Dynamics of Nocturnal Evapotranspiration and Its Biophysical Controls over a Desert Shrubland of Northwest China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Flux and Biophysical Measurements
2.3. Data Processing
2.4. Calculation of Bulk Parameters
2.4.1. Penman–Monteith Equation
2.4.2. Calculations of Extractable Water Content
2.5. Growing Season, Phenological Phase and Data Analysis
3. Results
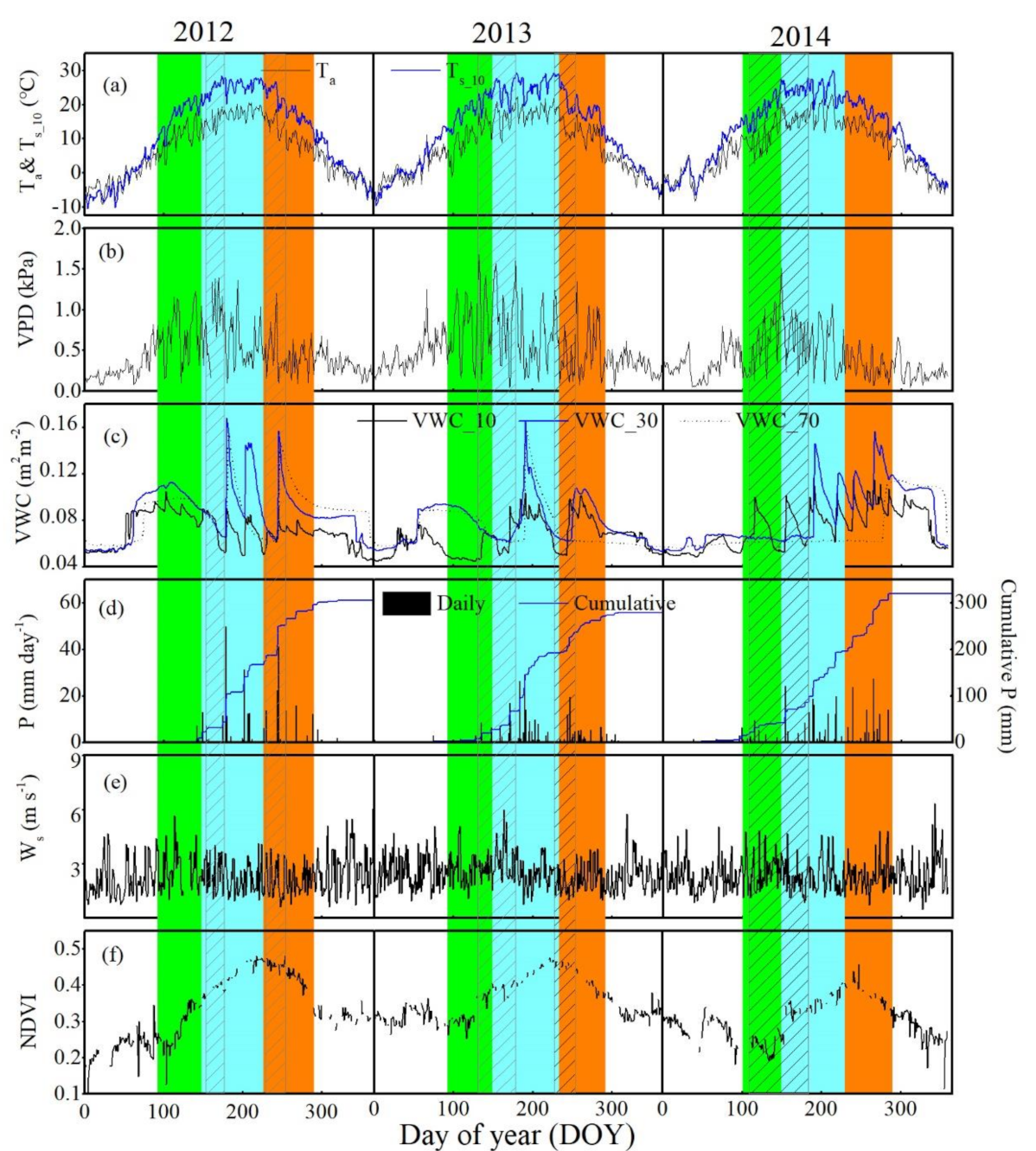
3.1. Biophysical Factors
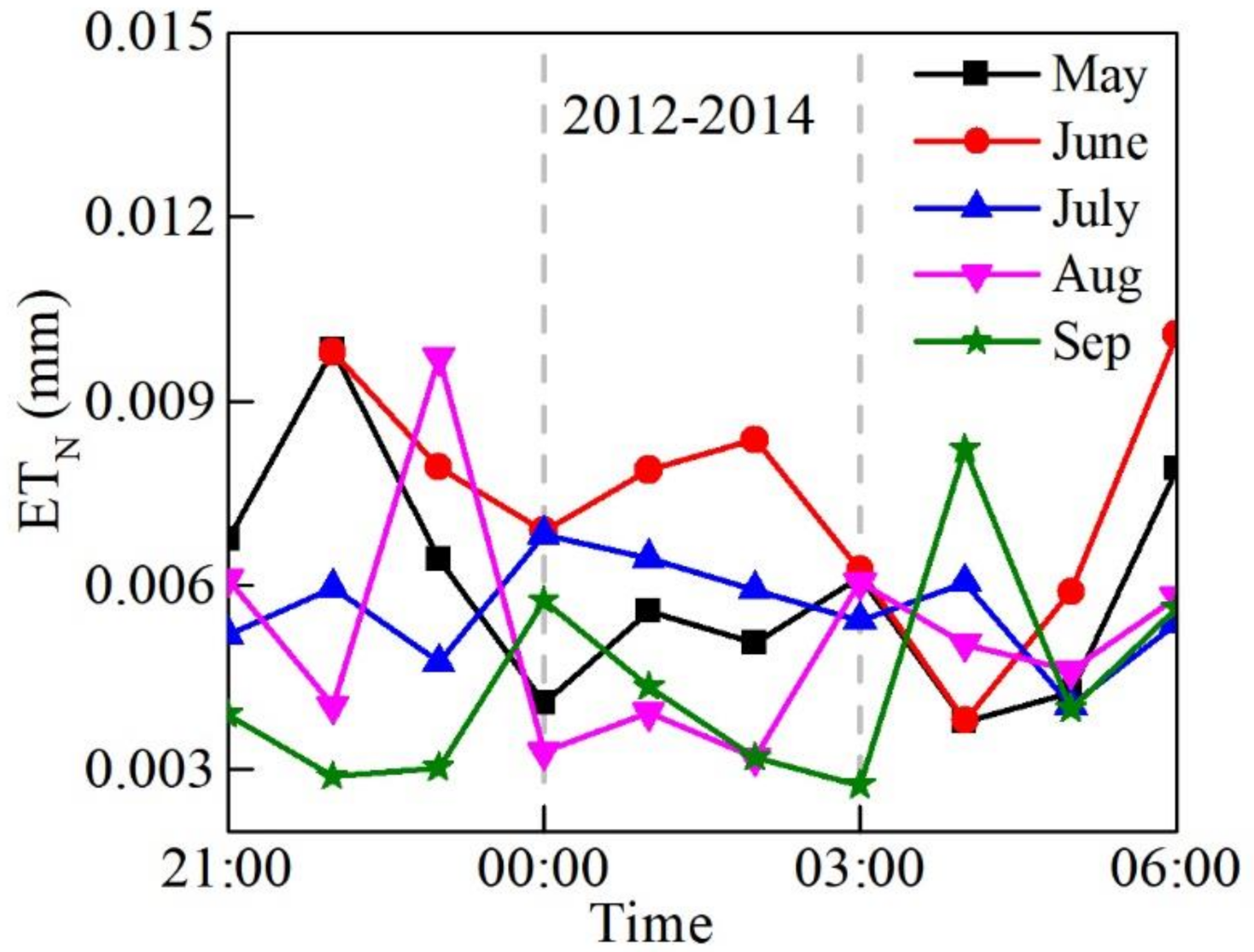
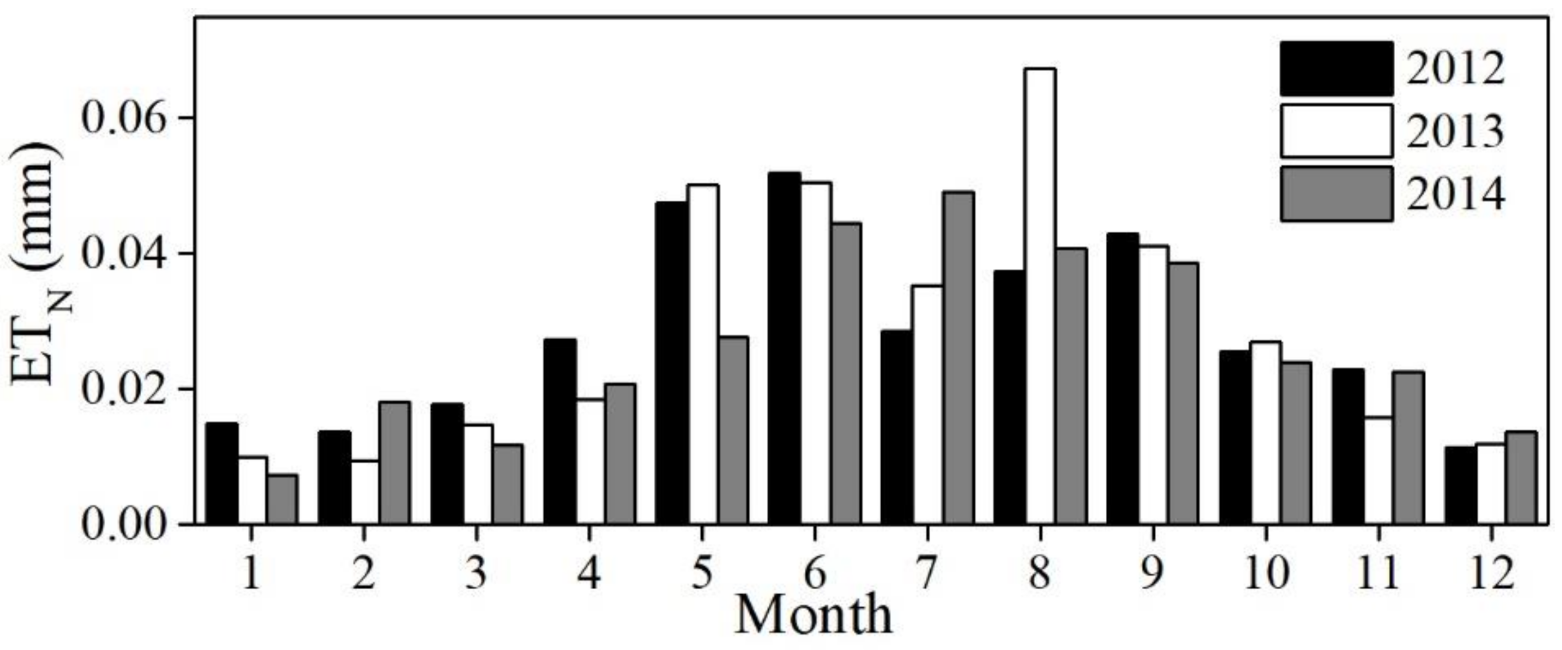
3.2. Diurnal, Seasonal and Interannual Variations in ETN
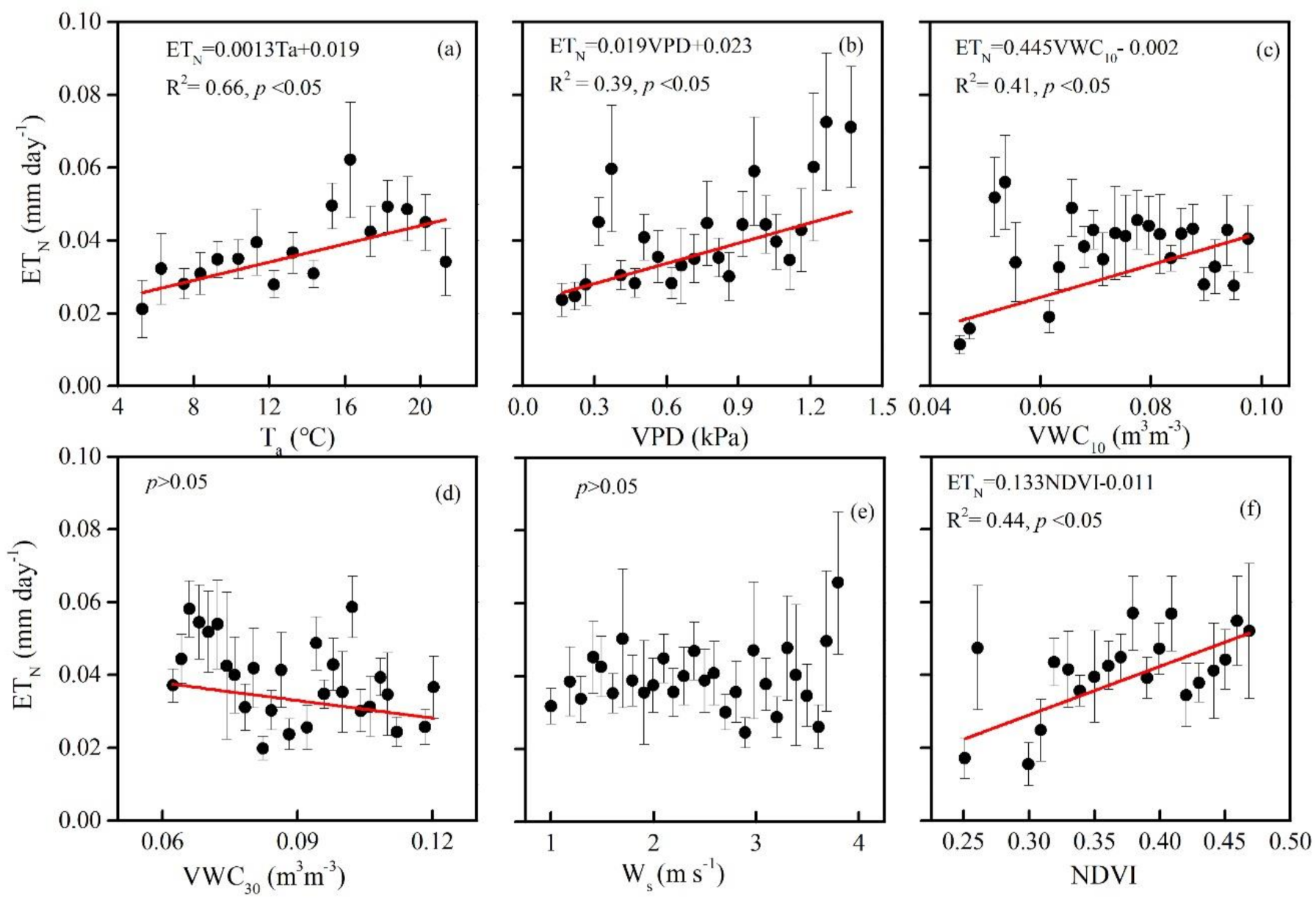
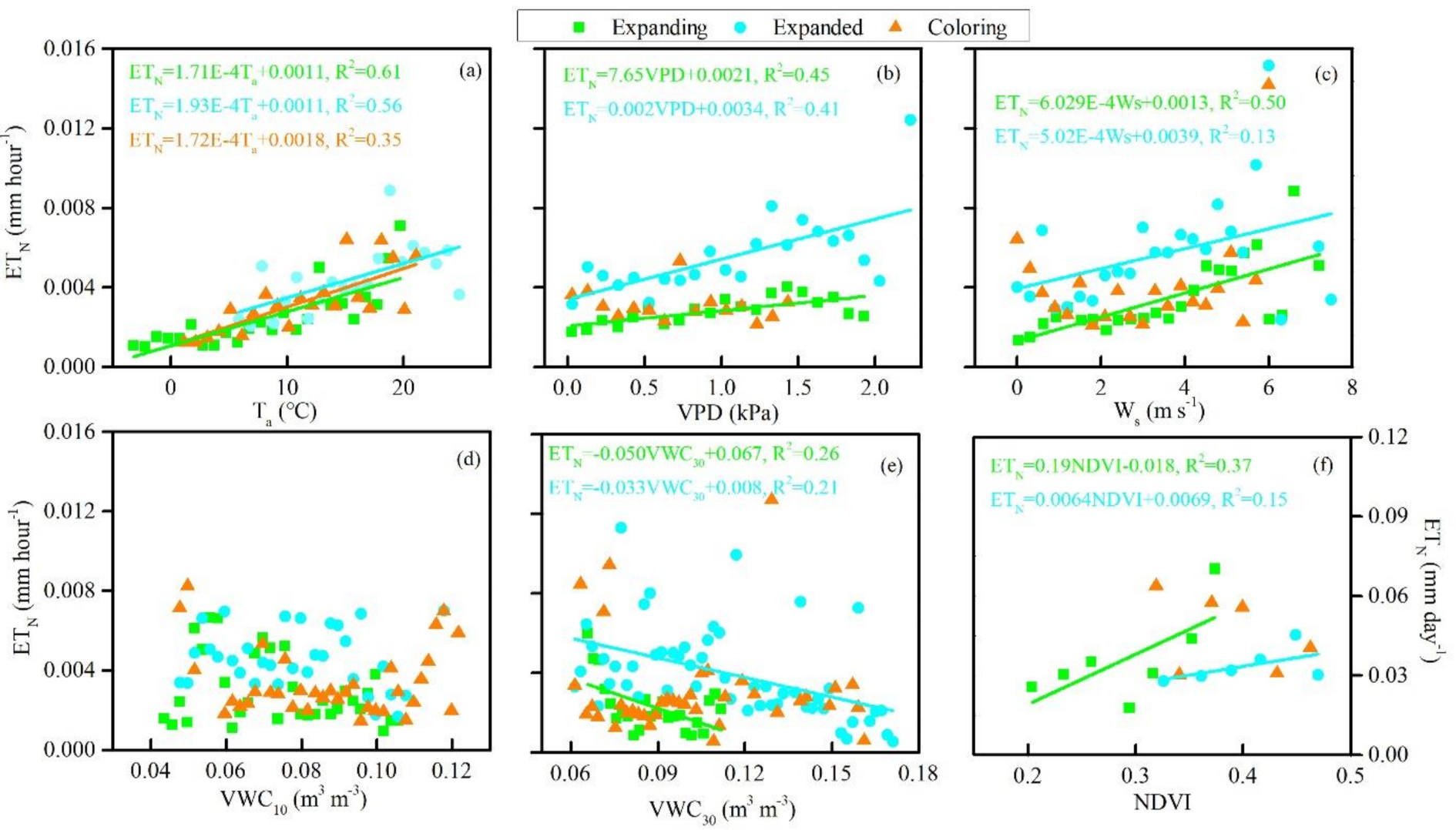
3.3. Biophysical Regulations on ETN
4. Discussion
4.1. Measurement Uncertainties
4.2. Magnitude of ETN
4.3. Biophysical Controls on Seasonal and Phenological Variation in ETN
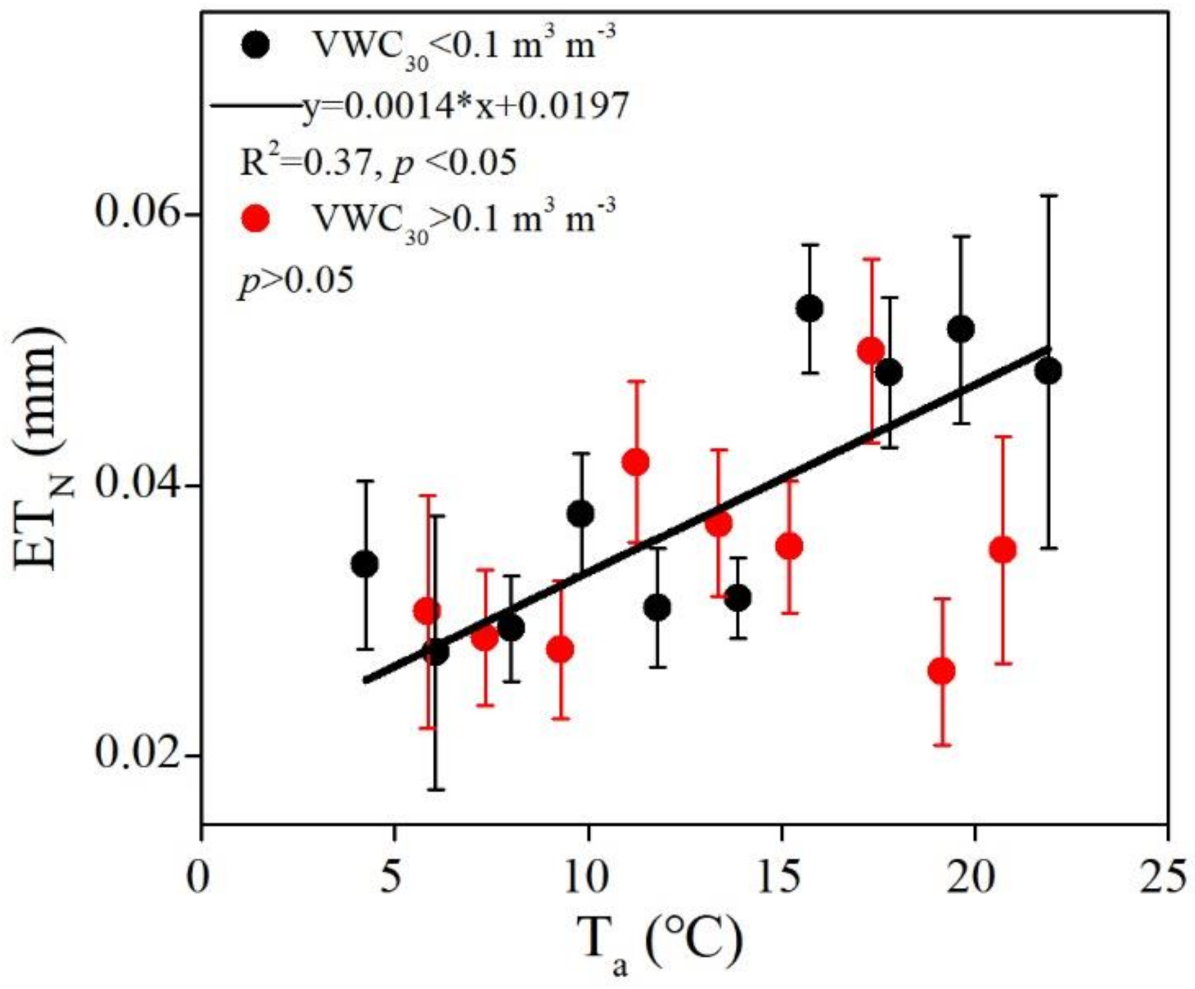
4.4. Effects of Drought on ET
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padron, R.S.; Gudmundsson, L.; Michel, D.; Seneviratne, S.I. Terrestrial water loss at night: Global relevance from observations and climate models. Hydrol. Earth Syst. Sci. 2020, 24, 793–807. [Google Scholar] [CrossRef] [Green Version]
- Fisher, J.B.; Melton, F.; Middleton, E.; Hain, C.; Anderson, M.; Allen, R.; McCabe, M.F.; Hook, S.; Baldocchi, D.; Townsend, P.A.; et al. The future of evapotranspiration: Global requirements for ecosystem functioning, carbon and climate feedbacks, agricultural management, and water resources. Water Resour. Res. 2017, 53, 2618–2626. [Google Scholar] [CrossRef]
- Lombardozzi, D.L.; Zeppel, M.J.B.; Fisher, R.A.; Tawfik, A. Representing nighttime and minimum conductance in CLM4.5: Global hydrology and carbon sensitivity analysis using observational constraints. Geosci. Model. Dev. 2017, 10, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Caird, M.A.; Richards, J.H.; Donovan, L.A. Nighttime stomatal conductance and transpiration in C3 and C4 plants. Plant. Physiol. 2007, 143, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Fricke, W. Night-time transpiration–favouring growth? Trends Plant Sci. 2019, 24, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Dios, V.; Chowdhury, F.I.; Granda, E.; Yao, Y.; Tissue, D.T. Assessing the potential functions of nocturnal stomatal conductance in C3 and C4 plants. New Phytologist. 2019, 223. [Google Scholar]
- Chen, Z.; Zhang, Z.; Sun, G.; Chen, L.; Xu, H.; Chen, S. Biophysical controls on nocturnal sap flow in plantation forests in a semi-arid region of northern China. Agric. For. Meteorol. 2020, 284, 107904. [Google Scholar] [CrossRef]
- Matyssek, R.; Günthardt-Goerg, M.S.; Maurer, S.; Keller, T. Nighttime exposure to ozone reduces whole-plant production in Betula pendula. Tree Physiol. 1995, 15, 159–165. [Google Scholar] [CrossRef]
- Schoppach, R.; Sinclair, T.R.; Sadok, W. Sleep tight and wake-up early: Nocturnal transpiration traits to increase wheat drought tolerance in a Mediterranean environment. Funct. Plant Biol. 2020, 47, 1117–1127. [Google Scholar] [CrossRef]
- Chen, J.M.; Liu, J. Evolution of evapotranspiration models using thermal and shortwave remote sensing data. Remote Sens. Environ. 2020, 237, 111594. [Google Scholar] [CrossRef]
- Thomas, S.C. Photosynthetic capacity peaks at intermediate size in temperate deciduous trees. Tree Physiol. 2010, 30, 555–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayat, M.; Zha, T.; Jia, X.; Iqbal, S.; Qian, D.; Bourque, C.P.A.; Khan, A.; Tian, Y.; Bai, Y.; Liu, P.; et al. A multiple-temporal scale analysis of biophysical control of sap flow in Salix psammophila growing in a semiarid shrubland ecosystem of northwest China. Agric. For. Meteorol. 2020, 288–289, 107985. [Google Scholar] [CrossRef]
- Hayat, M.; Iqbal, S.; Zha, T.; Jia, X.; Yang, R. Biophysical control on nighttime sap flow in Salix psammophila in a semiarid shrubland ecosystem. Agric. For. Meteorol. 2021, 300, 108329. [Google Scholar] [CrossRef]
- Folland, C.K.; Karl, T.R.; Jim Salinger, M. Observed climate variability and change. Weather 2002, 57, 269–278. [Google Scholar] [CrossRef]
- Peng, S.; Piao, S.; Ciais, P.; Myneni, R.B.; Chen, A.; Chevallier, F.; Dolman, A.J.; Janssens, I.A.; Penuelas, J.; Zhang, G. Asymmetric effects of daytime and night-time warming on Northern Hemisphere vegetation. Nature 2013, 501, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Groh, J.; Pütz, T.; Gerke, H.H.; Vanderborght, J.; Vereecken, H. Quantification and Prediction of Nighttime Evapotranspiration for Two Distinct Grassland Ecosystems. Water Resour. Res. 2019, 55, 2961–2975. [Google Scholar] [CrossRef]
- Fisher, J.B.; Baldocchi, D.; Misson, L.; Dawson, T.E.; Goldstein, A.H. What the towers don’t see at night: Nocturnal sap flow in trees and shrubs at two AmeriFlux sites in California. Tree Physiol. 2007, 27, 597–610. [Google Scholar] [CrossRef] [Green Version]
- Dios, V.D.; Roy, J.; Ferrio, J.P.; Alday, J.G.; Landais, D.; Milcu, A.; Gessler, A. Processes driving nocturnal transpiration and implications for estimating land evapotranspiration. Sci. Rep. 2015, 5, 10975. [Google Scholar] [CrossRef]
- Jia, X.; Zha, T.; Gong, J.; Zhang, Y.; Wu, B.; Qin, S.; Peltola, H. Multi-scale dynamics and environmental controls on net ecosystem CO2 exchange over a temperate semiarid shrubland. Agric. For. Meteorol. 2018, 259, 250–259. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Si, J.H.; Qi, F.; Yu, T.F.; Li, P.D. Comparative study of daytime and nighttime sap flow of Populus euphratica. Plant Growth Regul. 2017, 82, 1–10. [Google Scholar] [CrossRef]
- Novick, K.A.; Oren, R.; Stoy, P.C.; Siqueira, M.B.S.; Katul, G.G. Nocturnal evapotranspiration in eddy-covariance records from three co-located ecosystems in the Southeastern U.S.: Implications for annual fluxes. Agric. For. Meteorol. 2009, 149, 1491–1504. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Zha, T.; Gong, J.; Wang, B.; Zhang, Y.; Wu, B.; Qin, S.; Peltola, H. Carbon and water exchange over a temperate semi-arid shrubland during three years of contrasting precipitation and soil moisture patterns. Agric. For. Meteorol. 2016, 228–229, 120–129. [Google Scholar] [CrossRef]
- Guo, X.; Zha, T.; Jia, X.; Wu, B.; Feng, W.; Xie, J.; Gong, J.; Zhang, Y.; Peltola, H. Dynamics of Dew in a Cold Desert-Shrub Ecosystem and Its Abiotic Controls. Atmos 2016, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Zha, T.S.; Wu, B.; Zhang, Y.Q.; Gong, J.N.; Qin, S.G.; Chen, G.P.; Qian, D.; Kellomäki, S.; Peltola, H. Biophysical controls on net ecosystem CO2 exchange over a semiarid shrubland in northwest China. Biogeosciences 2014, 11, 4679–4693. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Jia, X.; Li, C.; Yu, H.; Xie, J.; Wang, B.; Wu, Y.; Wang, S.; Yang, X.M. Extreme rainfall impacts on soil CO2 efflux in an urban forest ecosystem in Beijing, China. Can. J. Soil Sci. 2016, 96, 504–514. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Zha, T.; Jia, X.; Wang, B.; Guo, X.; Zhang, Y.; Wu, B.; Yang, Q.; Peltola, H. Diurnal Freeze-Thaw Cycles Modify Winter Soil Respiration in a Desert Shrub-Land Ecosystem. Forests 2016, 7, 161. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Zha, T.S.; Jia, X.; Wu, B.; Zhang, Y.Q.; Qin, S.G. Soil moisture modifies the response of soil respiration to temperature in a desert shrub ecosystem. Biogeosciences 2014, 11, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Zha, T.S.; Gong, J.N.; Wu, B.; Zhang, Y.Q.; Qin, S.G.; Chen, G.P.; Feng, W.; Kellomäki, S.; Peltola, H. Energy partitioning over a semi-arid shrubland in northern China. Hydrol. Process. 2016, 30, 972–985. [Google Scholar] [CrossRef]
- She, W.; Bai, Y.; Zhang, Y.; Qin, S.; Feng, W.; Lai, Z.; Qiao, Y.; Liu, L.; Zhang, W.; Miao, C. Nitrogen-enhanced herbaceous competition threatens woody species persistence in a desert ecosystem. Plant Soil 2021, 460, 333–345. [Google Scholar] [CrossRef]
- Jia, X.; Mu, Y.; Zha, T.; Wang, B.; Tian, Y. Seasonal and interannual variations in ecosystem respiration in relation to temperature, moisture, and productivity in a temperate semi-arid shrubland. Sci. Total Environ. 2019, 709, 136210. [Google Scholar] [CrossRef]
- Schmid, H.P. Experimental design for flux measurements: Matching scales of observations and fluxes. Agric. For. Meteorol. 1997, 87, 179–200. [Google Scholar] [CrossRef]
- Zha, T.-S.; Wu, Y.J.; Jia, X.; Zhang, M.Y.; Bai, Y.J.; Liu, P.; Ma, J.Y.; Bourque, C.P.-A.; Peltola, H. Diurnal response of effective quantum yield of PSII photochemistry to irradiance as an indicator of photosynthetic acclimation to stressed environments revealed in a xerophytic species. Ecol. Indic. 2017, 74, 191–197. [Google Scholar] [CrossRef]
- Xie, J.; Zha, T.; Zhou, C.; Jia, X.; Yu, H.; Yang, B.; Chen, J.; Zhang, F.; Wang, B.; Bourque, C.P.A.; et al. Seasonal variation in ecosystem water use efficiency in an urban-forest reserve affected by periodic drought. Agric. For. Meteorol. 2016, 221, 142–151. [Google Scholar] [CrossRef]
- Misson, L.; Tang, J.; Ming, X.; Mckay, M.; Goldstein, A. Influences of recovery from clear-cut, climate variability, and thinning on the carbon balance of a young ponderosa pine plantation. Agric. For. Meteorol. 2005, 130, 207–222. [Google Scholar] [CrossRef]
- Foken, T.; Göockede, M.; Mauder, M.; Mahrt, L.; Amiro, B.; Munger, W. Post-field data quality control. In Handbook of Micrometeorology; Springer: Berlin/Heidelberg, Germany, 2004; pp. 181–208. [Google Scholar]
- Hollinger, D.Y.; Aber, J.; Dail, B.; Davidson, E.A.; Goltz, S.M.; Hughes, H.; Leclerc, M.Y.; Lee, J.T.; Richardson, A.D.; Rodrigues, C.; et al. Spatial and temporal variability in forest–atmosphere CO2 exchange. Glob. Chang. Biol. 2004, 10, 1689–1706. [Google Scholar] [CrossRef]
- Ma, J.; Jia, X.; Zha, T.; Bourque, C.P.A.; Tian, Y.; Bai, Y.; Liu, P.; Yang, R.; Li, C.; Li, C.; et al. Ecosystem water use efficiency in a young plantation in Northern China and its relationship to drought. Agric. For. Meteorol. 2019, 275, 1–10. [Google Scholar] [CrossRef]
- Papale, D.; Reichstein, M.; Aubinet, M.; Canfora, E.; Bernhofer, C.; Kutsch, W.; Longdoz, B.; Rambal, S.; Valentini, R.; Vesala, T. Towards a standardized processing of Net Ecosystem Exchange measured with eddy covariance technique: Algorithms and uncertainty estimation. Biogeosciences 2006, 3, 571–583. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zhang, Z.; Xiao, J.; Chen, J.; Zhu, M.; Cao, W.; Chen, Z. Environmental and canopy stomatal control on ecosystem water use efficiency in a riparian poplar plantation. Agric. For. Meteorol. 2020, 287, 107953. [Google Scholar] [CrossRef]
- Falge, E.; Baldocchi, D.; Olson, R.; Anthoni, P.; Aubinet, M.; Bernhofer, C.; Burba, G.; Ceulemans, R.; Clement, R.; Dolman, H. Gap filling strategies for long term energy flux data sets. Agric. For. Meteorol. 2001, 107, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Wilson, K.; Goldstein, A.; Falge, E.; Aubinet, M.; Verma, S. Energy balance closure at FLUXNET sites. Agric. For. Meteorol. 2002, 113, 223–243. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Gu, B.; Du, T.; Zhang, J. Crop coefficient and ratio of transpiration to evapotranspiration of winter wheat and maize in a semi-humid region. Agric. Water Manag. 2007, 59, 239–254. [Google Scholar] [CrossRef]
- Campbell, G.S.N.; Norman, J. An Introduction to Environmental Biophysics; Springer Science & Business Media: Dordrecht, The Netherlands, 1998. [Google Scholar]
- Zhou, J.; Zhang, Z.; Sun, G.; Fang, X.; Zha, T.; McNulty, S.; Chen, J.; Jin, Y.; Noormets, A. Response of ecosystem carbon fluxes to drought events in a poplar plantation in Northern China. For. Ecol. Manag. 2013, 300, 33–42. [Google Scholar] [CrossRef]
- Zha, T.; Barr, A.G.; Black, T.A.; McCaughey, J.H.; Bhatti, J.; Hawthorne, I.; Krishnan, P.; Kidston, J.; Saigusa, N.; Shashkov, A.; et al. Carbon sequestration in boreal jack pine stands following harvesting. Glob. Chang. Biol. 2009, 15, 1475–1487. [Google Scholar] [CrossRef]
- Guo, X.N.; Zha, T.S.; Jia, X.; Yang, Q.; Liu, P. Estimation of dewfall amount in a typical desert shrub ecosystem. J. Beijing For. Univ. 2016. [Google Scholar]
- Zhao, C.; Si, J.; Feng, Q.; Yu, T.; Li, P.; Forster, M.A. Nighttime transpiration of Populus euphratica during different phenophases. J. For. Res. 2019, 30, 435–444. [Google Scholar] [CrossRef]
- Phillips, N.G.; Lewis, J.D.; Logan, B.A.; Tissue, D.T. Inter- and intra-specific variation in nocturnal water transport in Eucalyptus. Tree Physiol. 2010, 30, 586–596. [Google Scholar] [CrossRef] [Green Version]
- Zeppel, M.; Logan, B.; Lewis, J.D.; Phillips, N.; Tissue, D. Why lose water at night? Disentangling the mystery of nocturnal sap flow, transpiration and stomatal conductance—When, where, who? Acta Hortic. 2013, 991, 307–312. [Google Scholar] [CrossRef]
- Reichstein, M.; Tenhunen, J.D.; Roupsard, O.; Ourcival, J.m.; Rambal, S.; Miglietta, F.; Peressotti, A.; Pecchiari, M.; Tirone, G.; Valentini, R. Severe drought effects on ecosystem CO2 and H2O fluxes at three Mediterranean evergreen sites: Revision of current hypotheses? Glob. Chang. Biol. 2002, 8, 999–1017. [Google Scholar] [CrossRef]
- Launiainen, S. Seasonal and inter-annual variability of energy exchange above a boreal Scots pine forest. Biogeosciences 2010, 7, 3921–3940. [Google Scholar] [CrossRef] [Green Version]
- Dawson, T.E.; Burgess, S.S.O.; Tu, K.P.; Oliveira, R.S.; Santiago, L.S.; Fisher, J.B.; Simonin, K.A.; Ambrose, A.R. Nighttime transpiration in woody plants from contrasting ecosystems. Tree Physiol. 2007, 27, 561–575. [Google Scholar] [CrossRef] [Green Version]


| Variable | Year | ||
|---|---|---|---|
| 2012 | 2013 | 2014 | |
| Ta (°C) | 8.11 | 9.50 | 9.34 |
| Ts_10 (°C) | 11.31 | 12.62 | 12.60 |
| VPD (kPa) | 0.67 | 0.78 | 0.73 |
| VWC10 (m3 m−3) | 0.070 | 0.065 | 0.073 |
| VWC30 (m3 m−3) | 0.086 | 0.077 | 0.082 |
| Ws (m s−1) | 2.8 | 3.1 | 3.1 |
| NDVI | 0.33 | 0.35 | 0.30 |
| P (mm) | 335 | 278 | 342 |
| ET (mm) | 331 | 260 | 230 |
| ETN (mm) | 9 | 11 | 10 |
| Maximum daily ETN (mm) | 0.21 | 0.17 | 0.14 |
| Number of days during droughts (mm) | 27 | 78 | 90 |
| Year | Equation | R2 | p for the Model | p for the Individual Variables |
|---|---|---|---|---|
| 2012 | ETN = 0.261Ta + 0.190VWC10 | 0.17 | <0.01 | <0.01(Ta) |
| <0.01(VWC10) | ||||
| 2013 | ETN = 0.473Ta | 0.23 | <0.01 | <0.01(Ta) |
| 2014 | ETN = 0.573Ta + 0.163VWC30 | 0.50 | <0.01 | <0.01(Ta) |
| <0.01(VWC30) | ||||
| 2012–2014 | ETN = 0.275Ta + 0.150VPD + 0.135VWC10 | 0.26 | <0.01 | <0.01(Ta) |
| <0.05(VPD) | ||||
| <0.05(VWC10) |
| Phenologies | Equation | R2 | p For The Model | p for the Individual Variables |
|---|---|---|---|---|
| Expanding | ETN = 0.496VWC30 + 0.316NDVI | 0.58 | <0.01 | <0.01(VWC30) <0.01(NDVI) |
| Expanded | ETN = 0.531VPD + 0.154VWC30 | 0.42 | <0.01 | <0.01(VPD) |
| <0.05(VWC30) | ||||
| Coloring | ETN = 0.732Ta − 0.297Ws | 0.30 | <0.01 | <0.01(Ta) |
| <0.015(Ws) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Shang, G.; Tian, Y.; Jia, X.; Zha, T.; Li, C.; Yang, H.; Zhang, X. Dynamics of Nocturnal Evapotranspiration and Its Biophysical Controls over a Desert Shrubland of Northwest China. Forests 2021, 12, 1296. https://doi.org/10.3390/f12101296
Guo X, Shang G, Tian Y, Jia X, Zha T, Li C, Yang H, Zhang X. Dynamics of Nocturnal Evapotranspiration and Its Biophysical Controls over a Desert Shrubland of Northwest China. Forests. 2021; 12(10):1296. https://doi.org/10.3390/f12101296
Chicago/Turabian StyleGuo, Xiaonan, Guofei Shang, Yun Tian, Xin Jia, Tianshan Zha, Cheng Li, Huicai Yang, and Xia Zhang. 2021. "Dynamics of Nocturnal Evapotranspiration and Its Biophysical Controls over a Desert Shrubland of Northwest China" Forests 12, no. 10: 1296. https://doi.org/10.3390/f12101296
APA StyleGuo, X., Shang, G., Tian, Y., Jia, X., Zha, T., Li, C., Yang, H., & Zhang, X. (2021). Dynamics of Nocturnal Evapotranspiration and Its Biophysical Controls over a Desert Shrubland of Northwest China. Forests, 12(10), 1296. https://doi.org/10.3390/f12101296






