Abstract
Drought is one of the most devastating climate factors in terms of its spatial extent and intensity. Therefore, a study was conducted to evaluate the water stress tolerance in young saplings of Syzygium cumini (L.) Skeels and Populus deltoides Marchall that are cultivated in the rain fed areas of Pakistan. Plants were subjected to three levels of moisture regimes: well-watered (WW, 90% of field capacity), mild stress (MS, 60% field capacity), and severe stress (SS, 30% of field capacity). Results showed that dry biomass production (leaf, stem, and root), chlorophyll a, b and carotenoid contents decreased significantly while osmolyte accumulation increased in both species, with the highest increase was evidenced in Populus deltoides saplings. A significant decrease was evidenced in CO2 assimilation rate and stomatal conductance that resulted in a significant increase in intrinsic water use efficiency in both species under MS and SS. In both the species, along with a significant increase in the production of hydrogen peroxide and superoxide radical, the antioxidants enzyme activities of superoxide dismutase, peroxidase, catalase, and ascorbate peroxidase also increased significantly in both species under MS and SS with highest activity evidenced in Syzygium cumini. The results suggest that Syzygium cumini saplings showed better a tolerance mechanism to water stress.
1. Introduction
Climatic extremes have resulted in a global fluctuation in water availability and increase in evaporative demand [1]. According to the Intergovernmental Panel on Climate Change (IPCC), average temperature is expected to rise by 1.8–4.0 °C in 2100 and drought would takeover numerous regions of the world [2]. The poor irrigation and irregular rainfalls in the rain fed areas lead has to inadequate water availability [3]. It has been estimated that 36% of the global area falls under arid to semi-arid climate where annual precipitation is between 50–150 mm [4]. South Asia is ranked 5th among the severely affected regions of the world due to climate change [5]. Pakistan is an agricultural country; however, most of the country falls under arid to semi-arid climate [6,7], with insufficient rainfall for growing of crops and trees. In Pakistan annual precipitation is between 150–250 mm, and the country is facing a persistent problem of water shortage [8]. The Global Climate Risk Index (GCRI) has graded Pakistan in the top 10 countries that are being adversely affected by climate change since October 2020. It has been projected that dry climate will accentuate water stress in the cultivated areas and the supply of irrigation water for crop growth [9]. By the end of the 21st century, drought spells are predicted to increase because of the forecasted increasing in the temperature [10]. Environmental stresses frequently affect plant growth and development. Plants are overstressed by biotic (viruses, insects, bacteria, fungi,) and abiotic (drought, salinity, temperature, light) stress factors that stimulate a negative impact on the productivity of agriculture and forestry system [11]. Among many abiotic factors, drought is a one of the most important hazards and is posing a challenge to plant survival in the arid and semi-arid areas of the world [12]. Drought stress adversely effects the plant growth and development in terms of decrease in plant height, stem diameter, number of leaves and total biomass [13]. Several disparaging effects in plants species have been observed due to drought with undesirable affects on plant functions, for example decreased turgor pressure, increased oxidative damage, changes in leaf gas exchange parameters, decrease in chlorophyll content and disruption of hormonal balance [14]. Such changes induce variation at cellular level and results in the inhibition of cell growth and development [13,15,16]. To sustain the cell turgor pressure and to allow cells mitigate the harmful effects of drought stress, plants increase the accumulation of proline, soluble sugar, total phenolic contents and other osmolytes [17]. Moreover, decrease in stomatal conductance under water deficit that may lead to CO2 starvation under sever water stress condition has been reported [15,16]. Enhanced the production of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), singlet oxygen (1O2), superoxide radical (O2−) and hydroxyl radicals (OH) has also been observed under water stress which is harmful to living tissues and macromolecules like DNA proteins, and lipids [18]. Plants scavenge the overproduction of ROS by increasing the production of antioxidant enzymes like superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and ascorbate peroxidase (APX) [19,20]. Numerous studies have reported that the balance between the production of ROS and antioxidant enzyme may help to determine the plants tolerance to various types of abiotic stress [14].
Populus species and hybrids have high economic value and are cultivated worldwide [21]. The fast-growing poplar genotypes are generally vulnerable to drought stress owing to high water requirements [22]. On the other hand, poplars are also acknowledged as significant contributor to carbon sequestration. It has been anticipated that due to climate change poplar plantations would experience intensified drought spells that could significantly cutback the overall productivity [23,24]. Moreover, Jamun (Syzygium cumini) is a large fruit tree which is native to South Asia and is also cultivated in Hawaii since 1871. It can tolerate low levels of salinity and can endure drought which makes it a species of interest to grow in different climatic regions [25,26]. Madani [27] has also documented that fruit (black plum) of Syzygium cumini contains higher concentrations of phenolics and antioxidants compound that are good for human health. Therefore, the current experiment was designed to study various morphological, physiological, and biochemical traits and to elucidate the relationship among these factors that derives the water stress tolerance in these two species.
2. Materials and Methods
2.1. Plant Material and Treatments
The pot experiment was laid out under completely randomized design (CRD) in the green house at the Forestry nursery (31°44′02.20″ N, 73°05′50.15″ W), University of Agriculture, Faisalabad, Pakistan. Maximum and minimum temperature in the green house was 25 ± 5 °C and the relative humidity was at 55 ± 5% throughout the experiment. 4–5 months old, healthy, and undamaged saplings (60 saplings, 10 saplings/treatment) of Syzygium cumini (L.) Skeels and Populus deltoides Marchall were grown in plastic pots (25 × 35 cm and weight 270 ± 5 g) filled with 9 kg mixture of sandy loam soil and farmyard manure (3:1; electric conductivity 2.5 dS m−1), nitrogen (0.78%), phosphorus (12 ppm), organic matter (8%) and pH (6.6). To optimize the nutrients deficiency, NPK fertilizer (15% N; 5% P2O5; 5% K2O) was added at the rate of 5 g/kg of soil. Five soil cores of 100 g were taken randomly and dried in a heated oven to calculate soil moisture contents at field capacity which was 28.6 g. Data of soil moisture contents were calculate the reference pot weight at 90%, 60% and 30% of field capacity, which was 8742 g, 7970 g and 7198 g, respectively. Three level of water stress were maintained: well-watered (WW, 90% of field capacity), mild stress (MS, 60% of field capacity) and severe stress (SS, 30% of field capacity) Rasheed et al. [18]. Every pot was watered back daily to the reference weight by weighing the pots and adding the amount of water lost during evapotranspiration. The duration of the experiment was 90 days.
2.2. Growth and Dry Biomass Traits Measurements
Plant height (cm), stem diameter (mm) was measured during the experiment. At the end of the experiment all plants were uprooted and separated into various plants section (leaves, stem, and roots), placed into paper bags, and dried at 70 °C for 72 h in oven DGH-9202 Series, Thermal Electric Thermostat drying oven). Dry weight of all the plant section (leaves, stem, and roots) was used to calculate root/shoot ratio (R:S) and total dry biomass Zafar et al. [15].
2.3. Chlorophyll a, b, Carotenoid Contents and Leaf Gas Exchange Measurements
Chlorophyll a, b and carotenoid content were measured from the healthy and undamaged leaves from each saplings using 80% acetone, following the protocol of Arnon [28]. CO2 assimilation rate (Ar, μmol CO2 m−2 s−1) and stomatal conductance (gs, mol m−2 s−1) were determined by using the portable infrared gas analyzer (CIRAS-3 Amesbury, USA). Mature and undamaged leaves was selected before the final harvesting of the experiment. All measurement were taken between the 10:00 am to 12:00 pm. Intrinsic water use efficiency (WUEi, μmol mol−1) was determined as the ratio of net CO2 assimilation rate and stomatal conductance following Rasheed et al. [29].
2.4. Determination of Proline, Soluble Sugar, Total Phenolic Content and Total Soluble Protein
The proline content was measured in leaves sample by using the ninhydrine method Bates et al. [30]. Total phenolic content (TPC) was determined according to the protocol of Ainsworth and Gillespie [31]. Soluble protein was measured following the method of Bradford [32] and soluble sugar was calculated by using the Anthrone method demonstrated by Yemm and Willis [33].
2.5. Determination of Malondialdehyde Contents (MDA) and Electrolyte Leakage (EL %)
Lipid peroxidation was determined in the form of malondialdehyde (MDA) content as the protocol described by Hodges [34]. Electrolyte leakage (EL %) was estimated as demonstrated by Nayyar [35]. 0.2 g samples were rinsed in tubes with 20 mL of deionized water and the tubes were placed in water bath at 32 °C. After two hours the initial electrical conductivity (ECi) calculated with digital EC meter (Model DJS-1C Model DJS-1C; Shanghai Analytical Instrument Co. Shanghai, China). Then all the samples were heated at 120 °C for 120 min, then the samples were cooled at room temperature and final electrical conductivity (ECf) was measured.
(EL %) = (ECi/ECf) × 100
2.6. Production of Reactive Oxygen Species (ROS) and Antioxidant Enzyme Activity
The production of hydrogen peroxide (H2O2) in the leaf samples were measured as demonstrated by Velikova [36] and the production superoxide radical (O2−) was estimated according to the Bai [37] Different antioxidant enzyme activity such as superoxide dismutase (SOD) was estimated by photochemical reduction of NBT (nitroblue tetrazolium) as demonstrated by Bayer [38]. Peroxidase (POD) enzyme activity was measured as demonstrated by Maehly and Chance [39]. The Catalase (CAT) activity was estimated according to the Knörzer et al., [40] and ascorbate peroxidase (APX) activity was determined according to the Nakano and Asada [41].
2.7. Data Analysis
All the data corresponding to growth attributes, biomass production, chlorophyll a, b, carotenoids contents, osmolytes accumulation, oxidants and antioxidants activity were analyzed using the two-way ANOVA for species effect (S-effect), treatments (T-effect) and their interaction (S × T effect). Significant difference between treatment means were compared with respect to their control using Dunnett’s test. All means are presented with their standard error (±SE) and all tests were taken significant at p < 0.05.
3. Results
3.1. Effect of Water Deficit on Growth and Dry Biomass
Water deficit significantly decreased the growth parameter and dry biomass production of both species Syzygium cumini and Populus deltoides saplings. Plant height and stem diameter in both species decreased under MS and SS treatment (Table 1) with the highest decrease evidenced in Populus deltoides saplings. R:S ratio increased significantly in Syzygium cumini saplings under MS and SS treatments but decreased significantly in Populus deltoides.

Table 1.
Growth parameter plant height, stem diameter and R:S ratio under various level of water deficit treatments in Syzygium cumini and Populus deltoides. The traits were tested using a two-way ANOVA for species effect (S-effects), treatment effect (T-effects) and interaction effects (S × T). Values represent the means (±SE) in various treatments. Small letters represent significant difference between treatments within each species tested using the Dunnett’s test. All tests were taken to be significant at p < 0.05.
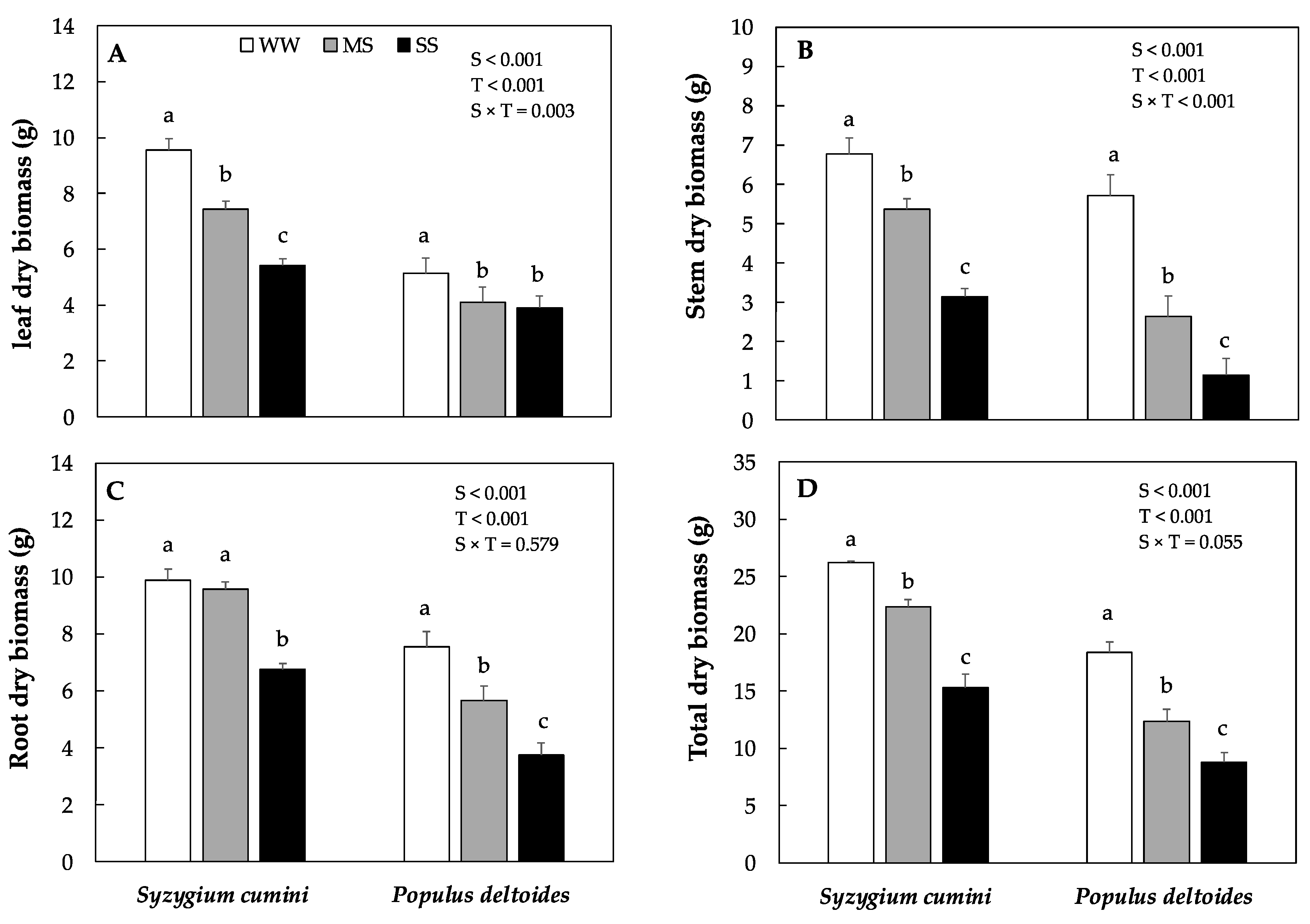
Leaf, stem, and total dry biomass decreased significantly in Syzygium cumini and Populus deltoides saplings under both MS and SS treatment, respectively. However, the highest was evidenced in Populus deltoides. In Populus deltoides, leaves, stem, root, and total dry biomass significantly decrease by 23%, 79%, 50% and 52% under SS as compared to WW treatment, respectively (Figure 1A–D). In Syzygium cumini, root dry biomass remained similar to WW under MS treatment but decreased significantly by 31% under HS.
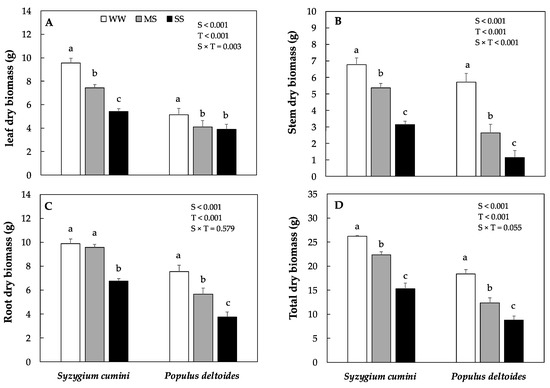
Figure 1.
Means dry biomass production of in leaf dry biomass (A), stem dry biomass (B), root dry biomass (C), and total dry biomass (D) under different level of water deficit treatment in Syzygium cumini and Populus deltoides. The traits were tested using a two-way ANOVA for species effect (S-effects), treatment effect (T-effects) and interaction effects (S × T). Values represent the means (±SE) in various treatments. Small letters represent significant difference between treatments within each species tested using the Dunnett’s test. All the tests were taken to be significant at p < 0.05.
3.2. Effect of Water Deficit on Chlorophyll a, b, Carotenoid and Gas Exchange
Chlorophyll a, b and carotenoid contents significant decrease significant under both MS and SS in Populus deltoides and Syzygium cumini (Table 2). The highest decrease was evidenced in Populus deltoides saplings. Water stress significantly decreased the CO2 assimilation rate (Ar) and stomatal conductance (gs) in both Syzygium cumini and Populus deltoides saplings under MS and SS treatment (Table 2). Under MS and SS treatment the Ar significantly decrease by 14% and 27% in Populus deltoides saplings as compared to WW, respectively. Under MS and HS treatment, gs in Syzygium cumini and Populus deltoides saplings also significantly decrease by 14%, 28%, 15% and 42%, respectively as compared to WW treatment respectively. The WUEi significantly increase under MS and SS treatment in both species however, the highest increase was evidenced in Syzygium cumini saplings. Under MS and SS, WUEi increased by 14% and 20% in Syzygium cumini saplings as compared to WW.

Table 2.
Chlorophyll a, b, carotenoid contents and gas exchange parameters (CO2 assimilation rate (Ar), stomatal conductance (gs) and water use efficiency (WUEi)) under various level of water deficit treatments in Syzygium cumini and Populus deltoides. The traits were tested using a two-way ANOVA for species effect (S-effects), treatment effect (T-effects) and interaction effects (S × T). Values represent the means (±SE) in various treatments. Small letters represent significant difference between treatments within each species tested using the Dunnett’s test. All tests were taken to be significant at p < 0.05.
3.3. Effect of Water Deficit on Osmolytes Accumulation
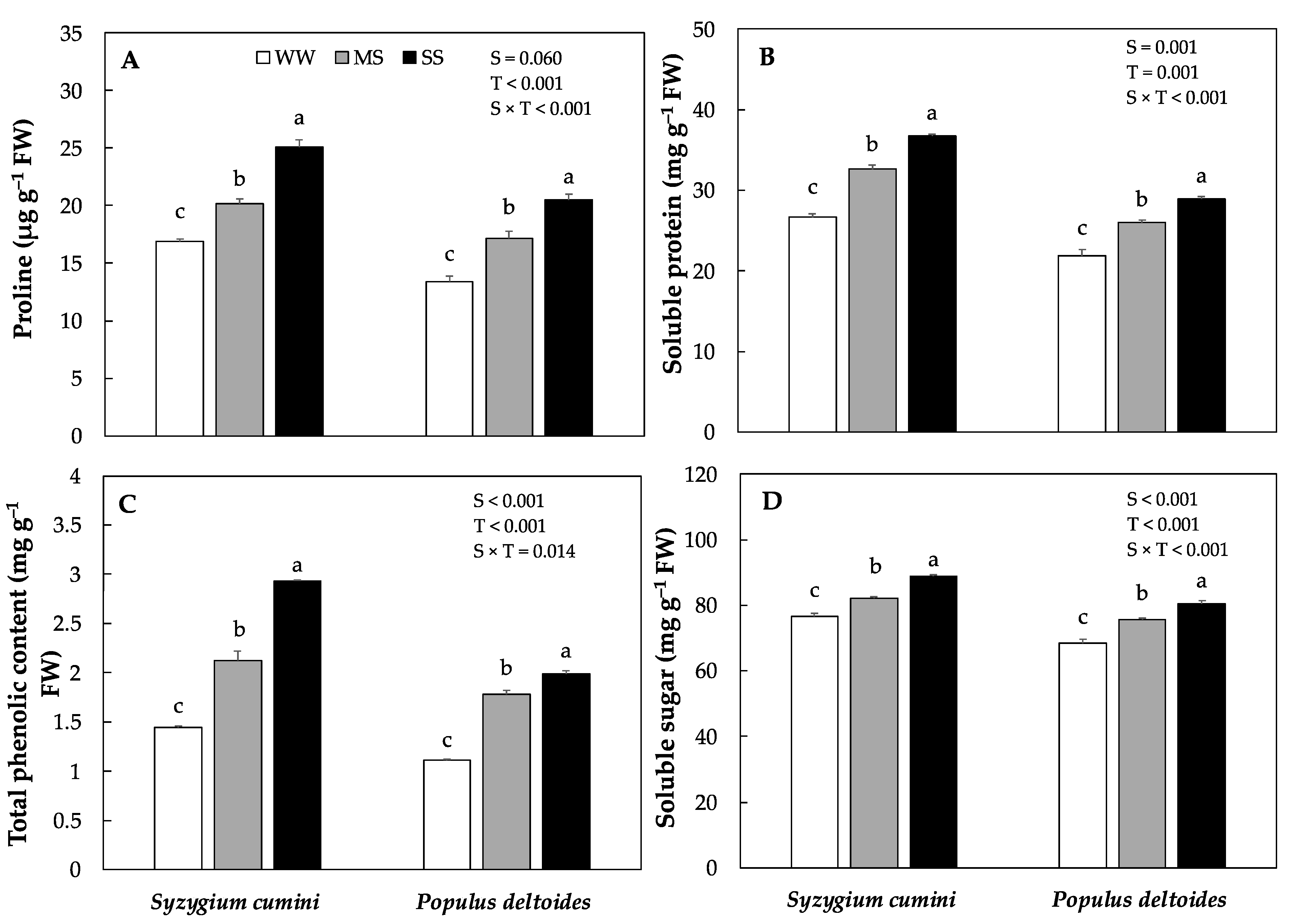
Water stress significantly increased the osmolytes accumulation in both the species under MS and SS treatment (Figure 2A). However, the highest was evidenced in Syzygium cumini. Proline content significantly increased by 19% and 48% in Syzygium cumini saplings under MS and SS treatments, respectively as compared to WW. The total phenolic content and soluble protein increased significantly in both species under MS and SS, the highest was evidenced in Syzygium cumini saplings, respectively (Figure 2B,C). Under MS and SS, the total phenolic content and soluble protein increased significantly by 47%, 103%, 60%, 79%, 22%, 37% and 18% and 31% in Syzygium cumini and Populus deltoides, respectively as compared to WW. The soluble sugar also increased significantly in both species under both water stress treatment, however the highest value was evidenced in Syzygium cumini saplings.
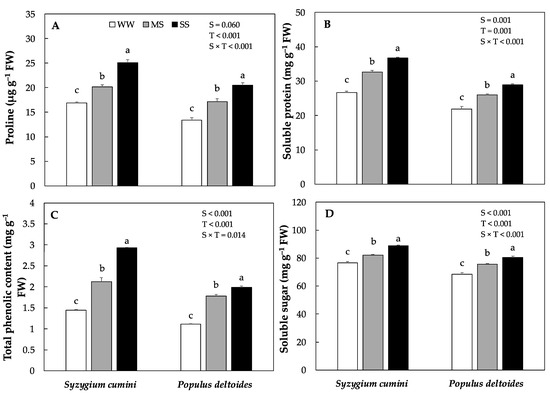
Figure 2.
Means of various concentration of Osmolytes (A) proline, (B) soluble protein, (C) total phenolic content and (D) soluble sugar under various level of water deficit treatments in Syzygium cumini and Populus deltoides. The traits were tested using a two-way ANOVA for species effect (S-effects), treatment effect (T-effects) and interaction effects (S × T). Values represent the means (±SE) in various treatments. Small letters represent significant difference between treatments within each species tested using the Dunnett’s test. All tests were taken to be significant at p < 0.05.
3.4. Effect of Water Deficit on the Production of Reactive Oxygen Species, (MDA) and Electrolyte Leakage (EL %)
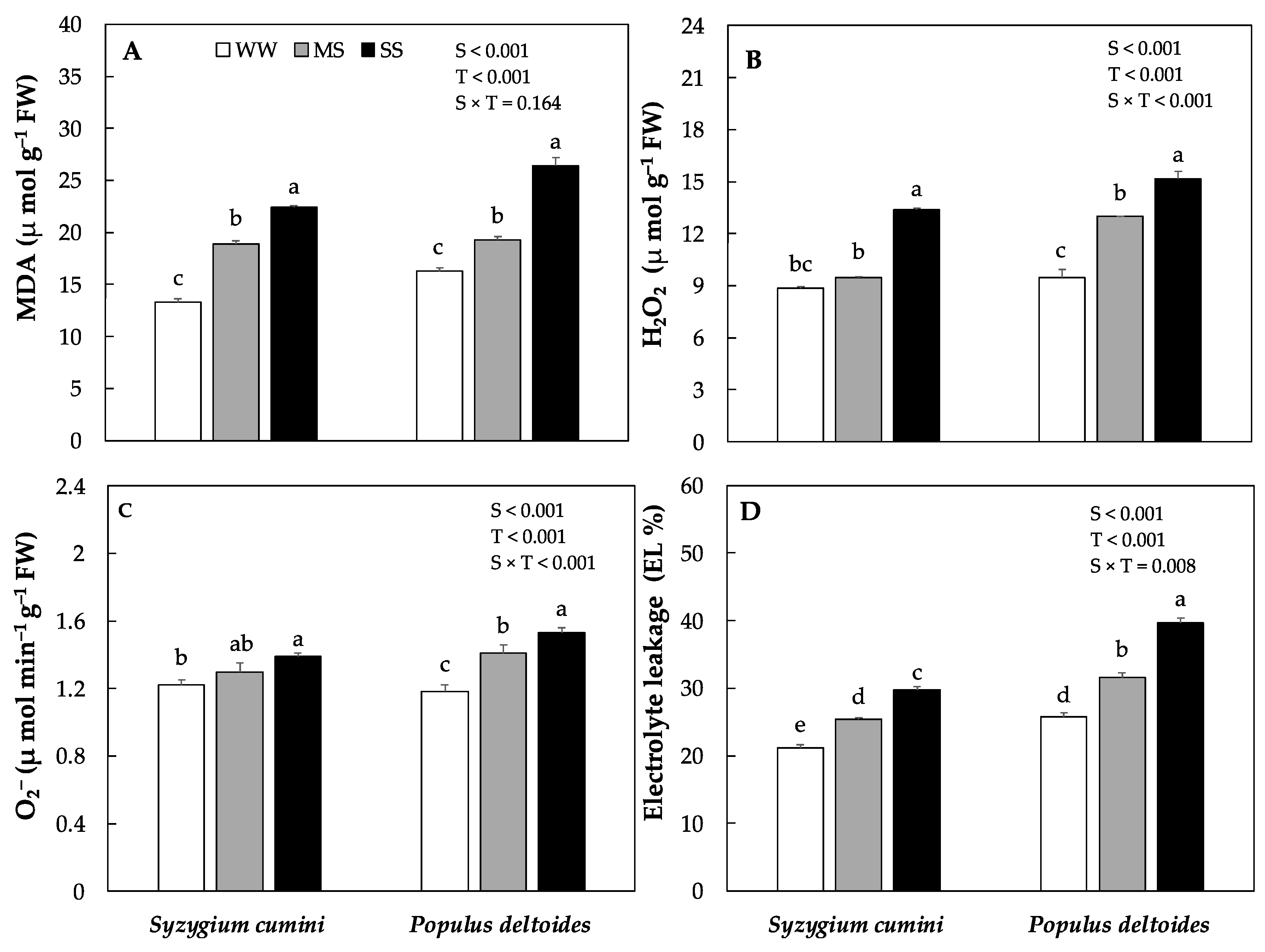
The production of reactive oxygen species ROS such as hydrogen peroxide (H2O2) and superoxide radical (O2−) along with MDA and electrolyte leakage (EL %) varied significantly between the species and treatments. In Syzygium cumini saplings the production of H2O2, O2−, MDA and EL% significantly increased by 6%, 6%, 42% and 20% under MS and by 50%, 13%, 68% and 41%, respectively under HS as compared to WW (Figure 3A–D). Similarly, in Populus deltoides saplings the production of H2O2, O2−, MDA and EL% significantly increased by under MS and SS treatment however, the highest values were evidenced under SS treatments.
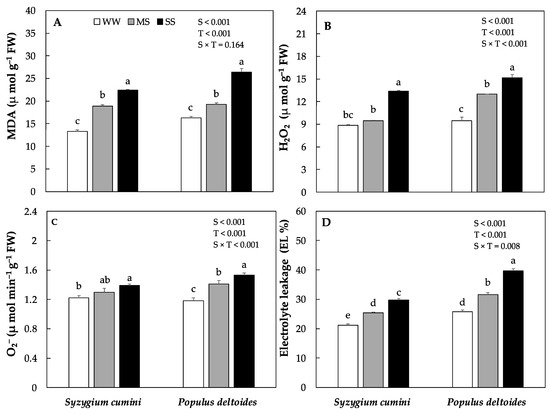
Figure 3.
Concentration of malondialdehyde contents, MDA, (A), various oxidants such as hydrogen peroxide, H2O2, (B) and superoxide radical O2− (C), along with Electrolyte leakage, EL% (D) under various level of water deficit treatments in Syzygium cumini and Populus deltoides. The traits were tested using a two-way ANOVA for species effect (S-effects), treatment effect (T-effects) and interaction effects (S × T). Values represent the means (±SE) in various treatments. Small letters represent significant difference between treatments within each species tested using the Dunnett’s test. All tests were taken to be significant at p < 0.05.
3.5. Effect of Water Deficit on Antioxidant Enzyme Activity
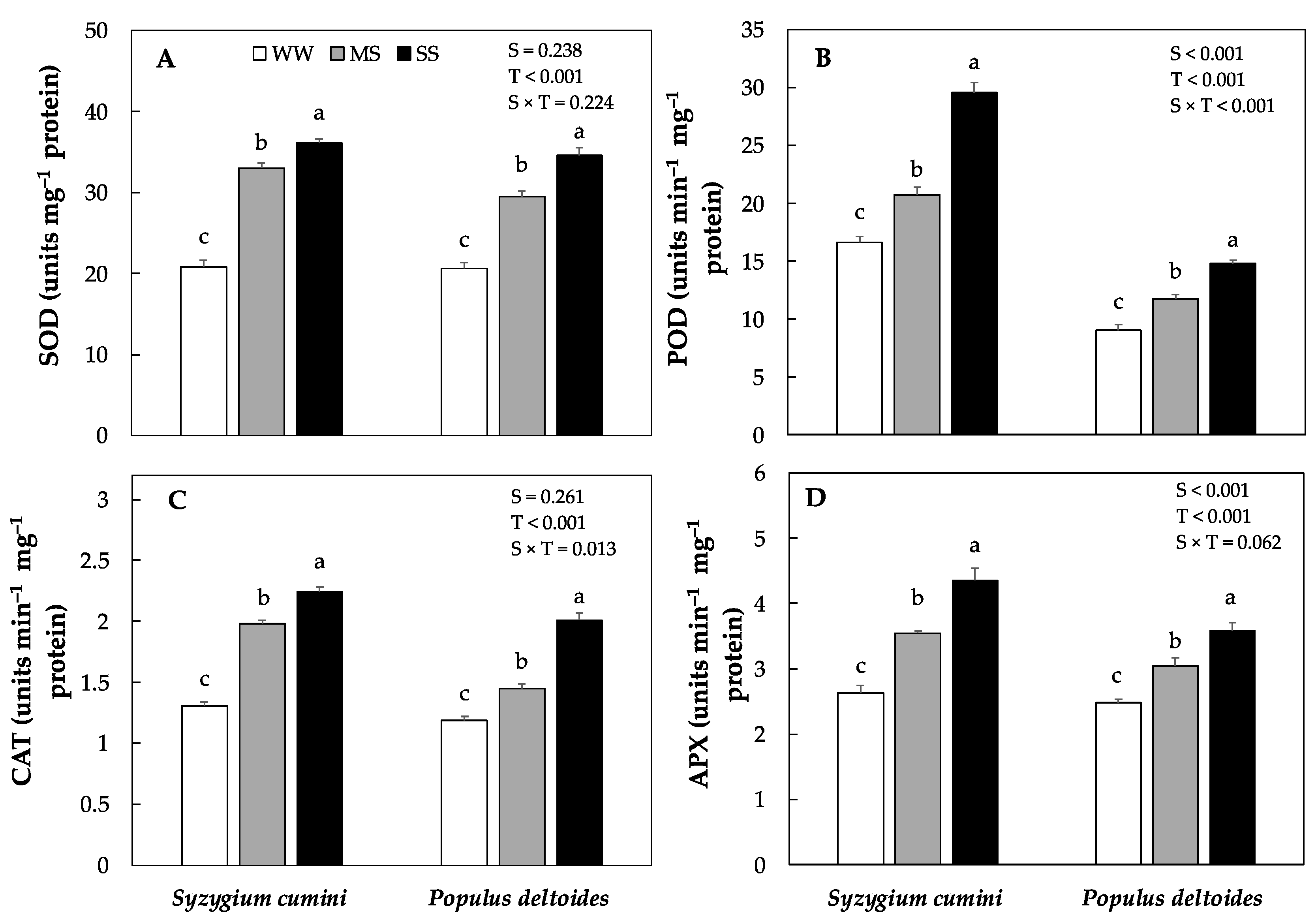
The activity of antioxidants enzymes such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and ascorbate peroxidase (APX) varied significantly between the species as well as across the treatments (Figure 4A–D). The highest antioxidants enzyme activity was evidenced in Syzygium cumini as compared to Populus deltoides saplings. In Syzygium cumini saplings the activity of antioxidants enzyme such as SOD, POD, CAT and APX progressively increased under MS and SS treatments. However, the highest increase was evidenced under SS (73%, 78%, 70% and 65%) as compared to WW. Similarly, in Populus deltoides saplings the antioxidants enzyme activity (SOD, POD, CAT and APX) increased significantly under MS and SS treatments respectively. As compared to WW, the antioxidants enzyme activity increased by (43%, 30%, 21% and 22%) under MS and (67%, 64%, 68% and 44%) under SS treatments.
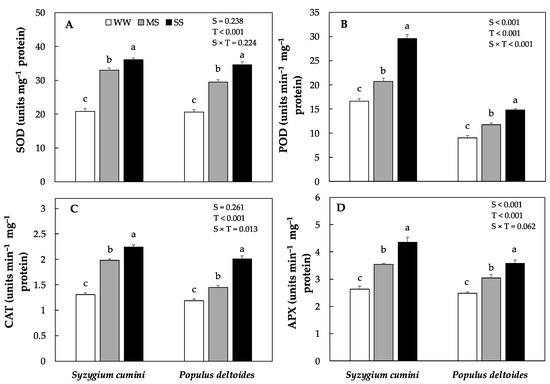
Figure 4.
Means of various antioxidants enzyme concentration (A) superoxide dismutase, (SOD), (B) peroxidase, (POD), (C) catalase, and (D) ascorbate peroxidase, (APX), under various level of water deficit treatments in Syzygium cumini and Populus deltoides. The traits were tested using a two-way ANOVA for species effect (S-effects), treatment effect (T-effects) and interaction effects (S × T). Values represent the means (±SE) in various treatments. Small letters represent significant difference between treatments within each species tested using the Dunnett’s test. All tests were taken to be significant at p < 0.05.
4. Discussion
In this study the water stress tolerance strategy to two important tree species of arid and semiarid environment was analyzed. Generally, plant growth is considered as the first morphological parameter that is negatively affected under water stress [20,42,43]. In the present study, growth traits revealed a variable response of the two species to water deficit. Considering the growth traits (plant height, stem diameter, leaf, stem, root, and total dry biomass production) as an indicator trait for water stress tolerance, Syzygium cumini saplings was found more resistant to water stress than Populus deltoides saplings (Table 1). Although both species showed a significant decrease in all the growth traits (stem diameter, plant height, leaf, stem root and total biomass), but the relative decrease in plant growth as compared to control was higher in Populus deltoides showing a superior performance of Syzygium cumini under MS and SS (Figure 1). Studies have shown that such decrease in plant growth is related to the inhibition of cell elongation which is mediated by an interlinked process where water uptake by the roots decrease, resulting in a decreased plant water potential and reduction in water transpired by the leaves through stomata [43,44]. Our findings agree with the previous studies on different tree species (Ficus, Syzygium cumini, Eucalyptus globulus, Conocarpus erectus, Populus deltoides, Betula alleghaniensis, Populus tremula and Betula pendula) where a significant reduction on growth and biomass production has been reported under water deficit condition [13,15,18,20,45,46]. R:S ratio is also considered as an important trait in determining the species tolerance to water stress [47]. Studies have demonstrated that an increase in R:S ratio under water stress condition indicates a greater investment of assimilated carbon towards the root development [48,49]. Therefore, increased R:S ratio depicts an increased capability of plants to absorb water from soil under water tress condition [18]. In this study although root biomass decreased in Syzygium cumini and Populus deltoides however, the relative decrease in root biomass in Syzygium cumini was lower than Populus deltoides. Resultantly, Syzygium cumini showed significant increase in R:S ratio under MS and SS. Based on the results, the superior performance of Syzygium cumini as compared to Populus deltoides may have been mediated by the increase in R:S ratio under MS and SS (Table 1). Similar results have been reported previously in Conocarpus erectus, Ficus benjamina, Syzygium cumini, Morus alba, Conocarpus erectus and Ziziphus nummularia saplings [13,15,20,50] under water stress treatment where a significant increase in R:S ratio was related to the increased stress tolerance. In plant species the water stress induces adjustments in the leaf gas exchange parameters which is mainly associated with the decrease in stomatal conductance and photosynthetic activity [51,52]. In the present experiment the CO2 assimilation rate decreased in both the species under water stress however, a greater decrease was evidenced in Populus deltoides as compared to Syzygium cumini saplings. Decrease in CO2 assimilation is mainly controlled by the stomatal aperture which allows the CO2 to diffuse in the intercellular spaces and fixed by rubisco through carboxylation [44,53]. In this study although the stomatal conductance decreased significantly in both the species under MS and SS, but the relative decrease was lower in Syzygium cumini as compared to Populus deltoides (Table 2). These results showed a high stomatal sensitivity in both Syzygium cumini and Populus deltoides even low water stress. Studies have reported that CO2 assimilation rate is highly resistant to abiotic stresses and that the water stress must be very high to negatively affect the CO2 assimilation rate [29]. As chlorophyll a and b content also decreased significantly in Syzygium cumini and Populus deltoides under MS and HS (Table 2), therefore, we argued that the observed decrease in CO2 assimilation rate might be related to the overall decrease in photosynthetic capacity rather to the decrease in stomatal conductance. These results agree with the previous studies where decrease in CO2 assimilation rate has been related to both decrease in chlorophyll contents and stomatal conductance in Arabidopsis [54], Populus euphratica [52], Torreya grandis [49], Betula alleghaniensis [18], Portulaca oleracea [55] under water deficit treatments. Increase in intrinsic water use efficiency (WUEi) is also considered an important physiological trait in determining drought tolerance index of a species [29]. Variation in WUEi depends upon the relative variation in both CO2 assimilation rate and stomatal conductance. In this study, WUEi increased in both the species under water deficit but the highest WUEi was evidenced in Populus deltoides saplings under SS condition. Lower WUEi in Syzygium cumini under both MS and SS shows that the decrease in stomatal conductance was lower in Syzygium cumini than in Populus deltoides that resulted in higher value of WUEi in Syzygium cumini under both MS and SS conditions. Presents study also confirms the hypothesis that productivity in Populus deltoides is primarily controlled by stomatal conductance which is closely related to water availability. Based on the results, it can be concluded that Syzygium cumini saplings were able to regulate the stomatal conductance under MS due to increase investment of assimilated carbon towards root development. Compatible solutes like proline, soluble sugars, total phenolic contents, and soluble proteins are considered important in protecting the subcellular structure under stress conditions [17,48,49,56]. In this study, concentration of all the compatible solutes increased under water stress (Figure 2). Increase in comparable solutes under water stress agree with the previous findings in poplar [52] in Conocarpus erectus, Morus alba, Syzygium cumini [15,20] olive and black polar [57,58]. In this study, highest increase in compatible solutes were evidenced in Syzygium cumini as compared to Populus deltoides. Previous studies have demonstrated that proline and soluble sugars are involved in maintaining the cell turgor and protection of plant cells under water stress conditions [59]. Likewise, studies have shown that phenolic compounds are the secondary metabolites that have a significant role in increasing water stress tolerance in plants as they act as antioxidants and protect plants under oxidative stress [60]. Therefore, higher concentration of compatible solutes under both MS and SS in Syzygium cumini as compared to Populus deltoides saplings confirms a better tolerance strategy in Syzygium cumini as compared to Populus deltoides. Based on the results it can be concluded that increased production of compatible solutes (proline, soluble sugars, total phenolic content and soluble proteins) helped in maintaining cellular osmotic status that translated in less productivity loss in Syzygium cumini as compared to Populus deltoides under MS and SS (Figure 2). Balance between the production of oxidants and the antioxidant enzyme activity have a significant role on determining the tolerance status of a plant species under abiotic stress [61]. Increased production of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) and super oxide radical (O2−) are involved in the cellular membrane damage, destroy protein and lipids [62,63]. In the present study the concentration of both hydrogen peroxide (H2O2) and super oxide radical (O2−) increased significantly in both Syzygium cumini and Populus deltoides saplings under MS and SS respectively (Figure 3). Previous studies have demonstrated that increase in ROS was related to decrease in productivity in, Populus nigra L. Syzygium cumini L., Conocarpus erectus, Acacia modesta, Salix tetrasperma, and Portulaca oleracea [20,55,62,63]. In this study, the observed increase in ROS and decrease in total biomass in Syzygium cumini and Populus deltoides under MS and SS supports this hypothesis. Moreover, increase in EL% and MDA have also been reported in Coffea canephora and Portulaca oleracea, Quercus ilex, Quercus pubescens and Quercus cerris, Quercus brantii, Acacia modesta, and Salix tetrasperma the studies due to increase in ROS that stimulates the peroxidation of lipid membrane [63,64,65,66,67]. Our results agree to this hypothesis as EL% and the concentration of MDA significantly increased in Syzygium cumini and Populus deltoides under MS and SS (Figure 3). However, a greater increase in ROS, EL% and MDA was observed in Populus deltoides as compared to Syzygium cumini that showed a higher vulnerability of Populus deltoides to water stress conditions. In response to the increased production of oxidants under abiotic stress, plants have a well-developed antioxidant defense mechanism. The most important antioxidant enzymes are SOD, POD, CAT and APX. These antioxidant enzymes scavenge the overproduction of oxidants and prevents the cellular damage in plants during stress conditions [61,68]. In this study, the enzyme activity of SOD, POD, CAT and APX increased significantly in Syzygium cumini and Populus deltoides saplings under MS and SS (Figure 4). However, the activity of antioxidants was found significantly higher in Syzygium cumini as compared to Populus deltoides. Previous studies have linked the increase in antioxidant enzyme activity to the increased stress tolerance in various species like Salix sinopurpurea and Salix suchowensis, Conocarpus erectus, Acacia modesta, and Salix tetrasperma, Syzygium cumini [20,63,69]. Such hypothesis is based on the activity of SOD that converts the O2− in to H2O2 which is further converted to H2O by the other antioxidants like CAT and POD [61,70]. In this study, a greater increase in the antioxidant enzyme activity, higher concentration of compatible solutes depicted a better water stress tolerance strategy in Syzygium cumini as compared to Populus deltoides saplings.
5. Conclusions
Water deficit had a significantly negative effect on both Populus deltoides and Syzygium cumini saplings as various growth attributes, dry weight production, and photosynthetic attributes (stomatal conductance, CO2 assimilation rate) decreased under stress conditions. The highest reduction was evidenced in Populus deltoides as compared to Syzygium cumini saplings. The concentration of MDA and EL% and the production of oxidants like H2O2 and O2−, increased significantly under both MS and SS treatments and was highest in Populus deltoides saplings. On the contrary, although the accumulation of osmolytes such as (proline, soluble sugar, total phenolic content, and soluble protein) increased significantly in both the species under MS and SS, however, was the highest in Syzygium cumini saplings. Moreover, the antioxidants enzyme activity of SOD, POD, CAT and APX was also found highest in Syzygium cumini. Therefore, it can be suggested that the Syzygium cumini saplings showed the higher tolerance to water deficit which can related to the increased accumulation of osmolytes and increased antioxidant enzyme activity however, further insight is required regarding the comparative behavior of these species under field conditions before final recommendations.
Author Contributions
Data correction, F.R. and M.N.; Formal analysis, Z.Z., M.M., Z.R. and F.R.; Supervision, F.R.; Validation, F.H.I., A.Q. and S.A.; writing original draft, Z.Z.; Writing—review and editing, F.R. and W.R.K. All authors have read and agreed to publish version of the manuscript.
Funding
This research received funding from the Office of Research, Innovation and Commercialization (ORIC) Grant no. A/C AO5213, University of Agriculture Faisalabad, Pakistan, and Universiti Putra Malaysia.
Data Availability Statement
Data is available on request from the corresponding author.
Acknowledgments
We are thankful to Muhammad Atif for providing laboratory facilities for biochemical analysis and Muhammad Haider for technical support during the experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IPCC. Executive Summary of the Intergovernmental Panel on Climate Change. February 2007. Available online: https://www.ipcc.ch/sr15/ (accessed on 18 May 2021).
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- Wang, F.Z.; Wang, Q.B.; Kwon, S.Y.; Kwak, S.S.; Su, W.A. Enhanced drought tolerance of transgenic rice plants expressing a peamanganese superoxide dismutase. J. Plant Physiol. 2005, 162, 465–472. [Google Scholar] [CrossRef]
- Eswaran, H.; Reich, P.; Beinroth, F. Global desertification tension zones. In Proceedings of the 10th International Soil Conservation Organization Meeting, West Lafayette, IN, USA, 24–29 May 2001. [Google Scholar]
- Aryal, J.P.; Sapkota, T.B.; Khurana, R.; Khatri-Chhetri, A.; Rahut, D.B.; Jat, M.L. Climate change and agriculture in South Asia: Adaptation options in smallholder production systems. Environ. Dev. Sustain. 2020, 22, 5045–5075. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, K.; Shahid, S.; Chung, E.S.; Wang, X.J.; Harun, S.B. Climate change uncertainties in seasonal drought severity-area-frequency curves: Case of arid region of Pakistan. J. Hydrol. 2019, 570, 473–485. [Google Scholar] [CrossRef]
- Liu, Y.; Hwang, Y. Improving drought predictability in Arkansas using the ensemble PDSI forecast technique. Stoch. Environ. Res. Risk Assess. 2015, 29, 79–91. [Google Scholar] [CrossRef]
- Anjum, S.; Saleem, M.; Cheema, M.; Bilal, M.; Khaliq, T. An assessment to vulnerability, extent, characteristics and severity of drought hazard in Pakistan. Pak. J. Sci. 2012, 64, 85–96. [Google Scholar]
- Food and Agriculture Organization (FAO). Drought Situation Report Pakistan 2021. Available online: https://reliefweb.int/report/pakistan/drought-situation-report-pakistan-volume-1-issue-ii-may-31-2021 (accessed on 18 August 2021).
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.T.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Kunz, J.N.; Voronine, D.V.; Lee, H.W.H.; Sokolov, A.V.; Scully, M.O. Rapid detection of drought stress in plants using femtosecond laser-induced breakdown spectroscopy. Opt. Express 2017, 25, 7251–7262. [Google Scholar] [CrossRef] [PubMed]
- Trenberth, K.E.; Dai, A.; Van der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Zafar, Z.; Rasheed, F.; Abdullah, M.; Salam, M.M.A.; Mohsin, M. Effects of water deficit on growth and physiology of Young Conocarpus erectus L. and Ficus benjamina L. saplings. Bangladesh J. Bot. 2019, 48, 1215–1221. [Google Scholar] [CrossRef]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and drought stresses in crop plants Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Zafar, Z.; Rasheed, F.; Atif, R.M.; Javed, M.A.; Maqsood, M.; Gailing, O. Foliar Application of Salicylic Acid Improves Water Stress Tolerance in Conocarpus erectus L. and Populus deltoides L. Saplings: Evidence from Morphological, Physiological, and Biochemical Changes. Plants 2021, 10, 1242. [Google Scholar] [CrossRef] [PubMed]
- Centritto, M.; Brilli, M.; Fodale, R.; Loreto, F. Different sensitivity of isoprene emission, respiration and photosynthesis to high growth temperature coupled with drought stress in black poplar (Populus nigra) saplings. Tree Physiol. 2011, 31, 275–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.; Sheng, M.; Tang, M. Effects of Rhizophagus irregularis on photosynthesis and antioxidative enzymatic system in Robinia pseudoacacia L. under drought stress. Front. Plant Sci. 2017, 8, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasheed, F.; Delagrange, S. Acclimation of Betula alleghaniensis Britton to moderate soil water deficit: Small morphological changes make for important consequences in crown display. Tree Physiol. 2016, 36, 1320–1329. [Google Scholar]
- Noctor, G.; Reichheld, J.P.; Foyer, C.H. ROS related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Zafar, Z.; Rasheed, F.; Atif, R.M.; Maqsood, M.; Gailing, O. Salicylic Acid-Induced Morpho-Physiological and Biochemical Changes Triggered Water Deficit Tolerance in Syzygium cumini L. Saplings. Forests 2021, 12, 491. [Google Scholar] [CrossRef]
- Feodorova, T.A.; Alexandrov, O.S. Comparative studying of leaf trichomes, teeth and glands in Populus nigra L., Populus deltoides W. Bartram ex Marshall and their hybrids. Forests 2020, 11, 1267. [Google Scholar] [CrossRef]
- Yi, L.; Li, B.; Korpelainen, H.; Yu, F.; Wu, L.; Tong, L.; Liu, M. Mechanisms of drought response in Populus. South For. 2020, 82, 359–366. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Xu, C.; Xu, H.; Zou, X.; Chen, H.Y.; Ruan, H. The abundance and community structure of soil arthropods in reclaimed coastal saline soil of managed poplar plantations. Geoderma 2018, 327, 130–137. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, H.Y.; Jin, L.; Wang, C.; Zhang, R.; Ruan, H.; Yang, J. Drought stress induced increase of fungi: Bacteria ratio in a poplar plantation. Catena 2020, 193, 104607. [Google Scholar] [CrossRef]
- Invasive Species Specialist Group. Species profile: Syzygium cumini. In Global Invasive Species Database; ISSG: Treviso, Italy, 2018. [Google Scholar]
- Wong, A. The Art of Botany: The Effect of Drought on Plant Anatomy. Horizons 2018, 3, 19. [Google Scholar]
- Madani, B.; Mirshekari, A.; Yahia, E.M.; Golding, J.B.; Hajivand, S.; Dastjerdy, A.M. Jamun (Syzygium cumini L. Skeels): A Promising Fruit for the Future. Hortic. Rev. 2021, 48, 275–306. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, F.; Dreyer, E.; Richard, B.; Brignolas, F.; Brendel, O.; Le Thiec, D. Vapour pressure deficit during growth has little impact on genotypic differences of transpiration efficiency at leaf and whole plant level: An example from Populus nigra L. Plant Cell Environ. 2015, 38, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Ainsworth, E.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [Green Version]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Nayyar, H. Accumulation of osmolytes and osmotic adjustment in water-stressed wheat (Triticum aestivum) and maize (Zea mays) as affected by calcium and its antagonists. Environ. Exp. Bot. 2003, 50, 253–264. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Bai, T.; Li, C.; Ma, F.; Feng, F.; Shu, H. Responses of growth and antioxidant system root-zone hypoxia stress in two Malus species. Plant Soil 2009, 327, 95–105. [Google Scholar] [CrossRef]
- Bayer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Maehly, A.C.; Chance, B. The assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Knörzer, O.C.; Burner, J.; Boger, P. Alterations in the antioxidative system of suspension-cultured soybean cells (Glycine max) induced by oxidative stress. Physiol. Plant. 1996, 97, 388–396. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Trabelsiab, L.; Gargouria, K.; Hassenaa, A.B.; Mbadraa, C.; Ghrab, M.; Ncube, B.; Staden, J.V.; Gargouri, R. Impact of drought and salinity on olive water status and physiology performance in an arid climate. Agric. Water Manag. 2019, 213, 749–759. [Google Scholar] [CrossRef]
- Muller, B.; Pantin, F.; Genard, M.; Turc, O.; Freixes, S.; Piques, M.; Gibon, Y. Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. J. Exp. Bot. 2011, 62, 1715–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, A. Plant water relations, plant stress and plant production. In Plant Breeding for Water-Limited Environments; Springer: New York, NY, USA, 2011; pp. 11–52. [Google Scholar]
- Correia, B.; Pintó-Marijuan, M.; Neves, L.; Brossa, R.; Dias, M.C.; Costa, A.; Castro, B.B.; Araujo, C.; Santos, C.; Chaves, M.M.; et al. Water stress and recovery in the performance of two Eucalyptus globulus clones: Physiological and biochemical profiles. Physiol. Plant. 2014, 150, 580–592. [Google Scholar] [CrossRef]
- Possen, B.J.H.M.; Oksanen, E.; Rousi, M.; Ruhanen, H.; Ahonen, V.; Tervahauta, A.; Heinonen, J.; Heiskanen, J.; Karenlampi, S.; Vapaavuori, E. Adaptability of birch (Betula pendula Roth) and aspen (Populus tremula L.) genotypes to different soil moisture conditions. For. Ecol. Manag. 2011, 262, 1387–1399. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, R.; Radhamani, J.; Bhandari, D.C. Exploring the potential of Ziziphus nummularia (Burm. f.) Wight et Arn. from drier regions of India. Genet. Resour. Crop. Evol. 2010, 57, 929–936. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought from genes to the whole plant. Funct. Plant. Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Hu, Y.; Du, X.; Li, T.; Tang, H.; Wu, J. Salicylic acid induces physiological and biochemical changes in Torreya grandis cv. Merrillii seedlings under drought stress. Trees 2014, 28, 961–970. [Google Scholar] [CrossRef]
- Arndt, S.; Clifford, S.; Wanek, W.; Jones, H.; Popp, M. Physiological and morphological adaptations of the fruit tree Ziziphus rotundifolia in response to progressive drought stress. Tree Physiol. 2010, 21, 705–715. [Google Scholar] [CrossRef]
- Bacelar, E.A.; Santos, D.L.; Moutinho-Pereira, J.M.; Lopes, J.I.; Goncalves, B.C.; Ferreira, T.C.; Correia, C.M. Physiological behaviour, oxidative damage and antioxidative protection of olive trees grown under different irrigation regimes. Plant Soil 2007, 292, 1–12. [Google Scholar] [CrossRef]
- Bogeat-Triboulot, M.B.; Mikael, B.; Jenny, R.; Laurent, J.; Didier, L.T.; Payam, F.; Basia, V.; Erwin, W.; Kris, L.; Thomas, T.; et al. Gradual soil water depletion results in reversible changes of gene expression, protein profiles eco-physiology and growth performance in Populus euphratica, a poplar growing in arid regions. Plant Physiol. 2007, 143, 876–892. [Google Scholar] [CrossRef] [Green Version]
- Tahjib-Ul-Arif, M.; Siddiqui, M.N.; Sohag, A.A.M.; Sakil, M.A.; Rahman, M.M.; Polash, M.A.S.; Mostofa, M.G.; Tran, L.S.P. Salicylic acid mediated enhancement of photosynthesis attributes and antioxidant capacity contributes to yield improvement of maize plants under salt stress. J. Plant Growth Regul. 2018, 37, 1318–1330. [Google Scholar] [CrossRef]
- Hummel, I.; Pantin, F.; Sulpice, R.; Piques, M.; Rolland, G.; Dauzat, M.; Christophe, A.; Pervent, M.; Bouteill, M.; Stitt, M.; et al. Arabidopsis plants acclimate to water deficit at low cost through changes of carbon usage: An integrated perspective using growth, metabolite, enzyme, and gene expression analysis. Plant Physiol. 2010, 154, 357–372. [Google Scholar] [CrossRef] [Green Version]
- Saheri, F.; Barzin, G.; Pishkar, L.; Boojar, M.M.A.; Babaeekhou, L. Foliar spray of salicylic acid induces physiological and biochemical changes in purslane (Portulaca oleracea L.) under drought stress. Biologia 2020, 75, 2189–2200. [Google Scholar] [CrossRef]
- White, D.A.; Turner, N.C.; Galbraith, J.H. Leaf water relations and stomatal behavior of four allopatric Eucalyptus species planted in Mediterranean southwestern Australia. Tree Physiol. 2000, 20, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.R.; Chaitanya, K.V.; Jutur, P.P.; Sumithra, K. Differential antioxidative responses to water stress among five mulberry (Morus alba L.) cultivars. Environ. Exp. Bot. 2004, 52, 33–42. [Google Scholar] [CrossRef]
- Dianat, M.; Saharkhiz, M.J.; Tavassolian, I. Salicylic acid mitigates drought stress in Lippia citriodora L. effects on biochemical traits and essential oil yield. Biocatal. Agric. Biotechnol. 2016, 8, 286–293. [Google Scholar] [CrossRef]
- Szabados, L.; Savoure, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [Green Version]
- Laxa, M.; Michael, L.; Wilena, T.; Kamel, C.; Karl, J.D. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Regier, N.; Streb, S.; Cocozza, C.; Schaub, M.; Cherubini, P.; Zeeman, S.C.; Rrey, B. Drought tolerance of two black poplar (Populus nigra L.) clones: Contribution of carbohydrates and oxidative stress defence. Plant Cell Environ. 2009, 32, 1724–1736. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, F.; Gondal, A.; Kudus, K.A.; Zafar, Z.; Nawaz, M.F.; Khan, W.R.; Abdullah, M.; Ibrahim, F.H.; Depardieu, C.; Pazi, A.M.M.; et al. Effects of soil water deficit on three tree species of the arid environment: Variations in growth, physiology, and antioxidant enzyme activities. Sustainability 2021, 13, 3336. [Google Scholar] [CrossRef]
- Lima, A.L.S.; Da Matta, F.M.; Pinheiro, H.A.; Totola, M.R.; Loureiro, M.E. Photochemical responses and oxidative stress in two clones of Coffea canephora under water deficit conditions. Environ. Exp. Bot. 2002, 47, 239–247. [Google Scholar] [CrossRef]
- Jin, R.; Shi, H.; Han, C.; Zhong, B.; Wang, Q.; Chan, Z. Physiological changes of purslane (Portulaca oleracea L.) after progressive drought stress and rehydration. Sci. Hortic. 2015, 194, 215–221. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Remorini, D.; Pellegrini, E.; Landi, M.; Massai, R.; Nali, C.; Guidi, L.; Lorenzini, G. Variations in physiological and biochemical traits of oak seedlings grown under drought and ozone stress. Physiol. Plant. 2016, 157, 69–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafarnia, S.; Akbarinia, M.; Hosseinpour, B.; Modarres, S.A.M.; Salami, S.A. Effect of drought stress on some growth, morphological, physiological, and biochemical parameters of two different populations of Quercus brantii. iFor.-Biogeosci. For. 2018, 11, 212–220. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Guo, K.; Fan, D.; Li, G.; Zheng, Y.; Yu, L.; Yang, R. Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China. Environ. Exp. Bot. 2011, 71, 174–183. [Google Scholar] [CrossRef]
- Jia, H.; Wang, L.; Li, J.; Sun, P.; Lu, M.; Hu, J. Physiological and metabolic responses of Salix sinopurpurea and Salix su-chowensis to drought stress. Trees 2020, 34, 563–577. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).