Patterns of Leaf Morphological Traits of Beech (Fagus sylvatica L.) along an Altitudinal Gradient
Abstract
1. Introduction
2. Materials and Methods
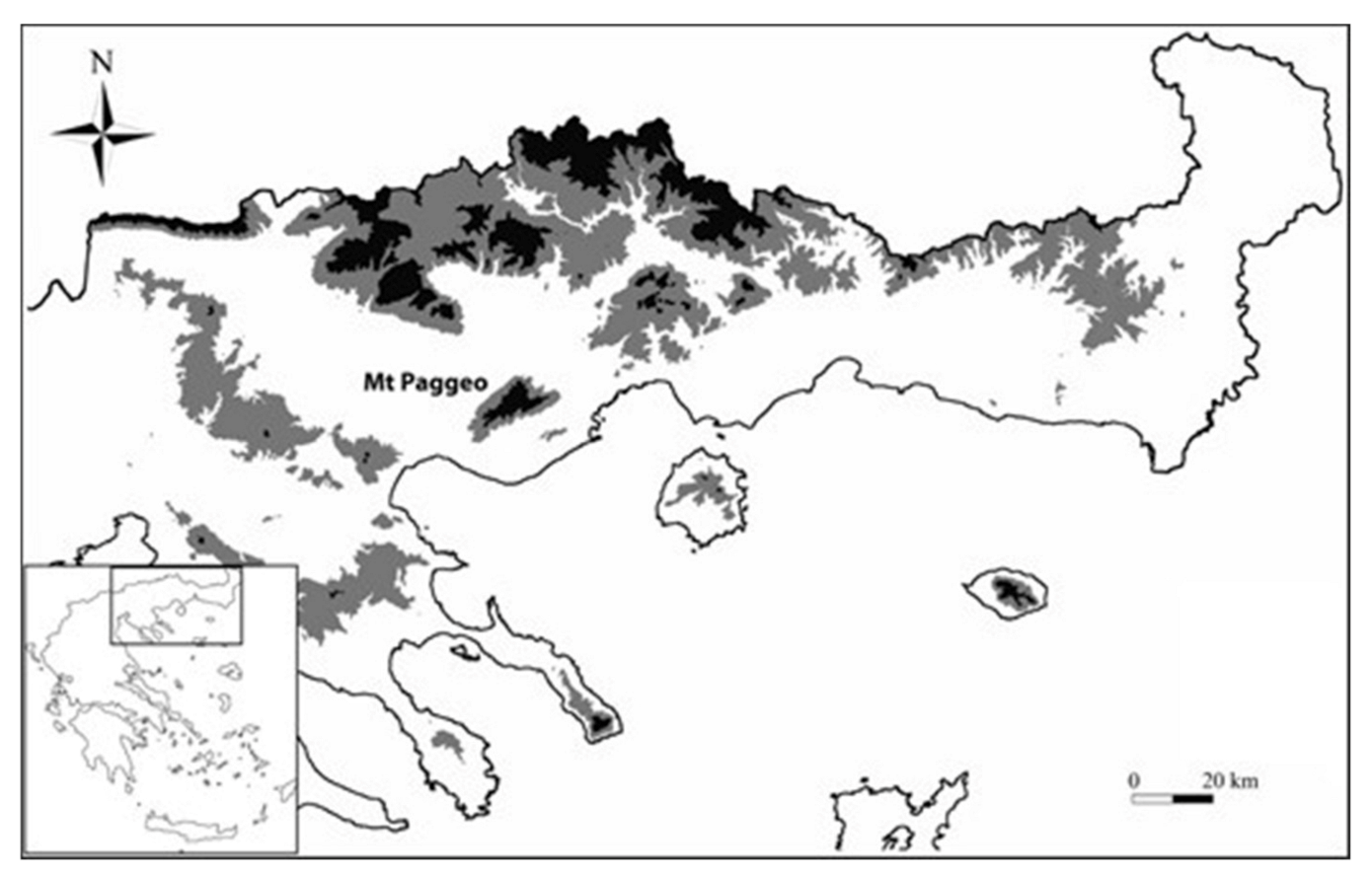
2.1. Area of the Study and Sampling
- Τhermophytic beech forests (Quercus frainetto Ten. woods—code: 9280) that mainly occur at the low elevations of the south-eastern slopes of the mountain, on gneiss–schist substrates and in a forest patch in the north-eastern part of the mountain on granite; these forests are similar to the units 5 and 6 according to Tsiripidis et al. [33], and to the F. sylvatica subsp. orientalis (Lipsky) Greuter & Burdet community [34];
- Beech forests that grow in a gorge on the north-eastern part of Mt. Paggeo, from 400 to 1600 m als (Tilio–Acerion forests of slopes, screes and ravines—code: 9180), that represent a rare vegetation type of high conservation value, belonging to the Tilio–Acerion alliance [32,33,35] and a possible local glacial refugium for beech [36].
2.2. Leaf Traits and Data Analysis
3. Results
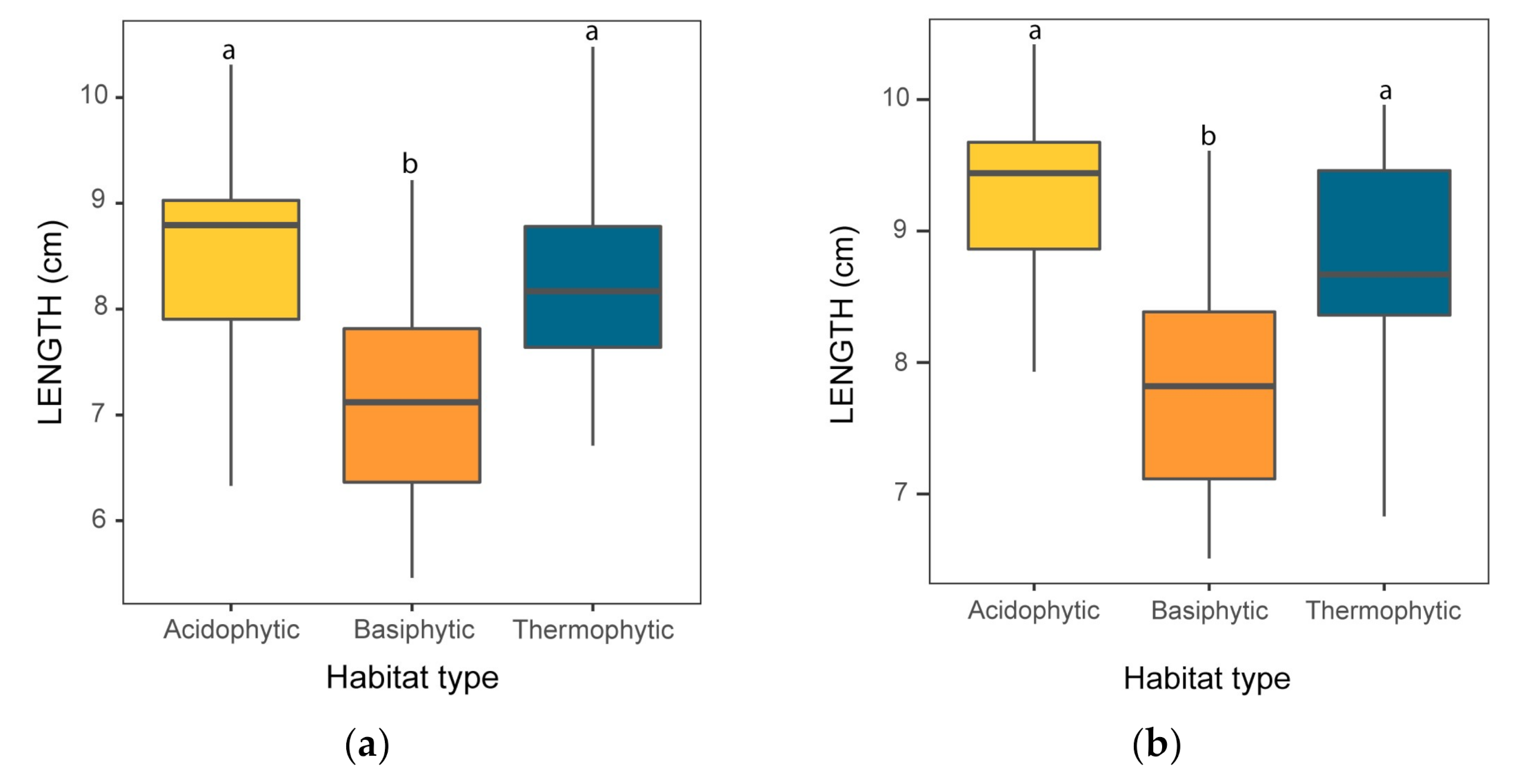
3.1. Light Leaves and Shade Leaves
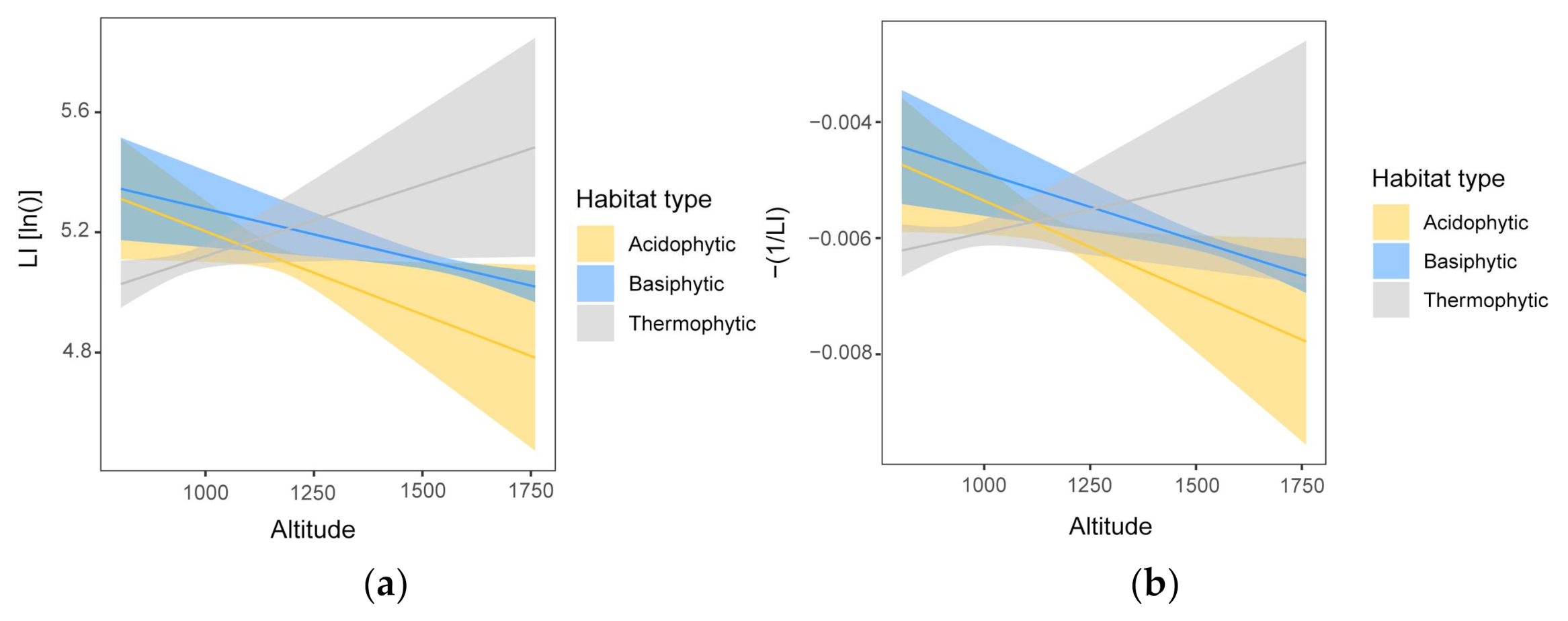
3.2. Generalized Linear Models
3.2.1. Size Traits
3.2.2. Shape Traits
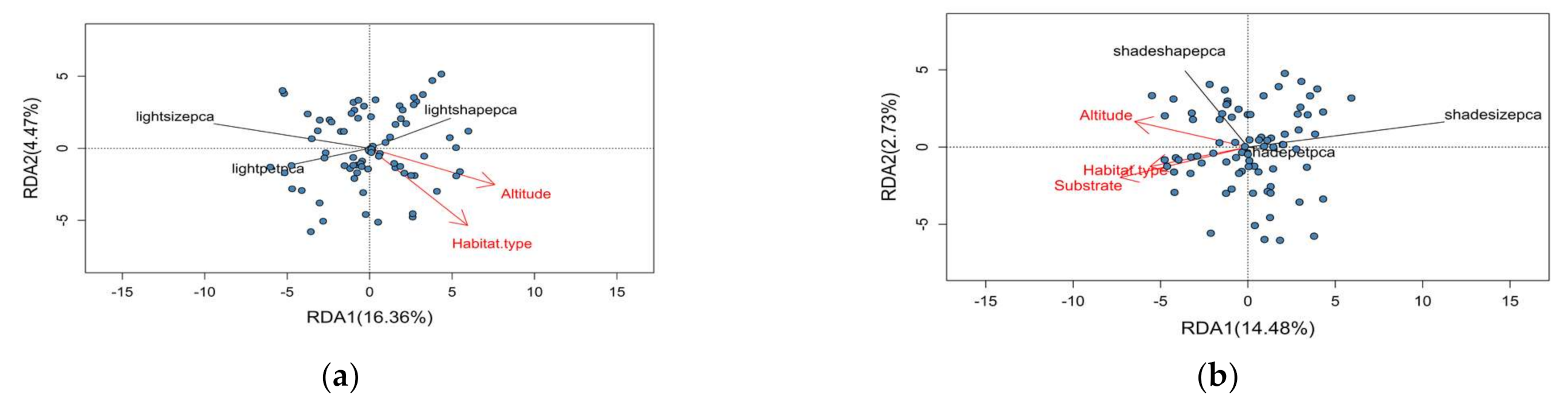
3.3. Redundancy Analysis
4. Conclusions
4.1. An Altitudinal Pattern in Leaf Morphology
4.2. Total Phenotypic Variation of Leaf Traits
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruschi, P.; Grossoni, P.; Bussotti, F. Within- and among-tree variation in leaf morphology of Quercus petraea (Matt.) Liebl. natural populations. Trees 2003, 17, 164–172. [Google Scholar] [CrossRef]
- Liu, Z.L.; Zhu, Y.; Li, G.R.; Jin, G.Z. Non-destructively predicting leaf area, leaf mass and specific leaf area based on a linear mixed-effect model for broadleaf species. Ecol. Indic. 2016, 78, 340–350. [Google Scholar] [CrossRef]
- Himanen, K.; Adem, G.D.; Van Lijsebettens, M. Genetic and epigenetic control of leaf size and shape. Int. J. Plant Develop. Biol. 2007, 1, 226–238. [Google Scholar]
- Viscosi, V.; Antonecchia, G.; Lepais, O.; Fortini, P.; Gerber, S.; Loy, A. Leaf shape and size differentiation in white oaks: Assessment of allometric relationships among three sympatric species and their hybrids. Int. J. Plant Sci. 2012, 173, 875–884. [Google Scholar] [CrossRef]
- Scartazza, A.; Di Baccio, D.; Bertolotto, P.; Gavrichkova, O.; Matteucci, G. Investigating the European beech (Fagus sylvatica L.) leaf characteristics along the vertical canopy profile: Leaf structure, photosynthetic capacity, light energy dissipation and photoprotection mechanisms. Tree Physiol. 2016, 36, 1060–1076. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.E.; Asner, G.P.; Bentley, L.P.; Shenkin, A.; Salinas, N.; Huaypar, K.Q.; Pillco, M.M.; Ccori Álvarez, F.D.; Enquist, B.J.; Diaz, S.; et al. Covariance of Sun and Shade Leaf Traits Along a Tropical Forest Elevation Gradient. Front. Plant Sci. 2020, 10, 1810. [Google Scholar] [CrossRef] [PubMed]
- Hagemeier, M.; Leuschner, C. Functional Crown Architecture of Five Temperate Broadleaf Tree Species: Vertical Gradients in Leaf Morphology, Leaf Angle and Leaf Area Density. Forests 2019, 10, 265. [Google Scholar] [CrossRef]
- Desmond, S.C.; Garner, M.; Flannery, S.; Whittemore, A.T.; Hipp, A.L. Leaf shape and size variation in bur oaks: An empirical study and simulation of sampling strategies. Am. J. Bot. 2021, 108, 1540–1554. [Google Scholar] [CrossRef]
- Dengler, N.G. The influence of light on leaf development. In Growth Patterns in Vascular Plants; Iqbal, M., Ed.; Dioscorides Press: Portland, OR, USA, 1994; pp. 100–136. [Google Scholar]
- McPherson, S.; Eamus, D.; Murray, B.R. Seasonal impacts on leaf attributes of several tree species growing in three diverse ecosystems of south–eastern Australia. Austr. J. Bot. 2004, 52, 293–301. [Google Scholar] [CrossRef]
- Thomas, S.C. Genetic vs. phenotypic responses of trees to altitude. Tree Physiol. 2011, 31, 1161–1163. [Google Scholar] [CrossRef]
- Cordell, S.; Goldstein, G.; Mueller-Dombois, D.; Webb, D.; Vitousek, P.M. Physiological and morphological variation in Metrosideros polymorpha, a dominant Hawaiian tree species, along an altitudinal gradient: The role of phenotypic plasticity. Oecologia 1998, 113, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Hovenden, M.J.; Vander Schoor, J.K. Nature vs nurture in the leaf morphology of Southern beech, Nothofagus cunninghamii (Nothofagaceae). New Phytol. 2003, 161, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Premoli, A.C.; Brewer, C.A. Environmental vs genetically driven variation in ecophysiological traits of Nothofagus pumilio from contrasting elevations. Aust. J. Bot. 2007, 55, 585–591. [Google Scholar] [CrossRef]
- Givnish, T.J. Comparative studies of leaf form: Assessing the relative roles of selective pressures and phylogenetic constraints. New Phytol. 1987, 106, 131–160. [Google Scholar] [CrossRef]
- Roderick, M.L.; Berry, S.L.; Noble, I.R. A framework for understanding the relationship between environment and vegetation based on the surface area to volume ratio of leaves. Funct. Ecol. 2000, 14, 423–437. [Google Scholar] [CrossRef]
- Pickup, M.; Westoby, M.; Basden, A. Dry mass costs of deploying leaf area in relation to leaf size. Funct. Ecol. 2005, 19, 88–97. [Google Scholar] [CrossRef]
- Ballian, D.; Jukić, B.; Balić, B.; Kajba, D.; von Wuehlisch, G. Fenološka varijabilnost obične bukve (Fagus sylvatica L.) u međunarodnom pokusu provenijencija. Šumar. List 2015, 139, 521–533. [Google Scholar]
- Körner, C.; Bannister, P.; Mark, A.F. Altitudinal variation in stomatal conductance, nitrogen content and leaf anatomy in different plant life forms in New Zealand. Oecologia 1986, 69, 577–588. [Google Scholar] [CrossRef]
- Pandey, M.; Pathak, M.L.; Shrestha, B.B. Morphological and wood anatomical traits of Rhododendron lepidotum Wall ex G. Don along the elevation gradients in Nepal Himalayas. Arct. Antarct. Alp. Res. 2021, 53, 35–47. [Google Scholar] [CrossRef]
- Milla, R.; Reich, P. Multi-trait interactions, not phylogeny, fine-tune leaf size reduction with increasing altitude. Ann. Bot. Lond. 2011, 107, 455–465. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Vilagrosa, A.; Alonso-Forn, D.; Ferrio, J.P.; Sancho-Knapik, D.; Gil-Pelegrín, E. Living in Drylands: Functional Adaptations of Trees and Shrubs to Cope with High Temperatures and Water Scarcity. Forests 2020, 11, 1028. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology, 4th ed.; Springer: Berlin, Germany, 2003; p. 254. [Google Scholar]
- Boutsios, S.; Vidalis, A.; Adamidis, G.C.; Hatziskakis, S.; Varsamis, G.; Tsiripidis, I.; Karanikola, P.; Papageorgiou, A.C. Diversity in shade and light leaf morphology in beech populations of South Rodopi mountains. Proc. Natl. Acad. Sci. India B 2021, 91, 53–61. [Google Scholar]
- Zunzunegui, M.; Cruz Díaz-Barradas, M.; Ain-Lhout, F.; Alvarez-Cansino, L.; Esquivias, M.P.; García Novo. F. Seasonal physiological plasticity and recovery capacity after summer stress in Mediterranean scrub communities. Plant Ecol. 2011, 212, 127–142. [Google Scholar] [CrossRef]
- Vitasse, Y.; Bresson, C.C.; Kremer, A.; Michalet, R.; Delzon, S. Quantifying phenological plasticity to temperature in two temperate tree species. Funct. Ecol. 2010, 24, 1211–1218. [Google Scholar] [CrossRef]
- Körner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Denk, T.; Grimm, G.; Stögerer, K.; Langer, M.; Hemleben, V. The evolutionary history of Fagus in western Eurasia: Evidence from genes, morphology and the fossil record. Plant System. Evol. 2002, 232, 213–236. [Google Scholar] [CrossRef]
- Papageorgiou, A.C.; Vidalis, A.; Gailing, O.; Tsiripidis, I.; Hatziskakis, S.; Boutsios, S.; Galatsidas, S.; Finkeldey, R. Genetic variation of beech (Fagus sylvatica L.) in Rodopi (N.E. Greece). Euro. J. Forest. Res. 2008, 127, 81–88. [Google Scholar] [CrossRef]
- Hatziskakis, S.; Tsiripidis, I.; Papageorgiou, A.C. Leaf morphological variation in beech (Fagus sylvatica L.) populations in Greece and its relation to their post-glacial origin. Bot. J. Linn. Soc. 2011, 165, 422–436. [Google Scholar] [CrossRef]
- European Commission/DG Environment. Interpretation Manual of European Union Habitats, EUR 28; European Commission/DG Environment: Brussels, Belgium, 2013; p. 146. [Google Scholar]
- Tsiripidis, I.; Bergmeier, E.; Dimopoulos, P. Geographical and ecological differentiation in Greek Fagus forest vegetation. J. Veg. Sci. 2007, 18, 743–750. [Google Scholar] [CrossRef]
- Tsiripidis, I.; Karagiannakidou, V.; Alifragis, D.; Athanasiadis, N. Classification and gradient analysis of the beech forest vegetation of the southern Rodopi (northeast Greece). Folia Geobot. 2007, 42, 249–270. [Google Scholar] [CrossRef]
- Bergmeier, E.; Dimopoulos, P. Fagus sylvatica forest vegetation in Greece: Syntaxonomy and gradient analysis. J. Veg. Sci. 2001, 12, 109–126. [Google Scholar] [CrossRef]
- Mastrogianni, A.; Kallimanis, A.S.; Chytrý, M.; Tsiripidis, I. Phylogenetic diversity patterns in forests of a putative refugial area in greece: A community level analysis. Forest Ecol. Manag. 2019, 446, 226–237. [Google Scholar] [CrossRef]
- Papageorgiou, A.C.; Tsiripidis, I.; Mouratidis, T.; Hatziskakis, S.; Gailing, O.; Eliades, N.G.H.; Vidalis, A.; Drouzas, A.D.; Finkeldey, R. Complex fine-scale phylogeographical patterns in a putative refugial region for Fagus sylvatica (Fagaceae). Bot. J. Linn. Soc. 2014, 174, 516–528. [Google Scholar] [CrossRef][Green Version]
- McCune, B.; Keon, D. Equations for potential annual direct incident radiation and heat load. J. Veg. Sci. 2002, 13, 603–606. [Google Scholar] [CrossRef]
- Fox, J. The R commander: A basic statistics graphical user interface to R. J. Stat. Softw. 2005, 14, 1–42. [Google Scholar] [CrossRef]
- Fox, J. Using the R Commander: A Point-and-Click Interface for R; Chapman and Hall/CRC Press: Boca Raton, FL, USA, 2017; p. 233. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: http://www.r-project.org/index.html (accessed on 16 June 2021).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 2nd ed.; Sage Publications: Thousand Oaks, CA, USA, 2011; p. 449. [Google Scholar]
- Breheny, P.; Burchett, W. Visualization of regression models using visreg. R J. 2017, 9, 56. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; p. 213. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-6. 2019. Available online: https://github.com/vegandevs/vegan (accessed on 10 July 2021).
- Halloy, S.R.P.; Mark, A.F. Comparative leaf morphology spectra of plant communities in New Zealand, the Andes and the European Alps. J. Royal Soc. N. Zeal. 1996, 26, 41–78. [Google Scholar] [CrossRef]
- Sun, S.; Jin, D.; Shi, P. The leaf size–twig size spectrum of temperate woody species along an altitudinal gradient: An invariant allometric scaling relationship. Ann. Bot. Lond. 2006, 97, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Poljak, I.; Idžojtić, M.; Šapić, I.; Korijan, P.; Vukelić, J. Diversity and structure of croatian continental and alpine-dinaric populations of grey alder (Alnus incana/L./Moench subsp. incana); Isolation by distance and environment explains phenotypic divergence. Šumar. List 2018, 142, 19–31. [Google Scholar]
- Zebec, M.; Idžojtić, M.; Poljak, I.; Modrić, I. Population variability of wych elm (Ulmus glabra Huds.) in the mountainous region of Croatia according to the leaf morphology. Šumar. List 2015, 139, 429–439. [Google Scholar]
- Paridari, I.C.; Jalali, S.G.; Sonboli, A.; Zarafshar, M.; Bruschi, P. Leaf macro-and micro-morphological altitudinal variability of Carpinus betulus in the Hyrcanian forest (Iran). J. Forest. Res. 2013, 24, 301–307. [Google Scholar] [CrossRef]
- McDonald, P.G.; Fonseca, C.R.; McC, J.; Westoby, M. Leaf-size divergence along rainfall and soil-nutrient gradients: Is the method of size reduction common among clades? Funct. Ecol. 2003, 17, 50–57. [Google Scholar] [CrossRef]
- Sack, L.; Melcher, P.J.; Liu, W.H.; Middleton, E.; Pardee, T. How strong is intracanopy leaf plasticity in temperate deciduous trees? Am. J. Bot. 2006, 93, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Meier, I.C.; Leuschner, C. Leaf size and leaf area index in Fagus sylvatica forests: Competing effects of precipitation, temperature, and nitrogen availability. Ecosystems 2008, 11, 655–669. [Google Scholar] [CrossRef]
- Fréjaville, T.; Vizcaíno-Palomar, N.; Fady, B.; Kremer, A.; Benito Garzón, M. Range margin populations show high climate adaptation lags in European trees. Glob. Chang. Biol. 2020, 26, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Miljković, D.; Stefanović, M.; Orlović, S.; Stanković Neđić, M.; Kesić, L.; Stojnić, S. Wild cherry (Prunus avium (L.) L.) leaf shape and size variations in natural populations at different elevations. Alp. Bot. 2019, 129, 163–174. [Google Scholar] [CrossRef]
- Tsukaya, H. A consideration of leaf shape evolution in the context of the primary function of the leaf as a photosynthetic organ. In The Leaf: A Platform for Performing Photosynthesis; Springer: Cham, Switzerland, 2018; pp. 1–26. [Google Scholar]
- De Casas, R.R.; Vargas, P.; Perez-Corona, E.; Manrique, E.; García-Verdugo, C.; Balaguer, L. Sun and shade leaves of Olea europaea respond differently to plant size, light availability and genetic variation. Funct. Ecol. 2011, 25, 802–812. [Google Scholar] [CrossRef]
- Denk, T. Phylogeny of Fagus, L. (Fagaceae) based on morphological data. Plant Syst. Evol. 2003, 240, 55–81. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Leigh, A.; Boyce, C.K.; Jones, C.S.; Niklas, K.J.; Royer, D.L.; Tsukaya, H. The evolution and functional significance of leaf shape in the angiosperms. Funct. Plant Biol. 2011, 38, 535–552. [Google Scholar] [CrossRef]
- Fritz, M.A.; Rosa, S.; Sicard, A. Mechanisms Underlying the Environmentally Induced Plasticity of Leaf Morphology. Front. Genet. 2018, 9, 478. [Google Scholar] [CrossRef]
- Li, Z.Q.; Yu, D. Factors affecting leaf morphology: A case study of Ranunculus natans C. A. Mey. (Ranunculaceae) in the arid zone of northwest China. Ecol. Res. 2009, 24, 1323–1333. [Google Scholar] [CrossRef]
- Guo, Z.; Lin, H.; Chen, S.; Yang, Q. Altitudinal patterns of leaf traits and leaf allometry in bamboo Pleioblastus amarus. Front. Plant Sci. 2018, 9, 1110. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C.; Döll, M.; Fietz, H.J.; Bach, T.; Kozel, U.; Meier, D.; Rahmsdorf, U. Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosynth. Res. 1981, 2, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Masarovicova, E.; Stefancik, L. Some ecophysiological features in sun and shade leaves of tall beech trees. Biol. Plant. 1990, 32, 347–374. [Google Scholar] [CrossRef]
- Vega, C.; González, G.; Bahamonde, H.A.; Valbuena-Carabaña, M.; Gil, L.; Fernández, V. Effect of irradiation and canopy position on anatomical and physiological features of Fagus sylvatica and Quercus petraea leaves. Plant Physiol. Biochem. 2020, 152, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Aranda, I.; Castro, L.; Pardos, M.; Gil, L.; Pardos, J.A. Effects of the interaction between drought and shade on water relations, gas exchange and morphological traits in cork oak Quercus suber L. seedlings. Forest. Ecol. Manag. 2005, 210, 117–129. [Google Scholar] [CrossRef]
- Wyka, T.P.; Oleksyn, J.; Zytkowiak, R.; Karolewski, P.; Jagodzinski, A.M.; Reich, P.B. Responses of leaf structure and photosynthetic properties to intra-canopy light gradients: A common garden test with four broadleaf deciduous angiosperm and seven evergreen conifer tree species. Oecologia 2012, 170, 1–14. [Google Scholar] [CrossRef]
- Proietti, P.; Palliotti, A.; Famiani, F.; Antognozzi, E.; Ferranti, F.; Andreutti, R.; Frenguelli, G. Influence of leaf position, fruit and light availability on photosynthesis of two chestnut genotypes. Sci. Hortic. Amst. 2000, 85, 63–73. [Google Scholar] [CrossRef]
- Poljak, I.; Idzojtić, M.; Zebec, M. Leaf morphology of the sweet chestnut (Castanea sativa Mill.); a methodological approach. Acta Hprtic. 2014, 1043, 211–218. [Google Scholar] [CrossRef]
- Bednorz, L. Morphological variability of leaves of Sorbus torminalis (L.) Crantz in Poland. Acta Soc. Bot. Pol. 2006, 75, 233–243. [Google Scholar] [CrossRef][Green Version]
- Jarni, K.; Westergren, M.; Kraigher, H.; Brus, R. Morphological variability of Fraxinus angustifolia Vahl in the north-western Balkans. Acta Soc. Bot. Pol. 2011, 80, 245–252. [Google Scholar] [CrossRef][Green Version]
- Desotgiu, R.; Cascio, C.; Pollastrini, M.; Gerosa, G.; Marzuoli, R.; Bussotti, F. Short and long term photosynthetic adjustments in sun and shade leaves of Fagus sylvatica L., investigated by fluorescence transient (FT) analysis. Plant. Biosyst. 2012, 146, 206–212. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Babani, F.; Langsdorf, G. Chlorophyll fluorescence imaging of photosynthetic activity in sun and shade leaves of trees. Photosynth. Res. 2007, 93, 234–244. [Google Scholar] [CrossRef]
- Tsiripidis, I.; Fotiadis, G.; Karagiannakidou, V.; Babalonas, D. Classification problems of forest vegetation in Greece: Transition from beech to deciduous oak zone. Bot. Chron. 2005, 18, 253–268. [Google Scholar]
- Lortie, C.J.; Aarssen, L.W. The specialization hypothesis for phenotypic plasticity in plants. Int. J. Plant. Sci. 1996, 157, 484–487. [Google Scholar] [CrossRef]
- Valladares, F. Light and plant evolution: Adaptation to the extremes versus phenotypic plasticity. In Advanced Studies in Plant Piology; Greppin, H., Ed.; University of Geneva: Geneva, Switzerland, 2000; pp. 341–355. [Google Scholar]
- Valladares, F.; Chico, J.M.; Aranda, I.; Balaguer, L.; Dizengremel, P.; Manrique, E.; Dreyer, E. The greater seedling highlight tolerance of Quercus robur over Fagus sylvatica is linked to a greater physiological plasticity. Trees 2002, 16, 395–403. [Google Scholar] [CrossRef]
- Hovenden, M.J.; Vander Schoor, J.K. The response of leaf morphology to irradiance depends on altitude of origin in Nothofagus cunninghamii. New Phytol. 2006, 169, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Denk, T. The taxonomy of Fagus in western Eurasia, 1: Fagus sylvatica subsp. orientalis (=F. orientalis). Feddes Repert. 1999, 110, 177–200. [Google Scholar] [CrossRef]
- Denk, T. The taxonomy of Fagus in western Eurasia, 2: Fagus sylvatica subsp. sylvatica. Feddes Repert. 1999, 110, 381–412. [Google Scholar] [CrossRef]
- Uhl, D. Variability of selected leaf traits in European beech (Fagus sylvatica) in relation to climatic factors—Some implications for palaeoenvironmental studies. Phytol. Balc. 2014, 20, 145–153. [Google Scholar]
- Gressler, E.; Jochner, S.; Capdevielle-Vargas, R.M.; Morellato, L.P.C.; Menzel, A. Vertical variation in autumn leaf phenology of Fagus sylvatica L. in southern Germany. Agr. Forest. Meteorol. 2015, 201, 176–186. [Google Scholar] [CrossRef]
- Papageorgiou, A.C.; Kostoudi, C.; Sorotos, I.; Varsamis, G.; Korakis, G.; Drouzas, A.D. Diversity in needle morphology and genetic markers in a marginal Abies cephalonica (Pinaceae) population. Ann. Forest. Res. 2015, 58, 217–234. [Google Scholar] [CrossRef]
- Shahba, M.A.; Bauerle, W.L. Growth temperature modulates the spatial variability of leaf morphology and chemical elements within crowns of climatically divergent Acer rubrum genotypes. Tree Physiol. 2009, 29, 869–877. [Google Scholar] [CrossRef][Green Version]
- Valladares, F.; Niinemets, Ü. Shade tolerance, a key plant feature of complex nature and consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef]
- Tsiripidis, I.; Athanasiadis, N. Contribution to the knowledge of the vascular flora of NE Greece: Floristic composition of the beech (Fagus sylvatica L.) forests in the Greek Rodopi. Willdenowia 2003, 33, 273–297. [Google Scholar] [CrossRef]
- Bresson, C.C.; Vitasse, Y.; Kremer, A.; Delzon, S. To what extent is altitudinal variation of functional traits driven by genetic adaptation in European oak and beech? Tree Physiol. 2011, 31, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Knutzen, F.; Meier, I.C.; Leuschner, C. Does reduced precipitation trigger physiological and morphological drought adaptations in European beech (Fagus sylvatica L.)? Comparing provenances across a precipitation gradient. Tree Physiol. 2015, 35, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zheng, L.; Qi, D. Variation in leaf traits at different altitudes reflects the adaptive strategy of plants to environmental changes. Ecol. Evol. 2020, 10, 8166–8175. [Google Scholar] [CrossRef]


| Forest Patch | Altitude Range (m) | Soil Substrate | Habitat Type | Number of Trees Sampled |
|---|---|---|---|---|
| 1 | 805–1100 | gneiss–schist | Thermophytic | 21 |
| 2 | 1100–1300 | gneiss–schist | Acidophytic | 20 |
| 3 | 1400–1500 | marble | Basiphytic | 19 |
| 4 | 16,601,760 | marble | Basiphytic | 20 |
| Trait | Unit | Method |
|---|---|---|
| LENGTH | cm | Length of leaf lamina |
| WIDTH | cm | Maximum width of leaf lamina |
| PERIMETER | cm | Lamina perimeter |
| Leaf Index (LI) | (LENGTH/WIDTH) × 100 | |
| Maximum Width Index (MWI) | (The distance between the lamina basis and the point of maximum width on the primary axis/LENGTH) × 100 | |
| RadiusRatio (RR) | The maximum distance possible connecting the center of the lamina with the perimeter/the minimum distance possible connecting the center of the lamina with the perimeter | |
| ROUNDNESS | PERIMETER/(4π × lamina area) | |
| AREABOX | The lamina area/the area of its imaginary bounding box | |
| DFINDEX | The longest caliper (feret) length within the lamina area/average caliper (feret) length within the lamina area | |
| Perimeter Ratio (PR) | Ratio of the convex perimeter of the lamina/ PERIMETER | |
| Fractal Dimension (FD) | The fractal dimension of the lamina’s outline | |
| ANGLE | degrees | Angle between the primary axis of the lamina and the first secondary nerve on the left side |
| VEINS | Number of secondary nerves of the lamina | |
| PETIOLE | cm | Length of the leaf petiole |
| Petiole Index (PI) | PETIOLE/LENGTH |
| Light Leaves | Shade Leaves | ANOVA | ||||
|---|---|---|---|---|---|---|
| Trait | Mean | CV% | Mean | CV% | F | p (>F) |
| LENGTH | 7.76 | 15.21 | 8.37 | 11.83 | 12.72 | <0.001 |
| WIDTH | 4.75 | 14.95 | 5.08 | 11.22 | 10.32 | 0.002 |
| PERIMETER | 19.43 | 14.46 | 20.87 | 10.92 | 12.65 | <0.001 |
| Leaf Index (LI) | 164.08 | 8.04 | 165.58 | 8.11 | 0.51 | 0.476 |
| Maximum Width Index (MWI) | 50.15 | 4.71 | 49.65 | 4.83 | 1.78 | 0.184 |
| RadiusRatio (RR) | 1.714 | 8.58 | 1.748 | 8.70 | 2.16 | 0.143 |
| ROUNDNESS | 1.144 | 3.58 | 1.161 | 3.79 | 5.969 | 0.016 |
| AREABOX | 0.679 | 2.06 | 0.670 | 2.09 | 16.51 | <0.001 |
| DFINDEX | 0.933 | 1.93 | 0.928 | 1.94 | 2.97 | 0.087 |
| Perimeter Ratio (PR) | 1.000 | 0.20 | 0.999 | 0.10 | 0.34 | 0.562 |
| Fractal Dimension (FD) | 1.006 | 0.10 | 1.006 | 0.10 | 1.51 | 0.221 |
| ANGLE | 35.95 | 8.71 | 37.61 | 8.67 | 10.73 | 0.001 |
| VEINS | 17.35 | 10.37 | 18.15 | 9.37 | 8.314 | 0.004 |
| PETIOLE | 0.950 | 17.89 | 0.770 | 22.08 | 48.63 | <0.001 |
| Petiole Index (PI) | 12.40 | 16.13 | 9.17 | 18.10 | 123.50 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamidis, G.C.; Varsamis, G.; Tsiripidis, I.; Dimitrakopoulos, P.G.; Papageorgiou, A.C. Patterns of Leaf Morphological Traits of Beech (Fagus sylvatica L.) along an Altitudinal Gradient. Forests 2021, 12, 1297. https://doi.org/10.3390/f12101297
Adamidis GC, Varsamis G, Tsiripidis I, Dimitrakopoulos PG, Papageorgiou AC. Patterns of Leaf Morphological Traits of Beech (Fagus sylvatica L.) along an Altitudinal Gradient. Forests. 2021; 12(10):1297. https://doi.org/10.3390/f12101297
Chicago/Turabian StyleAdamidis, George C., Georgios Varsamis, Ioannis Tsiripidis, Panayiotis G. Dimitrakopoulos, and Aristotelis C. Papageorgiou. 2021. "Patterns of Leaf Morphological Traits of Beech (Fagus sylvatica L.) along an Altitudinal Gradient" Forests 12, no. 10: 1297. https://doi.org/10.3390/f12101297
APA StyleAdamidis, G. C., Varsamis, G., Tsiripidis, I., Dimitrakopoulos, P. G., & Papageorgiou, A. C. (2021). Patterns of Leaf Morphological Traits of Beech (Fagus sylvatica L.) along an Altitudinal Gradient. Forests, 12(10), 1297. https://doi.org/10.3390/f12101297









