Abstract
Intermediate hawthorn (Crataegus × media Bechst.) is broadly distributed in Europe but very rarely examined by dendrochronologists. In NW Poland, it is one of three naturally occurring hawthorn species, growing mainly at forest margins, along roads, in mid-field woodlots, and on uncultivated land. Biocenotically, it is a very valuable species. This study aimed to determine the age of trees, tree-ring dynamics, and growth–climate relationship for intermediate hawthorn. Signature years were also determined. Samples for analysis were collected from 22 trees growing in a typical agricultural landscape in a monospecific mid-field woodlot comprised of several hundred specimens of various ages and forms (shrubs and trees). Using classic methods of dendrochronological dating, a 40-year long chronology spanning 1981–2020 was constructed. The radial growth rate of intermediate hawthorn is comparable to other tree species forming stands in NW Poland and equals 2.41 mm/year. Considerable intersubject variability is noted, from 1.48 to 4.44 mm/year. The chronology was also used for dendroclimatological analyses, including correlation and response function and signature years. Of the meteorological parameters analyzed, annual incremental growth in hawthorn is the most strongly shaped by precipitation totals from May to August of the current vegetation year: high rainfall favors the formation of wide tree-rings. Statistically significant growth–climate relationships were also obtained for winter months (December of the preceding vegetation year, January and February), for which period negative correlation and regression values are noted for air temperature and insolation. Furthermore, high precipitation, low-temperature and low insolation late in the preceding vegetation year (especially in August) make a positive influence on the condition of trees in the upcoming growing season. Signature year analysis clearly pointed to precipitation as the dominant factor in shaping tree-rings in the studied hawthorn population. As there are no dendrochronological papers concerning indigenous hawthorn species, future studies should be expanded to include diverse geographic locations and habitat conditions and should include all three species of hawthorn occurring in Poland.
1. Introduction
Intermediate hawthorn (Crataegus × media Bechst.) is a hybrid of two hawthorn species occurring naturally in Poland: one-seeded hawthorn (C. monogyna) and midland hawthorn (C. laevigata). In Poland, intermediate hawthorn occurs mainly in the west, whereas in Europe, it ranges from France and England in the west to Lithuania and Finland in the east, and from Italy and Spain in the south to southern Sweden, Norway and Finland in the north [1,2,3,4]. It is abundant in central Europe, including Denmark, Germany, Czech Republic and Poland [4,5,6]. Some hawthorn species (especially one-seeded hawthorn) are considered invasive in New Zealand, Australia and North America [7,8,9,10]. There, they hybridize with naturally occurring hawthorn species, intensely spread and threaten indigenous plant species.
Hawthorn species indigenous to Poland (C. monogyna, C. laevigata and C. × media) have small habitat requirements, prefer sun-lit sites (scarps, forest margins, roadsides, mid-field woodlots, or clearings), are resistant to drought and frost [2,11]. They are noted for their high biocenotic significance, as hawthorn gatherings are a source of nutrition for numerous animal taxa, provide shelter, and protect water and soils [12,13]. On the other hand, however, they are also the habitat of numerous pests that infest both hawthorns and orchard-grown trees (especially apple and pear trees). Hawthorns (both inflorescences and fruits) are also used in folk medicine and herbalism as a remedy to diarrhea, insomnia and for the treatment of cardiovascular diseases and digestive tract conditions. They are the source of numerous highly biologically active chemical compounds that display, for instance, anti-inflammatory, antibacterial and antioxidative effects [14,15,16,17].
A survey of publications included in Ref … [6] indicates there are few papers on intermediate hawthorn. Most studies focus on C. monogyna and C. laevigata. The prevailing study topics include pests and diseases, seed dispersal by birds and other animals, seed and fruit morphology, and seedling browsing by sheep and rabbits. In several papers, hawthorns are presented as invasive taxa, and numerous papers concern chemical eradication of hawthorn. Unfortunately, dendrochronological papers are lacking (this refers to all hawthorn species), although, in two papers, there are remarks on tree age, tree-rings and growth rate [7,18]. Out of approximately 50 hawthorn species occurring in Europe and Asia and 100 species occurring in North America, the study by Grissino-Mayer [19] concerning the potential of shrubs and trees for dendrochronology includes only C. azorolus L. (code-named CRAZ), which is given a cross-dating index (CDI) of 0, which means “species does not crossdate, or no information on crossdating for this species has been published. NO OR LITTLE IMPORTANCE IN DENDROCHRONOLOGY”. C. × media is also absent from Microscopic Wood Anatomy [20], which states only that subgenus Crataegus has no heatwood, and the anatomical structure of wood cannot serve as a basis for species identification. Hawthorn wood is diffuse-porous, and thus correct identification of ring boundaries is often problematic. The wood is very hard, with a yellow-light brown color.
The scarcity of publications on Crataegus × media Bechst. ecology and lack of dendrochronological data prompted the authors to determine the radial growth dynamics and growth–climate relationship for intermediate hawthorn. The present study aimed to (1) determine tree age and analyze radial growth dynamics; (2) examine the relationship between tree-ring width and meteorological conditions; and (3) determine signature years—especially favorable or unfavorable for tree development. The observations performed in this study will expand our understanding of the ecology of both C. × media and the parent taxa, both in areas of their natural occurrences and in areas where they are considered invasive plants.
2. Material and Methods
2.1. Study Area
The study area (N: 53°25′04.59′′ E: 14°24′17.12′′, 45 m a.s.l.) is located in NW Poland (about 2.2 km east from the Polish-German border, and about 5 km to the west of Szczecin city limits), on a morainic plateau composed of last glacial period sediments [21], in a typical agricultural landscape. On German topographic maps (including one from 1921, Figure 1A), the study area is traversed by a dirt road. Following World War II, the study area and the surrounding was plowed, and today it is sown on an annual basis. Only the study area was excluded from farming. The intermediate hawthorn gathering is derived probably from one or several individuals sown by birds next to the dirt road. At present, it is a monospecific mid-field woodlot, formed by trees and shrubs of intermediate hawthorn (Crataegus media Bechst.), composed of several hundred individuals of this species, of varying age. The woodlot is about 75 m long and 20–25 m wide, located on a SE-facing scarp rising up to 4 m above the surrounding. The substratum is composed of boulder clay, on which a cover of brown soils has developed. From all sides, the woodlot is surrounded by farmland (Figure 1B). During the 2020 vegetation season, the hawthorn trees were found to be infested by numerous individuals of orchard ermine (Yponomeuta padella), causing partial damage to the assimilation apparatus [22,23].
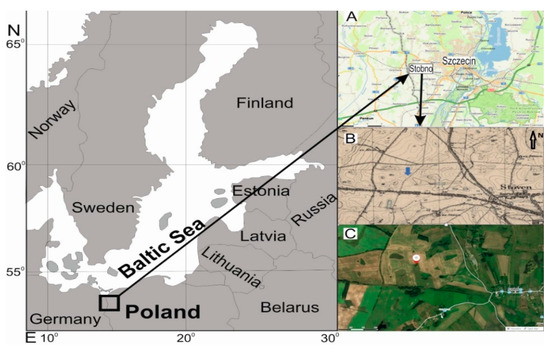
Figure 1.
Location of the study area: (A,C)—source of the photo: https://en.mapy.cz, (B)—Meßtischblatt 2552, Kreckow, 1921, scale 1:25,000.
Based on the following features: monospecific stand, no interspecific competition, no shade, likely little genetic diversity, mid-field location on a SE-facing slope, the study area was considered a typical intermediate hawthorn habitat for western Poland, and a prime example of climate-growth relationship examination.
2.2. Tree-Ring Data
Samples were taken using Pressler drills at a level of 1.3 m above ground. A total of 22 trees were sampled, including 30 trunks (numerous hawthorns had 2–3 trunks), yielding 52 wood cores. In the laboratory, samples were glued onto specially prepared mounting boards. Once dried, wood cores were cut using a sharp knife until ring boundaries could be clearly distinguished. Sample surfaces were smeared with chalk in order to enhance the ring boundaries. Tree-ring width was measured down to 0.01 mm under a stereomicroscope, using the Dendrometer software [24]. A total of 1990 tree-rings were measured. All abnormalities in the anatomical structure of tree-rings were noted during measurements, i.e., false rings (2–4 rows of cells) immediately adjacent to the tree-ring boundary and often pinching out were commonly observed. Tree-rings were assigned to calendar years using classic dating methods (cross-dating method). The tree-ring chronology verification, false ring identification was performed using Cofecha software [25,26]. Residual chronology (RES) was assembled using the Arstan program [27,28], emphasizing short-term variability and eliminating long-term trends and autocorrelation by applying a two-phase detrending technique, by fitting either a modified negative exponential curve or a regression line with a negative or zero slope [29,30]. Expressed population signal (EPS) index was also calculated in order to determine the chronology quality [31]. Dendroclimatic analyses were performed, including correlation and response function analysis, as well as signature year analysis. These analyses utilized air temperature (T), atmospheric precipitation (P) and insolation (IN) data from the period 1981–2020 (40 years). Correlation and response function analysis, performed using the Respo program [28], utilized residual chronology (RES) and meteorological data spanning 16 months, from June (pVI) of the year preceding the growth to September of the current vegetation year (IX). The analysis was performed separately for each meteorological parameter (T, P, IN), estimating a multiple regression determination coefficient (r2) for each parameter. Signature year analysis was carried out using TCS software [32] by calculating positive years (+) characterized by an increase in tree-ring width relative to the preceding year, and negative years (−), with a reduction in tree-ring width relative to the preceding year [33,34,35,36]. Signature years were calculated based on a minimum of 10 trees, assuming 90% as the minimum incremental trend consistency threshold. Student’s t-test value (computed using Prot, part of the Tree Rings software package) [37], and coherence coefficient Gleichläufigkeitswert (Gl, computed using the TCS program) [32] were calculated in order to determine the similarity of shared periods between ST chronology and local chronologies obtained for other tree taxa. Local chronologies for 11 tree species occurring in NW Poland in similar climatic conditions were selected for these comparisons.
2.3. Climate
The research area is characterized by cold climate—Dfb (without a dry season and warm summer) sensu Peel et al. [38], with a mean annual temperature of 7.1–10.9 °C (average 8.8 °C), annual precipitation sum of 347–796 mm (average 545 mm) and insolation of 1212–2186 h (average 1610 h). The warmest month is July with a mean temperature of 15.2–22.7 °C (average 18.1 °C), the coldest month is January with a mean temperature of −8.8–5.1 °C (average −0.3 °C). The most humid month is July, with an average precipitation sum of 71 mm, ranging from 5 to 193 mm. Precipitation is the lowest in February and March (31–33 mm). The highest insolation is noted in May (on average 234 h), but insolation is >200 h also from July to August. Insolation is the lowest in December, with an average of only 29 h per month, due to short day duration and high cloud cover. Due to the frequent advection of polar marine air masses, snow cover in winter is not permanent and is highly variable temporally.
Meteorological data (monthly mean temperatures, precipitation sums and insolation sums) come from the weather station Szczecin-Dąbie (12205), located 13 km to the east of the study area.
3. Results
3.1. Ring-Width Chronology
The highest number of measured tree-rings (60 and 58 tree-rings) were obtained for one of the trunks of a parent tree (ST22C). The curves obtained from this tree, however, were not included in the chronology due to the low similarity of the growth pattern to other samples. The chronology, code-named ST, was compiled based on dendrochronological curves derived from 20 trees, including 24 trunks. The ST chronology spans 56 years from 1965 to 2020 (Figure 2). It was, however, composed of 10 or more samples (EPS = 0.95) only for the period 1981–2020 (40 years), and therefore the data and analysed below concern this period only. The mean tree-ring width in the hawthorn population examined here was 2.41 mm, with a range of variation: from 1.48 mm for trees ST15 and ST6 to 4.44 mm for ST22. In recent years (since 2012), the mean tree-ring width displayed a decreasing trend (Figure 2). Standard deviation (STW) for the measured and indexed chronology (RES) equals 1.51 and 0.39, respectively, first-order autocorrelation (A1)—0.385 and 0.188, and mean sensitivity (MS)—0.558 and 0.455. At the boundary between late wood from 2003 and from 2004, discontinuous tree-rings (scars), or callus tissue and dark-colored wood tissue were observed in 27% of the studied trees (6 trees, 9 wood cores).
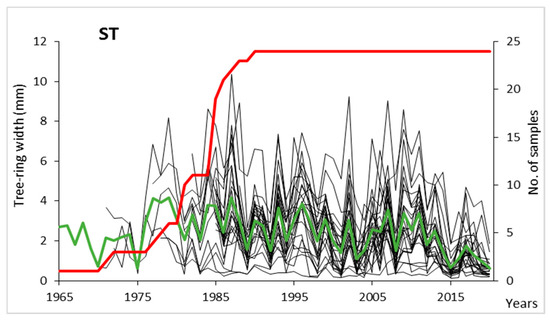
Figure 2.
Individual sequences of tree-ring width (thin black lines), making up the local hawthorn chronology (ST; green line), and number of samples included in the chronology (red line).
In order to estimate the radial growth rate for the studied hawthorn population, a comparison to 56-year long cambial age cumulative radial growth profiles of the main tree species in stands of NW Poland was made, including: Pinus sylvestris, Quercus spp., Picea abies, Fagus sylvatica and several admixture species (Taxus baccata, Sorbus torminalis, Aesculus hippocastanum) (Figure 3). Horse-chestnut at the avenue location had the highest rate of the radial growth, followed by very similar rated in beech, pine and oak—forest populations. Surprisingly, the hawthorn radial growth rate was comparable to the oak growth rate. Spruce, checker tree and yew had lower growth dynamics [39,40,41]. Among the 11 studied taxa occurring in NW Poland, the highest similarity to ST chronology was observed for a composite chronology based on indigenous oak species (Quercus robus L. and Q. petrea (Matt.) Liebl.): both t (3.63) and Gl (68%) attained the highest values for this chronology (Table 1). Student’s t-test values exceeded 3.0 for three other chronology pairs: ST/Quercus rubra, ST/Pinus sylvestris and ST/Aesculus hippocastanum. The Gl coefficient was ≥65% for two additional chronology pairs: ST/Quercus rubra and ST/Aesculus hippocastanum.
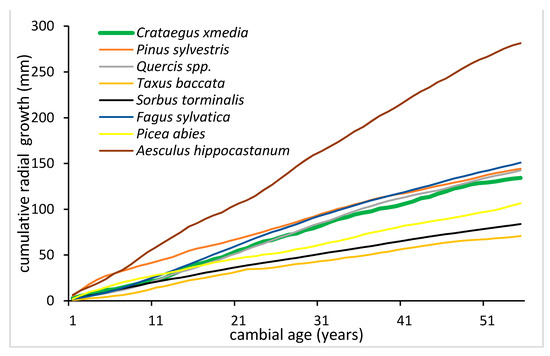
Figure 3.
Cumulative radial growth of Crataegus media and Pinus sylvestris, Quercus spp., Picea abies, Fagus sylvatica, Taxus baccata, Sorbus torminalis, Aesculus hippocastanum expressed in mm. Growth profiles for all species represent the mean values of the species population from the northwestern part of Poland.

Table 1.
Similarity between intermediate hawthorn (Crataegus media, ST) chronology and local chronologies for other tree species growing in NW Poland, expressed as Student’s t-test and Gl (Gleichläufigkeitswert) values.
3.2. Correlation and Response Function
Correlation and response function analysis indicated three periods of growth–climate relationship. Hawthorn growth dynamics were influenced by the end of the preceding vegetation year, the winter period, and the summer months of the current vegetation year (Figure 4). High precipitation, low-temperature and low insolation late in the preceding vegetation year (especially in August) had a positive influence on the tree condition in the upcoming growing season. For winter months (December, January and February), negative correlation and regression values were noted for air temperature and insolation. A period of positive growth-precipitation correlation was initiated in May of the current vegetation year (and lasts until August). Additionally, low temperatures and low insolation in July and August had a positive influence on tree-ring width. Of the three meteorological parameters analyzed, precipitation sum had the highest significance for shaping tree-ring growth in hawthorn, with the highest multiple regression coefficient (r2 = 49%). Insolation yielded r2 = 38%, and temperature yielded r2 = 34% (Figure 4).
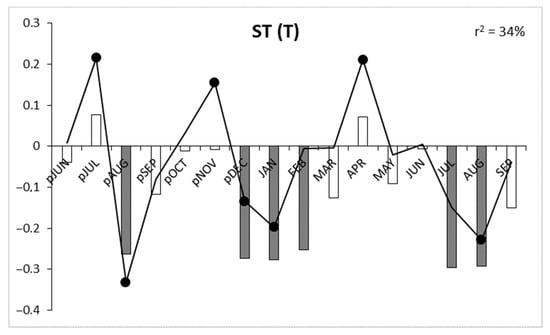
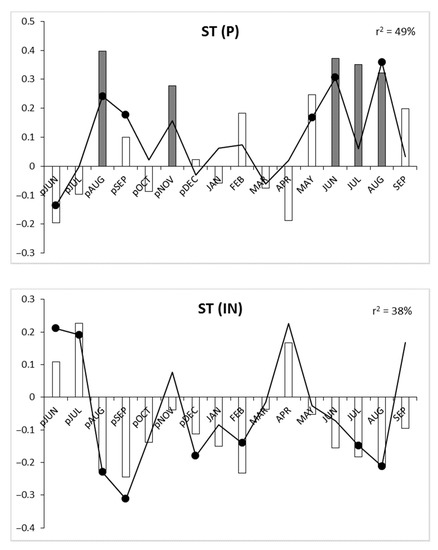
Figure 4.
Response function analysis for air temperature—T, precipitation—P and insolation—IN. Bars depict correlation coefficients, lines show regression coefficients, significant values (p ≤ 0.05) are indicated by gray bars and solid black dots, p—previous year, r2—multiple regression determination coefficients.
3.3. Signature Years
Nineteen signature years were calculated for the studied hawthorn population, including 8 positive years (+): 1984, 1987, 1990, 1993, 2002, 2009, 2011 and 2016, and 11 negative years (−): 1983, 1986, 1989, 1992, 1994, 1998, 2001, 2003, 2010, 2012 and 2015. An analysis of meteorological conditions in these years indicated that positive pointer years were characterized by higher than average precipitation in summer (June–August), lack of very high air temperatures in summer in conjunction with insufficient rainfall, and lower than usual insolation (Figure 5). In positive years, winter thermal conditions had no significant influence on tree-ring width, high precipitation in spring months favored the formation of broad tree-rings, and annual insolation, especially during summer months, was lower. Negative pointer years were often preceded by very dry years (e.g., for the year 1983—dry year 1982, for year 1986—dry year 1985, for year 1989—dry year 1988), or the given vegetation year was very dry (e.g., 1992, or the year 2003 which was the driest in a multiannual perspective, with a precipitation sum of 392 mm compared to multiannual norm equal to 545 mm, and year 2015). For all negative pointer years, insufficient precipitation occurred in summer months (1 to 3 months with low precipitation sums, Figure 5), often in conjunction with high air temperatures and high insolation. As in the case of positive pointer years, the weather conditions of the winter season preceding the pointer year were very varied. For 2003, a negative pointer year, scars, callus tissue, and discontinuous tree-rings were observed at the boundary between late wood from 2003 and early wood from 2004 in 27% of the examined trees. In these spots, the wood frequently had a dark color. This was most likely the effect of a fire late in the 2003 vegetation season. The summer of that year was unusually dry, warm and sunny (especially August, with a mean temperature of 19.1 °C, compared to a multiannual average of 17.5 °C, rainfall: 23 mm vs. 59 mm and insolation: 255 h vs. 219 h). Furthermore, September 2003 was warm and sunny. In such conditions, a fire started during farming or self-ignition of litter could had set the studied assemblage on fire, leading to the development of wounds on the trunks of the hawthorns examined here.
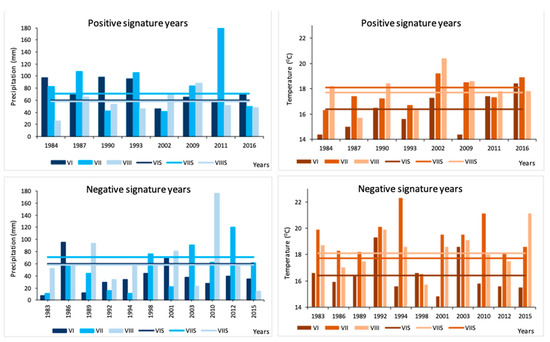
Figure 5.
Precipitation in June, July and August (blue bars) in positive and negative signature years in comparison to the average precipitation in June, July and August of the years 1948–2020 (blue lines). Temperature in June, July and August (orange bars) in positive and negative signature years in comparison to the average temperature in June, July and August of the years 1948–2020 (orange lines).
4. Discussion
The studied population of intermediate hawthorn is very young. The oldest tree, which is probably the parent specimen for the entire population, is about 65 years old (60 measured tree-rings). The age of the trees is corroborated by an analysis of topographic maps (both prewar German maps and postwar Polish maps) and the available aerial photographs. The hawthorn gathering arose along a dirt road that ceased to be used following the war. It was subsequently plowed and today is part of a field. The steepest section (currently a SE-facing scarp of 4 m relative elevation) became uncultivated land that, over the years, turned into a mid-field woodlot. Most of the studied trees are 40–45 years old. The tree-ring width in the studied population of Crataegus × media is high, equal to 2.41 mm. It is, however, subject to very high intersubject variability (from 1.48 to 4.44 mm). Due to their rather young age, the radial growth rate of the hawthorns was compared to growth profiles of other species based on cumulative radial growth. The results of this comparison confirm the high radial growth rate of the hawthorns, comparable to the growth rate of oaks, and slightly lower than the growth rate of pine or beech (i.e., the main forest-forming species in NW Poland). Notably, however, these are the first life decades of trees that are characterized by a high growth rate. Furthermore, the research by Good et al. [18] indicates different radial growth rates in hawthorns growing in northern Wales (Great Britain). Good et al. found these differences to arise from habitat fertility and intersubject differences between trees and shrubs growing in the same habitat that preclude an estimate of tree age based on trunk diameter. Radial growth rate decreases with tree age, and mortality significantly increases after an individual reaches the age of 80 years (although in that particular population, the oldest trees and shrubs were up to 120 years old) [18]. A variable growth rate was also noted in New Zealand (South Island), where the hawthorn is an invasive plant [7]. Depending on the habitat, hawthorn populations displayed tree-rings from 1.4 to 4.8 mm-wide. Such large differences were explained by sheep and cattle grazing. The maximum age of hawthorns was determined as 60 years [7].
A comparison of hawthorn chronology (ST) to other local chronologies for tree species occurring in NW Poland indicated that ST showed the highest similarity to a composite chronology for indigenous oak species (Quercus spp.), with t = 3.63 and Gl = 68%. These values are not high, and the chronology similarity may be too low to enable dendrochronological sample dating, and especially problematic for samples including fewer than 100 tree-rings. For this reason, further study is required to determine the potential of Crataegus media for paleoclimatic analyses and paleoenvironmental condition reconstructions.
The strongest growth–climate relationship is observed for summer months: June, July and August, when high precipitation, low air temperatures and low insolation favor the formation of wide tree-rings in intermediate hawthorn. Similar relationships also exist for other species, e.g., oaks, beeches, spruces or pines [42,43,44,45,46,47,48]. Shortage of water in the soil and high air temperatures through these months are a growth-limiting factor and may even cause the trees to wither [49]. Phenological studies on hawthorns indicate the end of the vegetation season as the period of seed and fruit ripening [1,50]. This process involves substantial metabolic resources of a plant, which is why unfavorable meteorological conditions in these months have such high significance and are reflected, for instance, in cambial activity and tree-ring width. Additional periods of growth–climate relationship appear in the winter season (December of the preceding growth season through February–March) when negative correlation and regression values are noted for temperature and insolation, and late in the preceding growth season (August–September), when high precipitation sums, low temperatures and low insolation make a positive influence on tree-ring width in hawthorns. The end of the preceding growth season is a period when trees prepare for winter dormancy. For trees that shed their assimilation apparatus for winter and regenerate it the next spring from stored metabolites, it is an unusually important period—determining the condition of trees in the upcoming growth season [41]. Furthermore, the analysis of meteorological conditions in the determined pointer years identified precipitation, especially in summer months, and water availability in habitat (also in the preceding vegetation year) as the factors contributing to the formation of wide or narrow tree-rings.
Although hawthorns are described as tolerant of drought and shortage of precipitation [2,11,13,51], the growth–climate relationship results obtained here point to water availability in habitat (especially in summer months) as the main factor determining radial growth in intermediate hawthorn.
The results presented in this study concern a single, small and young population of Crataegus × media, and the total lack of published dendrochronological studies points to an urgent need for future work to focus on other hawthorn species and for future analyses to include populations of varying ages, and diverse with respect to geographic location and habitat. Despite minor economic importance, intermediate hawthorn (and other hawthorn species) is very valuable biocenotically, as it increases habitat biodiversity and provides shelter and nutrition to insects, birds and mammals.
5. Conclusions
The radial growth rate of intermediate hawthorn described as a shrub or low tree is comparable to other species of trees forming stands in NW Poland and equals 2.41 mm/year. It displays a considerable intersubject variability, from 1.48 to 4.44 mm/year.
Three-ring width depends mostly on precipitation and temperature, and insolation in the summer months (June–August). The analyses, however, point also to the end of the preceding year’s vegetation season and winter months as periods of strong growth–climate relationship.
Negative pointer years occur most frequently in a year following a very dry period or in a very dry year (in particular, with insufficient precipitation in the summer period). Positive pointer years occur in years with humid summer months and lower than average mean summer temperature.
No dendrochronological studies were available for this species, and the resulted of the analysis based on a single assemblage of intermediate hawthorn indicate that future studies should be expanded to include other hawthorn species, different types of habitats, and occurrences in other regions.
Author Contributions
A.C., conceptualization; A.C., B.C., field collection, data analyses, draft preparation, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guitian, J.; Fuentes, M. Reproductive biology of Crataegus monogyna in northwestern Spain. Acta Ecol. 1992, 13, 3–11. [Google Scholar]
- Oklejewicz, K.; Chwastek, E.; Szewczyk, B.; Mitka, J. Chorological Aspects of the Occurrence of Hawthorn in the Polish Carpathians; Wydawnictwo Uniwersytetu Rzeszowskiego: Rzeszów, Poland, 2014; pp. 1–210. [Google Scholar]
- Hassler, M. World Plants: Synonymic Checklists of the Vascular Plants of the World (version Nov 2018). In 2019, Species 2000 & ITIS Catalogue of Life; Roskov, Y., Ower, G., Orrell, T., Nicolson, D., Bailly, N., Kirk, P.M., Bourgoin, T., DeWalt, R.E., Decock, W., Nieukerken, E., et al., Eds.; Naturalis: Leiden, The Netherlands, 2019; ISSN 2405-884X. Annual Checklist; Species 2000; Available online: www.catalogueoflife.org/annual-checklist/2019 (accessed on 9 November 2020).
- Fitzgerald, H.; Helpdesk, G.N. Nordic Crop Wild Relative (CWR) Checklist. Version 1.16. Nordic Genetic Resource Center (NORDGEN). 2020. Available online: https://www.gbif.org/dataset/8027d8d5-c8bc-4d54-bee9-f854f141b442 (accessed on 9 November 2020).
- Sobral, M.; Larrinaga, A.R.; Guitián, J. Do seed-dispersing birds exert selection on optimal plant trait combinations? Correlated phenotypic selection on the fruit and seed size of hawthorn (Crataegus monogyna). Evol. Ecol. 2010, 24, 1277–1290. [Google Scholar] [CrossRef]
- Annotated Bibliography on the Ecology and Management of Invasive Species: Common hawthorn (Crataegus monogyna). Prepared by Judith Cullington & Associates, Victoria, BC for the Garry Oak Ecosystems Recovery Team and the Nature Conservancy of Canada, March 2002. Available online: https://stewardshipcentrebc.ca/PDF_docs/GOERT/Publications/Inv_Bibliographies/Bib_cratmono.pdf (accessed on 9 November 2020).
- Williams, P.A.; Buxton, R.P. Hawthorn (Crataegus monogyna) Populations in Mid-Canterbury. N. Z. J. Ecol. 1986, 9, 11–17. [Google Scholar]
- Sallabanks, R. Fruit fate, frugivory, and fruit characteristics: A study of the hawthorn, Crataegus monogyna (Rosaceae). Oecologia 1992, 91, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Sallabanks, R. Fruiting plant attractiveness to avian seed dispersers: Native vs. Invasive Crataegus in western Oregon. Madrono 1993, 40, 108–116. [Google Scholar]
- Phipps, J.B. Introduction to the red-fruited hawthorns (Crataegus, Rosaceae) of Western North America. Can. J. Bot. 1998, 76, 1863–1899. [Google Scholar]
- Gostyńska-Jakuszewska, M.; Hrabetova-Uhrova, A. Distribution of Crataegus in Poland and Czechoslovakia. Preslia Praha 1983, 55, 9–24. [Google Scholar]
- Seneta, W. Drzewa i krzewy liściaste. Tom II; Wydawnictwo Naukowe PWN: Warszawa, Poland, 1994. [Google Scholar]
- Seneta, W.; Dolatowski, J. Dendrology; PWN: Warszawa, Poland, 2004; pp. 1–559. [Google Scholar]
- Özcan, M.; Hacıseferoğulları, H.; Marakoğlu, T.; Arslan, D. Hawthorn (Crataegus spp.) fruit: Some physical and chemical properties. J. Food Eng. 2005, 69, 409–413. [Google Scholar] [CrossRef]
- Kumar, D.; Arya, V.; Bhat, Z.A.; Khan, N.A.; Prasad, D.N. The genus Crataegus: Chemical and pharmacological perspectives. Rev. Bras. Farm. 2012, 22, 1187–1200. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A. Health-promoting potential of hawthorn fruits and flowers. Probl. Hig. Epidemiol. 2016, 97, 24–28. [Google Scholar]
- Nazhand, A.; Lucarini, M.; Durazzo, A.; Zaccardelli, M.; Cristarella, S.; Souto, S.B.; Silva, A.M.; Severino, P.; Souto, E.B.; Santini, A. Hawthorn (Crataegus spp.): An Updated Overview on Its Beneficial Properties. Forests 2020, 11, 564. [Google Scholar] [CrossRef]
- Good, J.E.G.; Bryant, R.; Carlill, P. Distribution, Longevity and Survival of Upland Hawthorn (Crataegus monogyna) Scrub in North Wales in Relation to Sheep Grazing. J. Appl. Ecol. 1990, 27, 272. [Google Scholar] [CrossRef]
- Grissino-Mayer, H.D. An updated list of species used in tree-ring research. Tree Ring Bull. 1993, 53, 17–43. [Google Scholar]
- Schweingruber, F.H. Microscopic Wood Anatomy. Swiss Federal Institute for Forest, Snow and Landscape Research; WSL: Birmensdorf, Switzerland, 1990; pp. 1–226. [Google Scholar]
- Kondracki, J. Geografia Fizyczna Polski; PWN: Warszawa, Poland, 1988; pp. 1–464. [Google Scholar]
- Abadi, H.S.N.; Soltani, A.; Saeidi, Z.; Zadeh, S.G. Study of Spatial Distribution of the Hawthorn (Crataegus monogyna) Trees Attacked by Orchard Ermine (Yponomeuta padella) in Bazoft Forests of Chaharmahal and Bakhtiari Province. Iran. J. Appl. Ecol. 2016, 4, 39–49. [Google Scholar] [CrossRef]
- Taggar, G.K.; Arora, R. Insect Biotypes and Host Plant Resistance. In Breeding Insect Resistant Crops for Sustainable Agriculture; Arora, R., Sandhu, S., Eds.; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Mindur, B. Dendrometer 1,0. 2000. Available online: www.ictinternational.com/support/software/ (accessed on 9 November 2020).
- Holmes, R.J. Computer-assisted quality control in tree-ring dating and measurement. Tree Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Grissino-Mayer, H.D. Evaluating Crossdating accuracy: A manual and tutorial for the compuret program COFECHA. Tree Ring Res. 2001, 57, 205–221. [Google Scholar]
- Cook, E.R.; Holmes, R.L. Guide for Computer Program ARSTAN. In The International Tree-Ring Data Bank Program Library Version 2.0 User’s Manual; Grissino-Mayer, H.D., Holmes, R.L., Fritts, H.C., Eds.; Laboratory of Tree-Ring Research: Tuscon, AZ, USA, 1996; pp. 75–87. [Google Scholar]
- Holmes, R.J. Dendrochronology Program Library. Users Manual. 1994. Available online: https://www.ltrr.arizona.edu/software.html (accessed on 9 November 2020).
- Fritts, H.C. Tree Rings and Climate; Academic Press: New York, NY, USA, 1976; pp. 1–582. [Google Scholar]
- Cook, E.R.; Kairiukstis, A. Methods of Dendrochronology; Kluwer Academic Publishers: New York, NY, USA, 1992; pp. 1–394. ISBN 0-7923-0586-8. [Google Scholar]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Clim. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Walanus, A. Instrukcja Obsługi Programu TCS. Program TCS do Obliczania Lat Wskaźnikowych. 2002. Available online: https://www.tcs.com/careers/ace-program/en-us (accessed on 9 November 2020).
- Meyer, F.D. Pointer years analysis in dendrochronology: A comparison of methods. Dendrochronologia 1997, 16–17, 193–204. [Google Scholar]
- Jetschke, G.; Van Der Maaten, E.; Van Der Maaten-Theunissen, M. Towards the extremes: A critical analysis of pointer year detection methods. Dendrochronologia 2019, 53, 55–62. [Google Scholar] [CrossRef]
- Schweingruber, F.H. Tree rings. In Basics and Applications of Dendrochronology; Kluwer Academic Publishers: New York, NY, USA, 1989; pp. 1–276. ISBN 978-0-7923-0559-0. [Google Scholar]
- Kaennel, M.; Schweingruber, F.H. Multilingual Glossary of Dendrochronology; WSL FNP: Haupt, Germany, 1990; pp. 1–467. ISBN 3-258-05259-X. [Google Scholar]
- Krawczyk, A. Program Komputerowy Tree Rings. 1995. Available online: https://www.ldeo.columbia.edu/tree-ring-laboratory/resources/software (accessed on 9 November 2020).
- Peel, M.C.; Finlayson, B.L.; Mcmahon, T.A. Updated world map of the Köppen-Geiger climateclassification. Hydrology and Earth System Sciences Discussions. Eur. Geosci. Union 2007, 4, 439–473. [Google Scholar]
- Cedro, A. Dendrochronologiczna analiza drzew rosnących w Puszczy Bukowej. W: (Red. Grażyna Domian i Krzysztof Ziarnek) Księga Puszczy Bukowej. In Tom I: Środowisko Przyrodnicze; PPH Zapol Dmochowski, Sobczyk sp.j.; Regionalna Dyrekcja Ochrony Środowiska w Szczecinie: Szczecin, Poland, 2010; pp. 122–128. [Google Scholar]
- Cedro, A. Dendrochronology of Yew in Poland and Western Ukraine; Zapol: Szczecin, Poland, 2012; pp. 1–230. ISBN 978-83-7518-386-3. [Google Scholar]
- Cedro, A. Dendrochronological Analysis of the Wild Service Tree (Sorbus torminalis L.) in Poland; Zapol: Szczecin, Poland, 2016; pp. 1–210. ISBN 978-83-7518-815-8. [Google Scholar]
- Koprowski, M.; Zielski, A. Dendrochronology of Norway spruce (Picea abies (L.) Karst.) from two range centres in lowland Poland. Trees 2006, 20, 383–390. [Google Scholar] [CrossRef]
- Koprowski, M. Dendrochronological analysis of European beech (Fagus sylvatica L.) tree rings in the Iława Forest District. Sylwan 2006, 5, 44–50. Available online: https://www.researchgate.net/publication/257046958_Dendrochronological_analysis_of_European_beech_Fagus_sylvatica_L_tree_rings_in_the_Ilawa_Forest_District (accessed on 9 November 2020).
- Friedrichs, D.A.; Büntgen, U.; Frank, D.C.; Esper, J.; Neuwirth, B.; Löffler, J. Complex climate controls on 20th century oak growth in Central-West Germany. Tree Physiol. 2009, 29, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Misi, D.; Puchałka, R.; Pearson, C.; Robertson, I.; Koprowski, M. Differences in the Climate-Growth Relationship of Scots Pine: A Case Study from Poland and Hungary. Forests 2019, 10, 243. [Google Scholar] [CrossRef]
- Scharnweber, T.; Heinze, L.; Cruz-García, R.; Van Der Maaten-Theunissen, M.; Wilmking, M. Confessions of solitary oaks: We grow fast but we fear the drought. Dendrochronologia 2019, 55, 43–49. [Google Scholar] [CrossRef]
- Dell’Oro, M.; Mataruga, M.; Sass-Klaassen, U.; Fonti, P. Climate change threatens on endangered relict Serbian spruce. Dendrochronologia 2020, 59, 125651. [Google Scholar] [CrossRef]
- Cedro, A. Climatic changes in Western Pomerania in the Light of Analysis of Tree-Ring Sequences of Scots Pine, Douglas Fir, and Native Species of Oak; Science Research: Szczecin, Poland, 2004; pp. 1–149. ISBN 83-89402-03-3. [Google Scholar]
- Buras, A.; Sass-Klaassen, U.; Verbeek, I.; Copini, P. Provenance selection and site conditions determine growth performance of pedunculate oak. Dendrochronologia 2020, 61, 125705. [Google Scholar] [CrossRef]
- Mijnsbrugge, K.V.; Janssens, A. Differentiation and Non-Linear Responses in Temporal Phenotypic Plasticity of Seasonal Phenophases in a Common Garden of Crataegus monogyna Jacq. Forests 2019, 10, 293. [Google Scholar] [CrossRef]
- Beloiu, M.B.; Stahlmann, R.S.; Beierkuhnlein, C.B. High Recovery of Saplings after Severe Drought in Temperate Deciduous Forests. Forests 2020, 11, 546. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).