Soil Biodiversity as Affected by Different Thinning Intensities in a Pinus laricio Stand of Calabrian Apennine, South Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Measurement of Microclimatic Variables
2.3. Experimental Design
2.4. Soil Chemical and Physical Analysis
2.5. Soil Microbial Analysis
2.6. Soil Fauna Determination
2.7. Data Analysis
3. Results and Discussion
3.1. Microclimate Variables
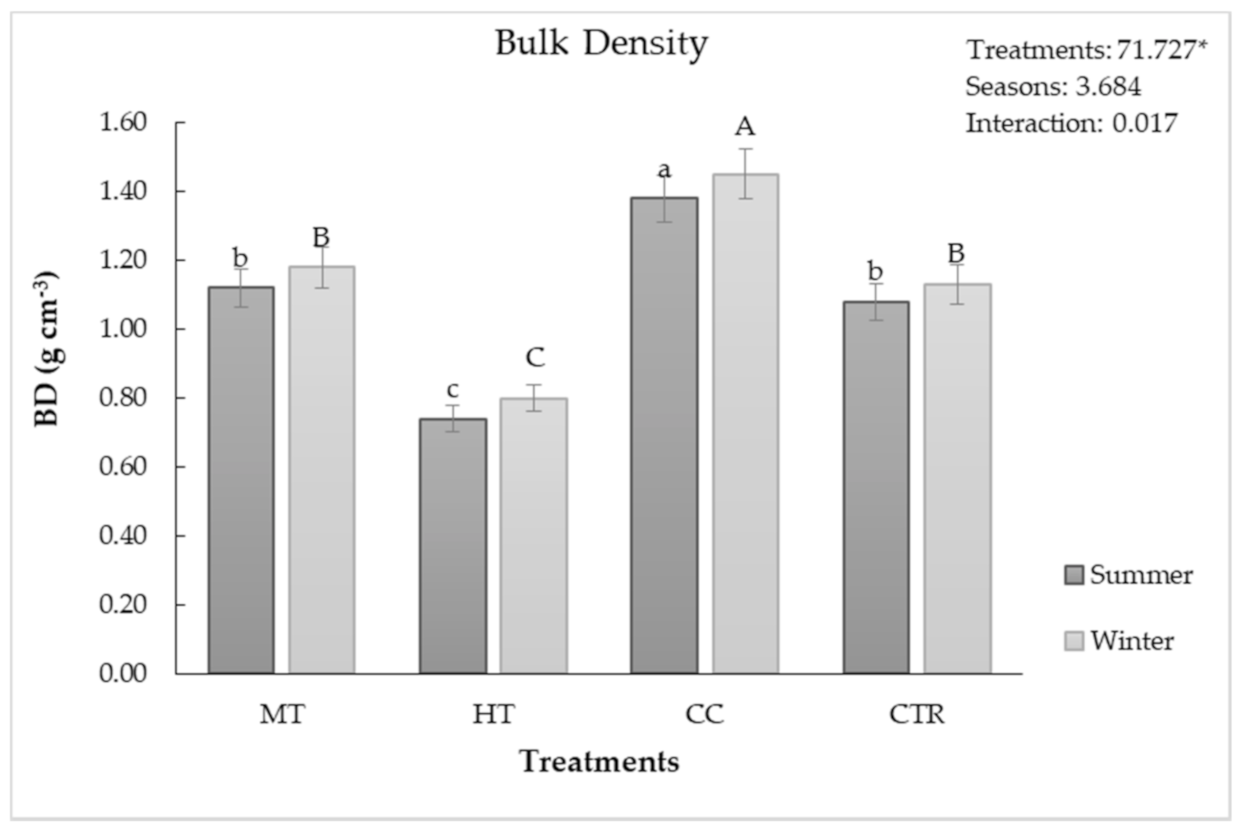
3.2. Soil Chemical and Physical Features
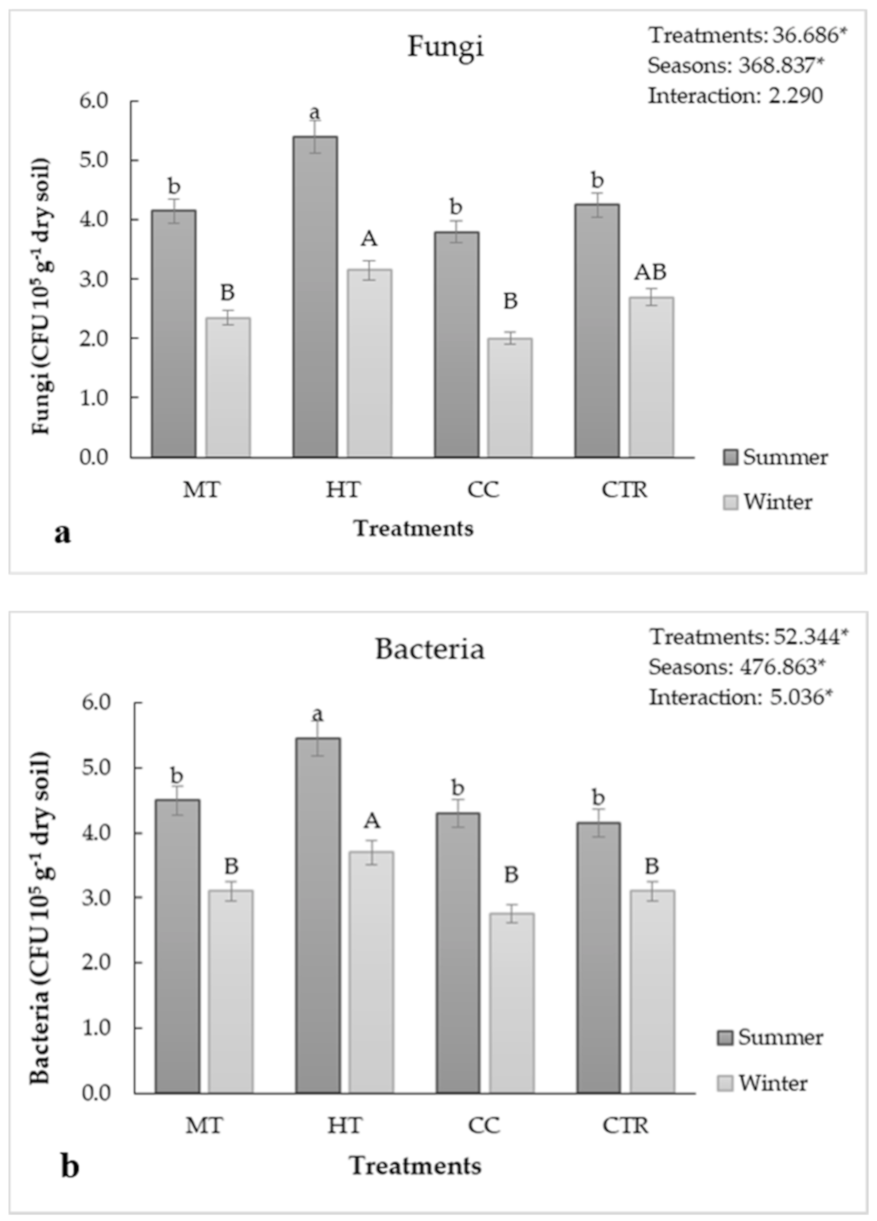
3.3. Soil Microbiological Features
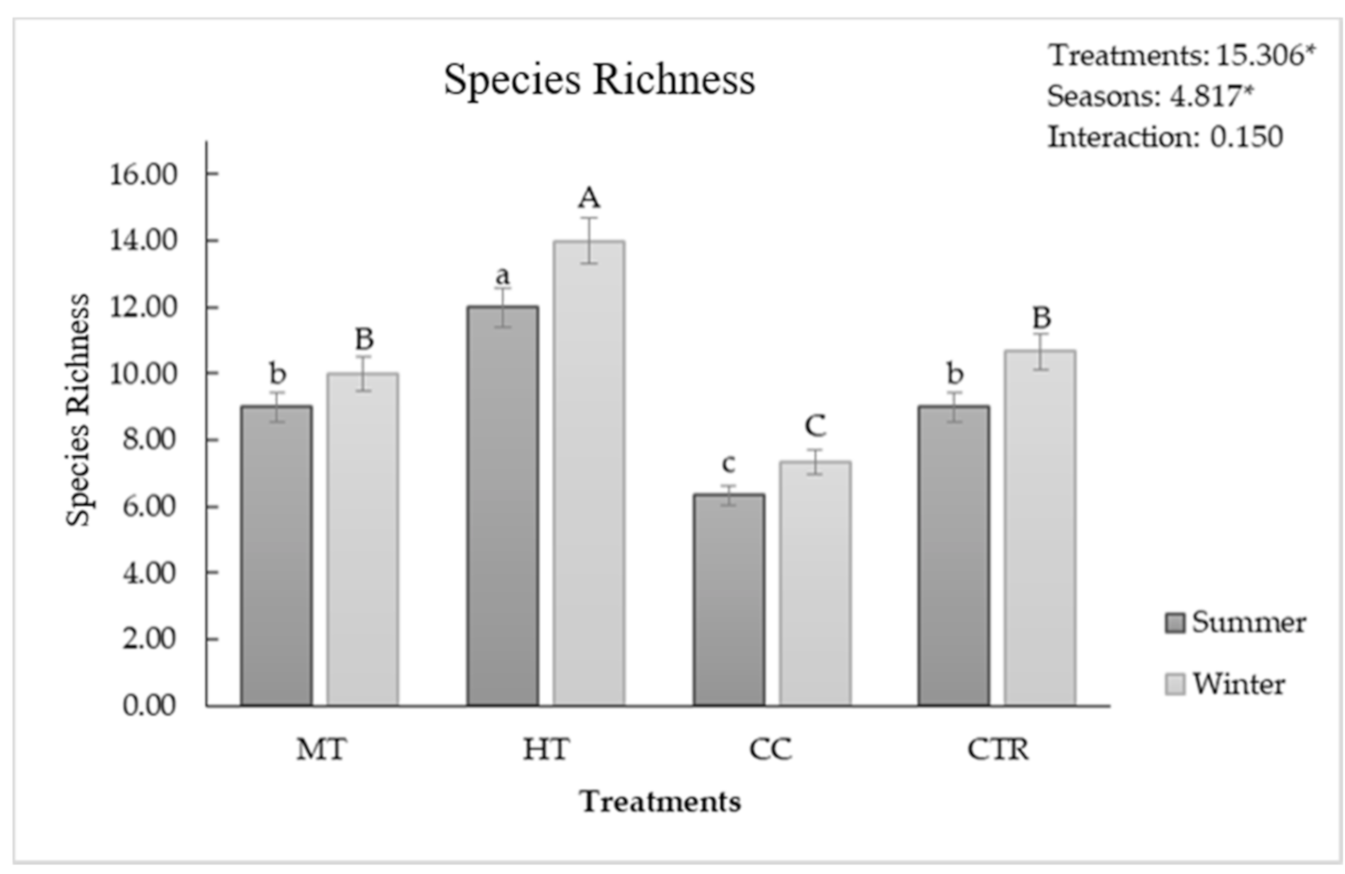
3.4. Soil Biodiversity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bianchi, L.; Paci, M.; Bresciani, A. Effetti del diradamento in parcelle sperimentali di pino nero in Casentino (AR): Risultati a otto anni dall’intervento. Forest 2010, 7, 73–83. [Google Scholar] [CrossRef]
- Cantiani, P.; Chiavetta, U. Estimating the mechanical stability of Pinus nigra Arn. using an alternative approach across several plantations in central Italy. iForest 2015, 8, 846–852. [Google Scholar] [CrossRef]
- Thom, D.; Rammer, W.; Dirnböck, T.; Müller, J.; Kobler, J.; Katzensteiner, K.; Helm, N.; Seidl, R. The impacts of climate change and disturbance on spatio-temporal trajectories of biodiversity in a temperate forest landscape. J. Appl. Ecol. 2017, 54, 28–38. [Google Scholar] [CrossRef]
- Kutnar, L.; Nagel, T.A.; Kermavnar, J. Effects of Disturbance on Understory Vegetation across Slovenian Forest Ecosystems. Forests 2019, 10, 1048. [Google Scholar] [CrossRef]
- Zhang, D.H.; Ye, Z.E.; Fan, B.Y.; Wei, T.L. Influence of thinning on soil fertility in artificial forests. Chin. J. App. Ecol. 2001, 12, 672–676. [Google Scholar]
- Goldmann, K.; Schöning, I.; Buscot, F.; Wubet, T. Forest Management Type Influences Diversity and Community Composition of Soil Fungi across Temperate Forest Ecosystems. Front. Microbiol. 2015, 6, 1300. [Google Scholar] [CrossRef]
- Ma, S.; Concilio, A.; Oakley, B.; North, M.; Chen, J. Spatial variability in microclimate in a mixed-conifer forest before and after thinning and burning treatments. Forest Ecol. Manag. 2010, 259, 904–915. [Google Scholar] [CrossRef]
- Wubet, T.; Christ, S.; Schöning, I.; Boch, S.; Gawlich, M.; Schnabel, B.; Fischer, M.; Buscot, F. Differences in soil fungal communities between European beech (Fagus sylvatica L.) dominated forests are related to soil and understory vegetation. PLoS ONE 2012, 7, e47500. [Google Scholar] [CrossRef]
- Muscolo, A.; Settineri, G.; Attinà, E. Early warning indicators of changes in soil ecosystem functioning. Ecol. Indic. 2015, 48, 542–549. [Google Scholar] [CrossRef]
- Muscolo, A.; Settineri, G.; Bagnato, S.; Mercurio, R.; Sidari, M. Use of canopy gap openings to restore coniferous stands in Mediterranean environment. iForest 2017, 10, 322–327. [Google Scholar] [CrossRef]
- Yang, Y.; Geng, Y.; Zhou, H.; Zhao, G.; Wang, L. Effects of gaps in the forest canopy on soil microbial communities and enzyme activity in a Chinese pine forest. Pedobiologia 2017, 61, 51–60. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Yang, W.; Wu, F.; Xu, Z.; Tan, B.; Zhang, L.; He, X.; Guo, L. Canopy gaps accelerate soil organic carbon retention by soil microbial biomass in the organic horizon in a subalpine fir forest. Appl. Soil Ecol. 2018, 125, 169–176. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, X.; Zhang, D.; Zhang, J. The effects of gap size and litter species on colonization of soil fauna during litter decomposition in Pinus massoniana plantations. Appl. Soil Ecol. 2020, 155, 103611. [Google Scholar] [CrossRef]
- Lassau, S.A.; Hochuli, D.F.; Cassis, G.; Reid, C. Effects of habitat complexity on forest beetle diversity: Do functional groups respond consistently? Divers. Distrib. 2005, 11, 73–82. [Google Scholar] [CrossRef]
- Perry, K.I.; Wallin, K.F.; Wenzel, J.W.; Herms, D.A. Forest disturbance and arthropods: Small-scale canopy gaps drive invertebrate community structure and composition. Ecosphere 2018, 9, e02463. [Google Scholar] [CrossRef]
- Romeo, F.; Settineri, G.; Sidari, M.; Mallamaci, C.; Muscolo, A. Responses of soil quality indicators to innovative and traditional thinning in a beech (Fagus sylvatica L.) forest. For. Ecol. Manag. 2020, 465, 118106. [Google Scholar] [CrossRef]
- Khatoon, H.; Solanki, P.; Narayan, M.; Tewari, L.; Rai, J. Role of microbes in organic carbon decomposition and maintenance of soil ecosystem. Int. J. Chem. Stud. 2017, 5, 1648–1656. [Google Scholar]
- David, J.F. The role of litter-feeding macroarthropods in decomposition processes: A reappraisal of common views. Soil Biol. Biochem. 2014, 76, 109–118. [Google Scholar] [CrossRef]
- Saha, S.; Sarkar, M.; Raychaudhuri, D. Assessing diversity and abundance of soil microarthropods in three discrete plots of Ramakrishna Mission Vivekananda Ashrama, Narendrapur, South 24 Parganas, West Bengal, India. World News Nat. Sci. 2020, 31, 58–69. [Google Scholar]
- Bagyaraj, D.; Nethravathi, C.; Nitin, K. Soil biodiversity and arthropods: Role in soil fertility. In Economic and Ecological Significance of Arthropods in Diversified Ecosystems; Springer: Berlin/Heidelberg, Germany, 2016; pp. 17–51. [Google Scholar]
- Chakravarthy, A.K.; Jayasimha, G.T.; Rachana, R.R.; Rohini, G. Insects as Human Food. In Economic and Ecological Significance of Arthropods in Diversified Ecosystems; Springer: Berlin/Heidelberg, Germany, 2016; pp. 133–146. [Google Scholar]
- Menta, C.; Renelli, S. Soil Health and Arthropods: From Complex System to Worthwhile Investigation. Insects 2020, 11, 54. [Google Scholar] [CrossRef]
- Andersen, A.N.; Majer, J.D. Ants show the way Down Under: Invertebrates as bioindicators in land management. Front. Ecol. Environ. 2004, 2, 291–298. [Google Scholar] [CrossRef]
- Muscolo, A.; Sidari, M.; Mercurio, R. Variations in soil chemical properties and microbial biomass in artificial gaps in Silver fir stands. Eur. J. For. Res. 2007, 126, 59–65. [Google Scholar] [CrossRef]
- Muscolo, A.; Bagnato, S.; Sidari, M.; Mercurio, R. A review of the roles of forest canopy gaps. J. For. Res. 2014, 25, 725–736. [Google Scholar] [CrossRef]
- Chen, X.L.; Wang, D.; Chen, X.; Wang, J.; Diao, J.J.; Zhang, J.Y.; Guan, Q.W. Soil microbial functional diversity and biomass as affected by different thinning intensities in a Chinese fir plantation. Appl. Soil Ecol. 2015, 92, 35–44. [Google Scholar] [CrossRef]
- Lewandowski, T.E.; Forrester, J.A.; Mladenoff, D.J.; Stoffel, J.L.; Gower, S.T.; D’Amato, A.W.; Balser, T.C. Soil microbial community response and recovery following group selection harvest: Temporal patterns from an experimental harvest in a US northern hardwood forest. For. Ecol. Manag. 2015, 340, 82–94. [Google Scholar] [CrossRef]
- Kwon, T.S.; Yang, H.M.; Shin, J.H.; Kim, S.K.; Yi, H.B. Effects of Thinning on Abundance and Community Structure of Arthropods in a Pinus koraiensis Plantation. Korean J. Appl. Entomol. 2010, 49, 187–198. [Google Scholar] [CrossRef][Green Version]
- Richards, L.A.; Windsorf, D.M. Seasonal variation of arthropod abundance in gaps and the understorey of a lowland moist forest in Panama. J. Trop. Ecol. 2007, 23, 169–176. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in tropical rain forests and coral reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- Pavari, A. Le classificazioni fitoclimatiche ed i caratteri della stazione [Phytoclimatic classifications and station characteristics]. Scr. Ecol. Selvic. Bot. For. 1959, 45–116. [Google Scholar]
- IUSS Working Group. World Reference Base for Soil Resources 2014; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2014. [Google Scholar]
- Gendron, F.; Messier, C.; Comeau, P.G. Comparison of various methods for estimating the mean growing season percent photosynthetic photon flux density in forests. Agric. For. Meteorol. 1998, 92, 55–70. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer method improved for making particle-size analyses of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Springer, U.; Klee, J. Prüfung der leistungsfähigkeit von einigen wichtigen verfahren zur bestimmung des kohlenstoffs mittels chromschwefelsaure sowie vorschlag einer neuen schnellmethode. J. Plant. Nutr. Soil Sci. 1954, 64, 1–26. [Google Scholar]
- Ciavatta, C.; Govi, M. Use of insoluble polyvinylpyrrolidone and isoelectric focusing in the study of humic substances in soils and organic wastes: A review. J. Chromatogr. 1993, 643, 261–270. [Google Scholar] [CrossRef]
- Elliott, M.L.; Des Jardin, E.A. Comparison of media and diluents for enumeration of aerobic bacteria from bermuda grass golf course putting greens. J. Microbiol. Methods 1999, 34, 193–202. [Google Scholar] [CrossRef]
- Picci, G.; Nannipieri, P. Metodi di Analisi Microbiologica del Suolo; Franco Angeli: Roma, Italy, 2003; p. 224. [Google Scholar]
- Eaton, A.; Clesceri, L.; Rice, E.; Greenberg, A.; Franson, M.A. Standard Methods for Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Berlese, A. Apparecchio per raccogliere presto ed in gran numero piccoli Artropodi. Redia 1905, 2, 85–90. [Google Scholar]
- Bano, R.; Roy, S. Extraction of Soil Microarthropods: A low cost Berlese-Tullgren funnels extractor. Int. J. Fauna Biol. 2016, 3, 14–17. [Google Scholar]
- Obrist, M.K.; Duelli, P. Rapid biodiversity assessment of arthropods for monitoring average local species richness and related ecosystem services. Biodivers. Conserv. 2010, 19, 2201–2220. [Google Scholar] [CrossRef]
- Angelini, P.; Fenoglio, S.; Isaia, M.; Jacomini, C.; Migliorini, M.; Morisi, A. Tecniche di Biomonitoraggio Della Qualità Del Suolo; ARPA Piemonte: Torino, Italy, 2002; p. 106. [Google Scholar]
- Ruiz, N.; Lavelle, P. Soil Macrofauna Field Manual, Technical Level; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2008; p. 100. [Google Scholar]
- Duyar, A. Seasonal Variatıon of Soil Arthropods (Arthropoda) in Fir (Abies bornmulleriana Mattf.) Ecosystems in Bolu-Aladag. Ph.D. Thesis, Science Institute, Istanbul University, Fatih, Turkey, 2014; p. 165. (In Turkish). [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1949; pp. 1–117. [Google Scholar]
- Heip, C.H.R.; Herman, P.M.J.; Soetaert, K. Indices of diversity and evenness. Océanis 1998, 24, 61–87. [Google Scholar]
- Whittaker, R.H. Evolution and Measurement of Species Diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 2nd ed.; W. H. Freeman: San Francisco, CA, USA, 1981; p. 859. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Stormont, J.C.; Farfan, E.; Coonrod, J.E.A. Total Soil Water Evaporation in a Riparian Environment: Model Development and Application. J. Hydrol. Eng. 2009, 14, 904–912. [Google Scholar] [CrossRef]
- Hornumg, M. Acidification of soils by trees and forests. Soil Use Manag. 2007, 1, 24–27. [Google Scholar] [CrossRef]
- Schindlbacher, A.; De Gonzalo, C.; Díaz-Pinés, E.; Pilar, G.; Matthews, B.; Inclán, R.; Zechmeister-Boltenstern, S.; Rubio, A.; Jandl, R. Temperature sensitivity of forest soil organic matter decomposition along two elevation gradients. J. Geophys. Res. 2010, 115, 1–10. [Google Scholar] [CrossRef]
- Muscolo, A.; Sidari, M.; Bagnato, S.; Mallamaci, C.; Mercurio, R. Gap size effects on above and below-ground processes in a silver fir stand. Eur. J. For. Res. 2010, 129, 355–365. [Google Scholar] [CrossRef]
- Settineri, G.; Mallamaci, C.; Mitrović, M.; Sidari, M.; Muscolo, A. Effects of different thinning intensities on soil carbon storage in Pinus laricio forest of Apennine South Italy. Eur. J. For. Res. 2018, 137, 131–141. [Google Scholar] [CrossRef]
- Chaudhari, P.R.; Ahire, D.V.; Ahire, V.D.; Chkravarty, M.; Maity, S. Soil bulk density as related to soil texture, organic matter content and available total nutrients of Coimbatore soil. Int. J. Sci. Res. Publ. 2013, 3, 1–8. [Google Scholar]
- Pizzeghello, D.; Francioso, O.; Concheri, G.; Muscolo, A.; Nardi, S. Land use affects the soil C sequestration in alpine environment, NE Italy. Forests 2017, 8, 197. [Google Scholar] [CrossRef]
- Ahad, T.; Kanth, T.A.; Nabi, S. Soil bulk density as related to texture, organic matter content and porosity in kandi soils of district Kupwara (Kashmir Valley). India Int. J. Sci. Res. 2015, 4, 198–200. [Google Scholar]
- Li, Y.; Tullberg, J.N.; Freebairn, D.M. Traffic and residue cover effects on infiltration. Aust. J. Soil Res. 2001, 39, 239–247. [Google Scholar] [CrossRef]
- Pollierer, M.M.; Scheu, S. Driving factors and temporal fluctuation of Collembola communities and reproductive mode across forest types and regions. Ecol. Evol. 2017, 7, 4390–4403. [Google Scholar] [CrossRef]
- Swenson, N.G.; Enquist, B.; Pither, J.; Kerkhoff, A.; Boyle, B.; Weiser, M.; Elser, J.J.; Fagan, F.W.; Forero, J.; Fyllas, N.; et al. The biogeography and filtering of woody plant functional diversity in North and South America. Global Ecol. Biogeogr. 2012, 21, 798–808. [Google Scholar] [CrossRef]
- Victorsson, J.; Jonsell, M. Ecological traps and habitat loss, stump extraction and its effects on saproxylic beetles. For. Ecol. Manag. 2013, 290, 22–29. [Google Scholar] [CrossRef]
- Taylor, A.R.; Victorsson, J. Short-term effects of stump harvesting on millipedes and centipedes on coniferous tree stumps. For. Ecol. Manag. 2016, 371, 67–74. [Google Scholar] [CrossRef]
- Legakis, A. Community structure and species richness in the Mediterranean-type soil fauna. In Plant-Animal Interactions in Mediterranean-Type Ecosystems; Arianoursou, M., Groves, R.H., Eds.; Kluwer Academic: Amsterdam, The Netherlands, 1994; pp. 37–45. [Google Scholar]
- Lionello, P.; Malanotte-Rizzoli, P.; Boscolo, R. Mediterranean Climate Variability; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–421. [Google Scholar]
- Mueller, K.E.; Eisenhauer, N.; Reich, P.B.; Hobbie, S.E.; Chadwick, O.A.; Chorover, J.; Dobies, T.; Hale, C.M.; Jagodzi´nski, A.M.; Kałucka, I. Light, earthworms, and soil resources as predictors of diversity of 10 soil invertebrate groups across monocultures of 14 tree species. Soil Biol. Biochem. 2016, 92, 184–198. [Google Scholar] [CrossRef]
- Santonja, M.; Fernandez, C.; Proffit, M.; Gers, C.; Gauquelin, T.; Reiter, I.M.; Cramer, W.; Baldy, V. Plant litter mixture partly mitigates the negative effects of extended drought on soil biota and litter decomposition in a mediterranean oak forest. J. Ecol. 2017, 105, 801–815. [Google Scholar] [CrossRef]
- Jiménez-Chacón, A.; Homet, P.; Matías, L.; Gómez-Aparicio, L.; Godoy, O. Fine Scale Determinants of Soil Litter Fauna on a Mediterranean Mixed Oak Forest Invaded by the Exotic Soil-Borne Pathogen Phytophthora cinnamomi. Forests 2018, 9, 218. [Google Scholar] [CrossRef]
- Tsurho, K.; Ao, B. Community analysis of soil Acarina in a Natural Forest and Jhum land ecosystem of Mokokchung, Nagaland. JOSR J. Appl. Phys. 2014, 6, 50–54. [Google Scholar] [CrossRef]
- Nsengimana, V.; Kaplin, B.A.; Francis, F.; Nsabimana, D. Use of soil and litter arthropods as biological indicators of soil quality in forest plantations and agricultural lands: A Review. Faun. Entomol. 2018, 7, 1–12. [Google Scholar]
- Wardle, D.A. The influence of biotic interactions on soil biodiversity. Ecol. Lett. 2006, 9, 870–886. [Google Scholar] [CrossRef]
- Dyer, L.A.; Walla, T.R.; Greeney, H.F.; Stireman, J.O.; Hazen, R.F. Diversity of interactions: A metric for studies of biodiversity. Biotropica 2010, 42, 281–289. [Google Scholar] [CrossRef]
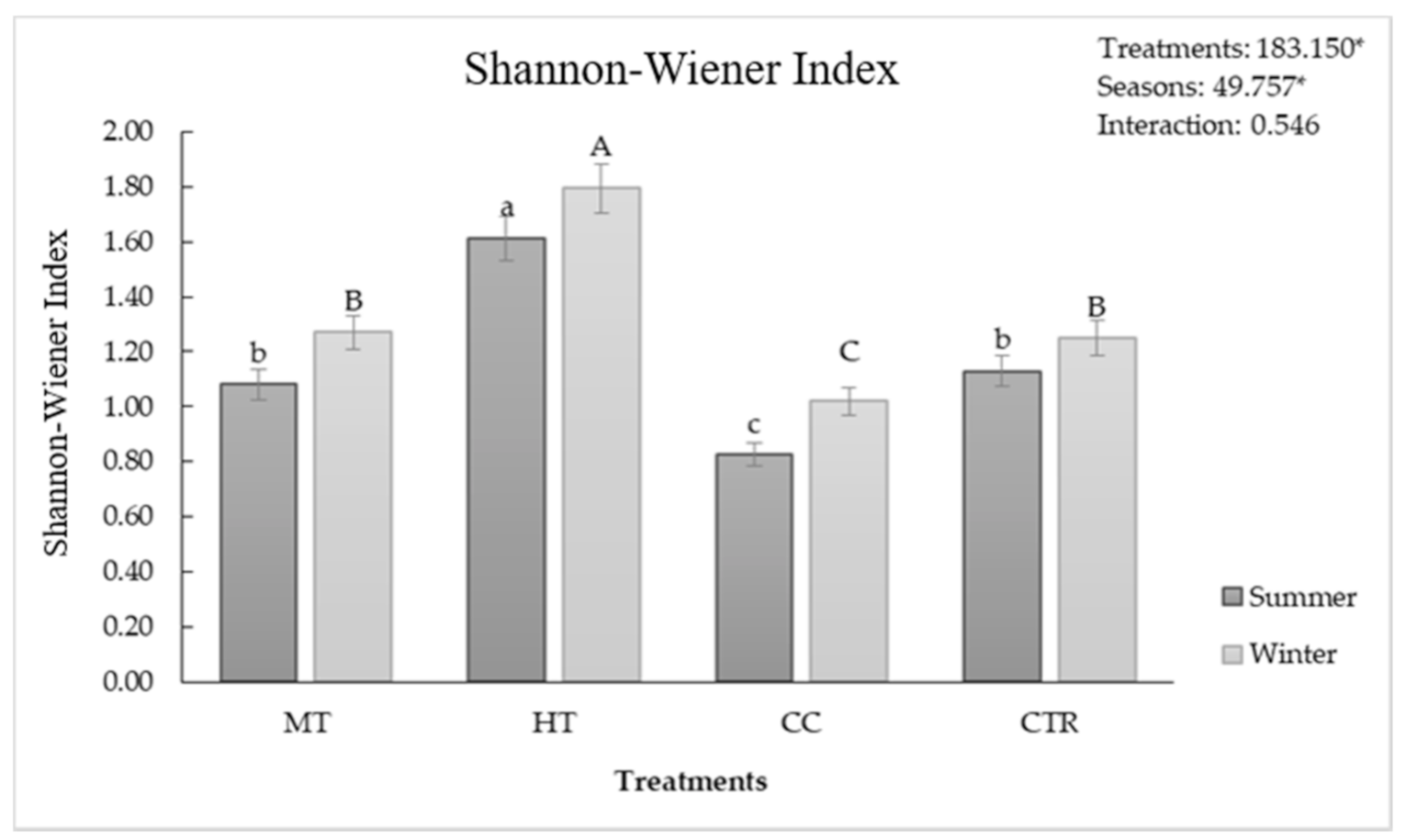

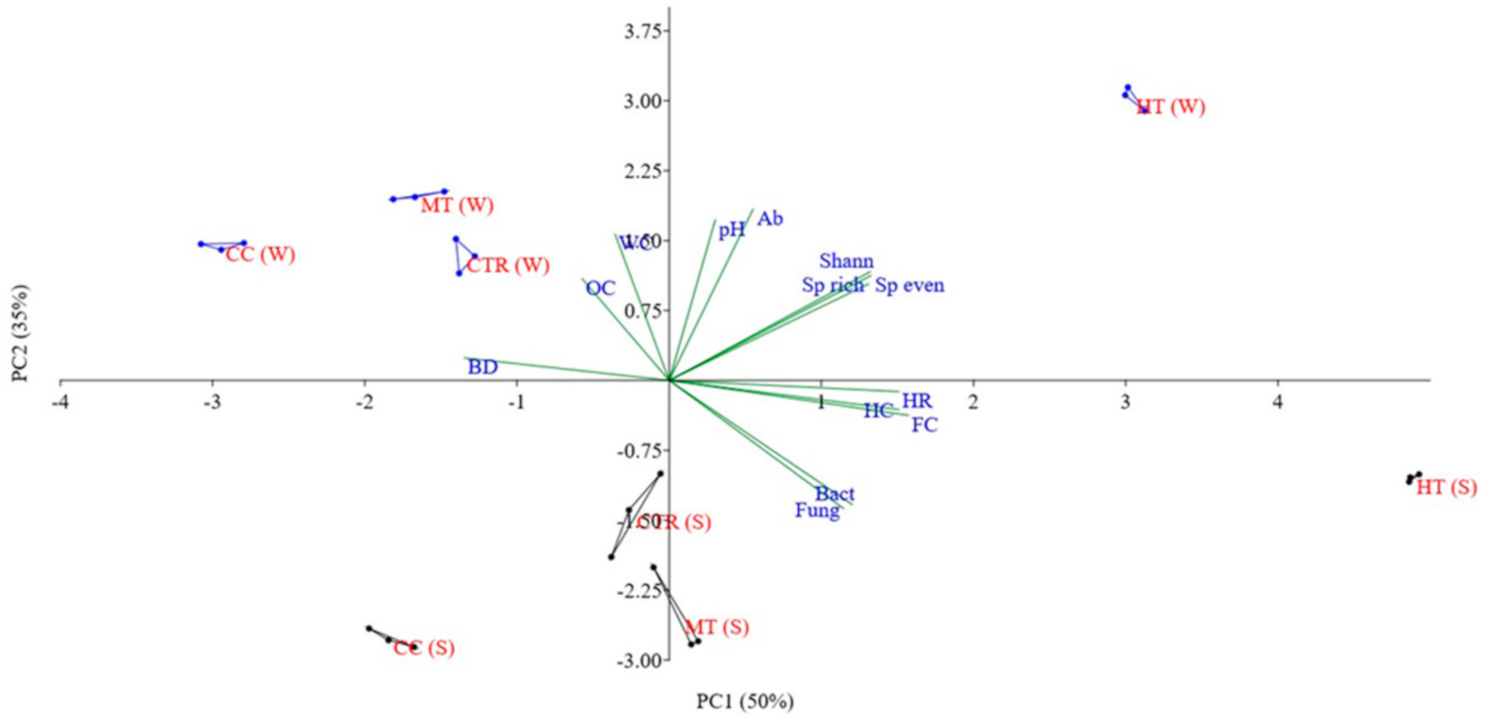
| Seasons | Parameters | HT | MT | CC | CTR |
|---|---|---|---|---|---|
| PAR transmittance (%) | 12.60 a (±2.72) | 2.3 b (±0.50) | 16.45 a (±3.43) | 1.42 b (±0.82) | |
| Air temperature (°C) | 12.48 b (±0.74) | 9.72 c (±0.50) | 16.74 a (±0.61) | 7.82 d (±0.73) | |
| Summer | Soil temperature (°C) | 11.52 b (±0.72) | 7.20 c (±0.94) | 15.78 a (±0.53) | 5.89 d (±0.75) |
| Litter thickness (cm) | 3.5 a (±0.11) | 1.11 b (±0.45) | 0.2 c (±0.05) | 1.5 b (±0.25) | |
| PAR transmittance (%) | 9.52 a (±1.25) | 1.21 b (±0.92) | 11.27 a (±1.48) | 0.81 b (±0.32) | |
| Air temperature (°C) | 7.58 a (±1.42) | 5.12 b (±0.53) | 5.24 b (±0.92) | 4.64 b (±0.71) | |
| Winter | Soil temperature (°C) | 6.32 a (±1.01) | 4.21 b (±0.94) | 3.54 b (±0.63) | 3.87 b (±1.02) |
| Litter thickness (cm) | 3.21 a (±0.62) | 1.05 b (±0.26) | 0.2 c (±0.07) | 1.27 b (±0.98) |
| Seasons | Management | WC (%) | pH | OC (%) | HC (%) | FC (%) | HR (%) |
|---|---|---|---|---|---|---|---|
| Summer | MT | 48 a ± 1.80 | 4.96 a ± 0.49 | 10.52 b ± 0.43 | 3.87 b ± 0.26 | 2.80 b ± 0.16 | 63.40 b ± 5.15 |
| HT | 51 a ± 1.40 | 5.39 a ± 0.39 | 12.81 a ± 0.61 | 5.56 a ± 0.24 | 4.28 a ± 0.32 | 76.81 a ± 4.88 | |
| CC | 38 b ± 1.25 | 5.10 a ± 0.36 | 7.59 c ± 0.58 | 3.11 c ± 0.13 | 1.82 c ± 0.28 | 64.95 b ± 5.70 | |
| CTR | 48 a ± 1.95 | 5.20 a ± 0.50 | 9.71 b ± 0.23 | 3.55 b,c ± 0.12 | 2.60 b ± 0.10 | 63.33 b ± 5.36 | |
| Winter | MT | 60 b ± 1.80 | 5.59 a ± 0.35 | 8.31 b ± 0.26 | 3.17 b ± 0.10 | 1.85 b ± 0.42 | 60.40 b ± 4.72 |
| HT | 65 b ± 1.20 | 5.64 a ± 0.31 | 9.40 a ± 0.43 | 3.98 a ± 0.16 | 3.14 a ± 0.12 | 75.74 a ± 5.31 | |
| CC | 82 a ± 1.00 | 5.37 a ± 0.29 | 8.08 b ± 0.22 | 2.91 b ± 0.33 | 1.62 b ± 0.13 | 56.06 b ± 5.23 | |
| CTR | 61 b ± 2.02 | 5.53 a ± 0.30 | 7.80 b ± 0.20 | 3.04 b ± 0.17 | 1.53 b ± 0.14 | 58.58 b ± 5.11 | |
| F-ratio | Treatments | 11.478 * | 0.625 | 70.611 * | 86.976 * | 82.725 * | 12.843 * |
| Seasons | 609.151 * | 5.565 * | 114.852 * | 80.687 * | 76.661 * | 4.360 | |
| Interaction | 87.075 * | 0.328 | 24.674 * | 12.653 * | 5.112 * | 0.617 |
| Summer | ||||||||
|---|---|---|---|---|---|---|---|---|
| MT | HT | CC | CTR | |||||
| Ab | Ab (%) | Ab | Ab (%) | Ab | Ab (%) | Ab | Ab (%) | |
| Acarina | 10,667 c ± 558 | 52.52 | 15,833 a ± 721 | 44.49 | 9417 d ± 65 | 71.56 | 13,417 b ± 172 | 62.46 |
| Araneidae | 333 b ± 18 | 1.64 | 833 a ± 79 | 2.34 | 0 | 0.00 | 250 b ± 9 | 1.16 |
| Coleoptera | 250 b ± 32 | 1.23 | 833 a ± 29 | 2.34 | 250 b ± 11 | 1.90 | 50 c ± 86 | 0.22 |
| Collembola | 7833 b ± 388 | 38.57 | 10,583 a ± 399 | 29.75 | 2500 d ± 84 | 18.99 | 5167 c ± 184 | 24.04 |
| Diplopoda | 250 b ± 16 | 1.23 | 500 a ± 37 | 1.40 | 30 d ± 51 | 0.22 | 116 c,d ± 87 | 0.53 |
| Diplura | 116 b ± 101 | 0.55 | 667 a ± 90 | 1.87 | 0 | 0.00 | 115 b ± 99 | 0.52 |
| Diptera | 171 d ± 118 | 0.81 | 2083 a ± 111 | 5.85 | 333 c ± 98 | 2.53 | 1167 b ± 105 | 5.43 |
| Hemiptera | 51 c ± 38 | 0.23 | 583 a ± 121 | 1.63 | 167 b,c ± 119 | 1.25 | 117 c ± 82 | 0.53 |
| Hymenoptera | 250 d ± 84 | 1.23 | 1000 a ± 148 | 2.81 | 417 c ± 87 | 3.17 | 667 b ± 124 | 3.10 |
| Isopoda | 79 a ± 35 | 0.36 | 53 a ± 19 | 0.15 | 0 | 0.00 | 51 a ± 20 | 0.23 |
| Protura | 333 b ± 102 | 1.64 | 750 a ± 145 | 2.11 | 53 c ± 26 | 0.39 | 52 c ± 33 | 0.23 |
| Pseudoscorpionida | 0 | 0.00 | 1833 a ± 124 | 5.16 | 0 | 0.00 | 333 b ± 97 | 1.55 |
| Symphyla | 0 | 0.00 | 30 ± 17 | 0.09 | 0 | 0.00 | 0 | 0.00 |
| Total | 20,333 | 100.00 | 35,581 | 100.00 | 13,167 | 100.00 | 21,502 | 100.00 |
| Winter | ||||||||
|---|---|---|---|---|---|---|---|---|
| MT | HT | CC | CTR | |||||
| Ab | Ab (%) | Ab | Ab (%) | Ab | Ab (%) | Ab | Ab (%) | |
| Acarina | 25,417 a ± 259 | 59.76 | 19,250 b ± 272 | 36.62 | 24,333 a ± 294 | 70.79 | 25,083 a ± 248 | 63.98 |
| Araneidae | 667 b ± 142 | 1.57 | 2083 a ± 214 | 3.96 | 208 c ± 124 | 0.60 | 83 c ± 43 | 0.21 |
| Chilopoda | 0 | 0.00 | 82 ± 32 | 0.15 | 0 | 0.00 | 0 | 0.00 |
| Coleoptera | 1167 b ± 174 | 2.59 | 2000 a ± 207 | 3.80 | 28 d ± 12 | 0.08 | 750 c ± 142 | 1.91 |
| Collembola | 9333 b ± 1142 | 22.07 | 16,167 a ± 1537 | 30.75 | 4667 d ± 942 | 13.58 | 6250 c ± 1021 | 15.94 |
| Diplopoda | 833 b ± 314 | 1.97 | 168 c ± 48 | 0.32 | 1333 a ± 523 | 3.88 | 167 c ± 51 | 0.43 |
| Diplura | 1417 a ± 231 | 3.35 | 1500 a ± 227 | 2.85 | 28 c ± 10 | 0.08 | 167 b ± 46 | 0.43 |
| Diptera | 2417 b ± 745 | 5.54 | 4583 a ± 974 | 8.72 | 1833 c ± 428 | 5.33 | 2417 b ± 681 | 6.16 |
| Hemiptera | 28 b ± 7 | 0.07 | 417 a ± 74 | 0.79 | 0 | 0.00 | 0 | 0.00 |
| Hymenoptera | 1083 c ± 179 | 2.54 | 2167 a ± 211 | 4.12 | 674 d ± 107 | 1.97 | 1417 b ± 183 | 3.61 |
| Isopoda | 167 b ± 62 | 0.39 | 417 a ± 183 | 0.79 | 119 c ± 54 | 0.35 | 124 c ± 53 | 0.32 |
| Protura | 28 d ± 12 | 0.07 | 1667 b ± 61 | 3.17 | 1159 c ± 324 | 3.35 | 1917 a ± 421 | 4.89 |
| Pseudoscorpionida | 30 c ± 17 | 0.08 | 1583 a ± 374 | 3.01 | 0 | 0.00 | 833 b ± 211 | 2.12 |
| Psocoptera | 0 | 0.00 | 115 ± 55 | 0.22 | 0 | 0.00 | 0 | 0.00 |
| Symphyla | 0 | 0.00 | 152 ± 42 | 0.09 | 0 | 0.00 | 0 | 0.00 |
| Thysanoptera | 0 | 0.00 | 333 ± 89 | 0.63 | 0 | 0.00 | 0 | 0.00 |
| Total | 42,586 | 100.00 | 52,583 | 100.00 | 34,381 | 100.00 | 39,208 | 100.00 |
| F-Ratio | |||
|---|---|---|---|
| Treatments | Seasons | Interaction | |
| Acarina | 2.967 | 871.066 * | 57.942 * |
| Araneidae | 418.725 * | 193.862 * | 83.547 * |
| Chilopoda | 0.921 | 0.882 | 0.921 |
| Coleoptera | 1.613 | 1.677 | 0.988 |
| Collembola | 3.345 | 0.029 | 1.696 |
| Diplopoda | 3.638 * | 4.735 * | 3.139 |
| Diplura | 8.040 * | 6.664 * | 6.868 * |
| Diptera | 2.167 | 17.107 * | 2.057 |
| Hemiptera | 6.525 * | 2.472 | 3.589 * |
| Hymenoptera | 1.203 | 5.387 * | 2.599 |
| Isopoda | 5.792 * | 8.116 * | 5.611 * |
| Protura | 5.135 * | 25.827 * | 11.417 * |
| Pseudoscorpionida | 5519.388 * | 61.935 * | 587.760 * |
| Psocoptera | 5.270 * | 5.049 * | 5.270 * |
| Raphidioptera | 0.921 | 0.882 | 0.921 |
| Symphyla | 1.729 | 0.109 | 0.113 |
| Thysanoptera | 531.951 * | 509.600 * | 531.951 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muscolo, A.; Settineri, G.; Romeo, F.; Mallamaci, C. Soil Biodiversity as Affected by Different Thinning Intensities in a Pinus laricio Stand of Calabrian Apennine, South Italy. Forests 2021, 12, 108. https://doi.org/10.3390/f12010108
Muscolo A, Settineri G, Romeo F, Mallamaci C. Soil Biodiversity as Affected by Different Thinning Intensities in a Pinus laricio Stand of Calabrian Apennine, South Italy. Forests. 2021; 12(1):108. https://doi.org/10.3390/f12010108
Chicago/Turabian StyleMuscolo, Adele, Giovanna Settineri, Federico Romeo, and Carmelo Mallamaci. 2021. "Soil Biodiversity as Affected by Different Thinning Intensities in a Pinus laricio Stand of Calabrian Apennine, South Italy" Forests 12, no. 1: 108. https://doi.org/10.3390/f12010108
APA StyleMuscolo, A., Settineri, G., Romeo, F., & Mallamaci, C. (2021). Soil Biodiversity as Affected by Different Thinning Intensities in a Pinus laricio Stand of Calabrian Apennine, South Italy. Forests, 12(1), 108. https://doi.org/10.3390/f12010108