; Li, Y.C.; Tolgor, B.; Zhu, X.T.; Zhao, Q.; Yang, Z.L. The genus Phylloporus (Boletaceae, Boletales) from China: Morphological and multilocus DNA sequence analyses. Fungal Divers. 2013, 58, 73–101. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, T.Y.; Kauff, F.; Schoch, C.; Matheny, P.B.; Hofstetter, V.; Cox, C.; Celio, G.; Gueidan, C.; Fraker, E.; Miadlikowska, J.; et al. Reconstructing the early evolution of the fungi using a six gene phylogeny. Nature 2006, 443, 818–822. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenies. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analyses program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Wu, G.; Feng, B.; Xu, J.P.; Zhu, X.T.; Li, Y.C.; Zeng, N.K.; Hosen, M.I.; Yang, Z.L. Molecular phylogenetic analyses redefine seven major clades and reveal 22 new generic clades in the fungal family Boletaceae. Fungal Divers. 2014, 69, 93–115. [Google Scholar] [CrossRef]
- Magnago, A.C.; Neves, M.A.; Silveira, R.M.B.D. Fistulinella ruschii sp. nov. and a new record of Fistulinella campinaranae var. scrobiculata for the Atlantic Forest, Brazil. Mycologia 2018, 109, 1003–1013. [Google Scholar]
- Binder, M.; Hibbett, D.S. Molecular systematics and biological diversification of Boletales. Mycologia 2006, 98, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Halling, R.E.; Nuhn, M.; Osmundson, T.; Fechner, N.; Trappe, J.M.; Soytong, K.; Arora, D.; Hibbett, D.S.; Binder, M. Affinities of the Boletus chromapes group to Royoungia and the description of two new genera, Harrya and Australopilus. Aust. Syst. Bot. 2012, 25, 418–431. [Google Scholar] [CrossRef]
- Drehmel, D.; James, T.; Vilgalys, R. Molecular phylogeny and biodiversity of the boletes. Fungi 2008, 1, 17–23. [Google Scholar]
- Smith, M.E.; Henkel, T.W.; Aime, M.C.; Fremier, A.K.; Vilgalys, R. Ectomycorrhizal fungal diversity and community structure on three co-occurring leguminous canopy tree species in a neotropical rainforest. New Phytol. 2011, 192, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Vadthanarat, S.; Lumyong, S.; Raspé, O. Cacaoporus, a new Boletaceae genus, with two new species from Thailand. MycoKeys 2019, 54, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Carriconde, F.; Gardes, M.; Bellanger, J.M.; Letellier, K.; Gigante, S.; Gourmelon, V.; Thomas Ibanez, T.; McCoy, S.; Goxe, J.; Read, J.; et al. Host effects in high ectomycorrhizal diversity tropical rainforests on ultramafic soils in New Caledonia. Fungal Ecol. 2019, 39, 201–212. [Google Scholar] [CrossRef]
- Kuo, M.; Ortiz-Santana, B. Revision of leccinoid fungi, with emphasis on North American taxa, based on molecular and morphological data. Mycologia 2020, 112, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Orihara, T.; Smith, M.E.; Shimomura, N.; Iwase, K.; Maekawa, N. Diversity and systematics of the sequestrate genus Octaviania in Japan: Two new subgenera and eleven new species. Persoonia 2012, 28, 85–112. [Google Scholar] [CrossRef] [PubMed]
- Tibpromma, S.; Hyde, K.D.; Jeewon, R.; Maharachchikumbura, S.S.N.; Liu, J.K.; Bhat, D.J.; Jones, E.B.G.; McKenzie, E.H.C.; Camporesi, E.; Bulgakov, T.S.; et al. Fungal diversity notes 491–602: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2017, 83, 184–190. [Google Scholar] [CrossRef]
- Hongo, T. The Agaricales of Japan 1–2. Rhodophyllaceae, Paxillaceae, Gomphidiaceae, Boletaceae and Strobilomycetaceae. Acta Phytotax. Geobot. 1960, 18, 97–112. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).